Abstract
Objective
The goal of this study was to examine the relationship between comorbid disorders and executive function (EF) in children diagnosed with Attention Deficit/Hyperactivity Disorder (ADHD).
Methods
Three hundred and fifty-five, 6–12 year old children clinically diagnosed with ADHD were included in the study. Comorbid anxiety disorders, Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) were examined. The EF domains were assessed using the Conners’ Continuous Performance Test (CPT), Wisconsin Card Sorting Test (WCST), Tower of London (ToL), Finger Windows (FW) and Self Ordered Pointing Test (SOPT).
Results
The findings indicate that children with comorbid anxiety disorders performed worse in domains measured by CPT and prior to controlling for age and sex, by FW. However, once sex was controlled for the results for FW were no longer significant. Children with CD obtained lower scores on WCST. Furthermore, a significant sex by CD interaction was observed.
Conclusion
These results indicate that comorbid disorders should be carefully examined as they play a significant role in EF performance and subsequently in day-to-day functioning of children with ADHD.
Keywords: ADHD, Executive Function, comorbid disorders, conduct disorder
Résumé
Objectif
Cette étude visait à examiner la relation entre les troubles comorbides et la fonction exécutive (FE) chez les enfants ayant reçu un diagnostic de trouble de déficit de l’attention avec hyperactivité (TDAH).
Méthodes
Trois cent cinquante-cinq enfants de 6 à 12 ans chez qui le TDAH a été cliniquement diagnostiqué ont été inclus dans l’étude. Les troubles anxieux, le trouble oppositionnel avec provocation (TOP) et le trouble des conduites (TC) comorbides ont été examinés. Les domaines de la FE ont été évalués à l’aide du test de performance continu de Conners (CPT), du test de tri de cartes du Wisconsin (WCST), du test de la Tour de Londres (ToL), du test Finger Windows (FW) et du test de pointage auto-imposé (SOPT).
Résultats
Les résultats indiquent que les enfants souffrant de troubles anxieux comorbides avaient un plus mauvais rendement dans les domaines mesurés par le CPT et avant le contrôle pour l’âge et le sexe, dans FW. Cependant, une fois le sexe contrôlé, les résultats de FW n’étaient plus significatifs. Les enfants souffrant du TC obtenaient des scores plus faibles au WCST. En outre, une interaction significative du sexe dans le TC a été observée.
Conclusion
Ces résultats indiquent que les troubles comorbides devraient être examinés avec soin car ils jouent un rôle significatif dans le rendement de la FE et subséquemment, dans le fonctionnement quotidien des enfants souffrant du TDAH.
Mots clés: TDAH, fonction exécutive, troubles comorbides, trouble des conduites
Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder, more commonly diagnosed in boys (Rucklidge, 2010). Children diagnosed with ADHD are at higher risk for educational failure, social difficulties, as well as high risk behaviour (Biederman et al., 2006). ADHD rarely occurs in isolation and there is now a well-established body of literature that has identified a range of frequently occurring comorbid psychiatric disorders that include oppositional defiant disorder (ODD), conduct disorder (CD) and anxiety disorders (Biederman et al., 2008; Schatz & Rostain, 2006). Rates of comorbid disorders range from 24% to 71%, varying across studies and across disorders (Jensen et al., 2001; Kadesjo & Gillberg, 2001; Robison, Sclar, Skaer & Galin, 1999). With about 50% of children meeting criteria for ODD or CD, and 25 to 33% for anxiety disorders, the presence of comorbidities plays a significant negative role in the degree of impairment and on the course of the disorder. This presents an additional challenge for the diagnosis and treatment of children with ADHD (Ollendick, Jarrett, Grills-Taquechel, Hovey & Wolff, 2008; Cuffe et al., 2015; Pliszka, Sherman, Barrow, & Irick, 2000).
In addition to comorbid disorders, children with ADHD often exhibit Executive Function (EF) deficits. EF represents a set of cognitive processes that integrate information from working memory with information about context in order to select optimal action (Willcutt, Doyle, Nigg, Faraone & Pennington, 2005). Among children diagnosed with ADHD, deficits have been reported in the EF domains of response inhibition and execution, vigilance, working memory, set and task-switching/cognitive flexibility and planning (Toplak, Bucciarelli, Jain & Tannock, 2009; Willcutt et al., 2005).
EF deficits are not specific to ADHD, but have also been associated with other psychiatric conditions such as symptoms of anxiety/depression (Emerson, Mollet & Harrison, 2005), obsessive compulsive disorder (Chamberlain et al., 2007) and CD (Toupin, Dery, Pauze, Mercier & Fortin, 2000; Pajer et al., 2008). Subsequently, several studies have examined EF deficit among children with a diagnosis of ADHD and comorbid disorders, however, these are limited in number and have shown inconsistent results (Doyle, 2006). For example, the presence of comorbid ODD or CD was reported not to affect EF performance as presence of ADHD accounted for EF deficit in children diagnosed with ADHD and ODD/CD (Kalff et al., 2002; Oosterlaan, Scheres and Sergeant, 2005). Similarly, in teens with disruptive behavioural disorders the presence of comorbid ADHD determined worse performance on EF tasks (Hummer et al., 2011). Rhodes et al. (Rhodes, Park, Seth, & Coghill, 2012) on the other hand found that compared to typically developing children, children with either ADHD or ODD or both performed worse on EF tasks. In adults, those with dual diagnosis of ADHD and CD showed poorer EF performance compared to ADHD only (Fischer, Barkley, Smallish & Fletcher, 2005).
Among children diagnosed with ADHD, those who reported anxiety have been shown to have/demonstrate better behavioural inhibition, while parent reported anxiety was not associated with neurocognitive dysfunction (Bloemsma et al., 2013). In a study examining working memory in four subgroups (children with anxiety, with ADHD, with ADHD and anxiety (N = 108) and a control group), children with dual diagnosis were found to display a similar impairment to children with only ADHD diagnosis, compared to both the control group and anxiety only group (Manassis, Tannock, Young & Francis-John, 2007).
In addition to EF differences seen among children presenting with various psychopathologies, sex differences have also been reported on some measures of EF (De Luca et al., 2003; Seidman et al., 2005), attention (Newcorn et al., 2001), and reaction time (Brocki & Bohlin, 2004). In general, girls have been found to be less impaired in such domains as compared to boys (Hasson & Fine, 2012). Moreover, sex differences have been well documented in the phenotypic expression of the ADHD, with girls demonstrating lower levels of disruptive behaviour and higher levels of inattentive symptoms (Stefanatos & Baron, 2007).
Given the frequently observed EF deficit and the high rate of comorbidities in ADHD population, the main aim of the current study is to examine the role comorbid disorders play on EF task performance in a large sample of children clinically diagnosed with ADHD. We examined the three most common comorbidities reported among elementary school age children. We hypothesised that the presence of anxiety disorders, CD or ODD will have an additive negative effect on EF, resulting in poorer performance compared to children without comorbid disorders. In addition, due to reported sex differences in ADHD and in EF we expected to find sex differences in EF performance among children diagnosed with ADHD.
This study will contribute to identifying the impact anxiety disorders, ODD and CD have on EF, and will allow clinicians to refine individual intervention by paying closer attention to neurocognitive delays that may interfere with the child’s functioning. In addition, this study addresses several of the limitations of previous studies, particularly with respect to sample size and the use of a thorough diagnostic and standardised assessment procedure.
Methods
Participants
Children ages six to 12 diagnosed with ADHD and referred to the ADHD clinic were recruited sequentially for this study. Children were excluded from the study if they met the DSM-IV criteria for psychosis, had a chronic medical condition, autism spectrum disorder, Gilles de la Tourette’s syndrome, or had an IQ less than 70. The final sample consisted of 355 children, 267 boys and 88 girls. The mean age for the group was 9.40 years (SD = 1.66), with no statistical difference in age between boys and girls.
Design and setting
The study was conducted at a university affiliated mental health institute. Internal Research Ethics Board approval was obtained for the study protocol. All participants signed the informed consent and children gave verbal ascent to participate in the study. Primary caregivers completed standardised interviews and questionnaires while children were assessed using EF measures. All medications were stopped prior to assessment and children underwent a minimum five-day medication washout period prior to completing the EF assessment. Two hundred thirty-nine children were not taking any medication when enrolled in the study, 105 children were taking medication at the time of participation. Of these children, 82 children were taking methylphenidate, 15 children were taking other commonly used stimulant or non-stimulant medication, and 12 children were taking risperidone (n=10), seroquel (n=1), clonidine (n=1) or haloperidole (n=1) alone or in combination. The washout period was determined by the team psychiatrist according to the type of medication the child was prescribed. Children taking neuroleptic medication had a washout period of two weeks. All EF measures were administered in the morning, to minimize the possible effects of fatigue on task performance.
Clinical Assessment
All children were diagnosed with ADHD and/or comorbidities by child psychiatrists according to the fourth edition of the DSM-IV interview based on school reports, observation of the child, and clinical interview with the family. To confirm the diagnosis as well as assess comorbidities a trained research assistant interviewed a parent, using The National Institutes of Mental Health Diagnostic Interview Schedule for Children 4th edition (DISC-IV: Shaffer, Fisher, Lucas, Dulcan & Schwab-Stone, 2000). DISC – IV is a highly structured, DSM-IV diagnostic interview which assesses most common mental disorders in children. The hierarchical nature of the interview allows for thorough assessment of each symptom, including duration, the frequency of occurrence, and interference with functioning. The following comorbid disorders were examined in this study: Oppositional Defiant Disorder (ODD), Conduct Disorder (CD), and anxiety disorders. DISC-IV was used to report on positive ADHD symptoms.
Child measures
Wechsler Intelligence Scale for Children – 3rd (WISC-III) or 4th (WISC-IV) editions was used to assess the children’s general cognitive ability (Wechsler, 1991). Children were assessed using the following measures: Conners Continuous Performance Test (CPT: Conners, Epstein, Angold & Klaric, 2003), Wisconsin Card Sorting Test (WCST: Heaton, Chelune, Talley, Kay, & Curtiss, 1993), Tower of London (ToL: Anderson, Anderson & Lajoie, 1996), Self-Ordered Pointing Test (SOPT: Petrides & Milner, 1982), and Finger Windows (FW: Sheslow & Adams, 2001).
Statistical Analysis
Statistical analyses were conducted using the SPSS software, Chicago, IL, USA. Continuous variables were expressed as means ± standard deviation (SD). Categorical variables were expressed as proportions (%). Chi Square tests were used to compare proportions between groups. SPSS General Linear Model procedure (GLM) multivariate analysis of covariance (MANCOVA) or univariate analysis of covariance (ANCOVA) were used to examine the effect of multiple fixed factors and control for confounding variables (sex and age). Separate MANCOVA analyses were conducted grouping variables according to domains they examine. CPT variables were examined together, WCST variables were examined together, and working memory measures (SOPT and FW) were examined together. As only one measure was available to assess planning (ToL total score) ANCOVA was used for this measure.
Results
Clinical Profile
Only 31% of children did not meet the criteria for any of the three examined comorbidities. Almost half of the group (44.5%) met the criteria for one or more anxiety disorders, 42.8% for ODD and 12.7% for CD. As many as 110 children were diagnosed with more than one comorbid disorder; 13.5% met the criteria for anxiety disorders only, 7.6% for anxiety disorders and CD and 23.5% for anxiety disorders and ODD (Table 1).
Table 1.
Distribution of comorbid disorders
| No comorbid disorders | 31% (N=110) |
| ANX only | 13.5% (N=48) |
| CD only | 5.1% (N=18) |
| ODD only | 19.4% (N=69) |
| ANX+CD | 7.6% (N=27) |
| ANX+ODD | 23.4% (N=83) |
Note: ANX = anxiety disorders; CD = Conduct Disorder; ODD = Oppositional Defiant Disorder.
No age (F(1,353) = .054, p = .816) or sex differences (χ2 (1, N = 355) = .527, p = .468) were found between children with and without comorbidities (Tables 2a, 2b and 2c). Children with anxiety disorders and ODD presented with more inattentive symptoms (anxiety: F(1,353) = 9.34, p = .002; ODD: F(1,353) = 11.45, p = .001) and hyperactive symptoms (anxiety: F(1,353) = 5.71, p = .017; ODD: F(1,353) = 27.22, p < .001), while children with CD more hyperactive symptoms (F(1.353) = 21.24, p < .001) compared to those without the comorbidities.
Table 2a.
Demographic and clinical characteristics of children with and without anxiety disorders
| With Anxiety n=158 |
Without Anxiety n=197 |
||||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | p | |
| Age | 9.43 | 1.68 | 9.37 | 1.65 | n.s |
| Percent boys | 74.1% (n=117) | 76.1% (n=150) | n.s | ||
| FSIQ | 96.99 | 12.32 | 97.4 | 13.49 | n.s |
| DISC-IV Inattentive symptoms | 7.29 | 2.16 | 6.56 | 2.28 | .002 |
| DISC-IV Hyperactive symptoms | 5.47 | 2.64 | 4.79 | 2.71 | .017 |
SD = Standard Deviation; FSIQ = Full Scale IQ.
Table 2b.
Demographic and clinical characteristics of children with and without ODD
| With ODD n=152 |
Without ODD n=203 |
||||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | p | |
| Age | 9.40 | 1.74 | 9.39 | 1.60 | n.s |
| Percent boys | 73.7% (n=112) | 76.4% (n=155) | n.s | ||
| FSIQ | 99.07 | 12.18 | 95.91 | 13.39 | .030 |
| DISC-IV Inattentive symptoms | 7.35 | 2.04 | 6.54 | 2.35 | .001 |
| DISC-IV Hyperactive symptoms | 5.93 | 2.43 | 4.47 | 2.72 | <.001 |
SD = Standard Deviation; FSIQ = Full Scale IQ; ODD=Oppositional Defiant Disorder
Table 2c.
Demographic and clinical characteristics of children with and without CD
| With CD n=45 |
Without CD n=310 |
||||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | p | |
| Age | 9.08 | 1.55 | 9.37 | 1.65 | n.s |
| Percent boys | 80% (n=36) | 74.5% (n=231) | n.s | ||
| FSIQ | 95.93 | 13.35 | 97.41 | 12.93 | n.s |
| DISC-IV Inattentive symptoms | 7.07 | 2.39 | 6.86 | 2.24 | n.s |
| DISC-IV Hyperactive symptoms | 6.78 | 1.92 | 4.85 | 2.71 | <.001 |
SD = Standard Deviation; FSIQ = Full Scale IQ; CD = Conduct Disorder
The Full Scale IQ for the group was within the average range (Tables 2a, 2b, and 2c). No significant IQ differences were found between boys and girls (FSIQ: F(1,325) = .081, p = .776). Children with ODD were found to have significantly higher scores on Full Scale IQ (F(1,325) = 4.77, p = .030), working memory (F(1,249) = 6.78, p = .010; with ODD: M = 95.64, SD =11.05; without ODD: M = 91.58, SD = 13.05), and processing speed (F(1,249) = 7.37, p = .007; with ODD: M = 99.59, SD =13.47; without ODD: M = 94.98, SD = 13.16) indices of WISC-IV compared to children without ODD .Significant differences were found between children with CD and without CD on verbal index when sex was used as a covariate (F(3,326) = 4.014, p = .046), children with CD having obtained lower scores compared to children without CD (with CD: M = 92.65, SD =14.02; without CD: M = 96.83, SD = 13.76).
Comorbid Disorders and Executive Function
CPT.
Table 3 presents the CPT data. The MANCOVA results showed a significant main effect of anxiety disorders (F(6,337) = 2.693, p = .014 ) and age (F(6,337) = 19.654, p <.001). Children with anxiety disorders obtained higher T scores on commission errors, RT standard error, and RT inter-stimuli-interval change. No significant multivariate effect of CD (F(6,338) = 1.574, p = .154) or ODD (F(6,340) = .767, p = .596) was observed.
Table 3.
CPT and comorbid disorders
| Anx | ODD | CD | Total | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| Yes | No | Yes | No | Yes | No | ||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Omission errors T score | 56.63 (14.81) | 56.74 (15.57) | 55.61 (14.49) | 57.49 (15.71) | 59.82 (15.82) | 56.28 (15.1) | 56.69 (15.21) |
| Commission errors T scores | 54.74* (8.01) | 53.4 (8.05) | 54.55 (8.52) | 53.59 (7.68) | 53.86 (7.1) | 54.02 (8.19) | 54 (8.05) |
| RT T scores | 52.31 (10.38) | 51.52 (11.48) | 51.06 (11.2) | 52.48 (10.83) | 53.26 (10.53) | 51.67 (11.06) | 51.87 (10.99) |
| RT Standard Error T scores | 58.66* (10.7) | 56.41 (10.3) | 57.23 (10.66) | 57.57 (10.45) | 60.17 (9.52) | 57.02 (10.62) | 57.42 (10.53) |
| RT Block Change T scores | 52.65 (12.62) | 51.82 (11.5) | 51.52 (11.75) | 52.69 (12.2) | 55.92 (11.43) | 51.65 (12.01) | 52.19 (12.01) |
| RT – ISI T scores | 56.44* (14.65) | 54.05 (12.07) | 55.29 (13.82) | 55.01 (12.98) | 55.77 (12.83) | 55.03 (13.42) | 55.13 (13.33) |
M = Mean; SD = Standard Deviation; RT = Reaction Time; ANX = anxiety disorders; CD = Conduct Disorder; ODD = Oppositional Defiant Disorder; No Com = no comorbid disorders; ISI = Inter –Stimuli – Interval.
= p<.05
WCST.
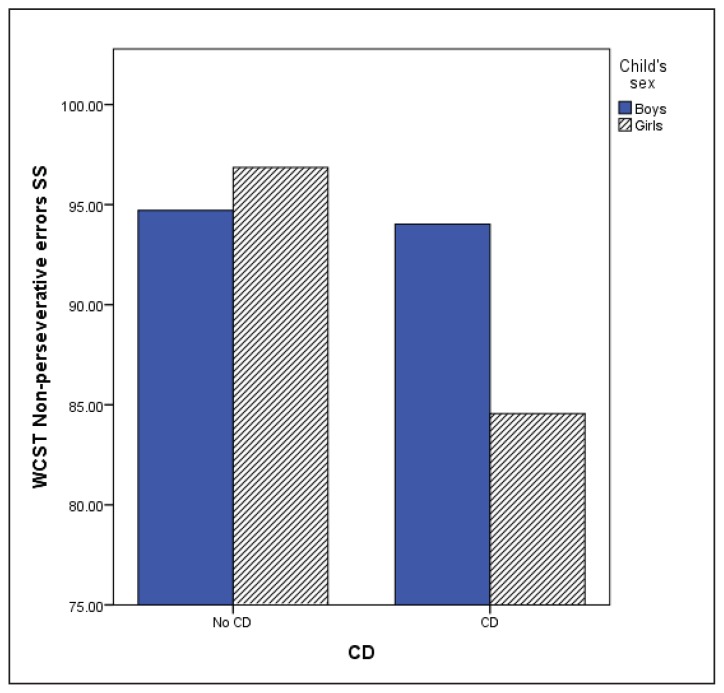
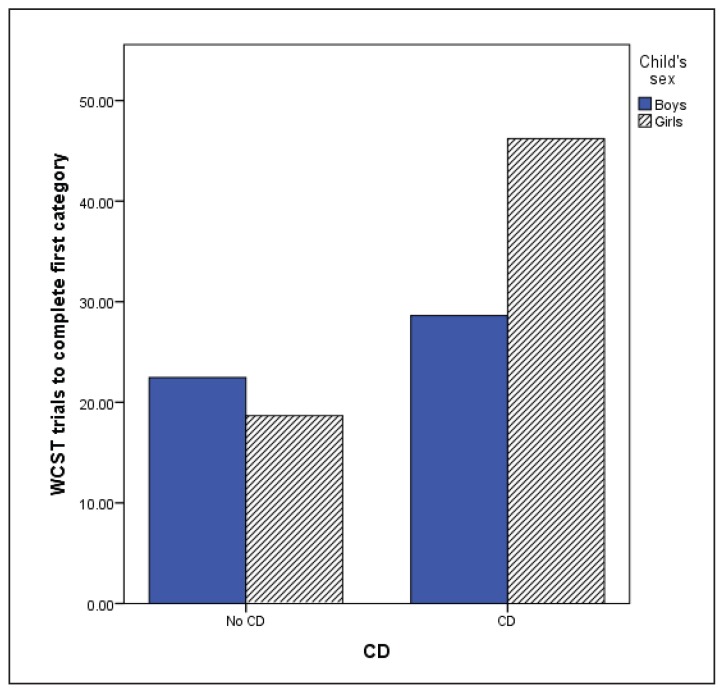
The MANCOVA using WCST variables (Table 4) showed no significant multivariate effect of anxiety disorders (F(6,348) = .645, p = .694) or ODD (F(6, 348) = 1.023, p = .410). However, significant multivariate CD (F(6,345) = 2.55, p = .020), age (F(6, 345) = 10.087, p < .001) and CD by sex interaction effects were observed (F(6, 345) = 2.173, p = .045). Children with CD made more perseverative and non-perseverative errors, completed fewer categories and required more trials to complete the first category indicating poorer performance. Sex by CD interaction was found for non-perseverative errors and number of trials to complete the first category. Boys with and without CD obtained similar scores on the non-perseverative errors (M = 94.02, SD = 16.17; M = 94.71, SD = 14.69), while girls with CD made more errors (M = 84.55, SD = 10.85) compared with girls without CD (M = 96.86, SD = 14.35) or boys in either group (p = .016; Figure 1). Girls with CD also required more trials to complete the first category compared to boys and girls without CD, or boys with CD (p = 0.14; Figure 2). This indicated that girls with CD had difficulty with initial concept formation.
Table 4.
WCST and comorbid disorders
| Anx | ODD | CD | Total | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Yes | No | Yes | No | Yes | No | ||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| WCST Perseverative responses SS | 97.2 | 98.99 | 98.6 | 97.9 | 94.9 | 98.66 | 98.2 |
| −13.54 | −13.7 | −15.26 | −12.32 | −13.08 | −13.67 | −13.64 | |
| WCST Perseverative Errors SSa | 96.92 | 99.18 | 99.18 | 97.43 | 94.51* | 98.71 | 98.18 |
| −13.43 | −12.4 | −13.46 | −12.43 | −12.98 | −12.82 | −12.89 | |
| WCST Non Perseverative Error SSa | 94.13 | 95.45 | 95.41 | 94.45 | 92.13* | 95.26 | 94.86 |
| −14.58 | −14.92 | −15.22 | −14.43 | −15.63 | −14.62 | −14.76 | |
| WCST number of categories completed SSa | 4.28 | 4.6 | 4.48 | 4.44 | 3.89* | 4.54 | 4.46 |
| −1.82 | −1.66 | −1.83 | −1.67 | −1.97 | −1.69 | −1.74 | |
| WCST Trials to complete first category SSb | 24.73 | 21.32 | 22.68 | 22.96 | 32.13* | 21.49 | 22.84 |
| −28.53 | −21.97 | −25.16 | −25.15 | −37.45 | −22.56 | −25.12 | |
| WCST Failure to maintain set SS | 1.55 | 1.56 | 1.53 | 1.58 | 1.51 | 1.57 | 1.56 |
| −1.46 | −1.38 | −1.27 | −1.52 | −1.51 | −1.4 | −1.42 | |
ANX = anxiety disorders; CD = Conduct Disorder; ODD = Oppositional Defiant Disorder; WCST = Wisconsin Card Sorting Test; SS = Standard Score; M = Mean; SD = Standard Deviation;
= p < .05;
lower scores on this measure indicates poor performance;
higher score on this measure indicates poor performance.
Figure 1.
WCST Non-perseverative errors
Higher scores correspond to better performance; WCST = Wisconcin Card Sorting Test; CD = Conduct Disorder
Figure 2.
WCST trials to complete the first category
Lower scores correspond to better performance; WCST = Wisconcin Card Sorting Test; CD = Conduct Disorder
ToL.
On the planning task, a significant main effect of sex was observed (F(1,350) = 11.127, p = .001). Boys, obtained higher scores (M = 111.28, SD = 13.57) than girls (M = 105.04, SD = 16.93). No significant effect of anxiety disorders (F(1, 353) = 1.856, p = .174), CD (F(1,353) = .026, p = .873) or ODD (F(1,353) = .080, p = .778) was observed.
Working Memory.
When sex and age were included in the MANCOVA model no significant effect of anxiety disorders was seen (F(2, 349) = 1.458, p=.234). However if confounding variables were not used, a significant effect of anxiety disorders was observed (F(2,351) =3.387, p = .035). Since FW scores are standardised the FW was examined without controlling for age or sex. Univariate analysis of variance showed that children with anxiety disorders obtained lower scores on the FW task (F(1,353) = 6.62, p = .010). However, when only sex was used in MANCOVA the significant multivariate effect was no longer present (F(2,350)=.1.46, p=.552). No other significant effects of comorbid disorders were observed (CD: F(2,352)=.497, p=.609; ODD: F(2,352)= .433, p= .649).
When multiple comorbidities were examined, such as anxiety disorders with CD or anxiety disorders with ODD, no significant differences were found between children with multiple comorbid disorders and those without multiple comorbid disorders.
Discussion
The current study examined attention, inhibition and EF in children with ADHD, both with and without comorbid disorders in a large sample of children clinically diagnosed with ADHD. The initial hypotheses were partially supported as our findings show that the presence of comorbid CD or anxiety disorders affect children’s EF performance. First, we found several differences in performance between children with and without anxiety disorders. Specifically, contrary to previous reports (Bloemsma, et al., 2013) children with anxiety disorders had more difficulty with response inhibition, resulting in higher number of commission errors on CPT. While their overall reaction time for correct responses (RTT) and change in reaction time across the test (RT-Block change) did not differ from children without anxiety disorders, children with anxiety disorders were more inconsistent in response speed (i.e. had a more erratic response style throughout the test as demonstrated by RT standard error) and as the speed between targets increased, their reaction time decreased (RT-ISI). Inconsistency in performance is now well recognized by clinicians working with the ADHD population. Our findings suggest that this inconsistency may be more pronounced in children who are also affected by anxiety disorders. Children with ADHD also have difficulties when faced with tasks that they deem challenging. In this study we observed that in laboratory setting, when the demands of the environment are increased, children with ADHD and anxiety disorders have difficulty adjusting to this change and as a result slow down their response. Contrary to other reports, we found no interaction between anxiety disorders and sex with respect to performance on the CPT task (Newcorn et al., 2001).
With respect to comorbid anxiety disorders and working memory our findings were in line with previous findings (Bedard & Tannock, 2008; Vance, Ferrin, Winther, Winther, & Gomez, 2013) as children with comorbid anxiety disorders had more working memory difficulties compared to those without anxiety disorders. This however was captured by only one of the working memory measures: the FW, and only when confounding variables (sex and age) were not included in the model. Difficulties with working memory may have a detrimental effect on children’s ability to learn and their functioning in the classroom or in day-to-day activities. These findings further emphasize the significance of comorbid anxiety disorders. Anxiety disorders should be routinely evaluated in children diagnosed with ADHD. Furthermore, if children present with ADHD and anxieties in clinical setting, clinicians should be encouraged to assess the child’s working memory abilities and if affected put in place intervention to decrease anxiety and accommodations allowing the child to compensate for anxieties as well as difficulties associated with working memory.
Presence of CD poses significant difficulties for children, their families and the society at large, due to associated risk factors. The findings of the current study bring to light potential deficits that may shape the phenotypic expression of the disorder. Contrary to other reports (Oosterlaan, Scheres & Sergeant, 2005), children in this study who were diagnosed with comorbid CD had more difficulties on the set-shifting task (WCST), making more perseverative and non-perseverative errors compared to their peers diagnosed with ADHD but without the comorbid CD. In addition, girls with CD had particular difficulties with initial concept formation and problem solving, as they required more trials to complete the first category on the WCST. In addition to differences on the set-shifting task, children with comorbid CD had lower verbal IQ as compared to children without CD. This finding supports the recent report by Murray and Farrington (2010), who found low IQ to be a predictor of CD.
It is well established that the presence of CD is associated with antisocial outcomes and substance use later in life (Pardini & Fite, 2010), thus identifying the clinical profile of children with ADHD and CD is important for prevention, early intervention and effective treatment. Further investigation is required to confirm whether low verbal IQ, in combination with increased hyperactivity symptoms, and difficulties with cognitive flexibility are specific risk factors for developing CD in childhood as was seen in our study. It is possible that having more severe ADHD symptoms combined with difficulties in verbal communication may exacerbate behavioural problems, resulting in the kinds of disruptive behaviour seen in a diagnosis of CD. Prevention and treatment programmes should focus on intervention targeting children’s language abilities, both expressive and receptive and cognitive flexibility, thus allowing children to develop necessary skills to respond in a socially adaptive manner to the demands of their environment.
In addition to sex differences seen between children with comorbid CD, we found sex differences in planning, as boys with ADHD performed better on the Tower of London task than did girls. Similar results were reported by O’Brien, Dowell, Mostofsky, Denckla and Mahone (2010) who compared girls and boys with ADHD using various EF tasks and found that girls demonstrated poorer performance on the planning test. The sex differences observed in this study may explain the differences in clinical presentation of ADHD between boys and girls, and could be explained by the neuroanatomical brain differences seen between boys and girls (Mahone & Wodka, 2008).
Finally, the overall clinical profile of the sample was similar to those reported in the literature. As expected, many children presented with at least one comorbid disorder (Gau et al., 2010). Multiple comorbidities were also frequent, which is similar to reports in the literature (Larson, Russ, Kahn, & Halfon, 2011), however having more than one comorbid disorder did not affect the EF task performance.
This study has several strengths, the first of which lays in addressing some of the methodological limitations in current literature. The large sample size allowed us to investigate subgroups of children with comorbidities, as well as examine sex differences. All the children in this study underwent a thorough diagnostic process, including clinical diagnosis by a child psychiatrist and vigorous parent interviews that included the level of impairment and interference with functioning in more than one setting as part of its diagnostic algorithm. The standardised EF assessment procedure allowed to control for potential effects of medication and fatigue on EF task performance.
Alongside these strengths, the study has limitations as well. As we did not include a control group, no comparisons can be made with typically developing children. While the assessments for the comorbid disorders were made using a thorough diagnostic tool used with parents, the study would have benefited from the inclusion of a child report measure of comorbid disorders. Finally, no teacher ratings of ADHD symptoms were included in this analysis and the ADHD symptoms were based only on parent report.
Conclusion
Our findings demonstrate that the presence of comorbidities, EF deficits, and sex differences may explain the heterogeneous nature of ADHD among elementary school-age children. These differences can also contribute to the inconclusive findings previously reported in the literature. Given the reported relationship between EF and social functioning (Miller & Hinshaw, 2010; Rinsky & Hinshaw, 2011) and EF and academic functioning (Miller & Hinshaw, 2010; Rogers, Hwang, Toplak, Weiss, & Tannock, 2011) in individuals with ADHD and comorbid disorders, identifying EF deficits should be an integral part of ADHD assessment. EF and cognitive deficits and comorbid disorders should also be central in intervention design for this population. In conclusion, to maximize treatment success, children diagnosed with ADHD should receive tailored treatment as opposed to one focusing solely on addressing ADHD symptoms. For example, children comorbid CD and with difficulties in the area of cognitive flexibility and language would benefit from skill training programme that will address these areas. Similarly, children whose working memory is affected can use strategies to compensate for this difficulty which will aid their functioning at school and in social settings. Further research is needed focusing on children with CD, particularly girls diagnosed with CD and ADHD and the developmental trajectories associated with development of CD in ADHD population.
Acknowledgments/Conflicts of Interest
The authors would like to thankfully acknowledge the Douglas Institute ADHD research team for data collection and data entry work, specifically Sandra Robinson and Jacqueline Richard for conducting the EF assessments, and Nicole Pawliuk for her assistance. This study was supported by the Canadian Institutes of Health Research (CIHR) operating grant and CIHR doctoral scholarship awarded to the first author. Most of all, we would like to thank all children and their families who graciously agreed to participate in this study.
References
- Anderson P, Anderson V, Lajoie G. The Tower of London test: Validation and standardization for pediatric populations. Clinical Neuropsychologist. 1996;10:54–65. [Google Scholar]
- Bedard AC, Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. Journal of Attention Disorders. 2008;11:546–557. doi: 10.1177/1087054707311213. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, …Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, Faraone SV. The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: Findings from a controlled 10-year prospective longitudinal follow-up study. Psychological Medicine. 2008;38(7):1027–1036. doi: 10.1017/S0033291707002668. [DOI] [PubMed] [Google Scholar]
- Bloemsma JM, Boer F, Arnold R, Banaschewski T, Faraone SV, Buitelaar JK, …Oosterlaan J. Comorbid anxiety and neurocognitive dysfunctions in children with ADHD. European Child and Adolescent Psychiatry. 2013;22:225–234. doi: 10.1007/s00787-012-0339-9. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: A dimensional and developmental study. Developmental Neuropsychology. 2004;26:571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- Cuffe SP, Visser SN, Holbrook JR, Danielson ML, Geryk LL, Wolraich ML, McKeown RE. ADHD and psychiatric comorbidity: Functional outcomes in a school-based sample of children. Journal of Attention Disorders. 2015;25 doi: 10.1177/1087054715613437. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2007;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. Journal of Abnormal Child Psychology. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C. Normative data from the CANTAB. I: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25(2):242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2006;67(Suppl 8):21–26. [PubMed] [Google Scholar]
- Emerson CS, Mollet GA, Harrison DW. Anxious-depression in boys: An evaluation of executive functioning. Archives of Clinical Neuropsychology. 2005;20:539–546. doi: 10.1016/j.acn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: Attention, inhibition, response perseveration, and the impact of comorbidity. Developmental Neuropsychology. 2005;27:107–133. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- Gau SS, Ni HC, Shang CY, Soong WT, Wu YY, Lin LY, Chiu YN. Psychiatric comorbidity among children and adolescents with and without persistent attention-deficit hyperactivity disorder. Australian and New Zealand Journal of Psychiatry. 2010;44:135–143. doi: 10.3109/00048670903282733. [DOI] [PubMed] [Google Scholar]
- Hasson R, Fine JG. Sex differences among children with ADHD on continuous performance tests: A meta-analytic review. Journal of Attention Disorders. 2012;16(3):190–198. doi: 10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Manual - Revised and Expanded. Lutz, FL: Psychological Assessment Resources, Inc; 1993. [Google Scholar]
- Hummer TA, Kronenberger WG, Wang Y, Dunn DW, Mosier KM, Kalnin AJ, Mathews VP. Executive functioning characteristics associated with ADHD comorbidity in adolescents with disruptive behavior disorders. Journal of Abnormal Child Psychology. 2011;39:11–19. doi: 10.1007/s10802-010-9449-3. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, …Vitiello B. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Kadesjo B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school-age children. Journal of Child Psychology and Psychiatry. 2001;42:487–492. [PubMed] [Google Scholar]
- Kalff AC, Hendriksen JG, Kroes M, Vles JS, Steyaert J, Feron FJ, Jolles J. Neurocognitive performance of 5- and 6-year-old children who met criteria for attention deficit/hyperactivity disorder at 18 months follow-up: Results from a prospective population study. Journal of Abnormal Child Psychology. 2002;30:589–598. doi: 10.1023/a:1020859629994. [DOI] [PubMed] [Google Scholar]
- Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127:462–470. doi: 10.1542/peds.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Wodka EL. The neurobiological profile of girls with ADHD. Developmental Disabilities Research Review. 2008;14:276–284. doi: 10.1002/ddrr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassis K, Tannock R, Young A, Francis-John S. Cognition in anxious children with attention deficit hyperactivity disorder: A comparison with clinical and normal children. Behavioral and Brain Functioning. 2007;3:4. doi: 10.1186/1744-9081-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Hinshaw SP. Does childhood executive function predict adolescent functional outcomes in girls with ADHD? Journal of Abnormal Child Psychology. 2010;38:315–326. doi: 10.1007/s10802-009-9369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Farrington DP. Risk factors for conduct disorder and delinquency: Key findings from longitudinal studies. Canadian Journal of Psychiatry. 2010;55:633–642. doi: 10.1177/070674371005501003. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Halperin JM, Jensen PS, Abikoff HB, Arnold LE, Cantwell DP, … Vitiello B. Symptom profiles in children with ADHD: Effects of comorbidity and sex. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:137–146. doi: 10.1097/00004583-200102000-00008. S0890-8567(09)60363-4 [pii] [DOI] [PubMed] [Google Scholar]
- O’Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology. 2010;25:656–670. doi: 10.1093/arclin/acq050. acq050 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick TH, Jarrett MA, Grills-Taquechel AE, Hovey LD, Wolff JC. Comorbidity as a predictor and moderator of treatment outcome in youth with anxiety, affective, attention deficit/ hyperactivity disorder, and oppositional/conduct disorders. Clinical Psychology Review. 2008;28:1447–1471. doi: 10.1016/j.cpr.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Scheres A, Sergeant JA. Which executive functioning deficits are associated with AD/HD, ODD/CD and comorbid AD/HD+ODD/CD? Journal of Abnormal Child Psychology. 2005;33:69–85. doi: 10.1007/s10802-005-0935-y. [DOI] [PubMed] [Google Scholar]
- Pajer K, Chung J, Leininger L, Wang W, Gardner W, Yeates K. Neuropsychological function in adolescent girls with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:416–425. doi: 10.1097/CHI.0b013e3181640828. [DOI] [PubMed] [Google Scholar]
- Pardini DA, Fite PJ. Symptoms of conduct disorder, oppositional defiant disorder, attention-deficit/hyperactivity disorder, and callous-unemotional traits as unique predictors of psychosocial maladjustment in boys: Advancing an evidence base for DSM-V. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1134–1144. doi: 10.1016/j.jaac.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Sherman JO, Barrow MV, Irick S. Affective disorder in juvenile offenders: A preliminary study. American Journal of Psychiatry. 2000;157:130–132. doi: 10.1176/ajp.157.1.130. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Park J, Seth S, Coghill DR. A comprehensive investigation of memory impairment in attention deficit hyperactivity disorder and oppositional defiant disorder. Journal of Child Psychology and Psychiatry. 2012;53:128–137. doi: 10.1111/j.1469-7610.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- Rinsky JR, Hinshaw SP. Linkages between childhood executive functioning and adolescent social functioning and psychopathology in girls with ADHD. Child Neuropsychology. 2011;17:368–390. doi: 10.1080/09297049.2010.544649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LM, Sclar DA, Skaer TL, Galin RS. National trends in the prevalence of attention-deficit/hyperactivity disorder and the prescribing of methylphenidate among school-age children: 1990–1995. Clinical Pediatrics (Philadelphia) 1999;38:209–217. doi: 10.1177/000992289903800402. [DOI] [PubMed] [Google Scholar]
- Rogers M, Hwang H, Toplak M, Weiss M, Tannock R. Inattention, working memory, and academic achievement in adolescents referred for attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology. 2011;17:444–458. doi: 10.1080/09297049.2010.544648. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Sex differences in attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America. 2010;33:357–373. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Valera E, Doyle AE, Faraone SV. Impact of sex and age on executive functioning: Do girls and boys with and without attention deficit hyperactivity disorder differ neuropsychologically in preteen and teenage years? Developmental Neuropsychology. 2005;27(1):79–105. doi: 10.1207/s15326942dn2701_4. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Schatz DB, Rostain AL. ADHD with comorbid anxiety: A review of the current literature. Journal of Attention Disorders. 2006;10(2):141–149. doi: 10.1177/1087054706286698. [DOI] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learning. Lutz, FL: PAR Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Stefanatos GA, Baron IS. Attention-Deficit/Hyperactivity Disorder: A neuropsychological perspective towards DSM-V. Neuropsychology Review. 2007;17:5–38. doi: 10.1007/s11065-007-9020-3. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: Performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychology. 2009;15:53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Toupin J, Dery M, Pauze R, Mercier H, Fortin L. Cognitive and familial contributions to conduct disorder in children. Journal of Child Psychology and Psychiatry. 2000;41:333–344. [PubMed] [Google Scholar]
- Vance A, Ferrin M, Winther J, Gomez R. Examination of spatial working memory performance in children and adolescents with attention deficit hyperactivity disorder, combined type (ADHD-CT) and anxiety. Journal of Abnormal Child Psychology. 2013;41(6):891–900. doi: 10.1007/s10802-013-9721-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Third Edition. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/ hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]