Abstract
Summary: Considerable data exist to support the use of positron emission tomography (PET) and single photon emission computed tomography (SPECT) scanning as biomarkers for Alzheimer’s disease (AD). The techniques are reasonably sensitive and specific in differentiating AD from normal aging, and recent studies with pathological confirmation show good sensitivity and specificity in differentiating AD from other dementias. These techniques also can detect abnormalities in groups of asymptomatic and presymptomatic individuals and may be able to predict decline to dementia. However, there are a number of existing questions related to the use of these techniques in samples that are fully representative of the spectrum of patients with dementia. For example, it is unclear how well PET and SPECT perform in comparison to a clinical diagnosis obtained in the same patient group, when autopsy is used as a gold standard. It will also be important to know what PET and SPECT add to the certainty of diagnosis in addition to the standard clinical diagnosis. Despite these unanswered questions, PET and SPECT may have application as biomarkers for AD in a number of clinical and research settings, especially in academic centers, where most of the existing studies have been done.
Keywords: PET, SPECT, functional imaging, molecular imaging, Alzheimer’s, glucose metabolism, blood flow
INTRODUCTION
Functional tomographic technology, or molecular imaging using high resolution, has been applied to the study of dementia for over two decades. The two main techniques of interest are positron emission tomography (PET) and single photon emission computed tomography (SPECT), both of which are capable of mapping the distribution of radionuclides in three dimensions, producing maps of brain biochemical and physiological processes. Early observations by neuroscience pioneers that whole brain blood flow was disturbed in dementia1 were followed by studies of regional changes in blood flow and oxygen metabolism2 and glucose metabolism (using the metabolic tracer [18F]fluorodeoxyglucose or FDG).3,4 These studies demonstrated that both blood flow and glucose metabolic reductions in Alzheimer’s disease (AD) were greatest in the temporal and the parietal neocortex, as did subsequent studies with SPECT perfusion imaging.5,6 Subsequent FDG-PET studies pointed out an equally severe and early involvement of posterior cingulate cortex.7
These observations have been repeatedly confirmed in studies of AD patients recruited from clinical samples, and have led to two general research themes in the literature on molecular imaging. One theme is the use of PET and SPECT to gain insight into fundamental biological mechanisms, clinical phenomenology, and pathophysiology. The second theme, and the subject of this review, is the use of molecular imaging as a diagnostic tool in dementia. The core of the argument for this approach is that the temporoparietal metabolic and perfusion reductions in AD may be viewed as a biomarker for the disease, thus providing clinicians with a tool that can be used in individual patients to help make a diagnosis. A corollary to this argument is that these functional abnormalities may be used to monitor both disease progression and response to treatment.
Over the years, as our understanding of AD and other dementias has improved, research on the use of molecular imaging as a biomarker for dementing disease has been refined. Current research provides considerable evidence supporting the application of PET and SPECT as biomarkers, but there are also a number of remaining questions. Although there are reasonable questions about what criteria need to be met to validate a biomarker, here we will concern ourselves with both the existing evidence supporting the use of these techniques as biomarkers and the remaining questions and concerns. This discussion will primarily concern itself with PET markers of glucose metabolism and SPECT markers of perfusion. Although a host of other tracers that measure neurochemistry are available and new tracers that may be able to detect amyloid deposition have recently been developed, these have not been widely applied to clinical series.
PET and SPECT in the diagnosis of clinical samples
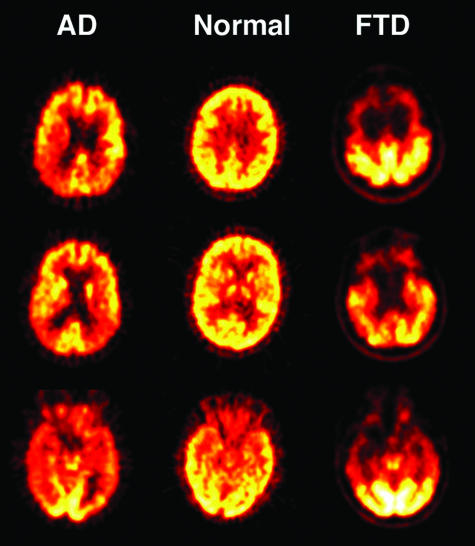
As noted, there is ample evidence that the pattern of temporal and parietal hypometabolism and hypoperfusion are reasonably sensitive and specific for AD. Initial studies comparing AD patients to controls using both PET and SPECT showed relatively high sensitivity and specificity (range, 85–90% for both) in differentiating AD patients from controls.8–10 Although differentiation of AD from normal aging may seem clinically irrelevant, the evidence for the ability of PET and SPECT to detect functional abnormalities in very mild dementia7,11 has considerable importance in early diagnosis, which is discussed in more detail below. A recent European multicenter PET study that used quantitative FDG-PET methodology found 93% sensitivity and 93% specificity in differentiating AD patients from controls, and 83% sensitivity and 93% specificity when only patients with a Mini-Mental State Examination score of 24 or greater were included.12 Figure 1 shows an example of the temporoparietal hypometabolism seen in AD in comparison to normal aging.
FIG. 1.
FDG-PET scans in normal aging, AD, and FTD. Glucose metabolism is presented on a heat scale, with brighter colors reflecting greater glucose utilization. Glucose metabolism is reduced in posterior cortical regions (temporal and parietal lobes) in patients with AD, in contrast to frontal and anterior temporal lobe reductions in FTD.
Other studies have evaluated the utility of PET and SPECT in differentiating AD from other dementias. There are clear limitations to such studies when autopsy confirmation of the diagnosis is lacking, especially in conditions like Dementia with Lewy Bodies (DLB) and vascular dementia, which are both difficult to diagnose and frequently coexist with AD. Nevertheless, compelling data show that the pattern of metabolic and perfusion abnormalities in AD is quite different from frontotemporal dementia (FTD), which is characterized by reduced frontal lobe perfusion and metabolism.13–15 Figure 1 shows frontal hypometabolism seen in a patient with FTD.
Other dementias that are clinically different from AD include DLB, which is characterized by temporoparietal hypofunction accompanied by abnormalities in both the primary visual cortex and the visual association cortex.16–18 Clinical studies of patients with vascular dementia have found variable results. In patients with large cortical infarcts a multifocal pattern of PET or SPECT hypofunction is frequently seen. However, some studies of vascular dementia report patterns that are not different from AD patients.19 A number of studies have suggested that subcortical vascular disease results in frontal hypofunction,20,21 although this has not been suggested to be diagnostically useful. As noted, conceptual and diagnostic challenges limit the interpretation of these studies. Finally, a host of other dementias appear to show patterns of metabolism and perfusion distinct from the findings in AD, including corticobasal degeneration, Huntington’s disease, multiple system atrophy, and spinocerebellar degeneration, 12 although these disorders are usually distinctive on other grounds.
An important feature of these studies in AD that has implications for the use of molecular imaging as a biomarker is the relationship between imaging and clinical disease features. Many but not all studies have found that the severity of the functional lesion is related to the severity and the nature of cognitive impairment,9,22–24 and longitudinal studies demonstrate metabolic reductions with disease progression.25 These parallels suggest that PET and SPECT are measuring a physiological process of fundamental importance to the pathophysiology of the disease.
Based on clinical series of patients, it might seem reasonable to conclude that PET and SPECT imaging may be useful biomarkers in diagnosing AD and distinguishing it from normal aging, DLB, and FTD. These findings should be supported by evidence that includes pathological confirmation. Fortunately, a number of studies have now been reported with pathological-imaging correlations.
PET and SPECT and autopsy-confirmed samples
Several studies have evaluated subjects with dementia caused by a range of different diseases using imaging followed by longitudinal observation and autopsy. Several SPECT series have been reported from the Oxford Project to Investigate Memory and Aging (OPTIMA), the largest of which included 118 patients and autopsied controls.26 The sample included a reasonable spectrum of dementia diagnoses confirmed pathologically, with 80 AD cases, 24 non-AD dementias, and 14 controls. This study reported 89% sensitivity for an AD diagnosis and 60% specificity. Another SPECT series of 54 subjects, 11 of whom had non-AD pathological diagnoses reported roughly comparable values, with 86% sensitivity and 73% specificity for AD diagnosis.27 A smaller series with 27 patients, 13 of whom had AD and seven of whom had FTD, found 85% sensitivity and 64% specificity.28
PET studies with autopsy confirmation include a series of 22 subjects who were difficult to diagnose clinically and followed to autopsy. Temporoparietal hypometabolism had 90% sensitivity and 65% specificity for the diagnosis of AD.29 In a recently published study of a large series, 138 patients were enrolled in multiple academic centers and followed to autopsy.30 A retrospective clinical-pathological analysis using visual interpretation of PET images found 93% sensitivity and 76% specificity for the prediction of pathological AD.
These imaging-pathological studies provide some insight into the utility of PET and SPECT as biomarkers for AD. They do not clearly define the circumstances in which PET and SPECT might be most useful (for example, they may be most effective in differentiating AD from FTD), nor do they provide clear guidance for how they should be used in clinical practice or how accurate imaging may be in comparison to a clinical diagnosis. One approach is to compare data from clinical-pathological studies with data from these imaging-pathological studies. In a recent review that established practice parameters,31 existing clinical criteria for probable AD had a sensitivity of 81% and a specificity of 70% when averaged over 13 reported studies.
Thus, one may argue that PET is at least as sensitive and specific as a clinical diagnosis. Although this may be true, there are problems with this interpretation. First, the samples in which clinical diagnosis and imaging diagnoses are compared may not, in fact, be comparable because some of these clinical series reflect community samples, whereas imaging studies more often are drawn from academic centers. Second, an imaging diagnosis is not likely to supplant a clinical evaluation in any circumstances. Another approach is therefore to compare clinical and imaging diagnoses in the same sample, asking whether imaging provides additional diagnostically useful information in addition to the clinical diagnosis. When the OPTIMA data were analyzed in this manner, it becomes clear that SPECT imaging can modestly increase diagnostic certainty over the clinical diagnosis.32 For example, a diagnosis of “probable AD” resulted in an 84% likelihood of pathological AD. If a SPECT scan was negative for AD, the likelihood fell to 70%, and if a SPECT scan was positive, the likelihood rose to 92%.
Effect of sample selection on diagnostic accuracy
To evaluate the utility of any biomarker, clear specification of the context in which the biomarker is effective is necessary.33 Because a likely application of an imaging biomarker is as an adjunct to clinical diagnosis, we must assess how well the existing data on the diagnostic accuracy of molecular imaging might generalize to patients presenting to a clinician. There are reasonable questions as to whether the existing published imaging data truly reflect the types of patients that a clinician will see in practice. Several factors in particular bear on this: the age of the patients reported, the presence of comorbid medical conditions, and the source of recruitment and any resulting selection bias. Because most imaging studies have been done at academic centers involved in dementia research, patients are often highly selected. In some studies, only AD patients who have met rigorous diagnostic criteria for AD are enrolled. Referral bias may also result in the inclusion of particularly young patients. In the largest imaging-pathological series to date, the mean age of dementia subjects was 67,30 whereas the mean age of patients with dementia seen in community samples is closer to 80.34,35
These factors are highly relevant to imaging studies. Age has been repeatedly shown to affect the pattern of metabolic and perfusion decline in AD, with younger patients demonstrating more severe temporoparietal hypoperfusion and hypometabolism than older patients.36–39 The presence of cerebrovascular disease also appears to reduce the prominence of temporoparietal abnormalities.40 Finally, education also has an effect on the severity of functional abnormalities, with more highly educated patients showing relatively more severe abnormalities.41 Thus, the inclusion of high proportions of young, highly educated, otherwise healthy, well-diagnosed dementia subjects will tend to produce results favorable toward the use of these techniques, when in reality results may not be as accurate in elderly and poorly educated patients with multiple medical problems including cerebrovascular disease.
This is perhaps demonstrated most clearly in the few existing studies that have used PET or SPECT in relatively unselected community samples. In one such study using FDG-PET in a sample of 93 subjects recruited from the Hispanic community with a high prevalence of diabetes and cerebrovascular disease, temporoparietal hypometabolism was not detected in a group of nine AD patients.42 Similarly, a SPECT perfusion study of a community dwelling sample from Amsterdam did not detect perfusion abnormalities in a subset diagnosed with AD.43 Although there are a variety of reasons for negative results in these studies, a distinct possibility is that the nature of the samples differ considerably from those selected from research clinics and academic practices.
Prediction, progression, and early detection
Biomarkers may be particularly useful and important in prediction of disease and decline. This may entail prediction of the rate of progression in clinically diagnosed individuals, or the prediction of the development of disease in those at risk. These are conceptually linked since presumably the detection and quantitation of a fundamental pathological process by a biomarker should be related to both the likelihood of disease and the rate of change over time.
Because dementia severity and longitudinal progression are associated with metabolic and perfusion abnormalities, it is not surprising that these abnormalities may have predictive ability. In two separate studies, both SPECT44 and PET45 at baseline were related to the rate of subsequent decline on the Mini-Mental State Examination score in patients with AD. SPECT perfusion imaging also predicts survival in AD.46
A number of studies have convincingly demonstrated that metabolic abnormalities can be detected with FDG-PET in groups of individuals who have genetic risks for AD but who do not have symptoms of disease. These studies have evaluated asymptomatic people homozygous for the apolipoprotein ɛ4 allele,47 as well as asymptomatic individuals who carry autosomal dominant genetic mutations of the presenilin 1 and amyloid precursor protein genes.48 In both of these groups of subjects, reductions in posterior cortical glucose metabolism in the typical regions seen in symptomatic AD have been detected. Similar studies have been performed in those at risk for AD by virtue of having mild cognitive impairment (MCI)49 or similar syndromes. Early observations noted that groups of patients with mild memory complaints, a family history of AD, and the ɛ4 allele showed lower parietal glucose metabolism than individuals without the ɛ4 genotype. There is some evidence that abnormalities in glucose metabolism can be found even earlier. Metabolic rates in the entorhinal cortex may be effective at discriminating MCI patients from controls,50 and normal subjects with lower entorhinal cortical metabolism are at increased risk of developing MCI.51
Finally, the ability of molecular imaging to predict the trajectory of individuals with MCI has received increasing recent attention. Parietal cortical metabolic rates at baseline predicted the rate of memory decline in a group of ɛ4 carriers with memory complaints.52 In a group of 20 MCI patients, baseline left temporoparietal FDG-PET was useful in discriminating between those who declined to AD and those who remained stable, using a retrospective analysis.53 A similar approach in a different subject group found that the right temporoparietal cortex was abnormal in MCI patients who converted to AD, compared with those who did not convert.54 A SPECT perfusion study found that individuals who subsequently converted to AD from MCI differed from normal controls in posterior and anterior cingulate, hippocampal-amygdaloid complex, and anterior thalamus.11 Although these studies in general used retrospective approaches in small samples, they suggest that molecular imaging has value in defining the likelihood of progression to dementia in those at risk.
Surrogate markers
Another potential use of a biomarker is as a surrogate measure of disease in monitoring the response to a drug or other treatment. Based on the evidence presented, molecular imaging has a number of features that make it a good candidate for a surrogate marker. Both PET and SPECT are reasonably sensitive and specific, at least in some patient populations, for the pathological diagnosis of AD. Thus, these techniques appear to reflect the pathological substrate of the disease. In addition to showing reasonable sensitivity and specificity for the presence of plaques and tangles, PET and SPECT reflect synaptic activity, a key pathological process in AD. Furthermore, PET and SPECT are correlated with cognitive function, and provide predictive information about the rate of subsequent deterioration, death, and conversion from MCI to dementia. However, simply predicting disease-course and correlating with pathology are not sufficient for establishing a technique as a surrogate marker. To do that, the laboratory measurement must be shown to be a good substitute for a clinically meaningful endpoint in a clinical trial, such that a treatment-induced change in this surrogate is reasonably likely to reflect changes in this clinically meaningful endpoint. With respect to Alzheimer’s disease, this use of a surrogate marker has particular relevance to the application to a disease-modifying treatment. Indeed, a potential advantage of a surrogate technique in AD is that a surrogate that reflects neuropathology may be able to disentangle symptomatic effects from disease-modification effects.
Although the lack of a disease-modifying drug makes this hypothesis presently untestable, there are some additional data that bear on the potential of molecular imaging as a surrogate marker. First is the issue of the effects of existing drugs on measurements of perfusion and metabolism in AD. Although relatively little data exist, a number of published studies show that PET and SPECT reflect clinical improvement. Cerebral glucose metabolism measured with PET remained stable in a group of subjects treated with donepezil for 24 weeks, but it declined in the placebo group.55 In another study, patients who improved clinically treated with the cholinesterase inhibitor metrifonate showed increases in glucose metabolism measured with PET.56 In a somewhat different study design, a group of subjects received SPECT perfusion imaging before and after donepezil treatment. Those who were stable over 15 months showed no perfusion decline, whereas those who deteriorated showed perfusion reductions.57 Similar SPECT findings were seen in comparing treated groups with placebo groups.58
A potential advantage of a surrogate marker is that it may be more sensitive than a clinical endpoint, allowing studies to be done with greater power or fewer subjects. Existing data support the use of FDG-PET as a surrogate from this perspective. For example, even a trial studying cognitively normal subjects with the apolipoprotein ɛ4 allele would be feasible with a PET surrogate marker, since sample sizes of 50–115 subjects could detect a 25% treatment effect in glucose utilization with 80% power.59 These optimistic results have been reported by more than one research group.52 When a similar approach was used with AD patients, the larger size of metabolic reductions over time resulted in still smaller estimates of necessary sample size—as low as 36 subjects per group to detect a 33% treatment effect with 80% power.25
Thus, molecular imaging reflects the biologically important and clinically meaningful effects of plaque and tangle pathology, synaptic loss, and clinical disease severity. It predicts decline, and is correlated with symptomatic improvement. It appears to be very sensitive for detecting longitudinal change in both symptomatic and at-risk subject groups. The remaining questions relate to whether PET and SPECT measurements can disentangle changes resulting from symptomatic improvement from those consequential to disease modification. This will remain an open question until disease modifying therapies are available for evaluation.
SUMMARY
Remaining questions
The information reviewed here provides a supportive perspective for the use of PET and SPECT as biomarkers in the diagnosis of dementia, particularly in relation to their ability to detect AD. Nevertheless, a number of questions remain, and future studies need to be performed to address several key issues.
The evidence favoring use of PET and SPECT as biomarkers is, as follows:
1) PET and SPECT are sensitive and specific (in the range of 85–90%) in differentiating patients with AD from normal older individuals.
2) A large imaging-autopsy series showed that PET has a sensitivity of 93% and a specificity of 76% for the prediction of pathological AD. Other PET and SPECT series of dementia patients with pathological validation of diagnosis show sensitivities on the order of 85–90%, and specificities in the range of 60–73%.
3) FDG-PET shows abnormalities in groups of asymptomatic individuals at risk for AD and both PET and SPECT have detected functional abnormalities in groups of individuals with mild cognitive impairment who are presymptomatic for AD.
4) Retrospectively analyzed data suggest that PET and SPECT can predict conversion from MCI to dementia.
5) PET and SPECT measures of perfusion and metabolism change in parallel with clinical findings in response to symptomatic therapy with cholinesterase inhibitors.
Despite these important findings, the following questions remain:
1) How do PET or SPECT perform in comparison to autopsy when compared with a clinical diagnosis obtained in the same patient cohort?
2) What do PET or SPECT imaging add to a clinical diagnosis? In other words, is diagnostic accuracy increased substantially by the addition of these techniques to the diagnostic armamentarium?
3) How helpful are PET and SPECT in the diagnosis of dementia when applied to unselected samples of subjects who are older, less educated, and with significant medical comorbidities, including cerebrovascular disease?
4) Are changes in a PET or SPECT scan consequent to therapy reasonably likely to reflect changes in the underlying disease?
5) Can PET or SPECT differentiate symptomatic effects from disease-modifying effects of therapy?
These questions are difficult to answer, and will require large-scale clinical trials. Nevertheless, it is worth noting that these techniques are not useless pending the resolution of these questions. For example, we know that PET is reasonably accurate in the diagnosis of AD in a specific type of patient cohort—that recruited from an academic medical center. Most subjects who participate in clinical trials are recruited from academic centers and are no more representative of the general population of AD patients than those who have been studied with PET.60 Therefore, studies that may wish to use PET to monitor therapy or assess diagnosis in these individuals are well justified. In essence, the utility of both PET and SPECT need to be evaluated with regard to the specific situation. Current data suggests that these techniques may serve as useful biomarkers for some aspects of AD in many situations, although their utility in other situations still needs to be validated.
Acknowledgments
This work was supported by National Institute on Aging Grant AG10129.
REFERENCES
- 1.Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. Res Publ Assoc Res Nerv Ment Dis 35: 31–45, 1956. [PubMed] [Google Scholar]
- 2.Frackowiak RSJ, Pozzili C, Legg NJ, Du Boulay GH, Marshall J, Lenzi L et al. Regional cerebral oxygen supply and utilization in dementia: a clinical and physiological study with oxygen-15 and positron tomography. Brain 104: 753–778, 1981. [DOI] [PubMed] [Google Scholar]
- 3.Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B et al. Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]Fluorodeoxyglucose. J Comput Assist Tomogr 7: 590–598, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Benson DF, Kuhl DE, Hawkins RA, Phelps ME, Cummings JL, Tsai SY. The fluorodeoxyglucose 18F scan in Alzheimer’s disease and multi-infarct dementia. Arch Neurol 40: 711–714, 1983. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KA, Mueller ST, Walshe TM, English RJ, Holman BL. Cerebral perfusion imaging in Alzheimer’s disease: use of single photon emission computed tomography and iofetamine hydrochloride I 123. Arch Neurol 44: 165–168, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Jagust WJ, Budinger TF, Reed BR. The diagnosis of dementia with single photon emission computed tomography. Arch Neurol 44: 258–262, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 42: 85–94, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KA, Holman BL, Rosen TJ, Nagel JS, English RJ, Growdon JH. Iofetamine I 123 single photon emission computed tomography is accurate in the diagnosis of Alzheimer’s disease. Arch Intern Med 150: 752–756, 1990. [PubMed] [Google Scholar]
- 9.Eberling JL, Jagust WJ, Reed BR, Baker MG. Reduced temporal lobe blood flow in Alzheimer’s disease. Neurobiol Aging 13: 483–491, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36: 1238–1248, 1995. [PubMed] [Google Scholar]
- 11.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A et al. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology 50: 1563–1571, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Herholz K. PET studies in dementia. Ann Nucl Med 17: 79–89, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Miller BL, Cummings JL, Villaneuva-Meyer J, Boone K, Mehringer CM, Lesser IM et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology 41: 1374–1382, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Ishii K, Sakamoto S, Sasaki M, Kitagaki H, Yamaji S, Hashimoto M et al. Cerebral glucose metabolism in patients with frontotemporal dementia. J Nucl Med 39: 1875–1878, 1998. [PubMed] [Google Scholar]
- 15.Santens P, De Bleecker J, Goethals P, Strijckmans K, Lemahieu I, Slegers G et al. Differential regional cerebral uptake of (18)F-fluoro-2-deoxy-d-glucose in Alzheimer’s disease and frontotemporal dementia at initial diagnosis. Eur Neurol 45: 19–27, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AAF. Fluorodeoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology 47: 462–466, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Imamura T, Ishii K, Sasaki M, Kitagaki H, Yamaji S, Hirono N et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease: a comparative study using positron emission tomography. Neurosci Lett 235: 49–52, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 56: 643–649, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Duara R, Barker W, Loewenstein D, Pascal S, Bowen B. Sensitivity and specificity of positron emission tomography and magnetic resonance imaging studies in Alzheimer’s disease and multi-infarct dementia. Eur Neurol 29(Suppl 3): 9–15, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Kwan LT, Reed BR, Eberling JL, Schuff N, Tanabe J, Norman D et al. Effects of subcortical cerebral infarction on cortical glucose metabolism and cognitive function. Arch Neurol 56: 809–814, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultzer DL, Mahler ME, Cummings JL, Van Gorp WG, Hinkin CH, Brown C. Cortical abnormalities associated with subcortical lesions in vascular dementia. Arch Neurol 52: 773–780, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Foster NL, Chase TN, Mansi L, Brooks R, Fedio P, Patronas NJ et al. Cortical abnormalities in Alzheimer’s disease. Ann Neurol 16: 649–654, 1984. [DOI] [PubMed] [Google Scholar]
- 23.Waldemar G, Walovitch RC, Andersen AR, Hasselbalch SG, Bigelow R, Joseph JL et al. 99mTc-bicisate (neurolite) SPECT brain imaging and cognitive impairment in dementia of the Alzheimer type: a blinded read of image sets from a multicenter SPECT trial. J Cereb Blood Flow Metab 14(Suppl 1): S99–S105, 1994. [PubMed] [Google Scholar]
- 24.Kawano M, Ichimiya A, Ogomori K, Kuwabara Y, Sasaki M, Yoshida T et al. Relationship between both IQ and Mini-Mental State Examination and the regional cerebral glucose metabolism in clinically diagnosed Alzheimer’s disease: a PET study. Dement Geriatr Cogn Disord 12: 171–176, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry 159: 738–745, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Jobst KA, Barnetson LPD, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer’s disease and the differential diagnosis of dementia: the use of NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and ApoE4 in medial temporal lobe dementias. Int Psychogeriatr 10: 271–302, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Bonte FJ, Weiner MF, Bigio EH, White CL. Brian blood flow in the dementias: SPECT with histopathologic correlation in 54 patients. Radiology 202: 793–797, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Read SL, Miller BL, Mena I, Kim R, Itabashi H, Darby A. SPECT in dementia: clinical and pathological correlation. J Am Geriatr Soc 43: 1243–1247, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med 41: 1920–1928, 2000. [PubMed] [Google Scholar]
- 30.Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 286: 2120–2127, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56: 1143–1153, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Jagust W, Thisted R, Devous MD Sr, Van Heertum R, Mayberg H, Jobst K et al. SPECT perfusion imaging in the diagnosis of Alzheimer’s disease: a clinical-pathologic study. Neurology 56: 950–956, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Consensus report of the Working Group on: Molecular and biochemical markers of Alzheimer’s disease. Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Neurobiol Aging 19: 109–116, 1998. [PubMed] [Google Scholar]
- 34.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 51: 169–177, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF. Mortal with dementia: results from a French prospective community-based cohort. Am J Epidemiol 154: 642–648, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Mielke R, Herholz K, Grond M, Kessler J, Heiss W-D. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging 1991; 13: 93–98 [DOI] [PubMed] [Google Scholar]
- 37.Grady CL, Haxby JV, Horwitz B, Berg G, Rapoport SI. Neuropsychological and cerebral metabolic function in early vs late onset dementia of the Alzheimer type. Neuropsychologia 25: 807–816, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Small GW, Kuhl DE, Riege WH, Fujikawa DG, Ashford JW, Metter J et al. Cerebral glucose metabolic patterns in Alzheimer’s disease: effect of gender and age at dementia onset. Arch Gen Psychiatry 46: 527–532, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Kemp PM, Holmes C, Hoffmann SM, Bolt L, Holmes R, Rowden J, et al. Alzheimer’s disease: differences in technetium-99m HMPAO SPECT scan findings between early onset and late onset dementia. J Neurol Neurosurg Psychiatry 74: 715–719, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeCarli C, Grady CL, Clark CM, Katz DA, Brady DR, Murphy DGM et al. Comparison of positron emission tomography, cognition and brain volume in Alzheimer’s disease with and without severe abnormalities of white matter. J Neurol Neurosurg Psychiatry 60: 158–167, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 32: 371–375, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Jagust WJ, Eberling JL, Wu CC, Finkbeiner A, Mungas D, Valk PE et al. Brain function and cognition in a community sample of elderly Latinos. Neurology 59: 378–383, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Scheltens P, Launer LJ, Barkhof F, Weinstein HC, Jonker C. The diagnostic value of magnetic resonance imaging and technetium 99m-HMPAO single photon emission computed tomography for the diagnosis of Alzheimer’s disease in a community dwelling elderly population. Alzheimer Dis Assoc Disord 11: 63–70, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe N, Reed BR, Eberling JL, Jagust WJ. Temporal lobe perfusion on single photon emission computed tomography predicts the rate of cognitive decline in Alzheimer’s disease. Arch Neurol 52: 257–262, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Jagust WJ, Haan MN, Eberling JL, Wolfe N, Reed BR. Functional imaging predicts cognitive decline in Alzheimer’s disease. J Neuroimaging 6: 156–160, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Jagust WJ, Haan MN, Reed BR, Eberling JL. Brain perfusion imaging predicts survival in Alzheimer’s disease. Neurology 51: 1009–1013, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ɛ4 allele for apolipoprotein E. N Engl J Med 334: 752–758, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy AM, Frackowiak RSJ, Newman SK, Bloomfield PM, Seaward J, Roques P et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer’s disease. Neurosci Lett 186: 17–20, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56: 303–308, 1999. [DOI] [PubMed] [Google Scholar]
- 50.De Santi S, deLeon MJ, Rusinek H, Convit A, Tarshish C, Roche A et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22: 529–539, 2001. [DOI] [PubMed] [Google Scholar]
- 51.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H et al. Prediction of cognitive decline in normal elderly subjects with 2- [(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci USA 98: 10966–10971, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA 97: 6037–6042, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. NeuroReport 12: 851–855, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology 60: 1374–1377, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Tune L, Tiseo PJ, Ieni J, Perdomo C, Pratt RD, Votaw JR et al. Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: results of a 24-week, double-blind, placebo-controlled study. Am J Geriatr Psychiatry 11: 169–177, 2003. [PubMed] [Google Scholar]
- 56.Mega MS, Cummings JL, O’Connor SM, Dinov ID, Reback E, Felix J et al. Cognitive and metabolic responses to metrifonate therapy in Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol 14: 63–68, 2001. [PubMed] [Google Scholar]
- 57.Nobili F, Koulibaly M, Vitali P, Migneco O, Mariani G, Ebmeier K et al. Brain perfusion follow-up in Alzheimer’s patients during treatment with acetylcholinesterase inhibitors. J Nucl Med 43: 983–990, 2002. [PubMed] [Google Scholar]
- 58.Nakano S, Asada T, Matsuda H, Uno M, Takasaki M. Donepezil hydrochloride preserves regional cerebral blood flow in patients with Alzheimer’s disease. J Nucl Med 42: 1441–1445, 2001. [PubMed] [Google Scholar]
- 59.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA 98: 3334–3339, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider LS, Olin JT, Lyness SA, Chui HC. Eligibility of Alzheimer’s disease clinic patients for clinical trials. J Am Geriatr Soc 45: 923–928, 1997. [DOI] [PubMed] [Google Scholar]