Abstract
Significance: High-mobility group protein 1 (HMGB1) is an evolutionarily conserved and multifunctional protein. The biological function of HMGB1 depends on its cellular locations, binding partners, and redox states. Extracellular HMGB1 is a mediator of inflammation during infection or tissue injury. Immune cells actively release HMGB1 in response to infection, which in turn orchestrates both innate and adaptive immune responses. Recent Advances: Hyperacetylation of HMGB1 within its nuclear localization sequences mobilizes HMGB1 from the nucleus to the cytoplasm and subsequently promotes HMGB1 release. The redox states of the cysteines in positions 23, 45, and 106 determine the biological activity of the extracellular HMGB1. Critical Issues: The full picture and the detailed molecular mechanisms of how cells regulate the posttranslational modifications and the redox status of HMGB1 during immune responses or under stress not only unravel the molecular mechanisms by which cells regulate the release and the biological function of HMGB1 but may also provide novel therapeutic targets to treat inflammatory diseases. Future Directions: It is important to identify the signaling pathways that regulate the posttranslational modifications and the redox status of HMGB1 and find their roles in host immune responses and pathogenesis of diseases. Antioxid. Redox Signal. 24, 620–634.
Introduction
High-mobility group protein 1 (HMGB1) is a 25-kDa protein ubiquitously expressed and evolutionarily highly conserved. HMGB1 is abundantly expressed in most kinds of mammalian cells. The amino sequence of HMGB1 is 98.5% identical in mammals (1). HMGB1 was first identified, 40 years ago, as a nonhistone chromatin-binding protein with high electrophoretic mobility (1, 33). The major structural features of HMGB1 are the two DNA-binding domains, termed box A and box B, and a negatively charged C-terminal acidic region. HMGB1 contains two nuclear localization sequences (NLS), which are recognized by the nuclear import complexes, resulting in a predominantly nuclear localization of HMGB1 under physiological conditions (1). Early studies demonstrated that nuclear HMGB1 stabilizes chromatin structure, modulates gene transcription, and promotes DNA damage repair by bending the DNA helical structure (6a, 33).
Another early study showed that the cytoplasm also contains considerable amounts of HMGB1, implicating a role of HMGB1 in the cytoplasm (36). Recently, Tang et al showed that cytoplasmic HMGB1 can bind Beclin-1 and promote mitophagy/autophagy and is thus involved in cell stress responses (77). Cell stress or infectious agents may stimulate HMGB1 translocation from the nucleus to the cytoplasm and subsequent release into the extracellular milieu (1, 76).
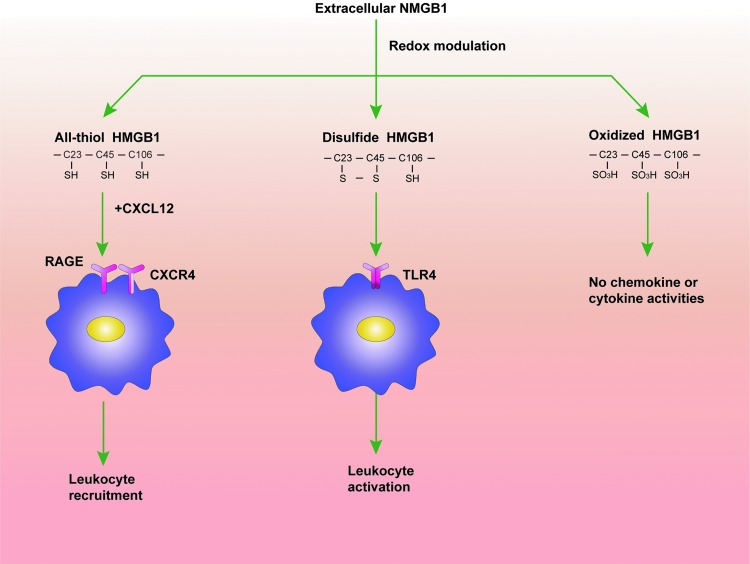
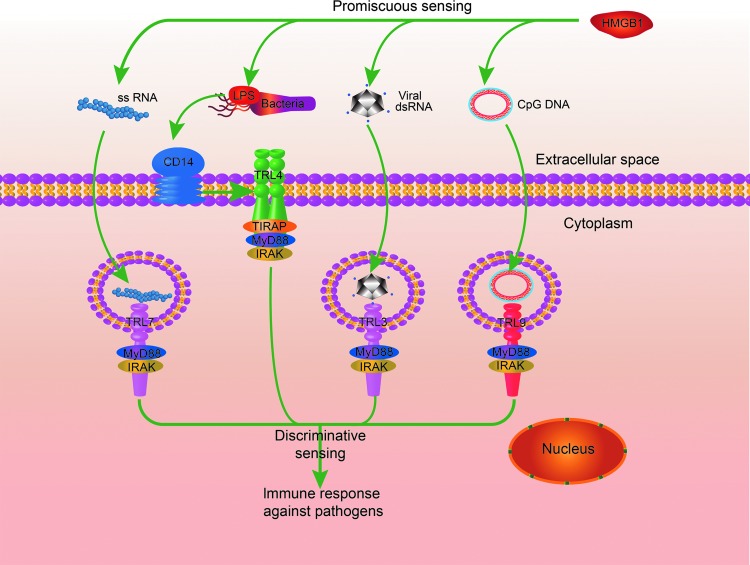
Numerous studies have focused on the biological role of extracellular HMGB1, since the protein is here involved in a variety of immune responses, acting as a prototypic damage-associated molecular pattern molecule (1). Extracellular HMGB1 was first identified as a late mediator of endotoxemia and sepsis. Unlike most classical cytokines that are released early during systemic inflammatory responses, HMGB1 is secreted in a delayed manner and maintains high levels in the circulation after the onset of sepsis (up to 100 ng/ml) (5, 85). Extracellular HMGB1 orchestrates the immune responses in a number of ways (1, 83, 90). For example, HMGB1 may act as a proinflammatory mediator that stimulates tumor necrosis factor (TNF) release during infection or sterile tissue injury (72, 91). HMGB1 may also promote migration of monocytes, dendritic cells, and neutrophils (15, 63, 83). The biological functions of extracellular HMGB1 are determined by its multiple reciprocal receptors, binding partners, and redox states (Fig. 1). In this context, fully reduced HMGB1 (HMGB1C23hC45hC106h), with all three cysteines expressing thiol groups, forms a complex with stromal cell-derived factor 1 (CXCL12) and synergistically promotes migration of immune cells via the chemokine receptor C-X-C chemokine receptor type 4 (CXCR4) and the receptor for advanced glycation end products (RAGE); partially oxidized HMGB1 with a disulfide bond between cysteine 23 and cysteine 45, termed disulfide HMGB1 (HMGB1C23-C45C106h), triggers inflammatory responses via Toll-like receptor (TLR) 4 (4, 82) but is unable to collaborate with CXCL12. The fully oxidized HMGB1, termed sulfonyl HMGB1 (HMGB1C23soC45soC106so), has no chemokine or cytokine activities. Intriguingly, HMGB1 may form complexes with almost all kinds of nucleic acids, as well as lipopolysaccharide (LPS), and increase the magnitude of immune responses to those agents (89, 94). In this regard, HMGB1 was recently proposed as a promiscuous receptor that broadly recognizes pathogen-derived molecules and amplifies immune responses initiated by pattern recognition receptors, including TLR3, TLR4, TLR7, and TLR9 (89) (Fig. 2).
FIG. 1.
The redox states of the cysteines in positions 23, 45, and 106 determine the biological activity of the extracellular HMGB1. The fully reduced HMGB1 forms a complex with CXCL12 and synergistically promotes migration of leukocytes via CXCR4 and RAGE; partially oxidized HMGB1 with a disulfide bond between cysteine 23 and cysteine 45 triggers inflammatory responses and the activation of leukocytes via TLR4 but is unable to collaborate with CXCL12. The fully oxidized HMGB1 has no chemokine or cytokine activities. CXCL12, stromal cell-derived factor 1; CXCR4, C-X-C chemokine receptor type 4; HMGB1, high-mobility group protein 1; RAGE, receptor for advanced glycation end products; TLR, Toll-like receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 2.
The role of extracellular HMGB1 in PAMP-induced immune responses. PRRs, such as TLR3, TLR4, TLR7, TLR9, and RIG-1, discriminatively sense distinct PAMPs and initiate immune responses, whereas extracellular HMGB1 promiscuously binds multiple PAMPs and broadly amplifies the PRR-induced immune responses. PAMPs, pathogen associated molecular patterns; PRRs, pattern recognition receptors. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
HMGB1 is an important player in adaptive immune responses. Early works showed that HMGB1 induces the maturation of dendritic cells and promotes the proliferation of T lymphocytes in an autocrine/paracrine manner (7, 14, 55). Activated dendritic cells release HMGB1 a few hours before its maturation. This process is critical for the efficient proliferation of antigen-specific T lymphocytes and their polarization toward a T helper-1 phenotype (14, 55). In addition, dendritic cell-released HMGB1 is important for the migration of dendritic cells to the draining lymph nodes, where dendritic cells engage antigen-specific T lymphocytes (7). HMGB1 released by dying tumor cells can also activate dendritic cells by signaling through TLR4 and is critical for the dendritic cell-mediated antitumor immunity during chemotherapy/radiotherapy (6). Notably, a TLR4 polymorphism that diminishes the HMGB1 and TLR4 interaction predicts early relapse after anthracycline-based chemotherapy in breast cancer patients (6). Because the biological activity of HMGB1 is determined by its redox status, it is not surprising that several environmental cues affect HMGB1-mediated immune responses. For example, reducing agents could prevent apoptosis-induced full oxidation of HMGB1 and thus reverse the immunological tolerance induction by apoptotic cells (41).
Although extracellular HMGB1 may play a crucial role in mounting sufficient immune responses against invading pathogens, excessive accumulation of HMGB1 in the circulation or the extracellular space in tissue deregulates homeostasis and may lead to diseases (1, 47). In addition to its proinflammatory effects, extracellular HMGB1 prevents the phagocytosis of Pseudomonas aeruginosa (16, 65). Accordingly, polyclonal or monoclonal antibodies that neutralize the activity of HMGB1 significantly improve survival in experimental sepsis and enhance bacterial clearance during P. aeruginosa infection (1, 16, 65). Administration of anti-HMGB1 antibodies or other HMGB1 antagonists, such as the recombinant HMGB1 box A peptide, significantly ameliorates the severity of experimental rheumatoid arthritis, colitis, and pancreatitis and prevents cognitive decline after experimental polymicrobial sepsis (1, 9, 38, 81). Importantly, in vivo knockdown of HMGB1 expression by synthesized short interfering RNAs (siRNA) in macrophages and dendritic cells dramatically reduced sepsis-induced mortality (93). These observations establish that deregulated HMGB1 release drives the pathogenesis of a number of infectious and autoimmune diseases, such as sepsis, rheumatoid arthritis, and colitis. While it is obvious that HMGB1 can be passively released from damaged or dying cells during tissue injury, it is yet not fully understood how HMGB1 is discharged from activated immune cells. This is a central question because the identification of key pathways that regulate HMGB1 release from activated immune cells may allow a design of novel therapeutics to treat many inflammatory diseases. It is known that posttranslational modifications of HMGB1 not only are essential for HMGB1 release from activated immune cells but also determine the biological activity of the released HMGB1. In this review, we focus on the recently identified signaling pathways controlling posttranslational modifications of HMGB1 and their roles in mediating HMGB1 release in host immune responses and the pathogenesis of diseases.
Regulation of HMGB1 Release from Activated Immune Cells
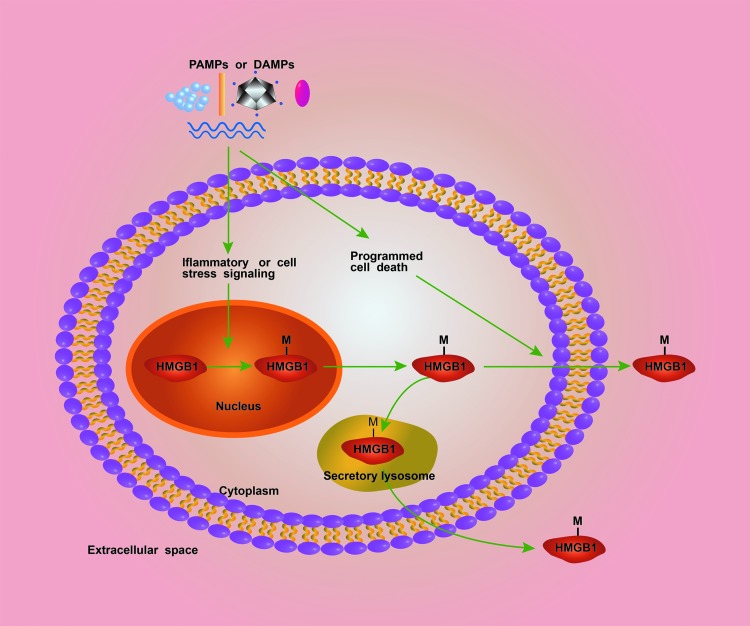
Most cytokines harbor a leader peptide that facilitates secretion through the endoplasmic reticulum (ER)—Golgi exocytotic route. HMGB1, which lacks a leader peptide, is released via unconventional protein secretion pathways (1, 49). Recent advance establishes two essential steps for HMGB1 release from activated immune cells. In the first step, infection or cellular stress induces the posttranslational modification of HMGB1 that culminates in HMGB1 cytoplasmic accumulation. In the second step, pathogens and endogenous danger signals induce rapid programmed cell death that mediates cytoplasmic HMGB1 release into the extracellular space or the formation of secretory lysosomes that deliver HMGB1 outside cells (49) (Fig. 3).
FIG. 3.
The mechanisms for HMGB1 release from activated immune cells. There are two steps for HMGB1 release from activated immune cells. In the first step, DAMPs or PAMPs stimulate the posttranslational modifications (M) of HMGB1 at the nuclear localization sequence sites in immune cells. This leads to the translocation of HMGB1 from the nucleus to the cytoplasm. In the second step, the DAMPs or PAMPs induce HMGB1 release into the extracellular space either through the formation of secretory lysosomes or through the activation of cell death programs, such as pyroptosis and necroptosis. DAMP, damage-associated molecular pattern molecule. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Regulation of HMGB1 cytoplasmic accumulation via posttranslational modifications of HMGB1 within the NLS sites
Protein shuttling between the nucleus and the cytoplasm depends on NLS and nuclear export sequences (NES), which are recognized by the nuclear import complex and nuclear export complex, respectively (23). HMGB1 contains two NLS sites and two nonclassical NES and therefore shuttles continually between the nucleus and the cytoplasm. However, the equilibrium is almost completely shifted toward a nuclear accumulation in most cells under physiological conditions (8). It is well known that posttranslational modifications of NLS or NES may block the interaction between these proteins and nuclear import complex or nuclear export complex and subsequently change the localization of intracellular proteins (23). These posttranslational modifications include acetylation, phosphorylation, and methylation. Infection or cell stress can induce these modifications and shift the equilibrium from a predominantly nuclear location toward a cytoplasmic accumulation and a subsequent release (8, 32, 95).
Acetylation is the most studied posttranslational modification that regulates HMGB1 cytoplasmic accumulation. The two NLS sites contain four and five lysines, respectively (8, 17). Acetylation of these lysines is determined by both histone acetylases (HATs) and histone deacetylases (HDACs). HDACs are a family of enzymes that remove acetyl groups and control the acetylation state of histones and additional intracellular proteins. HATs induce HMGB1 acetylation, whereas the activity of HDACs is conversely correlated with the extent of HMGB1 acetylation (8, 17). Bonaldi et al. first described that stimulation of LPS, TNF, or IL-1β uniformly induces HMGB1 hyperacetylation within NLS sites in monocytes (8). This study provided three strong pieces of evidence that HMGB1 hyperacetylation within NLS sites is critical for HMGB1 translocation from the nucleus to the cytoplasm as follows: (1) pharmacological inhibition of HDACs that induces HMGB1 hyperacetylation translocates HMGB1 from the nucleus to the cytoplasm; (2) mutation of six lysines to glutamine, which mimics acetylated lysines, also causes HMGB1 translocation from the nucleus to the cytoplasm; and (6a) mutation of the same six lysines to arginine, which cannot be acetylated, blocks HMGB1 translocation from the nucleus to the cytoplasm in response to HDAC inhibitors (8).
It should be noted that the ability to acetylate HMGB1 and mobilize it from the nucleus to the cytoplasm is not the privilege of immune cells only. Evankovich et al. demonstrated that liver ischemia/reperfusion (I/R) injury induces HMGB1 acetylation and cytoplasmic accumulation in an HDAC-dependent manner (17). During hypoxia, the levels of acetylated HMGB1 significantly increased with a concomitant decrease in total nuclear HDAC activity in hepatocytes (17). Among the HDAC family members, HDAC1 is a critical regulator of HMGB1 acetylation and translocation from the nucleus to the cytoplasm during oxidative stress. Oxidative stress increases the expression of HDAC1 in hepatocytes. Knockdown of HDAC1 by siRNA promoted HMGB1 cytoplasmic accumulation (17). Unlike immune cells, which actively release HMGB1 to modulate immune responses, hepatocytes mobilize HMGB1 from the nucleus to the cytoplasm for a different reason. Cytoplasmic HMGB1 binds Beclin-1 and promotes mitophagy/autophagy, self-protective processes that remove damaged and reactive oxygen species (ROS)-producing mitochondria (36, 37, 77). Accordingly, hepatocyte-specific HMGB1 knockout mice have significantly increased liver injury compared to control mice in response to I/R injury. The loss of intracellular HMGB1 in the hepatocytes exacerbates mitochondrial damage and ROS production (29). Interestingly, the oxidative stress, in return, could enhance HMGB1 translocation from the nucleus to the cytoplasm and release, possibly through the keap1/Nrf2 pathway, which is redox regulated and plays a key role in protecting cells against redox stress (21, 44, 78). Due to the oxidative stress, circulating HMGB1 released by damaged or necrotic hepatocytes during liver injury carries the signature of the posttranslational modifications (5, 20). There are several types of serum HMGB1 isoforms, including disulfide-bonded hyperacetylated HMGB1, disulfide-bonded nonacetylated HMGB1, and HMGB1 phosphorylated in serine 35, in liver diseases, such as alcoholic liver disease and drug-induced liver injury (5, 20). Notably, increased total and acetylated HMGB1 in serum were associated with worse prognosis in patients with liver diseases. Acetylated HMGB1 was a better predictor of outcome than the total HMGB1 (5).
Regulation of HMGB1 cytoplasmic accumulation by JAK/STAT1 pathway
We and others have previously established an important role of type 1 and type 2 interferons (IFN) and downstream janus kinase/signal transducers and activators of transcription (JAK/STAT1) signaling activation in mediating HMGB1 release (43, 69). In the light of these findings, we recently explored the role of JAK/STAT1 signaling in regulation of HMGB1 nuclear translocation to the cytoplasm. Indeed, pharmacological inhibition of JAK/STAT pathways or genetic deletion of STAT1 blocks LPS- or type 1 IFN-induced HMGB1 cytoplasmic accumulation (49). The regulation of HMGB1 subcellular location by the JAK/STAT1 is specific to LPS or IFN because pharmacological inhibition of JAK/STAT does not affect rapamycin or hydrogen peroxide-induced HMGB1 cytoplasmic accumulation (49). Mechanistically, JAK/STAT1 signaling is required for LPS- or IFN-induced hyperacetylation of HMGB1 within the NLS sites (49). In contrast, JAK/STAT1 signaling is dispensable for LPS- or IFN-induced HMGB1 intramolecular disulfide bond formation, a posttranslational modification that enables HMGB1 to initiate inflammatory responses by signaling through TLR4 and myeloid differentiation factor 2 (MD2) (49). These observations pinpoint an important role of the JAK/STAT1 in promoting HMGB1 acetylation and subsequent cytoplasmic accumulation. The JAK/STAT1 signaling is the common downstream pathway of type 1 and type 2 IFN (49). It is well known that type 1 and type 2 IFN are cytokines that confer protection against intracellular pathogens, such as virus and intracellular bacteria (62). These facts raise an intriguing possibility that HMGB1 cytoplasmic accumulation may be a host strategy to combat viral or intracellular bacterial invasion. This has elegantly been demonstrated by Taniguchi and colleagues to be the case (89).Cytoplasmic HMGB1 augments foreign nucleic acid-induced innate immune responses, which are essential for pathogen clearance (89). HMGB1 cytoplasmic accumulation also promotes autophagy, which is a well-known cellular strategy to remove intracellular bacteria and certain viruses. However, this seemly protective response may under certain conditions also be involved in the pathogenic pathways, leading to sepsis. Several studies demonstrate that pharmacological inhibition of the JAK/STAT1 pathway or genetic deletion of STAT1 significantly promotes survival in lethal endotoxemia as well as experimental sepsis. These observations suggest that the JAK/STAT1 pathway may provide a novel therapeutic target in sepsis (25, 43, 71).
The downstream mechanism by which JAK/STAT1 mediates HMGB1 acetylation is not fully understood. A recent study reveals an important role of interferon response factor (IRF) 1 for mediating HMGB1 acetylation during liver I/R injury (13). Hypoxia induces the nuclear upregulation of IRF1 in hepatocytes in a TLR4-dependent manner. Loss of IRF1 in hepatocytes significantly inhibits HMGB1 acetylation and release (13). Mechanistically, IRF1-mediated HMGB1 acetylation depends on the activity of histone acetyltransferase. Liver I/R induces the physical interaction between IRF1 and the nuclear histone acetyltransferase enzyme p300 (13). Notably, IRF1 is a downstream factor of the JAK/STAT1 signaling in macrophages (61). Stimulation of type 1 IFN significantly enhances IRF1 expression. Genetic deletion of IRF1 markedly reduces LPS-induced HMGB1 release in macrophages and significantly promotes survival during experimental sepsis (64). Thus, it is conceivable that IRF1 is one of the downstream factors of the JAK/STAT1 signaling that mediates HMGB1 acetylation and release in macrophages.
Regulation of HMGB1 cytoplasmic accumulation via phosphorylation or methylation of HMGB1 NLS sites
Acetylation is not the only type of posttranslational modification that regulates HMGB1 subcellular localization. Recent studies reveal that phosphorylation and methylation of HMGB1 within the NLS sites also importantly regulate the subcellular localization of HMGB1. Phosphorylation of several plant HMG family proteins has been observed and reported to modulate the interaction between these proteins and DNA molecules. In the light of these studies, Youn et al. found that stimulation of TNF or okadaic acid, a phosphatase inhibitor, induces HMGB1 phosphorylation (95). The important role of phosphorylation in regulating HMGB1 subcellular localization was demonstrated by a nuclear import assay, which showed that phosphorylated HMGB1 in the cytoplasm did not enter the nucleus (95). There are six serine residues within the two NLS sites. Substitution of these serine residues to glutamic acid, that mimics the phosphorylated serine, results in constitutively HMGB1 cytoplasmic accumulation. In contrast, replacement of these serine residues with alanine inhibits okadaic acid-induced HMGB1 cytoplasmic accumulation (95). Positively charged residues are abundant in the NLS and are necessary for binding to the nuclear importin proteins, such as the karyopherins (11). Thus, the underlying mechanism by which phosphorylation of HMGB1 within NLS sites relocates HMGB1 into the cytoplasm might be that the phosphorylation alters the charge of HMGB1 NLS sites and subsequently disrupts the interaction between HMGB1 and the nuclear importin. The upstream signaling pathway that regulates HMGB1 phosphorylation within NLS sites is not fully understood. A recent study revealed an important role of calcium/calmodulin-dependent protein kinase (CaMK) IV in the phosphorylation and release of HMGB1 from LPS-stimulated macrophages (97). CaMK IV-deficient macrophages display significantly decreased HMGB1 serine phosphorylation and release in response to LPS stimulation compared to the wild-type macrophages (97). CaMK IV also regulates HMGB1 phosphorylation and release in hepatocytes during I/R injury (80). However, whether CaMK IV directly mediates HMGB1 serine phosphorylation within NLS sites remains to be elucidated.
During infection or autoimmune reactions, neutrophils are an important source of extracellular HMGB1 (1). Unlike other types of immune cells, most HMGB1 locates in the cytoplasm rather in the nucleus (32). One explanation is that HMGB1 in neutrophil is monomethylated at lysine 42. The methylation at this site changes the conformation of HMGB1 and weakens its DNA-binding activity, causing it to accumulate in the cytoplasm by passive diffusion out of the nucleus (32). Thus, different types of cells might use distinct posttranslational modifications to regulate HMGB1 subcellular location.
Regulation of HMGB1 release into the extracellular space
As mentioned above, HMGB1 lacks a leader peptide and thus cannot be secreted by the conventional ER—Golgi exocytotic route (1). Many studies have reported that HMGB1 is actively released by immune cells during inflammation and proposed several separate pathways that deliver HMGB1 from the cytoplasm to the extracellular space. These unconventional protein secretion pathways include pyroptosis, necroptosis, apoptosis, NETosis, and secretory lysosome-mediated release.
HMGB1 release via pyroptosis
Despite the fact that HMGB1 can be released into the extracellular space through many different routes, a fundamental question in the field remains to be answered: What is the dominant pathway that mediates HMGB1 release in inflammatory diseases, such as sepsis? An early study unexpectedly demonstrated that pharmacological inhibition of caspase activity significantly blocked HMGB1 release and promoted survival in experimental sepsis (68). The improved survival rate in the caspase inhibitor-treated groups was, at least in part, due to a reduced HMGB1 release since HMGB1-neutralizing monoclonal antibodies dose dependently promoted survival in experimental sepsis (68). The pan-caspase inhibitor, used in this study, inhibited both caspase-3-mediated apoptosis and caspase-1/caspase-11-mediated pyroptosis, which is a proinflammatory programmed cell death form (46). This raises an intriguing question: Which is the dominant pathway that mediates HMGB1 release during experimental sepsis: apoptosis or caspase-1/caspase-11-mediated pyroptosis? Most transgenic caspase-1 knockout mice also lack caspase-11 and thus have a reduced capacity for the induction of pyroptosis. Lamkanfi et al. found that these caspase-1/caspase-11-double-deficient mice have markedly lower serum HMGB1 levels during lethal endotoxemia compared to wild-type mice (46). This observation clearly indicates that pyroptosis is the dominant pathway that mediates HMGB1 release during endotoxemia. Accordingly, caspase-1/caspase-11-double-deficient mice have markedly higher survival rate during lethal endotoxemia compared to their wild-type controls (46). In addition to its role in pyroptosis, caspase-1 is critical for the maturation of pro-IL-1β and pro-IL-18, both of which are well-established proinflammatory cytokines. Surprisingly, genetic deletion of both IL-1β and IL-18 did not confer any protection against lethal endotoxemia (46). In contrast, HMGB1 neutralization via HMGB1-specific antibodies significantly prevented endotoxemia-induced death (46). These observations underscore the importance of the interplay between caspase-1/caspase-11 and HMGB1 in the pathogenesis of sepsis.
Caspase-1 and caspase-11 are activated by canonical and noncanonical inflammasomes, respectively (39). The inflammasome is a key component for innate immune responses and a sentinel protein complex that surveys the intracellular environment. The canonical inflammasome consists of at least two distinct components, including procaspase-1, and an NOD-like receptor (NLR) molecule or a molecule of the pyrin and HIN-200 domain-containing family. In response to certain danger signals, these proteins assemble into functional inflammasome complexes and subsequently activate caspase-1, which in turn mediates the maturation of pro-IL-1β and pro-IL-18, as well as pyroptosis (74). Dixit and colleagues. recently discovered a noncanonical inflammasome, which activates caspase-11 rather than caspase-1 (39). Caspase-11 physically interacts with caspase-1, and contributes to the activation of caspase-1, upon activation by noncanonical stimuli, such as Escherichia coli bacteria (39, 86). Notably, caspase-11 may mediate pyroptosis and HMGB1 release in the absence of caspase-1. Genetic deletion of caspase-11, rather than caspase-1, protects mice from lethal endotoxemia (39). Recent studies reveal that intracellular LPS induces caspase-11 activation even in the absence of its known receptor TLR4 (22, 40) (Fig. 4).
FIG. 4.
The activation of caspase-11. Mouse caspase-11 and human caspase-4/5 are the receptors for intracellular LPS. During Gram-negative bacterial infection, the lysis of bacteria-containing phagosomes results in LPS translocation to the cytoplasm. This culminates in the LPS recognition by caspase-4/5/11, which subsequently forms oligomers and mediates pyroptosis that leads to HMGB1 release. LPS, lipopolysaccharide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
It is noteworthy that HMGB1 released via pyroptosis has specific translational modifications. Mass spectrometry analysis reveals that HMGB1 released via pyroptosis is hyperacetylated at the NLS sites, whereas HMGB1 released via freeze–thaw cycle-induced necrosis contains hypoacetylated NLS sites (27). Together with the finding that HMGB1 hyperacetylation at NLS sites is essential for HMGB1 translocation from the nucleus to the cytoplasm, which greatly facilitates subsequent HMGB1 release, these observations implicate that HMGB1 release upon inflammasome activation is a highly regulated process. Interestingly, different types of inflammasome activation may induce distinct posttranslational modifications of HMGB1. In this context, NLRP3 inflammasome stimuli, including adenosine triphosphate (ATP), monosodium uric acid (MSU), and adjuvant aluminum, induce HMGB1 disulfide bond formation between cysteine 23 and cysteine 45 (27). In contrast, activation of the NLRC4 inflammasome results in the release of the fully reduced all-thiol form of HMGB1 (35). One possible explanation for these phenomena is that the activation of the NLRP3 inflammasome, but not the NLRC4 inflammasome, is associated with mitochondrial ROS production, which may oxidize HMGB1 and induce the Cys23–Cys45 disulfide bond formation (98). Both the NLRP3 and the NLRC4 inflammasome mediate the maturation of IL-1β and IL-18. However, the distinct redox states of HMGB1 may fine-tune the immune responses against different invading pathogens. For example, the NLRP3 inflammasome can be activated by several types of RNA viruses (45), which can generate extracellular HMGB1 with a disulfide bond that can activate TLR4 cascades to initiate inflammation and IFN production (1) that will augment the antiviral immune responses (89). The NLRC4 inflammasome is an intracellular sentinel against Salmonella typhimurium infection (56). The recruitment of neutrophils is essential for clearance of S. typhimurium (56). Accordingly, the fully reduced HMGB1, whose release is mediated by the NLRC4 inflammasome, will markedly increase the migration of neutrophils by forming protein complexes with CXCL12 to signal through the CXCR4 receptor (63, 82).
HMGB1 release via necroptosis
Necrotic cells passively release HMGB1 and trigger sterile inflammation (63, 82). Genetic deletion of HMGB1 or neutralization of extracellular HMGB1 activity markedly reduces necrotic cell-induced TNF production, establishing a critical role for HMGB1 in sterile tissue injury (72, 91). It was previously thought that necrosis is not a programmed cell death. However, recent advances reveal that necrosis induced by cytokines (e.g., TNF) or some viral infection may reflect a highly regulated process termed necroptosis or programmed necrosis (10, 24). The protein kinase receptor-interacting protein 3 (RIP3) is a molecular switch between TNF-induced apoptosis and necrosis. In the presence of a pan-caspase inhibitor, TNF stimulation results in the formation of an RIP3–RIP1 protein complex, which executes the necrotic process (10, 24, 58). In addition to TNF, type 1 or type 2 IFN can also induce necroptosis when the adaptor protein fas-associated death domain is lost or the caspase activity is blocked, which, for example, may occur during energy deprivation. Stimulation of IFN markedly induces the formation of RIP3–RIP1 complexes via the JAK/STAT1 pathway (79). It is clear that mitochondria-derived ROS are involved in the necroptosis process and partially account for the ability of RIP3–RIP1 protein complexes to promote the programmed necrosis (96). Thus, it will be of great interest to investigate whether HMGB1 released via necroptosis contains the Cys23–Cys45 disulfide bond or not.
Regulation of HMGB1 release by apoptosis
Unlike necrosis, apoptotic cells do not significantly release HMGB1 at the early stage when HMGB1 is retained within the nucleus surrounded by a cell membrane (72). Bacterial LPS or polyinosinic–polycytidylic acid, a double-stranded RNA (dsRNA) mimetic, could induce apoptosis of mouse macrophage. Using this in vitro system, Jiang et al. observed that HMGB1 release is correlated with the percentage of cells undergoing apoptosis (34). Indeed, apoptotic cells release HMGB1 during the late stage of apoptosis, termed secondary necrosis, which may occur if the removal and degradation of apoptotic bodies are inadequate (81). In this context, HMGB1 remains bound to nucleosomes released at the late stage of apoptosis. HMGB1-containing nucleosomes from apoptotic cells could induce the production of proinflammatory cytokines, such as TNF and IL-1β, and the maturation of the dendritic cells (81). Notably, HMGB1–nucleosome complexes can be detected in plasma from patients with systemic lupus erythematosus (SLE) (81). Injection of HMGB1-containing nucleosomes from apoptotic cells induced anti-dsDNA and antihistone IgG responses in a TLR2-dependent manner in mice (81). These findings suggest that apoptotic cell-released HMGB1-containing nucleosomes may crucially contribute to the pathogenesis of SLE. Interestingly, apoptotic cells could stimulate macrophage to release HMGB1. Administration of a pan-caspase inhibitor, which blocks apoptosis, markedly reduced serum HMGB1 levels during experimental sepsis (68). One possible underlying mechanism is that apoptotic cells robustly secrete ATP, which in turn activates the NLRP3 inflammasome in immune cells, which subsequently results in HMGB1 release (79). Accordingly, overexpression of the antiapoptotic gene, bcl-2 gene, or pharmacological inhibition of apoptosis markedly reduces HMGB1 release and improves the survival in experimental sepsis (68). It is noteworthy that apoptotic cells also release HMGB1-containing microparticles (66, 67). Microparticles are small membrane-bound vesicles that are released from cells during cell death or cell activation (67). In vitro, bacterial LPS or polyinosinic–polycytidylic acid could stimulate macrophage-like RAW 267.4 cells to secrete HMGB1-containing microparticles, whose release is associated with apoptosis (66, 67). Growing evidence indicates that the microparticles exert proinflammatory and prothrombotic effects. These studies also suggest that microparticles released during systemic inflammation are markers and mediators of microvascular dysfunction, immunosuppression, and renal dysfunction and that HMGB1 might contribute to the proinflammatory activity of the microparticles (66, 67, 73).
Regulation of HMGB1 release by double-stranded RNA-dependent kinase (PKR)
PKR is a 65-kDa intracellular protein, which contains two RNA-binding domains at the C-terminal and a kinase domain at the N-terminal (12). Although PKR was originally identified as an intracellular dsRNA receptor and a key antiviral protein, recent studies implicate that it is also an intracellular stress-sensing molecule (88). In addition to dsRNA, bacterial components and free fatty acid induce PKR autophosphorylation (28, 59). In the light of these findings, we recently identified an important role of PKR in inflammasome activation and HMGB1 release (56). Genetic deletion or pharmacological inhibition of PKR markedly reduces inflammasome activation induced by various NLRP3 agonists and the NLRP1 agonist, anthrax lethal toxin (LTx) (56). Overexpression of PKR significantly enhances the activity of the reconstituted NLRP3 and NLRP1 inflammasomes in nonimmune cells. These findings clearly indicate that PKR is an important regulator of several inflammasomes and subsequent HMGB1 release. However, the mechanisms by which PKR regulates inflammasome activation remain unknown. It is unlikely that PKR regulates inflammasome activation through phosphorylation of its substrates, partly because we did not find any phosphorylation of the inflammasome components (56) and partly because a mutant PKR that lost its kinase activity still activated the NLRP3 and NLRP1 inflammasomes (our unpublished data). A recent study made similar observations to ours. Knockdown of PKR expression by siRNA in macrophages blocked LTx-induced inflammasome activation and subsequent pyroptosis (26). Addition of a PKR inhibitor, 7-desacetoxy-6,7-dehydrogedunin, significantly inhibited the NLRP3 and NLRP1 inflammasome agonist-induced apoptosis-associated speck-like protein containing a CARD oligomerization, caspase-1 activation, and pyroptosis in macrophages (26).
Intriguingly, PKR also participates in other types of programmed cell death, including apoptosis and necroptosis (28, 79). Bacteria or their components, such as LPS, induced robust PKR phosphorylation and apoptosis in macrophages (28). Genetic deletion of PKR dramatically inhibited bacteria-induced macrophage apoptosis (28). The proapoptotic actions of PKR are, at least in part, mediated via inhibition of protein synthesis and activation of IRF3 because elF-2a or IRF3 deficiency significantly inhibited bacteria-induced apoptosis (28). A recent study showed that PKR is also required for type 1 or type 2 IFN-induced necroptosis (79). IFN transcriptionally activated PKR, which physically interacted with RIP1 to facilitate RIP3–RIP1 protein complex formation (79). Together with the identification of PKR as an important regulator of inflammasome-mediated pyroptosis (26, 56), these observations establish PKR as a master regulator of multiple programmed cell death pathways.
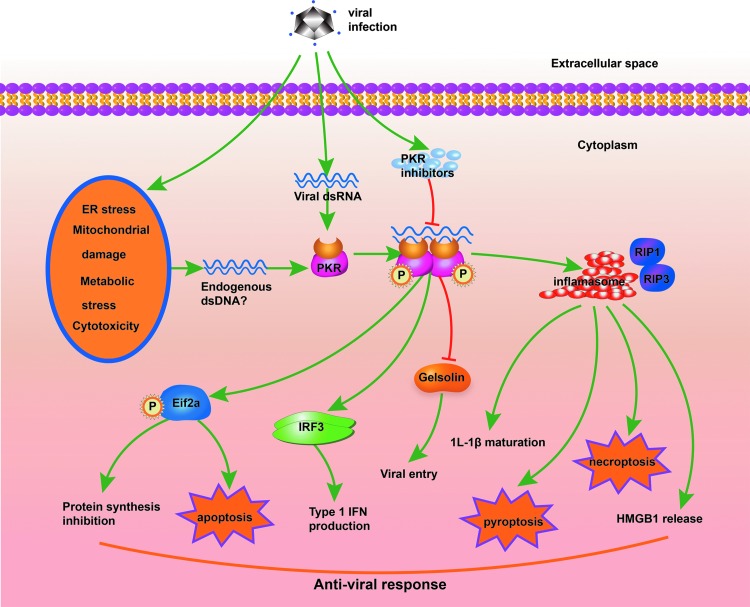
It is noteworthy that aforementioned facts provide novel insights into the comprehensive role of PKR in antiviral responses (Fig. 5). Numerous types of viruses inhibit PKR expression or activation, implicating important roles of PKR in antiviral responses (57). In addition to dsRNA molecules derived from RNA viruses, a variety of cellular stress processes, such as ER stress, mitochondrial damage, metabolic stress, and cytotoxicity, also induce PKR activation (12, 28, 56, 59, 88). In this context, the intact dsRNA-binding domain of PKR is required for PKR activation, implicating the existence of endogenous dsRNA molecules generated during cellular stress (59). Functioning as both a viral RNA receptor and a cellular stress response molecule, PKR may broadly detect all kinds of viral infections. Upon ligand recognition, PKR uncovers its intramolecular dimerization domain, culminating in PKR dimerization and its subsequent autophosphorylation (12). Phosphorylated PKR contributes to the antiviral responses in multiple ways as follows: (i) It recruits and phosphorylates eIF2a, resulting in inhibition of viral protein synthesis (12); (ii) PKR enhances type 1 IFN production by the interaction with IRF3 and nuclear factor-kappa B pathways (54, 88); (iii) PKR activation regulates actin dynamics and abrogates viral entry into cells by inhibiting gelsolin, a key actin-modifying protein (31); and (iv) PKR physically interacts with inflammasome components, RIP1, or IRF3 and broadly promotes programmed cell death, including apoptosis, pyroptosis, and necroptosis (26, 28, 56, 79). Apoptosis, pyroptosis, and necroptosis are important strategies for the host to directly eliminate the intracellular virus or in mounting sufficient antiviral immune responses (57). However, deregulated PKR expression and phosphorylation might contribute to the pathogenesis of a number of diseases (56). Studies show that PKR deficiency confers resistance to high-fat diet-induced obesity and insulin resistance, as well as Alzheimer's disease, suggesting PKR as a potential therapeutic target in these diseases (48, 59).
FIG. 5.
The role of PKR in antiviral responses. dsRNA molecules derived from RNA viruses and different forms of cellular stress can induce PKR dimerization and autophosphorylation. PKR contributes to the antiviral responses at many levels: (1) It recruits and phosphorylates eIF2a, resulting in inhibition of viral protein synthesis and apoptosis; (2) PKR enhances type 1 IFN production by interaction with IRF3 and NF-kB pathways; (3) PKR regulates actin dynamics and abrogates viral entry into cells by inhibiting gelsolin; and (4) PKR physically interacts with inflammasome components or RIP1 and promotes pyroptosis and necroptosis. dsRNA, double-stranded RNA; IFN, interferons; IRF, interferon response factor; NF-kB, nuclear factor-kappa B; RIP, receptor-interacting protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
HMGB1 release via NETosis
NETosis is a type of programmed cell death in activated neutrophils. In response to proinflammatory stimuli, such as LPS, TNF-α, and ROS, neutrophils release its DNA and numerous DNA-binding proteins, including HMGB1, into the extracellular milieu and form the neutrophil extracellular traps (NETs) (75). NETs play an important role in preventing microbial dissemination and promoting bacterial clearance (75). However, deregulated NET formation could contribute to the tissue damage and the pathogenesis of diseases, such as SLE and liver I/R injury (18, 30). For example, abnormal NET formation exerts a pathogenic role in SLE through the enhanced production of type 1 IFN (66). NETs are an important source of extracellular HMGB1 in this context. HMGB1 could significantly increase NET DNA-induced production of IFN-α and other cytokines in a TLR9-dependent manner in immune cells (75). Notably, HMGB1 could induce NET formation by signaling through the TLR4 and TLR9 and thus further increase the levels of extracellular HMGB1 (18, 30).
HMGB1 release via secretory lysosome
Activated immune cells release HMGB1 through two routes as follows: programmed cell death and exocytosis of secretory lysosome (50). Previous studies demonstrated that some leaderless proteins that cannot be secreted by the ER—Golgi exocytotic pathway—can be released into the extracellular space by the caspase-1-mediated unconventional protein secretion pathway (42). These proteins include caspase-1 and its binding partners, such as IL-1α and fibroblast growth factor-2 (42). Rubartelli and colleagues found that caspase-1 and its substrate IL-1β are packed into vesicle compartments, termed secretory lysosomes, before their release from activated monocytes (2). Unlike the conventional lysosomes, secretory lysosomes are specialized organelles that deliver their cargo into the extracellular space via exocytosis (2). Notably, cytoplasmic HMGB1 is also located in secretory lysosomes after LPS stimulation in human monocytes (19). Although both IL-1β and HMGB1 are released by secretory lysosomes, the upstream signals that trigger IL-1β or HMGB1 release are different. IL-1β secretion is induced earlier by ATP stimulation, which is followed by potassium efflux, whereas HMGB1 secretion is triggered by lysophosphatidylcholine (19). These observations provide evidence for those live immune cells that may actively release HMGB1 upon stimulation in addition to the already discussed pyroptotic or necroptotic pathways in dying cells. However, the intracellular signal pathways that control the formation of secretory lysosomes and the sequestration of cytoplasmic HMGB1 in secretory lysosomes remain largely unknown.
HMGB1 release during platelet activation
Early observations identified HMGB1 as an endogenous protein in platelets, which export HMGB1 from the intracellular compartment to the cell surface upon platelet activation (70). Maugeri et al. showed that platelets are an important source of the extracellular HMGB1 during vascular injury and that platelet-derived HMGB1 mediates platelet-induced neutrophil activation and NET formation by signaling through the RAGE (51, 53). In addition, platelet-derived HMGB1 promotes neutrophil survival, possibly through induction of autophagy and prevention of mitochondrial damage in neutrophils (53). Interestingly, the ROS generated by the activated neutrophils could enhance the proinflammatory activity of HMGB1 during vascular injury (53). Thus, the platelet-derived HMGB1 and the activated neutrophils form a positive feedback loop that amplifies inflammatory responses during sterile injury. It is noteworthy that decreased HMGB1 content and HMGB1 translocation to the outer leaflet of the plasma membrane were observed in circulating platelets of patients with systemic sclerosis compared to those of age-matched healthy controls, implying that platelet-derived HMGB1 contributes to pathogenesis of the disease (52). Recently, two studies independently showed that HMGB1 released by activated platelets is critical for regulating platelet activation, granule secretion, and adhesion by signaling through the TLR4 and guanylyl cyclase pathway (84, 92). Together, these studies establish that the platelet-derived HMGB1 is a critical player in both inflammation and thrombosis.
Regulation of HMGB1 Oxidation
HMGB1 contains three evolutionarily conserved cysteines (Cys23, Cys45, and Cys106). The redox states of these cysteines determine the biological function of extracellular HMGB1. Specifically, fully reduced HMGB1 forms a complex with CXCL12 and promotes the migration of immune cells via CXCR4 and RAGE. Partially oxidized HMGB1 with a disulfide bond between cysteine 23 and cysteine 45 initiates inflammatory responses by signaling through TLR4 (4, 82). The fully oxidized HMGB1, however, exerts neither chemotactic nor proinflammatory effect (41). While it is clear that redox modifications of HMGB1 importantly shape the innate immune responses, questions about how redox modifications of HMGB1 are regulated during immune responses remain largely unanswered at present.
Under physiological conditions, the majority of intracellular HMGB1 is fully reduced (49). The evidence that redox modifications of HMGB1 during immune responses are highly regulated processes comes from a study, which shows that a caspase- and mitochondria-dependent pathway mediates a terminal oxidation of HMGB1 in cells undergoing apoptosis (41). When the cellular apoptotic program is initiated, the activated caspases target mitochondria to produce excessive ROS (41). This event culminates in a terminal oxidation of HMGB1 expressing sulfonic groups, which renders HMGB1 immunologically inactive (41). Accordingly, unlike necrosis, which often results in inflammation and adaptive immune responses, apoptosis tends to be anti-inflammatory and promotes immune tolerance (41). Importantly, blocking sites of oxidation by mutation of the redox-sensitive cysteines in HMGB1 prevented immune tolerance induced by apoptotic cells (41). In addition, apoptotic cell-induced immune tolerance could be blocked when caspase-dependent ROS activity was blocked by scavenging or mutation of a mitochondrial caspase target, p75 NDUSF1 (41). Together, these observations not only indicate that redox modifications of HMGB1 critically orchestrate immune responses during sterile tissue injury but also clearly provide evidence that mitochondrial ROS mediate HMGB1 oxidation.
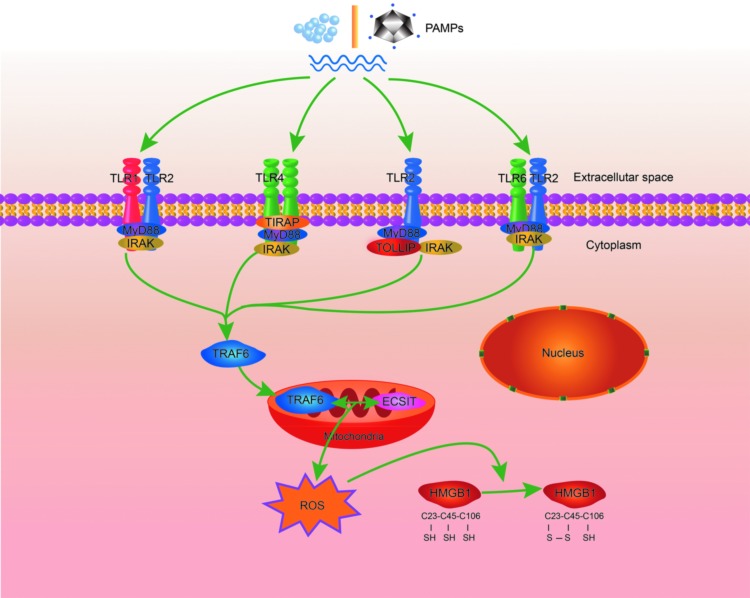
Intriguingly, extracellular ATP- and MSU-induced pyroptosis, a proinflammatory mode of cell death that is initiated by the activation of caspase-1 and involves robust mitochondrial ROS production (56), leads to the release of partially oxidized HMGB1 with a Cys23–Cys45 disulfide bond and a reduced Cys106. Unlike apoptotic cells, which restrain and condense intracellular proteins at the early stage of apoptosis, cells undergoing pyroptosis rapidly release their intracellular contents, including HMGB1 (39, 72, 74). This is likely the reason why HMGB1 released by pyroptotic cells is partially oxidized, while that released by apoptotic cells is terminally oxidized. Applequist and colleagues showed that the activation of caspase-1 does not mediate HMGB1 oxidation because the activation of the NLRC4 inflammasome by intracellular flagellin failed to induce HMGB1 oxidation (60). In contrast, we recently demonstrated that activation via TLR4 signaling induces HMGB1 Cys23–Cys45 disulfide bond formation (49). In line with this finding, a recent study showed that activation of TLR1, TLR2, and TLR4 augments mitochondrial ROS production (87). Mechanistically, activation of these TLRs results in the translocation of a TLR signaling adaptor protein, tumor necrosis factor receptor-associated factor 6 (TRAF6), to the mitochondria, where it interacts with the protein evolutionarily conserved signaling intermediate in Toll pathways (ECSIT). This is followed by ECSIT ubiquitination and enrichment in the mitochondria, culminating in significantly increased mitochondrial ROS generation (87). Thus, it is conceivable that this TLR–TRAF6–ECSIT–mitochondrial ROS signal pathway mediates HMGB1 oxidation during inflammation (Fig. 6).
FIG. 6.
Activation of TLR-mediated signaling may induce HMGB1 Cys23–Cys45 disulfide bond formation. Activation of TLRs results in the translocation of TRAF6 to mitochondria, where it interacts with the protein ECSIT. This is followed by ECSIT ubiquitination and enrichment in the mitochondria, culminating in significantly increased mitochondrial ROS generation. It is conceivable that this TLR–TRAF6–ECSIT–mitochondrial ROS signal pathway promotes HMGB1 oxidation during inflammation. ECSIT, evolutionarily conserved signaling intermediate in Toll pathway; ROS, reactive oxygen species; TRAF6, tumor necrosis factor receptor-associated factor 6. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Conclusions and Perspectives
In summary, HMGB1 is a multifunctional protein, and its biological activity and subcellular location are determined by distinct posttranslational modifications. These posttranslational modifications of HMGB1 in serum or tissue samples were analyzed by liquid chromatography–mass spectrometry (5, 20). Characterization of whole protein molecular weights, acetylated lysine residues, or redox modifications on cysteine residues within HMGB1 was performed by tandem mass spectrometry (5, 20). The data discussed in this review have identified some of the signaling pathways that control the posttranslational modifications of HMGB1 and their roles in mediating HMGB1 release in host immune responses and the pathogenesis of diseases. In this context, JAK/STAT1 signaling promotes HMGB1 acetylation within the NLS sites, leading to the translocation of HMGB1 from the nucleus to the cytoplasm. The TLR–mitochondrial ROS pathway induces partial oxidation of HMGB1 and formation of a Cys23–Cys45 disulfide bond, which enables HMGB1 to activate the TLR4–MD2 complex and initiate inflammation.
However, the molecular mechanisms of how cells regulate the posttranslational modifications of HMGB1 during immune responses or under stress are not fully understood. For example, certain stress influences may induce HMGB1 hyperacetylation in a JAK/STAT1-independent manner, which emphasizes that additional yet unresolved signal pathways regulate HMGB1 acetylation during stress responses. Another puzzle in this field is how the JAK/STAT1 pathway regulates HMGB1 acetylation, although it is likely that a downstream factor is either an acetylase or a partner molecule of an acetylase. In addition to acetylation, phosphorylation and methylation of HMGB1 within the NLS sites have also been demonstrated to promote HMGB1 translocation from the nucleus to the cytoplasm. How these separate posttranslational modifications of HMGB1 work in concert to determine HMGB1's subcellular localization remains still unknown. Finding the answers to these unresolved questions will not only unravel the molecular mechanisms by which cells regulate the posttranslational modifications of HMGB1 but may also provide novel therapeutic targets to treat inflammatory diseases.
Abbreviations Used
- ATP
adenosine triphosphate
- CaMK
calcium/calmodulin-dependent protein kinase
- CARD
caspase recruitment domain
- CXCL12
stromal cell-derived factor 1
- CXCR4
C-X-C chemokine receptor type 4
- Cys
cysteines
- DAMP
damage-associated molecular pattern molecule
- dsRNA
double-stranded RNA
- ECSIT
evolutionarily conserved signaling intermediate in Toll pathways
- ER
endoplasmic reticulum
- HATs
histone acetylases
- HDACs
histone deacetylases
- HMGB1
high-mobility group protein 1
- IFN
interferons
- IL-18
interleukin-18
- IL-1β
interleukin-1β
- IRF
interferon response factor
- I/R
ischemia/reperfusion
- JAK/STAT1
janus kinase/signal transducers and activators of transcription
- LPS
lipopolysaccharide
- LTx
anthrax lethal toxin
- MD2
myeloid differentiation factor 2
- MSU
monosodium urate monohydrate
- NES
nuclear export sequences
- NETs
neutrophil extracellular traps
- NF-kB
nuclear factor-kappa B
- NLR
NOD-like receptor
- NLRC4
the nucleotide-binding domain, leucine-rich repeat containing family caspase recruitment domain-containing 4
- NLRP1
NLR family pyrin domain-containing 1
- NLRP3
NLR family pyrin domain-containing 3
- NLS
nuclear localization sequences
- p75 NDUSF1
NADH dehydrogenase Fe-S protein-1
- PAMPs
pathogen associated molecular patterns
- PKR
double-stranded RNA-dependent kinase
- PRRs
pattern recognition receptors
- RAGE
receptor for advanced glycation end products
- RIP1
receptor-interacting protein 1
- RIP3
receptor-interacting protein 3
- ROS
reactive oxygen species
- siRNA
synthesized short interfering RNAs
- SLE
systemic lupus erythematosus
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRAF6
tumor necrosis factor receptor-associated factor 6
References
- 1.Andersson U. and Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Ann Rev Immunes 29: 139–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, and Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc Natl Acad Sci U S A 101: 9745–9750, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.This reference has been deleted
- 4.Antoine DJ, Harris HE, Andersson U, Tracey KJ, and Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med 20: 135–137, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, and Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol 56: 1070–1079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, André F, Delaloge S, Tursz T, Kroemer G, and Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13: 1050–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 6a.Austin M. and Neihart NK. Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell 16: 181–189, 1979 [DOI] [PubMed] [Google Scholar]
- 7.Bianchi ME. and Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220: 35–46, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, and Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551–5560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, Frankfurt M, Volpe BT, Tracey KJ, and Diamond B. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med 18: 930–937, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, and Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137: 1112–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti E, Uy M, Leighton L, Blobel G, and Kuriyan J. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin. Cell 94: 193–204, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Dey M, Cao C, Dar AC, Tamura T, Ozato K, Sicheri F, and Dever TE. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell 122: 901–913, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR, and Tsung A. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock 35: 293–301, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174: 7506–7515, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81: 84–91, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, and Mantell LL. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med 18: 477–485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, Klune JR, Zlotnicki J, Billiar T, and Tsung A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem 285: 39888–39897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, and Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3: 73ra20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, and Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3: 995–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge X, Antoine DJ, Lu Y, Arriazu E, Leung TM, Klepper AL, Branch AD, Fiel MI, and Nieto N. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem 289: 22672–22691, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Park MK, and Chang KC. β-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. Biochem Pharmacol 82: 769–777, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Hagar JA, Powell DA, Aachoui Y, Ernst RK, and Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341: 1250–1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao N, Budnik BA, Gunawardena J, and O'Shea EK. Tunable signal processing through modular control of transcription factor translocation. Science 339: 460–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He S, Wang L, Miao L, Wang T, Du F, Zhao L, and Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137: 1100–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Herzig D, Fang G, Toliver-Kinsky TE, Guo Y, Bohannon J, and Sherwood ER. STAT1-deficient mice are resistant to cecal ligation and puncture-induced septic shock. Shock 38: 395–402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hett EC, Slater LH, Mark KG, Kawate T, Monks BG, Stutz A, Latz E, and Hung DT. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat Chem Biol 9: 398–405, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, and Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, and Karin M. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428: 341–345, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Nace GW, McDonald KA, Tai S, Klune JR, Rosborough BR, Ding Q, Loughran P, Zhu X, Beer-Stolz D, Chang EB, Billiar T, and Tsung A. Hepatocyte specific HMGB1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular HMGB1 in cellular protection. Hepatology 59: 1984–1997, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, and Tsung A. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 62: 600–614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irving AT, Wang D, Vasilevski O, Latchoumanin O, Kozer N, Clayton AH, Szczepny A, Morimoto H, Xu D, Williams BR, and Sadler AJ. Regulation of actin dynamics by protein kinase R control of gelsolin enforces basal innate immune defense. Immunity 36: 795–806, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Ito I, Fukazawa J, and Yoshida M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem 282: 16336–16344, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Javaherian K, Liu JF, and Wang JC. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 199: 1345–1346, 1978 [DOI] [PubMed] [Google Scholar]
- 34.Jiang W, Bell CW, and Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol 178: 6495–6503, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, and Xiao TS. Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36: 561–571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT, and Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy 7: 1256–1258, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Kang R, Livesey KM, Zeh HJ, Loze MT, and Tang D. HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy 6: 1209–1211, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q, Bartlett DL, Whitcomb DC, Chang EB, Zhu X, Wang H, Lu B, Tracey KJ, Cao L, Fan XG, Lotze MT, Zeh HJ, 3rd, and Tang D. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 146: 1097–1107, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayagaki N, Warming S, Lamkanfi M, VandeWalle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, and Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, and Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, and Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29: 21–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller M, Rüegg A, Werner S, and Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132: 818–831, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Kim JH, Kim SJ, Lee IS, Lee MS, Uematsu S, Akira S, and Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol 182: 2458–2466, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Kim SR, Ha YM, Kim YM, Park EJ, Kim JW, Park SW, Kim HJ, Chung HT, and Chang KC. Ascorbic acid reduces HMGB1 secretion in lipopolysaccharide -activated RAW 264.7 cells and improves survival rate in septic mice by activation of Nrf2/HO-1 signals. Biochem Pharmacol 95: 279–289, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Kofoed EM. and Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamkanfi M, Sarkar A, VandeWalle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, and Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 185: 4385–4392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotze MT. and Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331–342, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, Sathler LB, Brito-Moreira J, Amaral OB, Silva CA, Freitas-Correa L, Espírito-Santo S, Campello-Costa P, Houzel JC, Klein WL, Holscher C, Carvalheira JB, Silva AM, Velloso LA, Munoz DP, Ferreira ST, and De Felice FG. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's β-amyloid oligomers in mice and monkeys. Cell Metab 18: 831–843, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Lu B, Antoine DJ, Kwan K, Lundbäck P, Wähämaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Harris EH, Chavan SS, Wang H, Andersson U, and Tracey KJ. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A 111: 3068–3073, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu B, Wang H, Andersson U, and Tracey KJ. Regulation of HMGB1 release by inflammasomes. Protein Cell 4: 163–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, Maiuri L, Maseri A, D'Angelo A, Bianchi ME, Rovere-Querini P, and Manfredi AA. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost 12: 2074–2088, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Maugeri N, Franchini S, Campana L, Baldini M, Ramirez GA, Sabbadini MG, Rovere-Querini P, and Manfredi AA. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity 45: 584–587, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Maugeri N, Rovere-Querini P, Baldini M, Baldissera E, Sabbadini MG, Bianchi ME, and Manfredi AA. Oxidative stress elicits platelet/leukocyte inflammatory interactions via HMGB1: a candidate for microvessel injury in systemic sclerosis. Antioxid Redox Signal 20: 1060–1074, 2014 [DOI] [PubMed] [Google Scholar]
- 54.McAllister CS. and Samuel CE. The RNA-activated protein kinase enhances the induction of interferon-beta and apoptosis mediated by cytoplasmic RNA sensors. J BiolChem 284: 1644–1651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, and Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 173: 307–313, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, and Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munir M. and Berg M. The multiple faces of protein kinase R in antiviral defense. Virulence 4: 85–89, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, and Alexander WS. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39: 443–453, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, and Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140: 338–348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nyström S, Antoine DJ, Lundbäck P, Lock JG, Nita AF, Högstrand K, Grandien A, Erlandsson-Harris H, Andersson U, and Applequist SE. TLR activation regulates damage-associated molecular pattern isoforms released during pyroptosis. EMBO J 32: 86–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohmori Y. and Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol 69: 598–604, 2001 [PubMed] [Google Scholar]
- 62.O'Shea JJ. and Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36: 542–550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, and Bianchi ME. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol 86: 617–623, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Pan PH, Cardinal J, Li ML, Hu CP, and Tsung A. Interferon regulatory factor-1 mediates the release of high mobility group box-1 in endotoxemia in mice. Chin Med J 126: 918–924, 2013 [PubMed] [Google Scholar]
- 65.Patel VS, Sitapara RA, Gore A, Phan B, Sharma L, Sampat V, Li JH, Yang H, Chavan SS, Wang H, Tracey KJ, and Mantell LL. High mobility group box-1 mediates hyperoxia-induced impairment of pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol 48: 280–287, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pisetsky DS, Gauley J, and Ullal AJ. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid Redox Signal 15: 2209–2219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pisetsky DS. The expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol Med 20: 158–163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, and Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 203: 1637–1642, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, and Wang H. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol 170: 3890–3897, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Rouhiainen A, Imai S, Rauvala H, and Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb Haemost 84: 1087–1094, 2000 [PubMed] [Google Scholar]
- 71.Sakaguchi S, Negishi H, Asagiri M, Nakajima C, Mizutani T, Takaoka A, Honda K, and Taniguchi T. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem Biophys Res Commun 306: 860–866, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Scaffidi P, Misteli T, and Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Souza AC, Yuen PS, and Star RA. Microparticles: markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int 87: 1100–1108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strowig T, Henao-Mejia J, Elinav E, and Flavell R. Inflammasomes in health and disease. Nature 481: 278–286, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, Pittet JF, Tracey K, Thannickal VJ, Abraham E, and Zmijewski JW. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol 304: L342–L349, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, 3rd, and Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol 190: 881–892, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, Zeh HJ, 3rd, and Lotze MT. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab 13: 701–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, and Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol 178: 7376–7384, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, and Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A 110: E3109–E318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, and Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204: 2913–2923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urbonaviciute V, Fürnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, and Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med 205: 3007–3018, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, and Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 209: 1519–1528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venereau E, Schiraldi M, Uguccioni M, and Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol 55: 76–82, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schäffer TE, Bohn E, Frick JS, Borst O, Münzer P, Walker B, Markel J, Csanyi G, Pagano PJ, Loughran P, Jessup ME, Watkins SC, Bullock GC, Sperry JL, Zuckerbraun BS, Billiar TR, Lotze MT, Gawaz M, and Neal MD. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest, 125: 4638–4654, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, and Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Wang S, Miura M, Jung YK, Zhu H, Li E, and Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92: 501–509, 1998 [DOI] [PubMed] [Google Scholar]
- 87.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, and Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472: 476–480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene 18: 6112–6120, 1999 [DOI] [PubMed] [Google Scholar]
- 89.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, and Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462: 99–103, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Yang H, Antoine DJ, Andersson U, and Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93: 865–873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, and Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107: 11942–11947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang X, Wang H, Zhang M, Liu J, Lv B, and Chen F. HMGB1: a novel protein that induced platelets active and aggregation via Toll-like receptor-4, NF-κB and cGMP dependent mechanisms. Diagn Pathol 10: 134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Ye C, Choi JG, Abraham S, Wu H, Diaz D, Terreros D, Shankar P, and Manjunath N. Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model. Proc Natl Acad Sci U S A 109: 21052–21057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Youn JH, Oh YJ, Kim ES, Choi JE, and Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol 180: 5067–5074, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Youn JH. and Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol 177: 7889–7897, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, and Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, and Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol 181: 5015–5023, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, and Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600, 2011 [DOI] [PubMed] [Google Scholar]