Abstract
Deep brain stimulation (DBS) targeting the subthalamic nucleus (STN) represents a powerful clinical tool for the alleviation of many motor symptoms that are associated with Parkinson's disease. Despite its extensive use, the underlying therapeutic mechanisms of STN-DBS remain poorly understood. In the present review, we integrate and discuss recent literature examining the network effects of STN-DBS for Parkinson's disease, placing emphasis on neuroimaging findings, including functional magnetic resonance imaging, positron emission tomography, and single-photon emission computed tomography. These techniques enable the noninvasive detection of brain regions that are modulated by DBS on a whole-brain scale, representing a key experimental strength given the diffuse and far-reaching effects of electrical field stimulation. By examining these data in the context of multiple hypotheses of DBS action, generally developed through clinical and physiological observations, we define a multitude of consistencies and inconsistencies in the developing literature of this rapidly moving field.
Key words: : deep brain stimulation, fMRI, functional connectivity, Parkinson's disease, PET, subthalamic nucleus, SPECT
Introduction
Deep brain stimulation (DBS) therapy has rapidly been established as a revolutionary treatment option for advanced Parkinson's disease (PD), as well as for other neurologic and neuropsychiatric disorders. With DBS, stimulating electrodes are implanted either unilaterally or bilaterally into a target nucleus or fiber tract, and electrical stimulation, generally at high frequencies, confers therapeutic effects. The symptom alleviation resulting from DBS often mirrors those observed from target lesioning, and, indeed, DBS was developed after the serendipitous observation that electrical stimulation of the ventral intermediate thalamus confers tremor reduction akin to thalamotomy (Benabid et al., 1987, 1991). Compared with target lesioning, DBS therapy has several appealing qualities, including its reversibility and the capacity to modulate the extent of stimulated tissue by altering stimulation parameters and active lead configuration. The latter advantage is particularly useful for tailoring the electric field to reduce the effect on off-target areas (McIntyre et al., 2004a; Xu et al., 2011). Based on such considerations, DBS has rapidly supplanted target lesioning as the premier neurosurgical technique for advanced, treatment-refractory motor disorders.
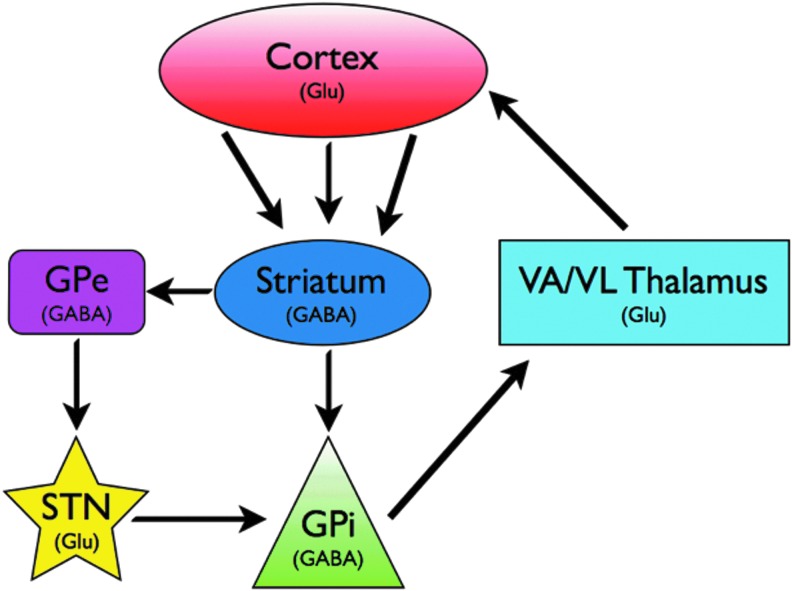
When used for the treatment of advanced Parkinson's disease, the subthalamic nucleus (STN) is most commonly chosen as the target for DBS. The STN is an input nucleus of the basal ganglia that receives direct cortical input and is also intimately connected with the dorsal pallidum or globus pallidus (Fig. 1) (Graybiel, 2000; Obeso et al., 2008b). DBS at this target (hereafter referred to as STN-DBS) can alleviate several hallmark parkinsonian symptoms, including tremor, bradykinesia, and rigidity (Fasano et al., 2012; Odekerken et al., 2013; Weaver et al., 2012). However, despite its efficacy and widespread clinical application, the underlying mechanisms of STN-DBS remain to be fully elucidated. One possible clue into the mechanisms of STN-DBS action lies in the stringent dependence on high pulse frequencies for therapeutic effects. Commonly, the STN is stimulated at 130 Hz (or above), and neuronal responses to such stimulation trains cannot often be readily predicted. Depolarization block, synaptic facilitation, and synaptic failure have been observed, and a multitude of hypotheses of DBS action have been built on these findings (Cagnan et al., 2009; Grill et al., 2004; McIntyre et al., 2004b; Vitek, 2002; Zheng et al., 2011). Interestingly, the local effects of high-frequency STN-DBS seem to converge to some extent with DBS at other target sites. Oscillatory activity in the beta band frequency range (13–30 Hz) has been detected in local field potential (LFP) recordings from various nodes within the dopamine-depleted cortico-basal ganglia-thalamo-cortical loop, and are widely believed to be pathological (Brittain and Brown, 2013; Engel and Fries, 2010; Weinberger et al., 2006). High-frequency stimulation at three nodes within this motor loop, the STN, internal globus pallidus, and substantia nigra pars reticulata, are each effective in treating motor symptoms, while concurrently suppressing local beta band rhythms (McConnell et al., 2012; Sutton et al., 2013; Whitmer et al., 2013; Wingeier et al., 2006). The establishment of a causal role for beta band rhythms in motor impairment will do much to strengthen this popular hypothesis of DBS action. However, it should be noted in this context that although high-frequency stimulation is most commonly applied, at some targets, low frequencies may be beneficial for treating movement disorders. Indeed, DBS at the pedunculopontine nucleus (PPN; a brainstem nuclei intimately connected with the basal ganglia and cerebellum) may alleviate parkinsonian symptoms of postural instability or freezing of gait, with therapeutic outcomes occurring at low frequencies (e.g., 35 Hz) (Follett and Torres-Russotto, 2012; Thevathasan et al., 2011). A small number of imaging studies have examined regional neural modulation by low-frequency PPN-DBS (Ceravolo et al., 2011; Schweder et al., 2010; Stefani et al., 2010). Thus, the critical nature of high frequencies for STN-DBS action remains to be adequately explained, and is not a universal DBS requirement, even for similar treatment effects, as exemplified by stimulation at the PPN.
FIG. 1.
Simplified model of the cortico-basal ganglia-thalamo-cortical loop. Both STN and GPi are common targets for Parkinson's therapy. Glu, glutamatatergic; GABA, GABAergic; GPe, globus pallidus external segment; GPi, globus pallidus internal segment; STN, subthalamic nucleus; VA/VL, ventral anterior/ventrolateral. Important circuit elements not shown include cortical innervation of STN (hyperdirect pathway), dopaminergic inputs to striatum and extrastriatal areas via the substantia nigra pars compacta, and the substantia nigra pars reticulata as a basal ganglia output nucleus.
The general lack of a mechanistic understanding of DBS is at least in part due to the inherently nonselective nature of electrical stimulation as a neuromodulatory tool. Specifically, DBS is likely to confer circuit changes both locally and long distances from the stimulating area, each of which could confer therapeutic effects. To better understand the global effects of STN-DBS, neuroimaging studies are often conducted, where relatively unbiased and noninvasive mapping of neural circuit changes due to DBS can be performed. Both functional magnetic resonance imaging (fMRI) and nuclear medicine approaches have been successfully applied to examine global brain activity changes during STN-DBS. Generally, these studies are conducted in off-medication patients and employ a within-subject design, where subjects are evaluated both ON and OFF DBS (DBS usually turned off before scanning in a washout period of 4–12 h) (Geday et al., 2009; Hilker et al., 2008), although between-subject studies with healthy controls are also common. In this context, fMRI allows for the time-locked evaluation of changes in neurovascular activity during DBS. Typically, blood-oxygen-level-dependent (BOLD) signals are acquired with fMRI, where oxygenated blood serves as an endogenous contrast agent to mark areas of neural stimulation or inhibition (Bandettini et al., 1992; Kwong et al., 1992; Ogawa et al., 1990, 1992). Nuclear medicine approaches, including positron emission tomography (PET) and single-photon emission computed tomography (SPECT), may also be used with DBS, and allow for relatively noninvasive imaging of multiple activity markers, such as glucose, blood flow, oxygen metabolism, and binding potential of various types of neurotransmitter receptors (Heiss and Herholz, 2006; Phelps, 2000). Nuclear medicine techniques are quite powerful, but they suffer from poor spatiotemporal resolution compared with fMRI. This is particularly problematic for resolving changes in activity within the small, discrete subcortical basal ganglia nuclei that are likely to be modulated by DBS. Further, time-locked activity changes to DBS cannot be studied, and, thus, separate within-subject baseline scanning sessions are often required. An additional point to be considered with regard to PET/SPECT imaging for DBS studies involves the use of tracers with very different uptake and clearance kinetics. Tracers used to measure cerebral blood perfusion (including H215O for PET and 99mTc-bicisate for SPECT) provide highly transient signals and, thus, reflect blood flow changes over short time periods (commonly 1 min). To achieve a sufficient number of trials, these tracers are often injected multiple times in a single session. In contrast, measurements of glucose metabolism using 18F-FDG PET allow for multiple scans to be conducted after a single bolus injection, as signals accumulate over a much longer time period (scan acquisition typically begins 30–60 min after injection) (Varrone et al., 2009). Thus, significant methodological variations exist within PET scanning procedures based on tracer kinetics, and may have implications for comparing data examining glucose metabolism and blood flow as markers of brain activity.
The overwhelming use of PET and SPECT techniques compared with fMRI is likely due to a number of safety concerns that are associated with the latter [reviewed in (Jech, 2008)]. Such safety considerations include the possibilities of thermal lesioning, electrode lead migration, and DBS hardware malfunction (Chhabra et al., 2010; Tagliati et al., 2009; Zekaj et al., 2013). In response to concerns about patient safety, fMRI studies of DBS are typically conducted using externalized pulse generators in the immediate days after lead implantation. Thus, patients in these studies may be experiencing microlesional effects and focal edema associated with the implantation procedure, and therapeutic stimulation parameters have likely not been optimized; all of these factors may influence the obtained fMRI response profile and reduce the relevance of such work as it pertains to chronically implanted patients (Jech, 2008). Recent studies describing fMRI in patients with fully implanted DBS hardware may pave the road for additional chronic DBS fMRI studies (Carmichael et al., 2007; Kahan et al., 2012). The concerns associated with DBS fMRI mentioned earlier are in addition to the already problematic MR artifacts at the tissue-lead interface. Such artifacts may preclude analysis of DBS-induced activity in the area surrounding the electrode (where DBS is most likely to confer activity changes). However, despite these concerns and when proper precautions are taken, DBS-fMRI has been shown to be a highly informative tool (Chhabra et al., 2010; Jech, et al., 2001).
The purpose of the present review is to highlight important recent findings in the expanding field of DBS neuroimaging. Neuroimaging techniques such as fMRI, PET, and SPECT allow for regionally unbiased evaluations of neural modulation by DBS, and are, thus, critical tools for the identification of novel or otherwise unanticipated loci of DBS-induced brain activity. Further, neuroimaging allows for the noninvasive evaluation of circuit-level hypotheses of DBS action, as generally developed using physiological and histological tools in experimental animal models. Here, we attempt to integrate such physiological data with the results of neuroimaging experiments, highlighting the resulting consistencies and inconsistencies in findings garnered with these toolsets. For this purpose, we focus on DBS effects related to intrinsic basal ganglia circuits as well as noncanonical motor areas, the latter of which have become of increasing interest in understanding the cognitive and emotional effects of STN-DBS.
Modulation of Intrinsic Basal Ganglia Circuitry by STN-DBS
Subthalamic nucleus
High-frequency stimulation undoubtedly modulates activity in the local environment surrounding the electrode, although the nature of these changes is poorly understood (Garcia et al., 2005). Early theories of STN-DBS action posited that DBS inhibits STN activity and functionally disconnects the nucleus from the basal ganglia circuitry [reviewed in (Dostrovsky and Lozano, 2002; Vitek, 2002)], an intuitive explanation given the clinical outcome similarities between subthalamotomy and DBS. Physiological data have been obtained in support of this hypothesis. Microelectrode recordings in human Parkinson's patients provide strong evidence for a long-lasting suppression of STN cell firing after STN-DBS (Filali et al., 2004; Toleikis et al., 2012). More comprehensive analyses conducted in rodent models further support a predominantly inhibitory local effect of high-frequency stimulation, including observations of depolarization blockade after a 1 min stimulation train in STN neurons recorded in vitro (Beurrier et al., 2001). Although this depolarization blockade is believed to be independent of synaptic inputs, neurotransmitter release within the STN has also been reported during DBS in anesthetized rats, resulting in increased extracellular glutamate concentrations within the nucleus (Lee et al., 2007). An earlier study employing sharp electrode intracellular recordings in brain slices detected excitatory postsynaptic potentials within the STN during stimulation, which were sensitive to glutamate receptor antagonists (Lee et al., 2004).
Although this finding may seem discrepant with the data from Beurrier and colleagues (2001), it should be emphasized that the excitatory potentials detected by Lee et al. occurred during, and not after the stimulation period. Indeed, consistent with both reports, the period after a high-frequency stimulation train was characterized by relative inactivity within the STN. Of additional note, when the STN was stimulated at 200 Hz, Lee et al. observed that STN neurons became incapable of firing action potentials, which was also consistent with a depolarization blockade.
The electrophysiological data described earlier provide a spatially limited picture of STN-DBS effects within the target nucleus. Single cell recordings have superior temporal resolution, yet only a small number of cells can be sampled, and the majority of such experiments have been done in slice preparations with compromised neural circuitry. Nonetheless, given the general consistency of inhibitory DBS effects at the STN in such studies, the spatially broad signal detected using neuroimaging modalities would similarly be expected to be negative (e.g., reduced blood flow or glucose metabolism). However, the opposite has been found to be true in an overwhelming majority of imaging studies to date (Asanuma et al., 2006; Boertien et al., 2011; Geday et al., 2009; Haslinger et al., 2005; Hilker et al., 2004, 2008; Jech et al., 2001). In one of the more direct evaluations of STN modulation by STN-DBS, Hilker and colleagues (2008) used 18F-FDG PET to measure changes in glucose consumption during bilateral stimulation in a group of 12 advanced Parkinson's patients and healthy age-matched control subjects (Hilker et al., 2008). Notably, the investigators included a presurgical baseline scan in addition to the more widely used OFF-stimulation baseline scan (post DBS lead implantation), which allowed for the observation of effects of electrode implantation alone. In these patients, glucose metabolism was reduced compared with presurgical baseline in the OFF-stimulation condition, and significantly increased during stimulation (when compared with the OFF-stimulation, but not presurgical baseline scans). These findings suggest that the STN has a reduced metabolic rate after DBS surgery, possibly indicating a microlesional effect, and that stimulation increases metabolism from this reduced baseline. STN activation has also been observed using blood flow PET imaging with H2150. Haslinger et al. (2005) reported frequency-dependent bilateral hyperperfusion within the STN in five of six subjects tested during DBS. These blood flow increases were maximal at 190 Hz, with the highest stimulation frequency tested (Haslinger et al., 2005). Finally, although BOLD fMRI does not permit direct observation of the STN around the electrode due to the electrode imaging artifact, the presence of BOLD activation in the area surrounding the electrode has been reported (Jech et al., 2001; Jech, 2008).
Several possibilities exist for this seeming discrepancy between single-cell and neuroimaging studies of STN modulation by STN-DBS. First, it is possible that the activity enhancements detected by PET and fMRI reflect activity in axons located within the region of the STN, rather than cell bodies. In addition to axons terminating within, or projecting from the STN, the area has recently been shown to include a large number of fibers of passage, at least in nonhuman primates (Mathai et al., 2013).
Neuroimaging techniques also differ tremendously in their spatiotemporal resolution when compared with single-cell recordings. While electrophysiological recordings can identify cell firing dynamics on a millisecond timescale, DBS effects detected by neuroimaging methods may reflect summed regional activity changes over seconds to minutes. In addition, neuroimaging modalities examine changes in neural activity only indirectly, for example, as changes in blood oxygenation, blood flow, or glucose metabolism. Thus, non-neuronal contributions to effects reported in these studies cannot be ruled out. Related to this point, when compared with multi-unit recordings or LFPs, the BOLD signal detected in fMRI is more greatly correlated with LFPs, reflecting a potential bias in fMRI for the detection of input activity rather than local cell spiking (Goense and Logothetis, 2008; Lippert et al., 2010; Logothetis et al., 2001; Yen et al., 2011). Although this relationship may not hold true at high spatial resolution (Kahn et al., 2013; Shih et al., 2013), it is worth noting that low-frequency LFP oscillations (1–1.5 Hz) may be increased during STN-DBS (Priori et al., 2006), with possible relevance for the activation profile detected with BOLD fMRI.
Caudate/putamen (striatum)
The striatum, along with the STN, represents the major center of cortical input to the basal ganglia. It is composed primarily of GABAergic medium spiny neurons (MSNs), which are segregated into two groups depending on their anatomical projections and expression of dopamine receptors and peptides. Specifically, MSNs projecting to the external globus pallidus (GPe) (i.e., the indirect pathway) express D2 dopamine receptors and enkephalin, whereas those neurons projecting to the internal globus pallidus (GPi) (the direct pathway) express D1 dopamine receptors and substance P. This description is greatly simplified, with caveats, including the innervation of GPe by direct pathway MSNs (Nambu, 2008; Surmeier et al., 2011). Nonetheless, this convenient scheme may be useful for understanding both convergent and parallel processing within the striatum. Although direct and indirect pathway MSNs are active during goal-directed movements (Cui et al., 2013), the effects of selective pathway manipulation on motor function are diametrically opposed. Using optogenetics to selectively activate D1 or D2 receptor expressing MSNs within the mouse dorsomedial striatum (caudate), Kravitz et al. (2010) observed that unilateral direct pathway stimulation induced ipsilateral turning behavior, whereas stimulating the indirect pathway resulted in contralateral turning (Kravitz et al., 2010). When bilaterally stimulating each pathway selectively, the effects were again opposing: Direct pathway stimulation increased ambulatory behavior, while indirect pathway stimulation promoted freezing behaviors. Lastly, given the facilitatory effects of direct pathway stimulation on movement, the authors examined the efficacy of this stimulation in the bilateral 6-hydroxydopamine (6-OHDA) mouse model of Parkinson's disease. For this model, an injection of the dopaminergic neurotoxin 6-OHDA into the dorsomedial striatum resulted in a near-complete loss of dopaminergic fiber innervation into the striatum. Behaviorally, these mice exhibited a variety of parkinsonian symptoms, such as bradykinesia and freezing of movement. Remarkably, selective stimulation of direct pathway MSN's was able to successfully rescue many of these deficits, including restoration of measures of ambulation and motor freezing behavior to prelesion values. Taken in the context of STN-DBS, such findings suggest that DBS-induced modulation of striatal MSNs (inhibition of indirect or stimulation of direct pathway MSN's) may be a possible downstream therapeutic mechanism.
Neuroimaging experiments to date have not successfully segregated activity changes within the spatially heterogeneous direct and indirect MSNs, although it is clear from these studies that STN-DBS may modulate striatal activity as a whole. In an fMRI case study of a 36-year-old Parkinson's patient (likely nonidiopathic PD) with comorbid depression, Stefurak and colleagues (2003) reported BOLD signal increases in striatum during STN-DBS (Stefurak et al., 2003). Due to the limitations associated with fMRI procedures, bipolar stimulation was used via an externalized pulse generator, and both leads used in the bilateral procedure were tested independently. DBS induced striatal BOLD signal increases (specifically within the putamen) with both leads, despite the fact that only the left lead effectively ameliorated motor symptoms (stimulation with the right lead resulted in profound emotional disturbances without motor effects). This result suggests that striatal activation may be necessary, but not sufficient for therapeutic motor effects. Similar results have also been obtained with PET, demonstrating DBS-induced striatal activation localized to the lentiform nucleus or putamen (Geday et al., 2009; Hilker et al., 2004).
Internal globus pallidus
The GPi represents one of the major output structures of the basal ganglia, along with the substantia nigra pars reticulata, and it provides tonic inhibitory drive to the motor thalamus under normal conditions. Interest in the GPi within the context of Parkinson's disease arises from many sources, including the therapeutic effects exerted from lesioning (pallidotomy) or DBS at this target (Follett and Torres-Russotto, 2012; Obeso et al., 2001; Pizzolato and Mandat, 2012; Rouaud et al., 2010). In classical models of Parkinson's disease, loss of striatal dopamine is predicted to result in hyperactivity within the GPi with consequent over-inhibition of thalamocortical motor relays (Obeso et al., 2008b; Wichmann et al., 2011). One possible mechanism of STN-DBS action may be the attenuation of such pathological GPi hyperactivity, and consequent restoration of thalamic motor relay fidelity (see next section).
A variety of experimental tools have provided converging evidence that DBS at the STN, indeed, modulates activity within the GPi, a finding which could be predicted by the intimate anatomical connectivity between these regions (Graybiel, 2000; Obeso et al., 2008a). However, the direction of this modulation is under debate. In normal monkeys, high-frequency burst stimulation of the STN predominantly inhibits GPi neurons, an effect that is sensitive to GABA antagonists and thus likely represents a polysynaptic response which is mediated by inhibitory inputs from theGPe (Kita et al., 2005). In addition to this inhibitory action, short-latency excitatory responses were also noted in that study, consistent with monosynaptic excitation via the STN. Additional data supporting recruitment of the glutamatergic STN-GPi projection include recordings obtained in MPTP-treated monkeys, demonstrating augmented GPi firing rates during therapeutically effective STN-DBS (Hashimoto et al., 2003). Further, in Parkinson's patients undergoing STN-DBS surgery, microdialysis recordings have revealed elevated cGMP levels within the GPi during STN-DBS, an effect most likely downstream of glutamate release from the STN (Stefani et al., 2005). Collectively, the data cited earlier present a complex picture of GPi modulation by STN-DBS, by which both excitatory and inhibitory effects may be observed.
A small number of neuroimaging studies have reported activity changes within the GPi during STN-DBS. In Parkinson's patients receiving bilateral STN-DBS, reductions in glucose metabolism (as detected by 18F-FDG PET) were observed in the left GPi (Asanuma et al., 2006). Of note, that same study reported similar metabolic decreases within the left GPi in a separate group of Parkinson's patients receiving levodopa infusions titrated for maximal therapeutic efficacy. Why this effect was consistently unilateral was not discussed, although it is possible that it reflects restoration of asymmetrically impaired motor circuit function. Supporting this possibility, SPECT analysis of radiolabeled dopamine transporter (DAT) density (an indirect measure of dopamine signaling, detected using DAT radioligand [123I]β-CIT) has revealed significant DAT reductions in the left, compared with right putamen in Parkinson's patients compared with healthy controls, at least for right-handed subjects (Scherfler et al., 2012). An additional 18F-FDG-PET study of Parkinson's patients with unilateral STN-DBS implantations revealed a discrete locus of hypometabolism within the contralateral, but not ipsilateral, GPi (Arai et al., 2008). Thus, it may be that downstream GPi inactivation is only required unilaterally for DBS-induced restoration of motor function. However, complicating matters, observations of enhanced GPi activity have also been made during therapeutic STN-DBS, including increases in blood flow (Ceballos-Baumann et al., 1999), glucose metabolism (Hilker et al., 2008), and BOLD signal enhancement (Jech et al., 2001). Therefore, reduction of GPi activity alone is incapable of explaining motor symptom alleviation by DBS.
Modulation of Basal Ganglia Input/Output Structures by STN-DBS
Substantia nigra pars compacta
A major question regarding the mechanisms of DBS efficacy lies in the possibility that stimulation promotes residual dopamine release from the substantia nigra pars compacta (SNpc), partially restoring dopaminergic tone within the basal ganglia. DBS at the STN has long been known to enable reductions in dopaminergic therapies such as levodopa, consistent with this hypothesis (Moro et al., 2010; Odekerken et al., 2013; Weaver et al., 2012). Levodopa responsiveness is also a strong predictor of DBS efficacy, further hinting that dopamine release may be an important downstream mechanism of STN-DBS action.
If STN-DBS is indeed recruiting SNpc dopamine cells, a straightforward anatomical means for such downstream stimulation has been described in the rat (Groenewegen and Berendse, 1990) and mouse (Watabe-Uchida et al., 2012) in which the STN monosynaptically and reciprocally innervates the SNpc (Cragg et al., 2004). In vivo recordings in normal, anesthetized rats have shown enhanced rates of SNpc firing during high-frequency STN stimulation (Benazzouz et al., 2000), although a recent report using longer pulse trains (>15 min) has failed to replicate this finding in slice recordings (Ledonne et al., 2012). In one of the more convincing demonstrations of dopamine release by STN-DBS to date, Shon and colleagues (2010) applied fast-scan cyclic voltammetry (FSCV) to measure extracellular dopamine concentrations in the striatum of normal pigs. FSCV benefits from its nanomolar-range chemical detection sensitivity and submillisecond temporal resolution in resolving dopamine release events in vivo (Robinson et al., 2003). Using this technique, dopamine concentrations in pig striatum were found to vary in a voltage and stimulation frequency-dependent manner during STN-DBS. Specifically, release was maximal at high frequencies (≥120 Hz) and voltages (7 V, highest tested). The use of a normal animal model weakens the therapeutic relevance of these findings, although microdialysis studies in rats with nigrostriatal lesions have also found increased striatal dopamine release by STN-DBS (Meissner et al., 2002, 2003).
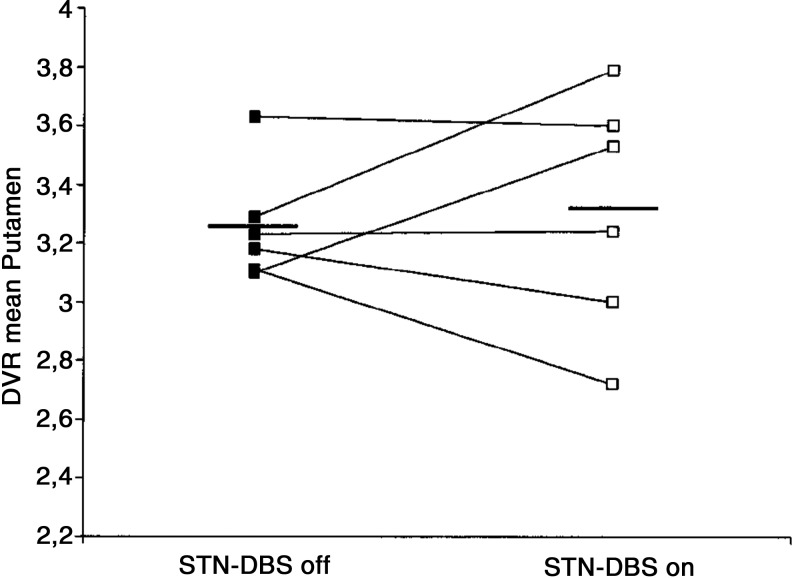
Confirmation of dopamine release by STN-DBS in human Parkinson's patients is possible using PET imaging to visualize displacement of radiolabeled dopamine receptor ligands by endogenous neurotransmitter (Thobois et al., 2004; Volkow et al., 1996, 2009). Despite the attractive simplicity of the dopamine hypothesis of STN-DBS action, the few receptor displacement studies conducted to date have generally not provided support for such a mechanism [though see (Nozaki et al., 2013)]. In a direct test of the DBS dopamine hypothesis, Hilker and colleagues (2003) measured DBS-induced striatal dopamine release using radiolabeled 11C-raclopride (reversible D2/D3 receptor agonist). In six PD patients with therapeutically effective bilateral STN-DBS implantations, no significant changes were observed in dopamine receptor binding by DBS in caudate/putamen, either ipsi-or contralateral to the most therapeutically effective stimulation side (Fig. 2). An additional study using radiolabeled L-DOPA, a dopamine precursor (18F-DOPA), in four subjects with unilateral DBS leads also failed to find differences in striatal dopamine levels on or off DBS, either ipsi- and contralateral to the stimulation side (Arai et al., 2008). Although these findings suggest that striatal dopamine release does not occur during STN-DBS, detection sensitivity may be an issue. For example, using 11C-raclopride displacement, an increase in dopamine concentrations in excess of 10% is required to visualize a change (Volkow et al., 1994). Thus, it remains possible that residual dopamine release may be an important component of STN-DBS action, but adequate testing of this hypothesis will require more sensitive (and likely more invasive) measures of endogenous transmitter release. It will also be interesting to test the ability of STN-DBS to promote dopamine release in extra-striatal targets of SNpc innervation, including the GPi, GPe, and STN itself (Rommelfanger and Wichmann, 2010).
FIG. 2.
Lack of effect of STN-DBS on endogenous striatal dopamine release within the putamen, as detected by positron emission tomography imaging of 11C-raclopride (D2/D3 receptor agonist) displacement. Figure displays raclopride displacement volume ratio (DVR) averaged within the putamen for each of six advanced Parkinson's patients, both during bilateral STN-deep brain stimulation (DBS) and after a nonstimulation period of at least 12 h. Figure modified from Hilker et al. (2003).
Ventral anterior/ventrolateral thalamus
The ventral anterior/ventrolateral (VA/VL) thalamic motor relay nuclei serve as intermediary structures to connect the basal ganglia output to cortical targets. Interest in the motor thalamus as a downstream target for DBS arises from many sources, including its intimate anatomical relationship with the basal ganglia and the presence of tremor cells within this region in Parkinson's patients. Modeling studies suggest that, in Parkinson's disease, overactivity within the GPi obstructs signal relay fidelity within the motor thalamus (Guo et al., 2008; Meijer et al., 2011). In these models, DBS at high, but not at low frequencies restores the relay fidelity of these thalamocortical cells. Physiological evidence suggests that VA/VL thalamic neurons alter their firing rates in response to STN-DBS, again suggesting a possible role for motor thalamus activity in therapeutic stimulation. The mechanisms by which STN-DBS affects thalamic activity have not been adequately worked out, although as mentioned earlier, the upstream GPi is likely an important player. Supporting this idea, microdialysis sampling of GABA content in the VA thalamus has revealed reductions in this transmitter during DBS (Stefani et al., 2011), possibly due to inhibition of pallidal afferents.
The outlined framework for DBS-induced restoration of motor thalamic relay fidelity predicts that the motor thalamus will show increased activity during DBS (due to reductions in inhibitory inputs). A wealth of neuroimaging data have been collected showing that this is generally the case (Arai et al., 2008; Hershey et al., 2003; Hilker et al., 2004; Jech et al., 2001; Karimi et al., 2008; Phillips et al., 2006). However, at least two studies have reported reduced ventral thalamic activity (regional cerebral blood flow [rCBF] decreases) during bilateral STN-DBS (Cilia et al., 2009; Geday et al., 2009). In one of these studies, Geday and colleagues (2009) examined blood flow changes in nine subjects with bilateral STN-DBS. Six scans were performed in the ON and OFF-stimulation conditions, randomized and separated by a 4 h stimulation recovery period (Geday et al., 2009). During PET scan sessions, subjects viewed emotionally salient stimuli, although all effects reported were independent of this manipulation. DBS was found to induce a wealth of rCBF changes, including deactivation of the left (but not right) VA/VL thalamus. In discussing this finding within the context of previous studies showing ventral thalamic activation during STN-DBS, the authors raise the possibility that activation of the neighboring STN may be the true source of some reported increases in thalamic blood flow and glucose metabolism. Interestingly, unmedicated Parkinson's patients at rest show hypermetabolism in the thalamus, part of the so-called Parkinson's Disease-Related Spatial Covariance Pattern (PDRP), which also includes GPi and primary motor cortex hyperactivity, as well as hypoactivity within supplementary motor cortices (Ma et al., 2007; Niethammer et al., 2012). In the context of the PDRP, the ventral thalamic inactivation reported by Geday et al. may represent a normalization of pathological hyperactivity within the thalamocortical motor circuit. However, other findings reported in that study, for example, reduced blood flow within supplementary motor cortex, are more difficult to reconcile with the PDRP profile.
Primary and premotor cortices
The contributions of sensorimotor cortex to STN-DBS effects are under intense debate, largely due to conflicting reports regarding the therapeutic efficacy of direct primary motor cortex stimulation (Arle et al., 2008; Brittain et al., 2013; Cilia et al., 2007, 2008; Dejean et al., 2009; Gradinaru et al., 2009; Gutierrez et al., 2009; Lefaucheur et al., 2004; McAllister et al., 2013; Strafella et al., 2007). A number of abnormal features have been observed in motor cortical circuits of PD patients, including hyperconnectivity between motor cortex and STN and disease-specific oscillatory interactions (Baudrexel et al., 2011; Shimamoto et al., 2013). Notably, electrophysiological recordings have consistently demonstrated that such pathological circuit activity can be corrected by STN-DBS, in both animal models (Dejean et al., 2009; Li et al., 2012, 2007) and Parkinson's patients (de Hemptinne et al., 2013; Shimamoto et al., 2013).
Many possible routes exist by which STN-DBS may be postulated to affect motor cortical activity. The canonical cortico-basal ganglia-thalamo-cortical loops (Alexander et al., 1986; DeLong and Wichmann, 2009) include an excitatory connection from the STN to the GPi, which influences sensorimotor cortical activity via the VA/VL thalamus. In addition to this feedforward route, DBS at the STN may recruit the motor cortex directly via antidromic spike propagation along the substantial corticosubthalamic projection (the “hyperdirect” route) (DeLong and Wichmann, 2009). The net effect of these antidromic spikes on motor cortical activity is likely complex, although modulation can be assumed to occur with a shorter latency than the polysynaptic thalamic route. A thorough characterization of antidromic spikes was recently conducted by Li and colleagues (2012), who ultimately demonstrated that high-frequency STN stimulation does, in fact, modulate intrinsic motor cortical activity via antidromic signals (Li et al., 2012). When these spikes were observed, they tended to result in an early suppression and delayed excitation of cellular activity. How such biphasic changes, which occurred on a millisecond timescale, affect the global response to DBS within motor cortex cannot be readily predicted.
Neuroimaging studies have generated conflicting data regarding how STN-DBS may modulate motor cortical activity. A number of PET studies have reported hypoactivation in motor cortex (primary and/or premotor) during stimulation, as indicated by either reduced rCBF or glucose metabolism compared with nonstimulated conditions (Asanuma et al., 2006; Ceballos-Baumann et al., 1999; Geday et al., 2009; Haslinger et al., 2005; Karimi et al., 2008; Trost et al., 2006). For example, in a prospective study conducted by Cilia et al. (2009), 21 Parkinson's patients underwent two sets of PET scans, one before and another 6 months after STN-DBS surgery (off-medication during both sessions) (Cilia et al., 2009). The majority of brain regions showing significant changes during stimulation (as compared with presurgery baseline) were characterized by hypoperfusion, including large decreases in rCBF within primary and premotor cortices. Similar results have been obtained in a number of smaller studies, including one report demonstrating that the extent of rCBF decreases in motor cortex during stimulation positively correlates with DBS-induced motor improvement (Haslinger et al., 2005). Remarkably, a similar study in a larger patient population and using SPECT found robust increases in rCBF within the premotor cortex that positively correlated with DBS efficacy (Paschali et al., 2013). Additional studies have similarly found DBS-induced rCBF increases within motor cortex, using both SPECT (Sestini et al., 2005, 2007) and PET modalities (Limousin et al., 1997). That the PET/SPECT data mentioned earlier so directly conflict, even considering methodological variation, is perplexing. One possible explanation for this discrepancy involves subtle variations in active electrode contacts within the STN that may occur between studies, a topic which has only recently been explored (Hill et al., 2013).
Neuroimaging findings of motor cortex recruitment by STN-DBS are further clouded when considering the small number of functional MRI studies examining DBS effects on BOLD signal. The first of such reports was made in 2001 by Jech and colleagues, where three patients with unilateral electrodes in the STN were assessed acutely, before subclavicular placement of an internal pulse generator (Jech et al., 2001). In that case study, motor cortical modulation by DBS was observed in only one subject, where stimulation resulted in BOLD increases. No subject showed BOLD decreases in any region during stimulation. A more recent study in a slightly larger patient population failed to observe any DBS-induced modulation of motor cortical regions during DBS, although robust activation of the neighboring insula was noted (Kahan et al., 2012).
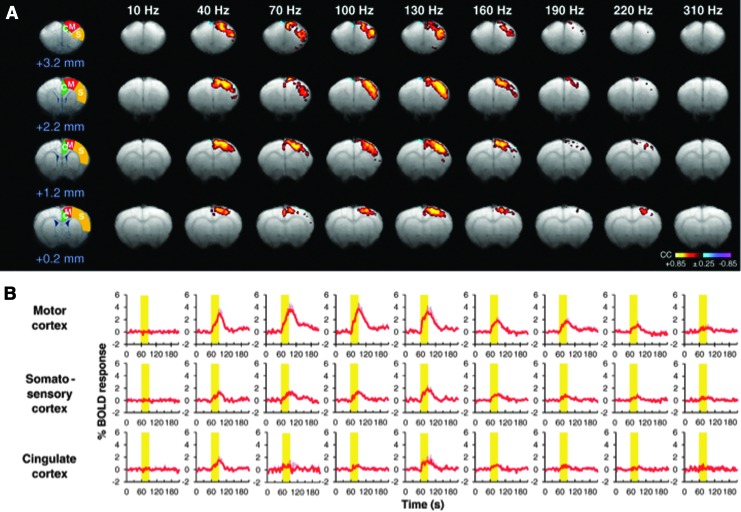
Although difficult to compare with human studies, a comprehensive analysis of global BOLD signal changes during STN-DBS has also been undertaken in animal models. In a recent report studying normal, unlesioned pigs, widespread, positive BOLD signal changes were noted during DBS, without any evidence of suppressed regional activity during stimulation (Min et al., 2012). The motor and premotor cortices represented the first and second largest areas of BOLD activity, respectively, and were similarly active during stimulation of the internal globus pallidus (another common DBS target for movement disorders). Lai and colleagues (2013) also found similar positive BOLD responses in the motor cortex of normal rats during high-frequency stimulation of either the STN or GPi. In this study, multiple DBS frequencies were tested (10–400 Hz), with the rationale that, similar to motor alleviation for Parkinson's, the neural correlates of effective DBS may demonstrate stringent frequency dependence. In line with this hypothesis, the largest BOLD signal enhancements during STN-DBS occurred within the motor cortex, peaking at 130 Hz (within the traditional therapeutic frequency range for STN-DBS) (Fig. 3). The relationship between these animal studies and the PET results mentioned earlier, generally demonstrating reduced activity in the motor cortices during STN-DBS, is not clear. However, the use of a normal animal model in the fMRI studies precludes all but casual comparison. Further, a study of fMRI signal changes induced by DBS in both humans and parkinsonian animal models is greatly needed.
FIG. 3.
STN-DBS evokes frequency-dependent blood-oxygen-level-dependent (BOLD) activation in sensorimotor cortex of normal rats. The amplitude of DBS-induced activation peaked at 130 Hz, and was largest in the ipsilateral motor cortex during unilateral stimulation. (A) BOLD activation maps in different brain slices with reference to bregma; (B) averaged traces of BOLD response profiles in cortical subregions depicted in A. Figure modified from Lai et al. (2013).
Modulation of limbic and executive circuits by STN-DBS
The modulation of nonmotor limbic and executive circuits by STN-DBS is apparent from a number of studies examining emotional and cognitive changes in PD patients (Denheyer et al., 2009; Strutt et al., 2012; Temel et al., 2006; Wolz et al., 2012; Zangaglia et al., 2009). Perhaps the greatest example of nonmotor circuit recruitment by STN-DBS exists in the recent targeting of the STN for the neurosurgical treatment of psychiatric disorders, primarily obsessive-compulsive disorder (OCD). This nontraditional application arose from the serendipitous observation that, in PD patients with comorbid OCD, STN-DBS could effectively treat symptoms arising from both pathologies (Fontaine et al., 2004; Mallet et al., 2002). Further evaluation in large clinical studies confirmed the therapeutic efficacy of STN-DBS for OCD (Mallet et al., 2008), ultimately resulting in FDA approval of DBS for this disorder.
The pathways by which STN-DBS contributes to emotional and cognitive processing are very poorly understood compared with its motor effects (Temel et al., 2005). Thus, regionally unbiased neuroimaging studies of nonmotor STN circuits are particularly valuable, as they hold the potential to identify unpredicted and behaviorally therapeutic circuits with the ultimate goal of DBS target refinement and extension. A number of PET studies have identified activity changes in canonical limbic/executive regions during STN-DBS in Parkinson's patients, including the dorsolateral prefrontal cortex and cingulate gyrus (Geday et al., 2009; Hilker et al., 2004; Limousin et al., 1997). Hilker and colleagues (2004) performed an 18F-FDG PET study in advanced Parkinson's patients with bilateral STN-DBS, both before and 4 months after the surgical procedure (Hilker et al., 2004). Although no regional differences in glucose metabolism were noted for the off-stimulation state before and after surgery, DBS evoked metabolic changes in a variety of brain areas, both canonically motor and limbic. With regard to limbic areas, the left anterior cingulate and right medial temporal lobe, among others, showed increased glucose metabolism during stimulation. The authors additionally administered a battery of neuropsychological tests before and after DBS surgery to determine the consequences of chronic DBS for emotional and cognitive function. Interestingly, although the majority of these tests showed no change between time points, performance was enhanced in two measures of delayed recall during DBS. This result suggests that long-term memory functions may be particularly influenced by stimulation, a finding in line with other studies (Halpern et al., 2009).
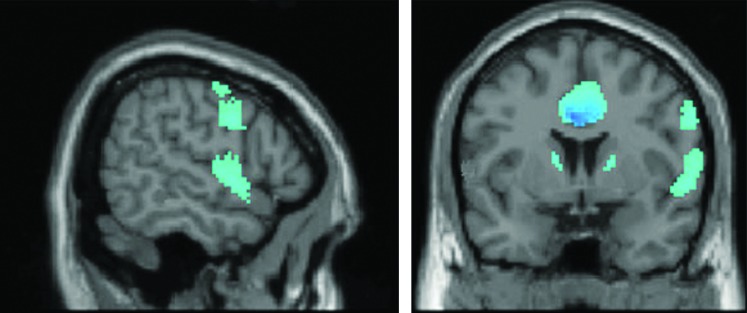
Following clinical success in treating OCD symptoms comorbid with Parkinson's disease using STN-DBS, recent studies have been extended to include STN stimulation as applied therapeutically for OCD alone. In a small sample of OCD patients with bilateral STN-DBS, PET imaging of glucose metabolism has been compared in the ON- and OFF-stimulation conditions (Le Jeune et al., 2010). The therapeutic efficacy of DBS in these patients was measured using the Yale–Brown Obsessive Compulsive Scale, demonstrating a mean reduction in OCD symptoms after chronic stimulation. Compared with the OFF-stimulation condition, DBS also resulted in significant loci of hypoactivity in the cingulate gyrus and orbitofrontal cortex, major hubs for emotional and executive functioning (Fig. 4). This finding is significant, because these same areas may be hyperfunctional in untreated OCD, hinting at normalization of prefrontal circuit activity as a possible mechanism of DBS action for this disorder (Bourne et al., 2012; Evans et al., 2004; Milad and Rauch, 2012).
FIG. 4.
Detection of prefrontal hypometabolism during STN-DBS for treatment-refractory obsessive-compulsive disorder. Bilateral STN-DBS (targeting the associative and limbic STN subregions) induced reduced glucose metabolism in the cingulate gyrus and left frontal lobe (among other regions) when compared with a within-subject DBS-OFF baseline. Figure modified from Le Jeune et al. (2010).
Although it is tempting to compare STN-DBS studies in Parkinson's and OCD patients, some major caveats need to be considered. First, although the sensorimotor territory of the STN is generally targeted for PD, surgeons often choose the associative or limbic regions of the STN for OCD treatment (Le Jeune et al., 2010). These areas of the STN function within distinct basal ganglia loops, and, thus, may be expected to differentially modulate global brain activity (DeLong and Wichmann, 2009). Second, the nonstimulated brain of the PD and OCD patient are likely to be quite dissimilar, reflecting unique pathological circuit activity, and may be predicted to differentially interact with electrical stimulation. Related to this, such patients are generally on very different medication regimens, which may be expected to induce persistent circuit plasticity. Thus, although limbic and executive cortical areas are subject to modulation by STN-DBS in both disorders, the nature of this activity and its therapeutic relevance may vary between them.
Conclusions and Future Directions
STN-DBS represents a powerful clinical tool for the treatment of advanced Parkinson's disease, although the mechanism(s) of therapeutic action are poorly understood. Although a large number of studies have been conducted to uncover its basic mechanisms, in both human patients and animal models, general inconsistencies in their conclusions continue to plague the STN-DBS literature. Notably, these differences in findings do not appear to vary according to the experimental technique used in any meaningful way. Neuroimaging methods benefit from their relatively noninvasive nature, and whole-brain responses to DBS can be detected generally with adequate sensitivity. Placed in the context of the extensive physiological data collected in animal models, imaging studies allow for the translational analysis of theories of DBS action. However, for most brain regions that have been identified, the direction of DBS modulation is ambiguous. Perhaps the one exception would be the STN, where activation is reported in most neuroimaging studies, though this finding is discrepant with physiological data showing inhibition at a single-cell level.
Several possibilities exist to explain such inconsistencies. Neuroimaging studies may be prone to spurious results, depending on basal neural activity, vascular reactivity and, with PET or SPECT imaging, possible mislocalized areas of DBS-induced activity changes. Furthermore, and not specific to imaging experiments, certain areas of modulation may be irrelevant for DBS effects, and these areas would be expected to vary in the presence and/or direction of modulation far more than therapeutic circuits. This point is highly pertinent to DBS as an extremely nonselective method of stimulation; it is quite unlikely that all areas affected downstream of the electrical field are clinically valuable, although some may represent the neural correlates of adverse or otherwise unintended stimulation effects (e.g., parasthesias, emotional and cognitive changes). Unfortunately, imaging studies are inherently correlative, and often not capable of distinguishing between such circuits. To understand which of these areas are truly therapeutically relevant will require experimental approaches that are capable of selective circuit manipulation during therapeutic DBS. In parkinsonian animal models, the tools of opto- and pharmacogenetics may plausibly be used to manipulate genetically defined circuits during DBS, as an extension of the DBS mimicry studies that have already been conducted with such techniques (Gradinaru et al., 2009; Kravitz et al., 2010). With these tools, it may be possible to isolate neural circuits where modulation is necessary for therapeutic DBS.
Ultimately, validation of any mechanism of DBS action will need to be performed in human subjects, not animal models. Although genetically based circuit manipulations are not possible in humans, recent developments in noninvasive functional connectivity analysis, the so-called resting-state connectivity profile, may provide powerful network-level information about DBS (Fox and Greicius, 2010; Fox and Raichle, 2007; Pawela et al., 2008). In resting-state analyses, spontaneous fluctuations in BOLD signals (generally below 0.1 Hz) are identified as the intrinsic markers for functional connectivity (Biswal et al., 1995). In the simplest form of this technique, a seed region is identified and correlated with other brain areas to determine areas of functional connectivity. The clinical utility of this approach comes from its capacity to identify aberrant functional connectivity in disease, and its amelioration by therapeutic intervention. While this technique has yet to be widely employed for the analysis of functional connectivity changes during STN-DBS, initial findings are promising. Vidal and colleagues (2013) recently reported an increase in connectivity within the premotor cortical hub during STN-DBS in 13 Parkinson's patients. Notably, the magnitude of this premotor hub connectivity was positively correlated with alleviation of disease symptoms (Vidal et al., 2013). A similar correlation between resting-state connectivity changes and therapeutic DBS outcomes has been obtained in patients receiving nucleus accumbens (NAc)-DBS for OCD (Figee et al., 2013). In these patients, DBS was found to normalize aberrant hyperconnectivity between the NAc and lateral and medial prefrontal cortices, and this reduction in connectivity correlated well with scores for OCD symptom relief. Based on such early observations, it is conceivable that resting-state functional connectivity analyses will yield a tremendous number of insights into network modulation by DBS.
Acknowledgments
The authors thank Manasmita Das, Heather Decot, and John Younce of the Shih Lab for their thoughtful comments and discussion in composing this review.
This work was supported by UNC Neurology and Biomedical Research Imaging Center startup funds to YYIS.
Author Disclosure Statement
The authors have nothing to disclose.
References
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381 [DOI] [PubMed] [Google Scholar]
- Arai N, Yokochi F, Ohnishi T, Momose T, Okiyama R, Taniguchi M, et al. . 2008. Mechanisms of unilateral STN-DBS in patients with Parkinson's disease: a PET study. J Neurol 255:1236–1243 [DOI] [PubMed] [Google Scholar]
- Arle JE, Apetauerova D, Zani J, Deletis DV, Penney DL, Hoit D, et al. . 2008. Motor cortex stimulation in patients with Parkinson disease: 12-month follow-up in 4 patients. J Neurosurg 109:133–139 [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, et al. . 2006. Network modulation in the treatment of Parkinson's disease. Brain 129:2667–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. 1992. Time course EPI of human brain function during task activation. Magn Reson Med 25:390–397 [DOI] [PubMed] [Google Scholar]
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, et al. . 2011. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage 55:1728–1738 [DOI] [PubMed] [Google Scholar]
- Benabid A, Pollak P, Louveau A, Henry S, De Rougemont J. 1987. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Stereotact Funct Neurosurg 50:344–346 [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Hoffmann D, Gervason C, Hommel M, Perret J, et al. . 1991. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337:403–406 [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gao D, Ni Z, Benabid A-L. 2000. High frequency stimulation of the STN influences the activity of dopamine neurons in the rat. Neuroreport 11:1593–1596 [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. 2001. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85:1351–1356 [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo—planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Boertien T, Zrinzo L, Kahan J, Jahanshahi M, Hariz M, Mancini L, et al. . 2011. Functional imaging of subthalamic nucleus deep brain stimulation in Parkinson's disease. Mov Disord 26:1835–1843 [DOI] [PubMed] [Google Scholar]
- Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN. 2012. Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. Front Integr Neurosci 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Brown P. 2013. Oscillations and the basal ganglia: motor control and beyond. Neuroimage [Epub ahead of print]; DOI: 10.1016/j.neuroimage.2013.05.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Probert-Smith P, Aziz TZ, Brown P. 2013. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol 23:436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnan H, Meijer HG, van Gils SA, Krupa M, Heida T, Rudolph M, et al. . 2009. Frequency-selectivity of a thalamocortical relay neuron during Parkinson's disease and deep brain stimulation: a computational study. Eur J Neurosci 30:1306–1317 [DOI] [PubMed] [Google Scholar]
- Carmichael DW, Pinto S, Limousin-Dowsey P, Thobois S, Allen PJ, Lemieux L, et al. . 2007. Functional MRI with active, fully implanted, deep brain stimulation systems: safety and experimental confounds. Neuroimage 37:508–517 [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, et al. . 1999. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol 56:997. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Brusa L, Galati S, Volterrani D, Peppe A, Siciliano G, et al. . 2011. Low frequency stimulation of the nucleus tegmenti pedunculopontini increases cortical metabolism in parkinsonian patients. Eur J Neurol 18:842–849 [DOI] [PubMed] [Google Scholar]
- Chhabra V, Sung E, Mewes K, Bakay RA, Abosch A, Gross RE. 2010. Safety of magnetic resonance imaging of deep brain stimulator systems: a serial imaging and clinical retrospective study. J Neurosurg 112:497–502 [DOI] [PubMed] [Google Scholar]
- Cilia R, Landi A, Vergani F, Sganzerla E, Pezzoli G, Antonini A. 2007. Extradural motor cortex stimulation in Parkinson's disease. Mov Disord 22:111–114 [DOI] [PubMed] [Google Scholar]
- Cilia R, Marotta G, Landi A, Isaias IU, Mariani CB, Vergani F, et al. . 2009. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case-control study. Clin Neurol Neurosurg 111:140–146 [DOI] [PubMed] [Google Scholar]
- Cilia R, Marotta G, Landi A, Isaias IU, Vergani F, Benti R, et al. . 2008. Cerebral activity modulation by extradural motor cortex stimulation in Parkinson's disease: a perfusion SPECT study. Eur J Neurol 15:22–28 [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD. 2004. Synaptic release of dopamine in the subthalamic nucleus. Eur J Neurosci 20:1788–1802 [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. . 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. . 2013. Exaggerated phase–amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A 110:4780–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean C, Hyland B, Arbuthnott G. 2009. Cortical effects of subthalamic stimulation correlate with behavioral recovery from dopamine antagonist induced akinesia. Cereb Cortex 19:1055–1063 [DOI] [PubMed] [Google Scholar]
- DeLong M, Wichmann T. 2009. Update on models of basal ganglia function and dysfunction. Parkinsonism Relat Disord 15 Suppl 3:S237–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denheyer M, Kiss ZH, Haffenden AM. 2009. Behavioral effects of subthalamic deep brain stimulation in Parkinson's disease. Neuropsychologia 47:3203–3209 [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Lozano AM. 2002. Mechanisms of deep brain stimulation. Mov Disord 17 Suppl 3:S63–S68 [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20:156–165 [DOI] [PubMed] [Google Scholar]
- Evans DW, Lewis MD, Iobst E. 2004. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive–compulsive disorder. Brain Cogn 55:220–234 [DOI] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. 2012. Treatment of motor and non-motor features of Parkinson's disease with deep brain stimulation. Lancet Neurol 11:429–442 [DOI] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, van Wingen G, de Kwaasteniet B, et al. . 2013. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 16:386–387 [DOI] [PubMed] [Google Scholar]
- Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. 2004. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res 156:274–281 [DOI] [PubMed] [Google Scholar]
- Follett KA, Torres-Russotto D. 2012. Deep brain stimulation of globus pallidus interna, subthalamic nucleus, and pedunculopontine nucleus for Parkinson's disease: which target? Parkinsonism Relat Disord 18:S165–S167 [DOI] [PubMed] [Google Scholar]
- Fontaine D, Mattei V, Borg M, von Langsdorff D, Magnie M-N, Chanalet S, et al. . 2004. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease: case report. J Neurosurg 100:1084–1086 [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. 2010. Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Garcia L, D'Alessandro G, Bioulac B, Hammond C. 2005. High-frequency stimulation in Parkinson's disease: more or less? Trends Neurosci 28:209–216 [DOI] [PubMed] [Google Scholar]
- Geday J, Ostergaard K, Johnsen E, Gjedde A. 2009. STN-stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp 30:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. 2008. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18:631–640 [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. 2009. Optical deconstruction of parkinsonian neural circuitry. Science 324:354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. 2000. The basal ganglia. Curr Biol 10:R509. [DOI] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. 2004. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15:1137–1140 [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. 1990. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol 294:607–622 [DOI] [PubMed] [Google Scholar]
- Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. 2008. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol 99:1477–1492 [DOI] [PubMed] [Google Scholar]
- Gutierrez JC, Seijo FJ, Alvarez Vega MA, Fernandez Gonzalez F, Lozano Aragoneses B, Blazquez M. 2009. Therapeutic extradural cortical stimulation for Parkinson's disease: report of six cases and review of the literature. Clin Neurol Neurosurg 111:703–707 [DOI] [PubMed] [Google Scholar]
- Halpern CH, Rick JH, Danish SF, Grossman M, Baltuch GH. 2009. Cognition following bilateral deep brain stimulation surgery of the subthalamic nucleus for Parkinson's disease. Int J Geriatr Psychiatry 24:443–451 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. 2003. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos-Baumann AO. 2005. Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson's disease. Neuroimage 28:598–606 [DOI] [PubMed] [Google Scholar]
- Heiss W-D, Herholz K. 2006. Brain receptor imaging. J Nucl Med 47:302–312 [PubMed] [Google Scholar]
- Hershey T, Revilla F, Wernle A, McGee-Minnich L, Antenor J, Videen T, et al. . 2003. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology 61:816–821 [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, et al. 2003. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord 18:41–48 [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weber T, Kracht L, Roggendorf J, Baudrexel S, et al. . 2008. STN-DBS activates the target area in Parkinson disease An FDG-PET study. Neurology 71:708–713 [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, et al. . 2004. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab 24:7–16 [DOI] [PubMed] [Google Scholar]
- Hill KK, Campbell MC, McNeely ME, Karimi M, Ushe M, Tabbal SD, et al. . 2013. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson's disease. Exp Neurol 241:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jech R. 2008. Functional imaging of deep brain stimulation: fMRI, SPECT, and PET. In: Tarsy D, Vitek JL, Starr PA, Okun MS. (eds.) Deep Brain Stimulation in Neurological and Psychiatric Disorders. Totowa, NJ: Humana Press; pp. 179–201 [Google Scholar]
- Jech R, Urgosik D, Tintera J, Nebuzelsky A, Krasensky J, Liscak R, et al. . 2001. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson's disease. Mov Disord 16:1126–1132 [DOI] [PubMed] [Google Scholar]
- Kahan J, Mancini L, Urner M, Friston K, Hariz M, Holl E, et al. . 2012. Therapeutic subthalamic nucleus deep brain stimulation reverses cortico-thalamic coupling during voluntary movements in Parkinson's disease. PLoS One 7:e50270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Knoblich U, Desai M, Bernstein J, Graybiel AM, Boyden ES, et al. . 2013. Optogenetic drive of neocortical pyramidal neurons generates fMRI signals that are correlated with spiking activity. Brain Res 1511:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, et al. . 2008. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain 131:2710–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. 2005. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci 25:8611–8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. . 2010. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. . 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89:5675–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H-Y, Younce JR, Albaugh DL, Kao Y-CJ, Shih Y-YI. 2013. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage 84C:11–18 [DOI] [PubMed] [Google Scholar]
- Le Jeune F, Verin M, N'Diaye K, Drapier D, Leray E, Du Montcel ST, et al. . 2010. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry 68:1016–1022 [DOI] [PubMed] [Google Scholar]
- Ledonne A, Mango D, Bernardi G, Berretta N, Mercuri NB. 2012. A continuous high frequency stimulation of the subthalamic nucleus determines a suppression of excitatory synaptic transmission in nigral dopaminergic neurons recorded in vitro. Exp Neurol 233:292–302 [DOI] [PubMed] [Google Scholar]
- Lee KH, Chang S-Y, Roberts DW, Kim U. 2004. Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus. J Neurosurg 101:511–517 [DOI] [PubMed] [Google Scholar]
- Lee KH, Kristic K, van Hoff R, Hitti FL, Blaha C, Harris B, et al. . 2007. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res 1162:121–129 [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. 2004. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson's disease. Clin Neurophysiol 115:2530–2541 [DOI] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, et al. . 2012. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76:1030–1041 [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. 2007. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol 98:3525–3537 [DOI] [PubMed] [Google Scholar]
- Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. 1997. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol 42:283–291 [DOI] [PubMed] [Google Scholar]
- Lippert MT, Steudel T, Ohl F, Logothetis NK, Kayser C. 2010. Coupling of neural activity and fMRI-BOLD in the motion area MT. Magn Reson Imaging 28:1087–1094 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157 [DOI] [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. 2007. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab 27:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L, Mesnage V, Houeto J-L, Pelissolo A, Yelnik J, Behar C, et al. . 2002. Compulsions, Parkinson's disease, and stimulation. Lancet 360:1302–1304 [DOI] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter M-L, Fontaine D, et al. . 2008. Subthalamic nucleus stimulation in severe obsessive–compulsive disorder. N Engl J Med 359:2121–2134 [DOI] [PubMed] [Google Scholar]
- Mathai A, Wichmann T, Smith Y. 2013. More than meets the Eye-Myelinated axons crowd the subthalamic nucleus. Mov Disord [Epub ahead of print]; DOI: 10.1002/mds.25603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CJ, Ronnqvist KC, Stanford IM, Woodhall GL, Furlong PL, Hall SD. 2013. Oscillatory Beta activity mediates neuroplastic effects of motor cortex stimulation in humans. J Neurosci 33:7919–7927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. 2012. Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J Neurosci 32:15657–15668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. 2004a. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol 115:589–595 [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. 2004b. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115:1239–1248 [DOI] [PubMed] [Google Scholar]
- Meijer HG, Krupa M, Cagnan H, Lourens MA, Heida T, Martens HC, et al. . 2011. From Parkinsonian thalamic activity to restoring thalamic relay using deep brain stimulation: new insights from computational modeling. J Neural Eng 8:066005. [DOI] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenstern R, et al. . 2002. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett 328:105–108 [DOI] [PubMed] [Google Scholar]
- Meissner W, Harnack D, Reese R, Paul G, Reum T, Ansorge M, et al. . 2003. High-frequency stimulation of the subthalamic nucleus enhances striatal dopamine release and metabolism in rats. J Neurochem 85:601–609 [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. 2012. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 16:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HK, Hwang SC, Marsh MP, Kim I, Knight E, Striemer B, et al. . 2012. Deep brain stimulation induces BOLD activation in motor and non-motor networks: an fMRI comparison study of STN and EN/GPi DBS in large animals. Neuroimage 63:1408–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. . 2010. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord 25:578–586 [DOI] [PubMed] [Google Scholar]
- Nambu A. 2008. Seven problems on the basal ganglia. Curr Opin Neurobiol 18:595–604 [DOI] [PubMed] [Google Scholar]
- Niethammer M, Feigin A, Eidelberg D. 2012. Functional neuroimaging in Parkinson's disease. Cold Spring Harb Perspect Med 2:a009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki T, Sugiyama K, Yagi S, Yoshikawa E, Kanno T, Asakawa T, et al. . 2013. Effect of subthalamic nucleus stimulation during exercise on the mesolimbocortical dopaminergic region in Parkinson's disease: a positron emission tomography study. J Cereb Blood Flow Metab 33:415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, et al. . 2008a. The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol 64 Suppl 2:S30–S46 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez MC, Guridi J, Alvarez L, Alvarez E, Macias R, et al. . 2001. Lesion of the basal ganglia and surgery for Parkinson disease. Arch Neurol 58:1165–1166 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, et al. . 2008b. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord 23 Suppl 3:S548–S559 [DOI] [PubMed] [Google Scholar]
- Odekerken VJJ, van Laar T, Staal MJ, Mosch A, Hoffmann CFE, Nijssen PCG, et al. . 2013. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12:37–44 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee T, Kay A, Tank D. 1990. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, et al. . 1992. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 89:5951–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschali A, Constantoyannis C, Angelatou F, Vassilakos P. 2013. Perfusion brain SPECT in assessing motor improvement after deep brain stimulation in Parkinson's disease. Acta Neurochir (Wien) 155:497–505 [DOI] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, et al. . 2008. Resting-state functional connectivity of the rat brain. Magn Reson Med 59:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME. 2000. PET: the merging of biology and imaging into molecular imaging. J Nucl Med 41:661–681 [PubMed] [Google Scholar]
- Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, et al. . 2006. Parkinson disease: pattern of Functional MR imaging activation during deep brain stimulation of subthalamic nucleus—initial experience1. Radiology 239:209–216 [DOI] [PubMed] [Google Scholar]
- Pizzolato G, Mandat T. 2012. Deep brain stimulation for movement disorders. Front Integr Neurosci 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Ardolino G, Marceglia S, Mrakic-Sposta S, Locatelli M, Tamma F, et al. . 2006. Low-frequency subthalamic oscillations increase after deep brain stimulation in Parkinson's disease. Brain Res Bull 71:149–154 [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. 2003. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem 49:1763–1773 [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T. 2010. Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat 4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud T, Dondaine T, Drapier S, Haegelen C, Lallement F, Peron J, et al. . 2010. Pallidal stimulation in advanced Parkinson's patients with contraindications for subthalamic stimulation. Mov Disord 25:1839–1846 [DOI] [PubMed] [Google Scholar]
- Scherfler C, Seppi K, Mair KJ, Donnemiller E, Virgolini I, Wenning GK, et al. . 2012. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson's disease. Brain 135:3348–3354 [DOI] [PubMed] [Google Scholar]
- Schweder PM, Joint C, Hansen PC, Green AL, Quaghebeur G, Aziz TZ. 2010. Chronic pedunculopontine nucleus stimulation restores functional connectivity. Neuroreport 21:1065–1068 [DOI] [PubMed] [Google Scholar]
- Sestini S, Pupi A, Ammannati F, Silvia R, Sorbi S, Castagnoli A. 2007. Are there adaptive changes in the human brain of patients with Parkinson's disease treated with long-term deep brain stimulation of the subthalamic nucleus? A 4-year follow-up study with regional cerebral blood flow SPECT. Eur J Nucl Med Mol Imaging 34:1646–1657 [DOI] [PubMed] [Google Scholar]
- Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. 2005. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson's disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med 46:1444–1454 [PubMed] [Google Scholar]
- Shih YY, Chen YY, Lai HY, Kao YC, Shyu BC, Duong TQ. 2013. Ultra high-resolution fMRI and electrophysiology of the rat primary somatosensory cortex. Neuroimage 73:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. 2013. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson's disease. J Neurosci 33:7220–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon YM, Lee KH, Goerss SJ, Kim IY, Kimble C, Van Gompel JJ, et al. . 2010. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci Lett 475:136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Fedele E, Galati S, Pepicelli O, Frasca S, Pierantozzi M, et al. . 2005. Subthalamic stimulation activates internal pallidus: evidence from cGMP microdialysis in PD patients. Ann Neurol 57:448–452 [DOI] [PubMed] [Google Scholar]
- Stefani A, Fedele E, Pierantozzi M, Galati S, Marzetti F, Peppe A, et al. . 2011. Reduced GABA content in the motor thalamus during effective deep brain stimulation of the subthalamic nucleus. Front Syst Neurosci 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Pierantozzi M, Ceravolo R, Brusa L, Galati S, Stanzione P. 2010. Deep brain stimulation of pedunculopontine tegmental nucleus (PPTg) promotes cognitive and metabolic changes: a target-specific effect or response to a low-frequency pattern of stimulation? Clin EEG Neurosci 41:82–86 [DOI] [PubMed] [Google Scholar]
- Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, et al. . 2003. Deep brain stimulation for Parkinson's disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord 18:1508–1516 [DOI] [PubMed] [Google Scholar]
- Strafella AP, Lozano AM, Lang AE, Ko JH, Poon YY, Moro E. 2007. Subdural motor cortex stimulation in Parkinson's disease does not modify movement-related rCBF pattern. Mov Disord 22:2113–2116 [DOI] [PubMed] [Google Scholar]
- Strutt AM, Simpson R, Jankovic J, York MK. 2012. Changes in cognitive-emotional and physiological symptoms of depression following STN-DBS for the treatment of Parkinson's disease. Eur J Neurol 19:121–127 [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Carrillo-Reid L, Bargas J. 2011. Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience 198:3–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AC, Yu W, Calos ME, Smith AB, Ramirez-Zamora A, Molho ES, et al. . 2013. Deep brain stimulation of the substantia nigra pars reticulata improves forelimb akinesia in the hemiparkinsonian rat. J Neurophysiol 109:363–374 [DOI] [PubMed] [Google Scholar]
- Tagliati M, Jankovic J, Pagan F, Susatia F, Isaias IU, Okun MS, et al. . 2009. Safety of MRI in patients with implanted deep brain stimulation devices. Neuroimage 47 Suppl 2:T53–T57 [DOI] [PubMed] [Google Scholar]
- Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. 2005. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol 76:393–413 [DOI] [PubMed] [Google Scholar]
- Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. 2006. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord 12:265–272 [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, et al. . 2011. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69:1248–1253; discussion 1254. [DOI] [PubMed] [Google Scholar]
- Thobois S, Jahanshahi M, Pinto S, Frackowiak R, Limousin-Dowsey P. 2004. PET and SPECT functional imaging studies in Parkinsonian syndromes: from the lesion to its consequences. Neuroimage 23:1–16 [DOI] [PubMed] [Google Scholar]
- Toleikis JR, Metman LV, Pilitsis JG, Barborica A, Toleikis SC, Bakay RA. 2012. Effect of intraoperative subthalamic nucleus DBS on human single-unit activity in the ipsilateral and contralateral subthalamic nucleus. J Neurosurg 116:1134–1143 [DOI] [PubMed] [Google Scholar]
- Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, et al. . 2006. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage 31:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Nagren K, et al. . 2009. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 36:2103–2110 [DOI] [PubMed] [Google Scholar]
- Vidal J, Fukushima FB, Boas PV. 2013. Deep-brain stimulation for Parkinson's disease. N Engl J Med 368:482–484 [DOI] [PubMed] [Google Scholar]
- Vitek JL. 2002. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 17 Suppl 3:S69–S72 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Logan J, Wang G-J, Ding Y-S, et al. . 1996. PET evaluation of the dopamine system of the human brain. J Nucl Med 37:1242. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. 2009. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56 Suppl 1:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. . 1994. Imaging endogenous dopamine competition with [11C] raclopride in the human brain. Synapse 16:255–262 [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. 2012. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873 [DOI] [PubMed] [Google Scholar]
- Weaver FM, Stroupe KT, Cao L, Holloway RG, Vickrey BG, Simuni T, et al. . 2012. Parkinson's disease medication use and costs following deep brain stimulation. Mov Disord 27:1398–1403 [DOI] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, et al. . 2006. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol 96:3248–3256 [DOI] [PubMed] [Google Scholar]
- Whitmer D, Solages C, Hill BC, Yu H, Bronte—Stewart H. 2013. Resting beta hypersynchrony in secondary dystonia and its suppression during pallidal deep brain stimulation in DYT3+ lubag dystonia. Neuromodulation 16:200–205 [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR, Guridi J, Obeso JA. 2011. Milestones in research on the pathophysiology of Parkinson's disease. Mov Disord 26:1032–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM. 2006. Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson's disease. Exp Neurol 197:244–251 [DOI] [PubMed] [Google Scholar]
- Wolz M, Hauschild J, Fauser M, Klingelhofer L, Reichmann H, Storch A. 2012. Immediate effects of deep brain stimulation of the subthalamic nucleus on nonmotor symptoms in Parkinson's disease. Parkinsonism Relat Disord 18:994–997 [DOI] [PubMed] [Google Scholar]
- Xu W, Miocinovic S, Zhang J, Baker KB, McIntyre CC, Vitek JL. 2011. Dissociation of motor symptoms during deep brain stimulation of the subthalamic nucleus in the region of the internal capsule. Exp Neurol 228:294–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CC, Fukuda M, Kim SG. 2011. BOLD responses to different temporal frequency stimuli in the lateral geniculate nucleus and visual cortex: insights into the neural basis of fMRI. Neuroimage 58:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangaglia R, Pacchetti C, Pasotti C, Mancini F, Servello D, Sinforiani E, et al. . 2009. Deep brain stimulation and cognitive functions in Parkinson's disease: a three-year controlled study. Mov Disord 24:1621–1628 [DOI] [PubMed] [Google Scholar]
- Zekaj E, Saleh C, Menghetti C, Servello D. 2013. Does magnetic resonance imaging induce tissue damage due to DBS lead heating? Acta Neurochir (Wien) 155:1677–1678 [DOI] [PubMed] [Google Scholar]
- Zheng F, Lammert K, Nixdorf-Bergweiler BE, Steigerwald F, Volkmann J, Alzheimer C. 2011. Axonal failure during high frequency stimulation of rat subthalamic nucleus. J Physiol 589:2781–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]