Abstract
Osteocalcin is secreted by osteoblasts and improves insulin sensitivity in vivo, although mechanisms remain unclear. We tested the hypothesis that osteocalcin directly modulates cell biology in insulin-targeted peripheral tissues. In L-6 myocytes, osteocalcin stimulated glucose transport both in the absence (basal) and presence of insulin. Similarly, in primary cultured adipocytes, both carboxylated and uncarboxylated osteocalcin increased basal and insulin-stimulated glucose transport as well as insulin sensitivity. Osteocalcin also increased basal and insulin-stimulated glucose oxidation, though there was no effect on fatty acid synthesis or lipolysis. In primary-cultured adipocytes, both forms of osteocalcin suppressed secretion of tumor necrosis factor alpha into the media; however, only carboxylated osteocalcin suppressed interleukin 6 release, and neither form of osteocalcin modulated monocyte chemoattractant protein-1 secretion. Both carboxylated and uncarboxylated osteocalcin increased secretion of adiponectin and the anti-inflammatory cytokine interleukin 10. In conclusion, both carboxylated and uncarboxylated osteocalcin directly increase glucose transport in adipocytes and muscle cells, while suppressing proinflammatory cytokine secretion and stimulating interleukin 10 and adiponectin release. Thus, these results provide a mechanism for the insulin-sensitizing effects of osteocalcin and help elucidate the role that bone plays in regulating systemic metabolism.
Keywords: glucose homeostasis, metabolism, adipocyte biology
Introduction
A connection between the skeleton and glucose metabolism is an emerging area of intense scientific scrutiny. Recent studies have indicated that the osteoblast-derived protein, osteocalcin, regulates energy metabolism, providing a link between these 2 seemingly unrelated systems. However, processes by which osteocalcin regulates systemic metabolism are not well understood and studies to elucidate the underlying mechanisms are limited.
In 2007, Lee et al. reported that osteocalcin-deficient mice were glucose intolerant, insulin resistant, obesity prone, and characterized by decreased secretion of insulin and adiponectin [1]. Although the mechanisms responsible for these systemic effects on metabolism have not been fully elucidated, a number of subsequent studies have consistently demonstrated a relationship between osteocalcin and glucose homeostasis. For example, consistent with observations in osteocalcin null mice, a mouse model lacking Foxo1 in osteoblasts displayed an increase in osteocalcin coupled with enhanced insulin secretion and action, increased adiponectin secretion, decreased fat mass, and protection from diet-induced obesity [2]. These metabolic effects were shown to be dependent upon the increase in osteocalcin, resulting from the loss of Foxo1-mediated suppression of osteocalcin expression. In addition, osteocalcin administration to wild type mice was observed to regulate glucose metabolism and fat mass, both in vivo and in vitro via increased expression of insulin and markers of pancreatic β-cell proliferation, as well as upregulation of adiponectin and PGC-1α in adipose tissue [3]. Low levels of serum osteocalcin have been reported in autoimmune nonobese diabetic mice and streptozotocin-induced diabetic mice [4], and, importantly, circulating osteocalcin levels are decreased in human patients with type 2 diabetes and insulin resistance [5] [6] [7] [8]. While these results consistently demonstrate an association between osteocalcin and glucose homeostasis, the mechanisms that explain these relationships have not been clarified.
The potential metabolic actions of osteocalcin are further complicated in that osteocalcin undergoes post-translational modifications that could differentially affect its bioavailability and bioactivity. Osteocalcin is post-translationally modified by vitamin K-dependent carboxylation of 3 glutamate residues into high calcium-affinity Gla residues. Fully carboxylated osteocalcin (cOC) as well as uncarboxylated or undercarboxylated osteocalcin (ucOC), which is carboxylated at less than 3 sites, are all present in the circulation. While there are reports that ucOC, but not cOC, is responsible for the metabolic actions of osteocalcin [1] [2] [3] [9], the question of whether ucOC and cOC exert differential effects has not been rigorously addressed in cell culture systems assessing direct actions of both osteocalcin isoforms.
Alterations in muscle and adipose tissue function, including insulin resistance, inflammation, and dysregulated cytokine secretion, appear prior to and are pivotal in the development of type 2 diabetes. In the current study, we have examined whether osteocalcin directly modulates these critical aspects of insulin-targeted peripheral tissues in order to assess a potential role for osteocalcin in the development of metabolic disease. Here we report that osteocalcin has a direct effect on myocytes and adipocytes to regulate the insulin-responsive glucose transport system. In adipocytes, osteocalcin promotes the secretion of proinflammatory and anti-inflammatory cytokines, and the production of adiponectin. Further, our results demonstrate that both cOC and ucOC are active and directly modulate insulin sensitivity in vitro.
Materials and Methods
Experimental animals
Ten week old male C57BL/6 J mice and 150–180 g male Wistar rats were purchased from Harlan (Indianapolis, Indiana). Animals were housed in standard conditions (12-h light/dark cycle; 22°C) and fed a standard diet. Animal care and treatments were approved by the UAB Institutional Animal Use and Care Committee.
Materials
DMEM was purchased from Invitrogen (Carlsbad, CA). Tumor necrosis factor alpha (TNFα, interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and interleukin 10 (IL-10) ELISA kits were obtained from BD Biosciences (San Jose, CA). Adiponectin radioimmunassay kit was obtained from Linco Research, Inc (St. Charles, MO). 2-Deoxy-d-[2,6-3H]glucose (2-DOG), and d-[U-14C]glucose were acquired from Amersham (Uppsala, Sweden).
Osteocalcin
cOC and ucOC were kind gifts from Dr. Caren M. Gundberg, Yale University School of Medicine. cOC was purified from mouse and cow bone by demineralization, gel filtration, ion exchange, and high pressure chromatography to homogeneity as previously described [10]. Purity was confirmed by electrospray mass spectroscopy and N-terminal sequencing. Concentration was determined by amino acid analysis of 2 separate samples from the stock material. ucOC was synthesized at the Keck Facility, Yale University. Purity was confirmed by mass spectrometry and concentration by amino acid analysis as above. Protein was stored at −80 °C. For reference, the molecular weight of carboxylated vs. uncarboxylated bovine osteocalcin is 5 850 Da and 5 731 Da, respectively, and for mouse osteocalcin 5 249 Da and 5 117 Da, respectively.
Adipocyte isolation
Adipocytes were isolated from periepididymal fat from rats or mice by collagenase digestion [11]. Minced fat pads were digested with EHB1 (Earle’s salts, 25 mM HEPES, 4% bovine serum albumin, 5 mM glucose) and 1.25 mg/mL type II collagenase at 37 °C for 30 min with gentle orbital shaking. Cells were filtered through nylon mesh (400 μm), washed 3 times with EHB2 (Earle’s salts, 20 mM HEPES, 1% bovine serum albumin, 2 mM sodium pyruvate, and 4.8 mM NaHCO3), and resuspended to a final 5% (vol/vol) cell concentration. Adipocyte viability was determined by trypan blue exclusion as described [11] and cell sizing was performed by microscopic measurement of cell diameter.
Effects of Osteocalcin on Glucose Transport Activity
Glucose transport assay
Measurements of 2-deoxyglucose uptake were performed as previously described [12]. Briefly, isolated adipocytes at a 5% (vol/vol) concentration were pre-incubated with 1 ng/ml osteocalcin for 1 h, followed by incubation for 30 min±insulin at indicated concentrations. Cells were pulsed with [3H]-2-deoxyglucose (0.2 μCi/tube, 0.1 mM final concentration). The reaction was stopped after 3 min by centrifuging 300 μl of cells per reaction through silicone for 30 s at 14 000×g. Each sample was assayed in duplicate. Radiolabeled l-glucose was used to determine the rate of glucose entry into cells by simple diffusion. Glucose uptake was measured by liquid scintillation counting. Values were normalized by cell surface area.
Osteocalcin dose-response
The osteocalcin dose-response was determined by pre-incubating isolated rat adipocytes with 0–50 ng/ml bovine cOC for 1 h, followed by treatment with ± 10 nM insulin for 30 min.
Insulin dose-response
Isolated rat adipocytes were pre-incubated with 1 ng/ml bovine cOC for 1 h, followed by treatment with 0–10 nM insulin for 30 min.
L6 muscle cells
L6 muscle cells were obtained from ATCC (Manassas, VA, USA). L6 myoblasts stably expressing Glut-4 with an exofacial myc epitope (L6-Glut4myc) were a kind gift from Dr. A. Klip, University of Toronto Hospital for Sick Children [13]. Glucose transport assays in L6 cells were performed as previously described [14]. Briefly, L6-Glut4myc cells were pre-treated with 20 ng/ml cOC for 1 h and then incubated with±100 nM insulin for 30 min at 37°C to measure basal and maximally-stimulated glucose transport rates. Cell-associated radioactivity was determined by lysing the cells with 0.05 N NaOH, followed by liquid scintillation counting.
Incorporation of d-[U-14C]glucose into lipids and oxidation to 14CO2
A 5% (vol/vol) adipocyte suspension was prepared in Krebs–Ringer phosphate buffer (pH 7.4) containing 1% bovine serum albumin and 5 mM glucose, incubated with 20 ng/ml of osteocalcin for 1 h, and then saturated with carbogen gas (95% O2, 5% CO2). Next, 450 μl aliquots were added to 25 μl of d-[U-14C]glucose (0.1 μCi/tube) and 2.0 mM of d-glucose±10 nmol/l insulin, and incubated for 1 h at 37 °C with gentle orbital shaking. At the end of incubation, a 0.5 ml Eppendorf tube containing a small, loosely folded piece of filter paper (2×2.5 cm) moistened with 0.2 ml of 2-phenylethylamine/methanol (1:1, vol/vol) was suspended in the center of the reaction tube. The incubation medium was acidified with 0.2 ml of 8 N H2SO4 to lyse cells and release 14CO2. After 1 h, the filter paper was transferred into scintillation vials and counted. The remaining reaction mixture was treated with 5 ml of Dole’s reagent (isopropanol/n-heptane/H2SO4, 4:1:0.25, vol/vol/vol) for lipid extraction [15], and counts in the lipid phase represented glucose incorporation into lipid, that is, lipogenesis. Oxidation and lipogenesis were calculated from the known concentration and specific activity of d-glucose.
Lipolysis and anti-lipolysis assay
A 5% (vol/vol) adipocyte suspension was prepared, and aliquots were incubated with 20 ng/ml osteocalcin for 1 h. The reactions were treated with adenosine deaminase (ADA) for 5 min, and treated for 1 h with either (i) 10 μM isoproterenol, a lipolytic agent and nonselective β-adrenoreceptor agonist, (ii) 0.5 nM insulin, an anti-lipolytic hormone, (iii) both insulin and isoproterenol, or (iv) buffer only for the basal control. The tubes were centrifuged at 3 000×g for 10 min and the infranatant was collected and used for glycerol measurement (Free Glycerol Determination Kit, Sigma, St. Louis, MO, USA) as a lipolytic index.
Adipokine measurements in cultured cells and tissue
Primary cultured isolated adipocytes and whole organ adipose tissue from Wistar rats were incubated±20 ng/ml cOC or ucOC for 1 h at 37 °C with 5% CO2. Adipocytes were isolated as described, and 5×105 cells/ml were incubated in 2 ml of culture media (EHB2). The media was collected and stored at −20 °C for subsequent adipokine measurements. Whole tissue culture was performed as previously described [16]. Briefly, peri-epididymal fat was excised and placed in sterile DMEM containing 20 mM HEPES pH 7.4, 5 mM glucose, 1% (w/vol) bovine serum albumin, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Tissue was trimmed of visible vessels and blood clots, minced into 2–4 mm3 pieces, mixed gently and distributed into 125 ml Erlenmeyer flasks containing culture media at a proportion of 1 ml/100 mg fat. Adipokine assays were performed according to manufacturer protocols.
Statistical analysis
Statistical analyses were performed (GraphPad Prism; GraphPad, Inc.) using Student’s t-test when 2 conditions were compared and one-way ANOVA followed by Bonferroni post-hoc test for multiple comparisons. Results are represented as mean±SEM unless stated otherwise, and the total number of independent experiments, as well as p-values, are specified in each figure legend. p-Values less than 0.05 were considered significant.
Results
Carboxylated (cOC) and uncarboxylated (ucOC) osteocalcin enhance adipocyte glucose transport and insulin sensitivity
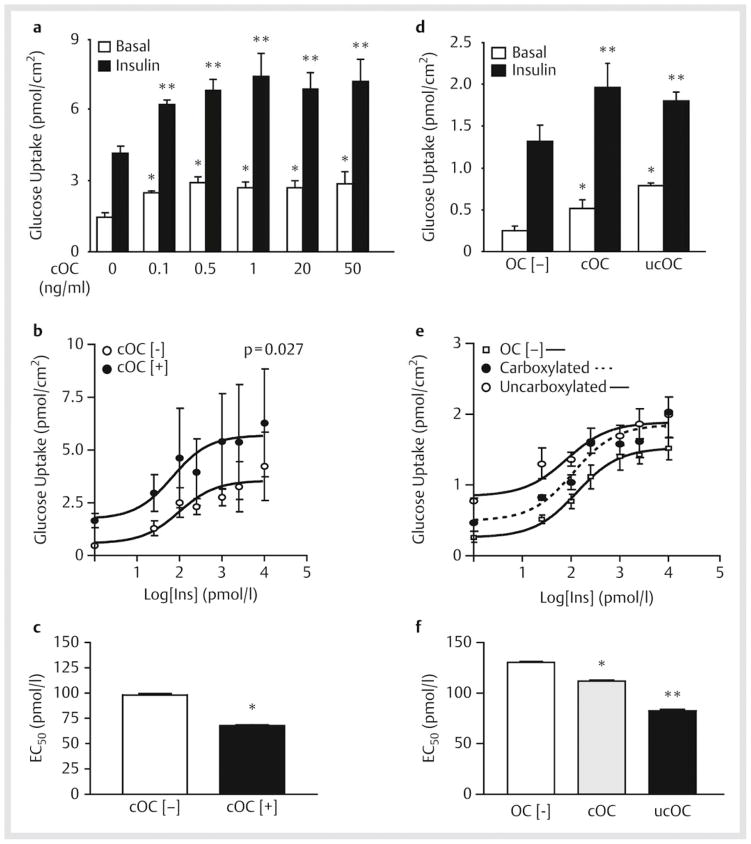
We first examined whether pretreatment with bovine cOC at increasing concentrations could modulate basal and insulin-stimulated glucose transport activity in primary cultured rat adipocytes. As shown in [Fig. 1a], cOC significantly increased both basal and maximal insulin-stimulated glucose transport activity. This effect was dose-dependent and achieved at even the lowest concentration of osteocalcin assayed (0.1 ng/ml), greater than 100-fold lower than circulating levels in wild-type animals [17]. The maximal effect was achieved at 1 ng/ml of cOC which produced a 102% increase in the basal glucose transport rate (1.45±0.18 vs. 2.93±0.19 pmol/cm2; p<0.05) and a 77% increase in the insulin-stimulated rate (4.19±0.24 vs. 7.42±0.98 pmol/cm2). We further determined the effect of cOC on the dose-response curve for insulin-stimulated glucose transport ([Fig. 1b, c]). Pre-incubation with cOC increased insulin sensitivity demonstrated by a leftward shift in the dose-response curve. The insulin EC50 was decreased by 31% when cells were pre-incubated with cOC (98.3±0.53 vs. 67.9±1.14 pmol/l; p<0.001). Taken together, these data indicate that cOC increases basal and insulin-stimulated glucose transport rates and increases insulin sensitivity in adipocytes.
Fig. 1.
Effects of osteocalcin on glucose transport and insulin sensitivity. a Isolated adipocytes were treated with bovine carboxylated osteocalcin (cOC) at the indicated concentrations, stimulated in the absence (basal) or presence (insulin) of a maximally-effective insulin concentration (10 nM), and then [3 H]-2-deoxyglucose transport was measured. Data represent the means of results from 3 experiments with triplicate determinations in each experiment. *p<0.05, significantly different from corresponding basal control value in cells not treated with cOC; **p<0.05, significantly different from corresponding insulin-stimulated value in cells not treated with cOC. b Isolated adipocytes treated with cOC were stimulated with varying concentrations of insulin to generate a dose-response curve. Data represent the means of results from 3 experiments with triplicate determinations in each experiment, p=0.027. c EC50 from insulin dose-response curves, *p<0.001. d Glucose transport using isolated adipocytes treated with murine cOC or uncarboxylated osteocalcin (ucOC) and then stimulated in the absence or presence of 10 nM insulin. Data represent the means of results from 5 experiments. *p<0.05, significantly different from basal OC [−]; **p=0.02, significantly different from insulin OC [−] e Isolated adipocytes treated with cOC or ucOC were stimulated with insulin at the indicated doses to generate a dose-response curve. Data represent the mean of results from 4 experiments each performed in triplicate. f EC50 from insulin dose-response curves. * and **p<0.001, significantly different from OC [−].
Zoom ImageZoom Image Fig. 1 Effects of osteocalcin on glucose transport and insulin sensitivity. a Isolated adipocytes were treated with bovine carboxylated osteocalcin (cOC) at the indicated concentrations, stimulated in the absence (basal) or presence (insulin) of a maximally-effective insulin concentration (10 nM), and then [3 H]-2-deoxyglucose transport was measured. Data represent the means of results from 3 experiments with triplicate determinations in each experiment. *p<0.05, significantly different from corresponding basal control value in cells not treated with cOC; **p<0.05, significantly different from corresponding insulin-stimulated value in cells not treated with cOC. b Isolated adipocytes treated with cOC were stimulated with varying concentrations of insulin to generate a dose-response curve. Data represent the means of results from 3 experiments with triplicate determinations in each experiment, p=0.027. c EC50 from insulin dose-response curves, *p<0.001. d Glucose transport using isolated adipocytes treated with murine cOC or uncarboxylated osteocalcin (ucOC) and then stimulated in the absence or presence of 10 nM insulin. Data represent the means of results from 5 experiments. *p<0.05, significantly different from basal OC [−]; **p=0.02, significantly different from insulin OC [−] e Isolated adipocytes treated with cOC or ucOC were stimulated with insulin at the indicated doses to generate a dose-response curve. Data represent the mean of results from 4 experiments each performed in triplicate. f EC50 from insulin dose-response curves. * and **p<0.001, significantly different from OC [−].
In the experiments described above, bovine cOC was used to treat rat adipocytes. To determine whether the effects were species-specific and whether carboxylation of osteocalcin was required for the observed bioeffects, mouse adipocytes were treated with 1 ng/ml murine cOC or ucOC. Pre-incubation with both forms of osteocalcin significantly increased both basal and maximal insulin-stimulated glucose transport, again shifting the insulin dose-response curve to the left ([Fig. 1d–f]). However, ucOC was more potent than cOC in increasing both basal transport in the absence of insulin as well as insulin sensitivity manifested as a more pronounced lowering of the insulin EC50 (82.6±0.16 pmol/l vs. 111.9±0.17 pmol/l, respectively; p<0.01).
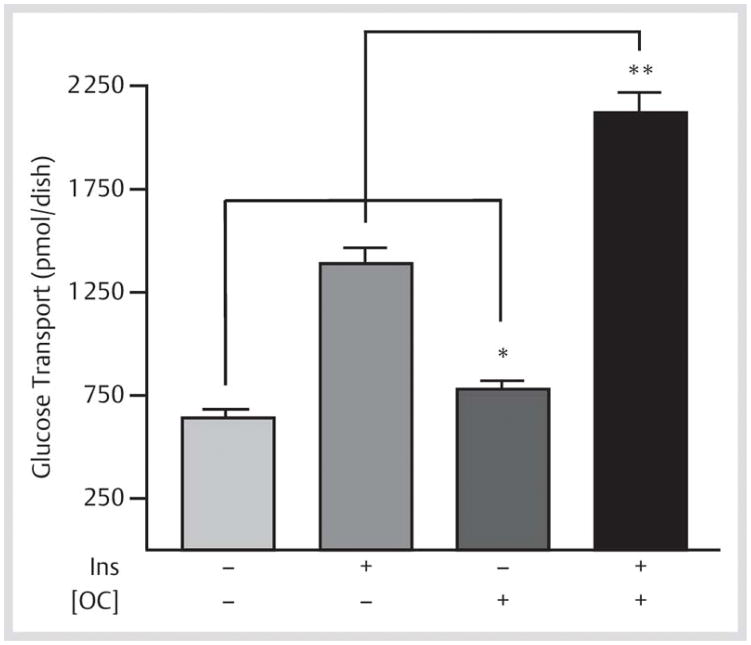
Since glucose transport is the rate-limiting factor for insulin-mediated glucose sensitivity in muscle, we tested for the direct effects of osteocalcin in differentiated L6 skeletal myocytes ([Fig. 2]). Pre-treatment with bovine cOC stimulated basal glucose transport 1.2-fold when compared with controls (782±40.6 pmol/dish vs. 640±40.6 pmol/dish, respectively; p=0.047). Osteocalcin also enhanced maximal insulin-stimulated glucose transport rates to a level that was 53% greater than observed with insulin alone (2 121±97 pmol/dish vs. 1 389±76.2 pmol/dish, respectively; p=0.0001).
Fig. 2.
Osteocalcin enhances glucose transport activity in L6 skeletal muscle cells. Differentiated L6 myotubes (L6-Glut4myc cells) were pre-treated with bovine carboxylated osteocalcin (cOC) and then±100 nM insulin to determine basal and maximally-stimulated glucose transport rates. Results are mean±SEM. *p=0.047; **p=0.0001 (n=5).
Zoom ImageZoom Image Fig. 2 Osteocalcin enhances glucose transport activity in L6 skeletal muscle cells. Differentiated L6 myotubes (L6-Glut4myc cells) were pre-treated with bovine carboxylated osteocalcin (cOC) and then±100 nM insulin to determine basal and maximally-stimulated glucose transport rates. Results are mean±SEM. *p=0.047; **p=0.0001 (n=5).
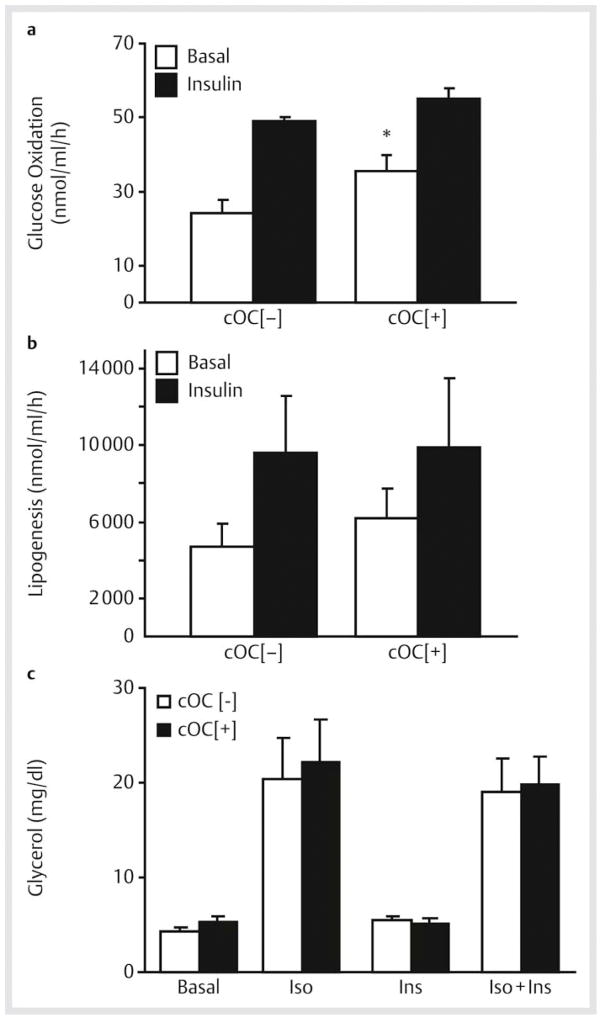
Osteocalcin increases glucose oxidation in adipocytes, but does not effect lipogenesis, lipolysis, or anti-lipolysis
Using primary rat cultured adipocytes, we assessed the effects of cOC on intracellular substrate metabolism downstream of glucose transport. Treatment with cOC significantly increased basal glucose oxidation compared with control (35.5±4.4 vs. 24.3±3.4 nmol/ml/h, respectively; p=0.01) with no effects on insulin-stimulated glucose oxidation ([Fig. 3a]). In contrast, cOC had no effect on basal or insulin-stimulated lipogenesis, that is, glucose incorporation into lipids ([Fig. 3b]). Rates of lipolysis and the anti-lipolytic effect of insulin were also assessed, since these processes are important determinants of cellular lipid content and fat cell size. Lipolysis was measured as the ability of isoproterenol, a non-selective β-adrenoreceptor agonist, to promote cellular release of glycerol from triacylglycerol breakdown. When compared with control cells, the basal lipolytic response was not altered by treatment with cOC, nor was isoproterenol-induced lipolysis. Similarly, cOC did not influence the ability of insulin to inhibit isoproterenol-induced lipolysis, that is, anti-lipolysis ([Fig. 3c]).
Fig. 3.
Effects of carboxylated osteocalcin (cOC) on basal and insulin-stimulated rates of glucose oxidation, lipogenesis, lipolysis and anti-lipolysis in rat adipocytes. Primary cultured rat adipocytes were incubated±cOC, followed by±10 nM insulin. a Rates of glucose oxidation were assessed as 14C glucose label released as 14CO2. *p=0.01 compared with basal without cOC treatment. b Rates of lipogenesis were assessed as 14C glucose label incorporated into lipids. Data from a, b represent the mean ± SEM of results from 3 experiments with each experiment performed in triplicate. c Lipolysis and insulin-mediated anti-lipolysis were measured by additional incubation of cOC-treated cells in the presence of 10 μM isoproterenol (Iso) alone, 0.5 nM insulin (Ins) alone, or isoproterenol + insulin (Iso + Ins). Rates of lipolysis were assessed as the cellular release of glycerol reflecting deacylation of triglyceride. Data represent the mean ± SEM of results from 8 experiments. For a–c, open bars represent no osteocalcin treatment (cOC [−]) and black bars represent osteocalcin-treated cells (cOC [+]).
Zoom ImageZoom Image Fig. 3 Effects of carboxylated osteocalcin (cOC) on basal and insulin-stimulated rates of glucose oxidation, lipogenesis, lipolysis and anti-lipolysis in rat adipocytes. Primary cultured rat adipocytes were incubated±cOC, followed by±10 nM insulin. a Rates of glucose oxidation were assessed as 14C glucose label released as 14CO2. *p=0.01 compared with basal without cOC treatment. b Rates of lipogenesis were assessed as 14C glucose label incorporated into lipids. Data from a, b represent the mean ± SEM of results from 3 experiments with each experiment performed in triplicate. c Lipolysis and insulin-mediated anti-lipolysis were measured by additional incubation of cOC-treated cells in the presence of 10 μM isoproterenol (Iso) alone, 0.5 nM insulin (Ins) alone, or isoproterenol + insulin (Iso + Ins). Rates of lipolysis were assessed as the cellular release of glycerol reflecting deacylation of triglyceride. Data represent the mean ± SEM of results from 8 experiments. For a–c, open bars represent no osteocalcin treatment (cOC [−]) and black bars represent osteocalcin-treated cells (cOC [+]).
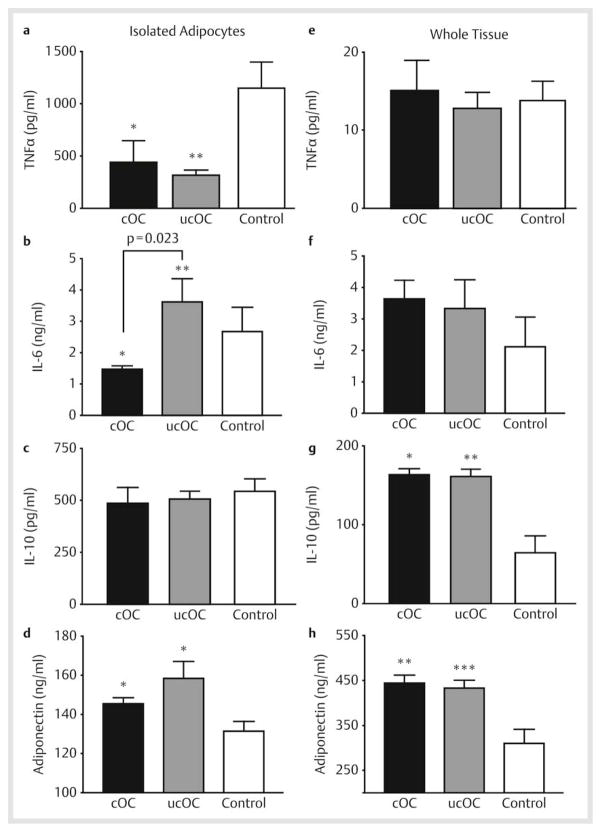
Osteocalcin directly modulates adipocytokine production
To better understand the mechanism for the insulin-sensitizing effects of osteocalcin in vivo, we assessed whether cOC and ucOC were able to alter adipokine secretion in both primary-cultured isolated rat adipocytes and in whole organ adipose tissue culture. We assessed the pro-inflammatory cytokines TNFα, IL-6, and MCP-1 and the anti-inflammatory cytokine, IL-10. In isolated adipocytes, both cOC and ucOC decreased TNFα secretion by 62% and 72%, respectively (p<0.05) ([Fig. 4a]). IL-6 was differentially affected by osteocalcin treatment, with cOC significantly decreasing IL-6 secretion in isolated adipocytes while ucOC had no effect ([Fig. 4b]). In contrast, MCP-1 was not affected by treatment with either form of osteocalcin (data not shown). Neither form of osteocalcin modulated the secretion of pro-inflammatory cytokines in whole tissue ([Fig. 4e, f], data not shown). Interestingly, although neither form of osteocalcin affected secretion of the anti-inflammatory cytokine IL-10 in isolated adipocytes, both forms resulted in significantly increased secretion from whole tissue ([Fig. 4c, g]).
Fig. 4.
Carboxylated (cOC) and uncarboxylated (ucOC) osteocalcin act differentially to modulate adipokine secretion. Primary cultured rat adipocytes and whole organ adipose tissue were incubated with cOC or ucOC, the culture media was then collected and assayed for the pro-inflammatory cytokines a, e TNFα, *p<0.05, **p=0.024 and b, f IL-6, *p<0.05, **p=0.023, indicates significance between results using cOC vs. ucOC, c, g the anti-inflammatory cytokine IL-10, *p<0.05, **p=0.015, and d, h the insulin-sensitizing hormone adiponectin, *p<0.05, **p=0.006; ***p=0.008. Results are the mean of 3 experiments ± SEM each performed in triplicate samples. All p-values are vs. control except where indicated.
Zoom ImageZoom Image Fig. 4 Carboxylated (cOC) and uncarboxylated (ucOC) osteocalcin act differentially to modulate adipokine secretion. Primary cultured rat adipocytes and whole organ adipose tissue were incubated with cOC or ucOC, the culture media was then collected and assayed for the pro-inflammatory cytokines a, e TNFα, *p<0.05, **p=0.024 and b, f IL-6, *p<0.05, **p=0.023, indicates significance between results using cOC vs. ucOC, c, g the anti-inflammatory cytokine IL-10, *p<0.05, **p=0.015, and d, h the insulin-sensitizing hormone adiponectin, *p<0.05, **p=0.006; ***p=0.008. Results are the mean of 3 experiments ± SEM each performed in triplicate samples. All p-values are vs. control except where indicated.
We also investigated whether osteocalcin has a direct ability to regulate adiponectin secretion, since this hormone has been shown to play a crucial role in the regulation of metabolism and insulin sensitivity and may mediate the insulin-sensitizing effects of osteocalcin in intact mice [1]. Adiponectin secretion was significantly increased by both cOC and ucOC in both isolated adipocytes and whole tissue ([Fig. 4d, h]). Thus, isolated adipocytes and adipose tissue respond differentially to the different forms of osteocalcin. However, overall, both cOC and ucOC act directly to decrease proinflammatory cytokine secretion while increasing the secretion of the anti-inflammatory cytokine IL-10 and the insulin-sensitizing hormone adiponectin.
Discussion and Conclusions
Several studies support the contention that osteocalcin plays an important role in glucose homeostasis and insulin sensitivity. First, null mutant mice in osteocalcin are glucose intolerant and insulin resistant [1]. Second, osteocalcin expression was increased in a mouse model lacking Foxo1 in osteoblasts that displayed enhanced insulin secretion and action, decreased fat mass, and protection from diet-induced obesity [2]. Third, osteocalcin administration to wild type mice was observed to regulate glucose metabolism and fat mass via increased expression of insulin, markers of pancreatic β-cell proliferation, and adiponectin and PGC-1α in adipose tissue [3]. Further, serum levels of osteocalcin are inversely correlated with insulin resistance in both a mouse model and in humans [4] [5] [6] [7] [8]. However, the underlying molecular mechanisms of action largely remain unknown.
Here, we tested the hypothesis that osteocalcin has a direct effect on peripheral insulin target tissues, specifically muscle and adipose tissue. Our results demonstrate that osteocalcin increases basal and insulin-stimulated glucose transport in cultured myocytes and isolated adipocytes. In adipocytes, osteocalcin also increases basal and insulin-stimulated glucose oxidation rates and lowers the corresponding ED50 for this insulin action. Further, osteocalcin increases secretion of the anti-inflammatory adipokine IL-10 and the insulin-sensitizing hormone adiponectin, while decreasing secretion of the pro-inflammatory adipokines TNFα and IL-6. Taken together, these results demonstrate that osteocalcin acts directly on peripheral tissues to regulate glucose metabolism, insulin sensitivity, and adipokine secretion.
Recent data suggests that these protective effects of osteocalcin may in part be mediated by its ability to decrease ER stress in liver, muscle, and adipose tissue in vivo and in vitro [18]. In this study, mice fed a high fat diet with osteocalcin showed, 1) an increased expression of insulin target genes, 2) an increased number and area of mitochondria, 3) decreased phosphorylation or expression of ER stress proteins, and 4) increased phosphorylation of IRS-1 and Akt in all 3 tissues when compared to mice fed a high fat diet without osteocalcin. Further, in vitro studies using the Fao liver cell, L6 muscle cell, and 3T3-L1 adipocyte cell lines also demonstrated decreased phosphorylation or expression of ER stress proteins and this effect was mediated by the PI3K/Akt/NF-kB signaling pathway. Our data would be consistent with these findings and further studies are needed to clarify.
It is well known that osteocalcin undergoes post-translational modifications that could differentially affect its bioactivity and bioavailability. Currently, there is no consensus regarding which is the biologically active form of osteocalcin, with previous reports suggesting that it is either the uncarboxylated form [1] [3] [19] [20], the carboxylated form [6] [21], or the ratio of the 2 or total osteocalcin [5] [7] [22] that is biologically active in metabolism. It has been proposed that the conflicting reports may be species dependent with ucOC playing a role in rodent models vs. cOC in humans, or that they may exert differential effects in glucose metabolism with ucOC affecting β cell function and cOC affecting insulin sensitivity [21]. However, a recent study by Brennan-Speranza et al. demonstrated that the effects of the uncarboxylated and carboxylated forms of osteocalcin were similar in an in vivo mouse model [23]. Heterotopic expression of both forms of osteocalcin reduced the effects of glucocorticoid treatment in similar fashion. Both forms resulted in an increase in lean mass, decrease in fat mass, and decrease in serum triglycerides and cholesterol; improved glucocorticoid-induced insulin resistance and glucose tolerance; decreased lipid deposition in the liver and increased insulin receptor phosphorylation. Our results are consistent with these findings, demonstrating that cOC and ucOC are both able to directly regulate aspects of myocyte and adipocyte biology. Even so, while we have demonstrated that both osteocalcin forms are bioactive, carboxylated and uncarboxylated osteocalcin exert differential effects with respect to potency and specific cellular actions. Thus, the different forms of osteocalcin add complexity regarding metabolic regulation, and further studies will be necessary to fully elucidate the role of each osteocalcin isoform in glucose homeostasis in vivo.
In conclusion, to our knowledge this is the first report that both carboxylated and uncarboxylated forms of osteocalcin act directly on myocytes and adipocytes to increase glucose transport and improve insulin sensitivity. In adipocytes, these effects are associated with an increase in secretion of adiponectin and the anti-inflammatory adipokine IL-10 as well as a decrease in secretion of the anti-inflammatory cytokines TNFα and IL-6. Taken together, our data support that both carboxylated and uncarboxylated osteocalcin play a protective role against the development of insulin resistance through anti-inflammatory mechanisms.
Acknowledgments
Osteocalcin was kindly donated by Dr. C. Gundberg, and the L6-Glut4-myc cells were kindly donated by Dr. A. Klip. This work was supported by grants from the National Institutes of Health (DK038765, DK083562) and the Merit Review program of the Department of Veterans Affairs to WTG; and by a grant from the Society of University Surgeons to JG. We also acknowledge core facility support from the UAB Diabetes Research Center (P60 DK079626).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rached M-T, Kode A, Silva BC, Jung DY, Gray S, Ong H, Paik J-H, DePinho RA, Kim JK, Karsenty G, Kousteni S. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120:357–368. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates β-cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 5.Kindbloom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten Å, Smith U, Mellström D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785–791. doi: 10.1359/jbmr.081234. [DOI] [PubMed] [Google Scholar]
- 6.Shea MK, Gundberg CM, Meigs JB, Dallal GE, Saltzman E, Yoshida M, Jacques PF, Booth SL. γ-Carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90:1230–1235. doi: 10.3945/ajcn.2009.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin, and insulin resistance in obese children before and after weight loss. Int J Obesity. 2010;34:852–858. doi: 10.1038/ijo.2009.282. [DOI] [PubMed] [Google Scholar]
- 8.Saleem U, Mosley TH, Kullo IJ. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2010;30:1474–1478. doi: 10.1161/ATVBAHA.110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundberg CM, Hauschka PV, Lian JB, Gallop PM. Osteocalcin-isolation characterization and detection. Methods in Enzymol. 1984;107:526–544. doi: 10.1016/0076-6879(84)07036-1. [DOI] [PubMed] [Google Scholar]
- 11.Digirolamo M, Medlinger S, Fertig JW. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971;221:850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- 12.Garvey WT, Olefsky JM, Matthaei S, Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes: a new mechanism of insulin resistance. J Biol Chem. 1987;262:189–197. [PubMed] [Google Scholar]
- 13.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayor P, Maianu L, Garvey WT. Glucose and insulin chronically regulate insulin action via different mechanisms in BC3H1 myocytes: effects on glucose transporter gene expression. Diabetes. 1992;41:274–285. doi: 10.2337/diab.41.3.274. [DOI] [PubMed] [Google Scholar]
- 15.Lima FB, Bao S, Garvey WT. Biologic actions of insulin are differentially regulated by glucose and insulin in primary cultured adipocytes: chronic ability to increase glycogen synthase activity. Diabetes. 1994;43:53–62. doi: 10.2337/diab.43.1.53. [DOI] [PubMed] [Google Scholar]
- 16.Mick GJ, Wang X, Ling Fu C, McCormick KL. Inhibition of leptin secretion by insulin and metformin in cultured rat adipose tissue. Biochim Biophys Acta. 2000;1502:426–432. doi: 10.1016/s0925-4439(00)00074-0. [DOI] [PubMed] [Google Scholar]
- 17.Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res Commun. 2010;397:691–696. doi: 10.1016/j.bbrc.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-kB signaling pathway. Endocrinology. 2013;154:1055–1068. doi: 10.1210/en.2012-2144. [DOI] [PubMed] [Google Scholar]
- 19.Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose intolerance and enhanced β-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25:768–772. doi: 10.1002/dmrr.1045. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa I, Yamaguchi T, Yamaguch M, Yamamoto M, Kruioka S, Yano S, Sugimoto T. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22:187–194. doi: 10.1007/s00198-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 21.Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, Bassali RW, Davis CL. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with β-cell function. J Clin Endocrinol Metab. 2011;96:E1092–E1099. doi: 10.1210/jc.2010-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab. 2010;95:3502–3506. doi: 10.1210/jc.2009-2557. [DOI] [PubMed] [Google Scholar]
- 23.Brennan-Speranza TC, Henneicke H, Gasparini SJ, Blankenstein KI, Heinevetter U, Cogger VC, Svistounov D, Zhang Y, Cooney GJ, Buttgereit F, Dunstan CR, Gundberg C, Zhou H, Seibel MJ. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest. 2013;122:4172–4189. doi: 10.1172/JCI63377. [DOI] [PMC free article] [PubMed] [Google Scholar]