Abstract
Objectives
To examine the association between clozapine treatment and frequency of cannabis use in adolescents with co-occurring psychotic and cannabis use disorder in a retrospective cohort chart review.
Method
We conducted a retrospective cohort chart review of patients diagnosed with a psychotic disorder and concurrent cannabis use disorder admitted to a tertiary care youth inpatient unit from 2010–2012. Longitudinal exposure and outcome data was coded month-by-month. Frequency of cannabis use was measured using a 7-point ordinal scale. Severity of psychosis was measured on a 3-point ordinal scale. Mixed effects regression modeling was used to describe the relationship between exposure and outcome variables.
Results
Thirteen patients had exposure to clozapine and fourteen had no exposure to clozapine. Cannabis use decreased in patients treated with clozapine, compared to patients treated with other antipsychotics (OR 2.8; 95% CI 0.97–7.9). Compared to no medication, clozapine exposure was associated with significantly less cannabis use (OR 7.1; 95% CI 2.3–22.3). Relative to treatment with other antipsychotics, clozapine exposure was significantly associated with lower severity of psychotic symptoms (OR 3.7; 95% CI 1.2–11.8).
Conclusions
Clozapine may lead to decreased cannabis use and psychotic symptoms in adolescents with concurrent psychosis and substance use. Clinical trials are warranted.
Keywords: Clozapine, cannabis, addiction, psychosis, adolescent
Résumé
Objectifs
Examiner l’association entre le traitement par clozapine et la fréquence d’utilisation de cannabis chez des adolescents souffrant d’un trouble psychotique et d’un trouble lié à l’utilisation de cannabis co-occurrents dans une revue rétrospective des dossiers d’une cohorte.
Méthode
Nous avons mené revue rétrospective des dossiers d’une cohorte de patients ayant reçu un diagnostic de trouble psychotique et d’un trouble co-occurrent lié à l’utilisation de cannabis et hospitalisés dans un service de soins tertiaires pour adolescents de 2010 à 2012. Les données d’exposition longitudinale et de résultats étaient codées mois après mois. La fréquence d’utilisation de cannabis était mesurée à l’aide d’une échelle ordinale en 7 points. La gravité de la psychose était mesurée par une échelle ordinale en 3 points. Les effets mixtes du modèle de régression ont été utilisés pour décrire la relation entre l’exposition et les variables des résultats.
Résultats
Treize patients avaient été exposés à la clozapine et 14 n’y avaient pas été exposés. L’utilisation de cannabis diminuait chez les patients traités par clozapine, comparativement aux patients traités par d’autres antipsychotiques (RC 2,8; IC à 95% 0,97 à 7,9). Comparativement à l’absence de médicament, l’exposition à la clozapine était associée à une utilisation significativement moindre de cannabis (RC 7,1; IC à 95% 2,3 à 22,3). Relativement au traitement par d’autres antipsychotiques, l’exposition à la clozapine était significativement associée à une gravité plus faible des symptômes psychotiques (RC 3,7; IC à 95% 1,2 à 11,8).
Conclusions
La clozapine peut entraîner une utilisation réduite de cannabis et une diminution des symptômes psychotiques chez les adolescents souffrant de psychose et d’utilisation de substance co-occurrentes. Des essais cliniques sont justifiés.
Mots clés: clozapine, cannabis, dépendance, psychose, adolescent
Introduction
Schizophrenia is a devastating mental illness associated with functional impairment, social disability, suicide, and increased all-cause mortality rates (Tandon, Nasrallah, & Keshavan, 2009). Early-age of onset and comorbid cannabis use are associated with each other (Dekker et al., 2012) and each are predictive of worse outcome (Tandon et al., 2009). Twenty-eight percent of patients in a first episode psychosis clinic were found to meet Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria for cannabis dependence (Green et al., 2004). In patients with a psychotic disorder, cannabis use disorder is associated with increased morbidity, increased rates of relapse (Linszen, Dingemans, & Lenior, 1994), noncompliance with treatment, increased rates of hospitalizations (Van Dijk, Koeter, Hijman, Kahn, & Van den Brink, 2012) and poorer overall functioning compared to non-cannabis users (Green, Burgess, Dawson, Zimmet, & Strous, 2003). Treatments that facilitate cessation of cannabis in people with schizophrenia may favourably impact their prognosis.
Clozapine is an atypical antipsychotic that has been thought to be the most effective intervention in adults with treatment-resistant schizophrenia (Leucht et al., 2013; McEvoy et al., 2006; Wahlbeck, Cheine, Essali, & Adams, 1999); however, its use is limited by potential side effects such as weight gain, sedation and risk of agranulocytosis. Conversely, there is a recent meta-analysis that calls into question clozapine’s superior efficacy (Samara et al., 2016). There are three small randomized trials (Kumra et al., 1996; Kumra et al., 2008; Sporn et al., 2007) suggesting superior efficacy in children and adolescents with refractory schizophrenia, in keeping with the more traditional view of the role of clozapine in adult populations.
In adult samples, multiple observational studies have shown an association between clozapine treatment and reductions of substance use (Brunette, Drake, Xie, McHugo, & Green, 2006; Drake, Xie, McHugo, & Green, 2000; Green et al., 2003; Zimmet, Strous, Burgess, Kohnstamm, & Green, 2000). There are two small, randomized controlled trials testing the role of clozapine treatment in outpatient adults with co-occurring schizophrenia and cannabis use disorder. Brunette et al. (2011) randomized 31 patients to clozapine versus continuing other antipsychotic treatment. Those receiving clozapine ended up using about 4.5 “joints” less cannabis per week relative to controls, but the difference did not reach statistical significance, possibly due to lack of power. Conversely, Schnell et al. (2014) randomized 30 such patients to receive clozapine or ziprasidone. All participants were offered an array of psychosocial treatments as well. They found that clozapine and ziprasidone were equally effective in reducing cannabis use. This null finding could be due to “floor effects” as most had drastically reduced their use within the first month after randomization and gains were maintained over 12 months. High drop-out rates limit the implications of this study. We did not find literature on the role of clozapine for concurrent schizophrenia and cannabis used disorders in the adolescent age group.
Our group conducted a retrospective cohort study through chart review to examine the association between clozapine treatment and frequency of cannabis use in a clinical sample of adolescents. Our primary hypothesis was that relative to other antipsychotics, clozapine treatment would be associated with a decrease in extent of cannabis use. Our secondary hypothesis was that relative to other antipsychotics, clozapine treatment would be associated with lower severity of psychotic symptoms.
Methods
The sample was derived from 127 patients consecutively admitted to a tertiary care inpatient psychiatric unit for youth from 2010 to 2012. Of these, 27 met inclusion criteria for the study. Inclusion criteria were adolescents aged 15–18 at the time of admission who met DSM-5 criteria for cannabis use disorder, and DSM-IV criteria for schizophrenia, schizoaffective disorder, bipolar disorder (if psychotic features were present), or a provisional diagnosis of schizophrenia. Bipolar disorder was included as clozapine is a third line medication option for bipolar disorder (Yatham et al., 2013), and many youth on the unit initially diagnosed with bipolar disorder are eventually diagnosed with schizoaffective disorder. These diagnoses were made by clinical impression of the treating psychiatrist (DBC). The psychiatry residents (ST, AA) reviewing the charts determined the clinical diagnosis of cannabis use disorder. DSM-5 criteria were applied retrospectively given that DSM-IV was the diagnostic system being used when patients were being treated. The principal investigator (ST) oversaw consistency of retrospective cannabis use disorder diagnoses in the chart review. Patients with a primary diagnosis of borderline personality disorder and/or psychotic symptoms secondary to trauma were excluded, as clozapine is typically not indicated for these syndromes.
The chart review was conducted in keeping with a nine-step formal process (Gearing, Mian, Barber, & Ickowicz, 2006). Two investigators (ST and AA) used a chart-auditing tool to code information on exposure and outcome variables based on the text written in the charts and medication records. The principle investigator (ST) was present to oversee the coding of all data to increase rating consistency. For admitted patients, data was collected for each week hospitalized. For patients who had been discharged and followed as an outpatient, data was recorded from each follow up visit. This data was then summarized into month-by-month increments and entered into a longitudinal database. Any data that was not available at specific time points was recorded as missing.
Baseline Demographics
Age, sex, race, level of care, medication type and dose, and duration of treatment were coded as part of the audit tool. Baseline motivation to stop cannabis use was also recorded using a 3-point ordinal scale where: 1 represented low motivation to stop using; 2 represented medium motivation; and, 3 represented high motivation.
Exposure Variable
Exposure to medication was categorized as “on clozapine”, “on another antipsychotic” or “no antipsychotic medication”. The individual had to be taking medication for at least 75% of the month to be considered for exposure. If patients were taking multiple antipsychotics, and one of these was clozapine, the patient would be categorized as “on clozapine”.
Primary Outcome Measurements
The primary outcome measure was frequency of cannabis use, i.e. tetrahydrocannabinol (THC) level recorded according to a 7-point ordinal scale based on text written in progress notes. Frequency of use was coded as 0 = zero use; 1 = less than once a month; 2 = once per month; 3 = two to three times per month; 4 = once to twice a week; 5 = three to four times per week; 6 = greater than five times a week. Baseline cannabis use, defined as the reported frequency of cannabis use in the three months prior to hospitalization, was recorded based on information in the admission note.
Secondary Outcome Measurements
Level of psychosis at each time point was measured on a 3-point ordinal scale, where: 0 represented absence of psychotic symptoms; 1 represented residual symptoms that were not distressing or not severely impairing to patient; and, 2 represented active psychotic symptoms that were highly distressing or severely impairing. Research Ethics Board approval was obtained from the Institute of Mental Health Research in Ottawa for this study.
Analysis
Baseline categorical data was described using proportions, and exposure groups were compared using Fisher’s exact tests. Baseline ordinal and continuous data was described with medians and inter-quartile ranges, and exposure groups were compared using Wilcoxon Rank-Sum tests given the non-parametric nature of the data. “Peak dose” was defined as the maximum dose a specific patient was prescribed over the observation period.
We set up a database to conduct an unbalanced longitudinal mixed effects regression analysis to allow for comparisons: (1) within-group, prior to initiating clozapine; and, (2) to a control group not exposed to clozapine over the observation period. We wished to make a common reference point for both exposure groups. For those exposed to clozapine, month “0” was the month that clozapine was initially started and reached a dose of 150mg or more. Months “−12” to “−1” correspond to the months prior to clozapine initiation. Months “+1” to “+12” correspond to months after starting clozapine. For those not exposed to clozapine, month “0” corresponds to the last observation found in the chart, as there was the potential for clozapine to have been initiated thereafter. Months “−12” to “−1” represent the 12 months prior to the last observation. The timepoint “0” was solely used to create a reference point for the database; if patients exposed to clozapine stopped their medication thereafter, the exposure variable would still be coded as “no medication” for the month they were off medication. This arrangement allowed us to adjust for the passage of time in analyzing the outcomes.
To test the primary hypothesis, longitudinal analysis was conducted using ordinal logistic regression mixed effects modeling with degree of cannabis use (THC level) as the outcome. As there were three medication categories, the first exposure variable (with a co-efficient of β1) represents the degree to which a “no medication” status for a particular month contributes to the outcome relative to clozapine; and the second exposure variable (with the co-efficient β2) represents the degree to which “non-clozapine antipsychotic medication” status for a particular month contributes to the outcome relative to clozapine. Month of treatment was entered as a covariate, and placement (inpatient versus outpatient) was treated as a time-varying covariate; despite being admitted to the inpatient unit, many patients still ended up using cannabis while on passes. Thus the equation being tested was:
The null hypothesis was that β2=0; this would indicate that clozapine exposure is not associated with changes in cannabis use relative to treatment with other antipsychotics. A positive β2 would suggest that clozapine use is associated with decreased cannabis use relative to treatment with other antipsychotics.
To test the secondary hypothesis, ordinal logistic regression mixed effects modeling was used with degree of psychosis (psychosis level) as the outcome. The medication-exposure independent variables were identical to the primary analysis. Month of treatment was entered as a covariate, and degree of cannabis use was entered as a time-varying covariate. Thus the equation for the secondary analysis being tested was:
The null hypothesis was that β2=0; this would represent that there is no association between clozapine and degree of psychosis relative to other antipsychotics. A positive β2 would suggest that clozapine use is associated with decreased psychotic symptoms relative to other antipsychotics.
Likelihood ratio tests were used to compare models, checking for clozapine’s importance as a contributing factor in the outcome.
Results
Of the 27 participants included in the study, ten had polysubstance abuse. Of these, six were concurrent alcohol and marijuana use disorders; two were polysubstance use disorders involving ecstasy, over-the-counter medications, and/ or cocaine; and two were recorded as having polysubstance use but the specific substances besides marijuana were not recorded.
Relative to controls, clozapine-exposed patients were more likely to be younger and more likely to have been in treatment for a longer period of time (see Table 1). Within the clozapine-exposed group (n=13), four had varying persistent exposure to additional antipsychotics (including risperidone, aripiprazole, olanzapine, quetiapine, ziprasidone and paliperidone palmitate). The mean peak dose of clozapine was 385mg/d; the range of peak doses was 225mg–600mg/d.
Table 1.
Sample Characteristics
| Total (n=27) | Tried on clozapine (n=13) | Not tried on clozapine (n=14) | Fisher’s exact | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Categorical Variable | n | % | n | % | n | % | |
| Gender | |||||||
| Male | 22 | 81.5 | 12 | 92 | 10 | 71 | 0.33 |
| Female | 5 | 18.5 | 1 | 8 | 4 | 29 | |
| Race | |||||||
| Caucasian | 14 | 52 | 6 | 46 | 8 | 57 | 0.58 |
| Afro-carribean | 3 | 11 | 2 | 15 | 1 | 7 | |
| Aboriginal | 4 | 15 | 1 | 8 | 3 | 21 | |
| Other (eg. Iranian, South East Asian, Mixed race) | 6 | 22 | 4 | 31 | 2 | 14 | |
| Diagnosis | |||||||
| Schizophrenia/Schizoaffective Disorder | 21 | 12 | 7 | 0.06 | |||
| Bipolar Disorder with psychotic symptoms | 3 | 0 | 3 | ||||
| Psychosis NOS | 5 | 1 | 4 | ||||
| Level of Psychosis at First Data Entry | |||||||
| 0 = residual symptoms | 5 | 2 | 3 | 1.00 | |||
| 1= partial symptoms | 5 | 2 | 3 | ||||
| 2 = florid symptoms | 17 | 9 | 8 | ||||
| Placement Status at First Data Entry | |||||||
| Inpatient | 17 | 8 | 9 | 1.00 | |||
| Outpatient | 10 | 5 | 5 | ||||
|
| |||||||
| Continuous/ Ordinal Variable | Wilcoxon Rank-Sum test | ||||||
|
| |||||||
| Age at First Data Entry | |||||||
| 15 | 2 | 7.4 | 2 | 15 | 0 | 0 | 0.03* |
| 16 | 11 | 40.7 | 7 | 54 | 4 | 29 | |
| 17 | 14 | 51.9 | 4 | 31 | 10 | 71 | |
| Motivation to reduce use at last observation prior “time zero”. | |||||||
| Low | 19 | 73.1 | 10 | 83 | 9 | 64 | 0.24 |
| Medium | 2 | 7.7 | 1 | 8 | 1 | 7 | |
| High | 5 | 19.2 | 1 | 8 | 4 | 26 | |
| Severity of THC use at baseline | |||||||
| 0 = hardly ever | 1 | 0 | 1a | 0.60 | |||
| 1 = less than once a month | 1 | 1a | 0 | ||||
| 2 = once a month | 3 | 2 | 1 | ||||
| 3 = 2–3 times per month | 4 | 2 | 2 | ||||
| 6 = > 5 times a week | 18 | 8 | 10 | ||||
|
| |||||||
| Median | IQR | Med | IQR | Med | IQR | ||
|
| |||||||
| Duration of Treatment (months) | 14 | 9–23 | 21 | 14–23 | 11.5 | 9–16 | 0.02* |
These patients had met criteria for cannabis use disorder prior to first data entry and so were still included in our sample as there was high potential for relapse.
indicates p<0.05
For the longitudinal analysis, a total of 293 patient-months were observed across 27 patients. There was a median of 10 observations per patient (range 1–21). There were 223 patient-months recorded for the analysis with respect to the primary hypothesis (regarding cannabis use), and 215 patient-months for the secondary analysis (regarding degree of psychosis).
In comparison to patients treated with other antipsychotics, cannabis use decreased in patients treated with clozapine with a p-value of 0.06 and an odds ratio (OR) of 2.8 (95% confidence interval (95% CI) 0.97–7.9) (see Table 2 and Figure 1). Comparing this model and a model omitting the medication variables using a likelihood ratio, the difference between models yielded a p-value of <0.01, indicating that the medication effects are significant.
Table 2.
Factors contributing to risk of less frequent cannabis use in ordinal regression analysis.
| Variable | β | Odds Ratio (95%CI) | p-value |
|---|---|---|---|
| No medication relative to clozapine | 1.96 | 7.1 (2.3–22.3) | 0.001* |
| Other antipsychotics relative to clozapine | 1.02 | 2.8 (0.97–7.9) | 0.06 |
| Time (in months) | −0.09 | 0.91 (0.84–0.98) | 0.02* |
| Placement | −0.38 | 0.69 (0.32–1.47) | 0.33 |
Indicates p<0.05
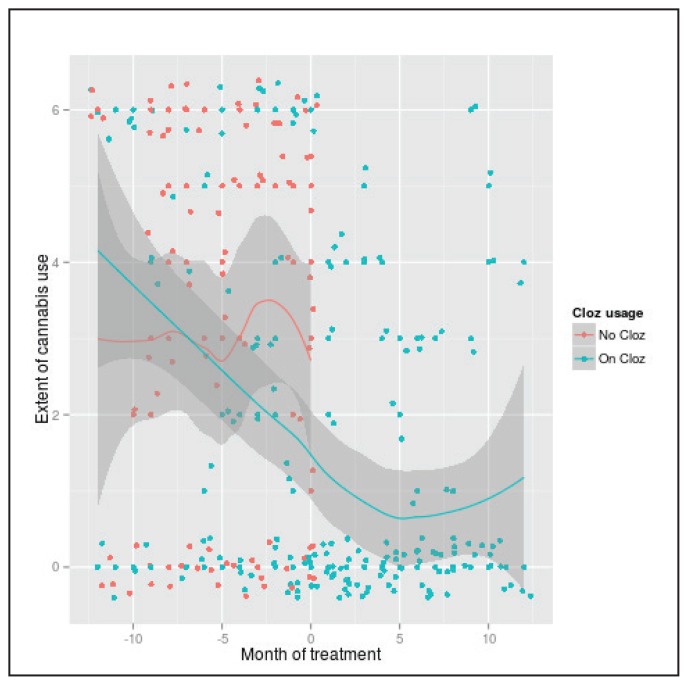
Figure 1.
Degree of cannabis use in an outpatient setting, as a function of time and clozapine exposure.
Data points were “jittered” to show as distinct points on the plot. Jittering was used to better visualize how many data points are positioned on one value depicted in the graph; the true value of data points is one of 0–6. Frequency of use was coded as 0 = zero use; 1= less than once a month; 2 = once per month; 3 = two to three times per month; 4 = once to twice a week; 5 = three to four times per week; 6 = greater than five times a week.
Relative to treatment with other antipsychotics, clozapine exposure was significantly associated with lower severity of psychotic symptoms (p=0.01) (see Table 3). Again, comparing this model against a model without medication effects using a likelihood ratio indicated that the medication effects were significant (p<0.03). Many patients exposed to clozapine did experience some adverse effects (see Table 4).
Table 3.
Factors contributing to risk of less psychosis in ordinal regression analysis.
| Variable | β | Odds Ratio (95%CI) | p-value |
|---|---|---|---|
| No medication relative to clozapine | 1.32 | 3.73 (1.35–9.99) | 0.03* |
| Other antipsychotics relative to clozapine | 1.30 | 3.67 (1.18–11.83) | 0.01* |
| Time (in months) | −0.09 | 0.91(0.85–0.99) | 0.03* |
| Extent of cannabis use | 0.22 | 1.24(1.03–1.49) | 0.02* |
Indicates p<0.05
Table 4.
Adverse events in clozapine-exposed as detected by chart review (n=13).
| Adverse event | Number (%) |
|---|---|
| Persistent tachycardia (heart rate > 100bpm) | 8 (62%) |
| Sialorrhea | 8 (62%) |
| Weight gaina (defined as >5kg increase) | 7 (54%) |
| Constipation | 5 (38%) |
| Gastrointestinal upset (reflux/vomiting) | 4 (31%) |
| Persistent sedation | 3 (23%) |
| Seizure | 2 (15%) |
| Increased triglycerides (>1.7 mmol/L) | 1 (8%) |
| Hypertension (SBP >140) | 1 (8%) |
| Blurry vision | 1 (8%) |
| Fever | 1 (8%) |
As adolescent patients are still growing, absolute weight gains may have been within normal depending upon the time frame.
Discussion
Our findings were consistent with our hypothesis, but did not reach statistical significance. The OR of 2.8 (95% CI 0.97–7.9) suggests that investment in future studies to further explore the relationship between clozapine and cannabis use is merited. Our results are consistent with results observed in the previously described randomized controlled trial in adults (Brunette et al., 2011).
Interestingly, Brunette et al. did not find that clozapine treatment led to improved symptoms of psychosis or improved functioning relative to other antipsychotics. This null finding may be due to the “moderate severity” of psychosis at baseline (as they were outpatients), and to the fact that participants were not required to have treatment-resistant schizophrenia for this trial (Brunette et al., 2011). In contrast, our study found that clozapine was significantly associated with reduced level of psychosis compared to other antipsychotic treatment or no antipsychotic treatment. Although the efficacy of clozapine as a treatment for refractory psychosis is well known in the literature (Leucht et al., 2013; McEvoy et al., 2006), we are not aware of any other reports on the effectiveness of clozapine compared to other antipsychotics specifically in adolescents with concurrent psychosis and cannabis use disorder.
The mechanism by which clozapine may reduce cannabis use is unclear. Other investigators speculate that the complex mechanism of action of clozapine on various neurotransmitter systems, particularly the noradrenergic system, may be directly responsible for dampening the urge to use cannabis (Green, Zimmet, Straus, & Schildkraut, 2009). It is also possible that the reduction in cannabis use is mediated by the improvement in psychosis. Cannabis contains two psychoactive compounds of interest: (1) THC, thought to promote psychosis (D’Souza, 2004): and, (2) cannabidiol thought to have antipsychotic properties alleviating the symptoms (Leweke et al., 2012). If patients are using cannabis for the antipsychotic properties, a potentially more effective antipsychotic (like clozapine) may decrease the need to use cannabis and subsequently decrease exposure to THC. Alternatively, clozapine may improve executive functioning (Meltzer & McGurk, 1999) and impulse control (Dursun, Szemis, Andrews, Whitaker, & Reveley, 2000), making it easier for motivated patients to maintain abstinence from cannabis use.
Adolescence is thought to be a “critical period” where cannabis use can have specific effects on neurodevelopment, increasing the risk of psychotic symptoms (Caballero & Tseng, 2012; Gleason, Birnbaum, Shukla, & Ghose, 2012). As such, there is potential for greater short-term and long-term gains if clozapine treatment curbs cannabis use in adolescents with schizophrenia.
Strengths and Limitations
Our study design allowed for data analysis on a population having multiple barriers to data collection, including patient factors such as lack of insight and limited engagement. We also used a repeated measures analysis, optimizing the power of the study, and allowed us to observe how clozapine exposure was related to reductions in substance use as a function of time.
Despite these strengths, sample size is a significant limit in our study. As a retrospective chart review, this study also has many inherent limitations. In the collection of data, chart information may have been limited and may not have been recorded in an orderly and consistent way. We are not aware of any validated scale that can measure the level of psychosis retrospectively from chart reviews, and we were thus limited in using a 3-point non-validated scale for measurement. The study design also renders the results susceptible to misclassification bias with respect to the coding of the cannabis use. Furthermore, the selection of patients was not random or blind to treatment assignment; there were no clear standard set of questions; and drug combinations and prevalence of co-morbidities or the impact of psychosocial measures could all have been underestimated. The study population was also not homogenous with different environmental variables such as routines, structure, consistency, and availability of marijuana.
Conclusions
This retrospective cohort chart review suggests that clozapine is associated with decreased cannabis use in adolescents with concurrent psychosis and substance use. Clinical trials in adolescents to test potential causality as a reason for this association are warranted.
Acknowledgements/Conflicts of Interest
We gratefully acknowledge the contributions of Dr. Catherine Dalzell for assisting with the statistical analysis, and to Nathan Parker (research assistant) for formatting the tables. This project was not funded. The Institute of Mental Health Research in Ottawa supported Dr. Dalzell’s statistical consultation. The authors have no financial relationships to disclose.
References
- Brunette MF, Dawson R, O’Keefe CD, Narasimhan M, Noordsy DL, Wojcik J, Green AI. A Randomized Trial of Clozapine Versus Other Antipsychotics for Cannabis Use Disorder in Patients with Schizophrenia. Journal of Dual Diagnosis. 2011;7(1–2):50–63. doi: 10.1080/15504263.2011.570118. http://doi.org/10.1080/15504263.2011.570118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette MF, Drake RE, Xie H, McHugo GJ, Green AI. Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophrenia Bulletin. 2006;32(4):637–643. doi: 10.1093/schbul/sbl003. http://doi.org/10.1093/schbul/sbl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng KY. Association of cannabis use during adolescence, prefrontal CB1 receptor signaling, and schizophrenia. Frontiers in Pharmacology. 2012;3(1) doi: 10.3389/fphar.2012.00101. http://doi.org/10.3389/fphar.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, … Krystal JH. The psychotomimetic effects of intravenous delta-9 tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Dekker N, Meijer J, Koeter M, van den Brink W, van Beveren N, Kahn RS, …Myin-Germeys I. Age at onset of non-affective psychosis in relation to cannabis use, other drug use and gender. Psychological Medicine. 2012;42(09):1903–1911. doi: 10.1017/S0033291712000062. http://doi.org/10.1017/S0033291712000062. [DOI] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophrenia Bulletin. 2000;26(2):441. doi: 10.1093/oxfordjournals.schbul.a033464. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Szemis A, Andrews H, Whitaker P, Reveley MA. Effects of clozapine and typical antipsychotic drugs on plasma 5-HT turnover and impulsivity in patients with schizophrenia: A cross-sectional study. Journal of Psychiatry and Neuroscience. 2000;25(4):347–352. [PMC free article] [PubMed] [Google Scholar]
- Gearing RE, Mian IA, Barber J, Ickowicz A. A Methodology for Conducting Retrospective Chart Review Research in Child and Adolescent Psychiatry. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2006;15(3):126–134. [PMC free article] [PubMed] [Google Scholar]
- Gleason KA, Birnbaum SG, Shukla A, Ghose S. Susceptibility of the adolescent brain to cannabinoids: Long-term hippocampal effects and relevance to schizophrenia. Translational Psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.122. http://doi.org/10.1038/tp.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: Effects of clozapine vs. risperidone. Schizophrenia Research. 2003;60(1):81–85. doi: 10.1016/s0920-9964(02)00231-1. http://doi.org/10.1016/S0920-9964(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Green AI, Tohen MF, Hamer RM, Strakowski SM, Lieberman JA, Glick I, Clark WS. First episode schizophrenia-related psychosis and substance use disorders: Acute response to olanzapine and haloperidol. Schizophrenia Research. 2004;66(2–3):125–135. doi: 10.1016/j.schres.2003.08.001. http://doi.org/10.1016/j.schres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Straus RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: Do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harvard Review of Psychiatry. 1999;6(6):287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, …Rapoport JL. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Archives of General Psychiatry. 1996;53(12):1090–1097. doi: 10.1001/archpsyc.1996.01830120020005. [DOI] [PubMed] [Google Scholar]
- Kumra S, Kranzler H, Gerbino-Rosen G, Kester HM, DeThomas C, Kafantaris V, …Kane JM. Clozapine and “High-Dose” Olanzapine in Refractory Early-Onset Schizophrenia: A 12-Week Randomized and Double-Blind Comparison. Biological Psychiatry. 2008;63(5):524–529. doi: 10.1016/j.biopsych.2007.04.043. http://doi.org/10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, …Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. http://dx.doi.org/10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, …Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. http://dx.doi.org/10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Archives of General Psychiatry. 1994;51(4):273–279. doi: 10.1001/archpsyc.1994.03950040017002. http://doi.org/10.1016/1353-1131(95)90097-7. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman Ja, Stroup TS, Davis SM, Meltzer HY, Rosenheck Ra, …Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry. 2006;163(4):600–610. doi: 10.1176/ajp.2006.163.4.600. http://doi.org/10.1176/appi.ajp.163.4.600. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia bulletin. 1999;25(2):233–256. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Samara MT, Dold M, Gianatsi M, Nikolakopoulou A, Helfer B, Salanti G, Leucht S. Efficacy, Acceptability, and Tolerability of Antipsychotics in Treatment-Resistant Schizophrenia: A Network Meta-analysis. JAMA psychiatry. 2016;73(3):199–210. doi: 10.1001/jamapsychiatry.2015.2955. [DOI] [PubMed] [Google Scholar]
- Schnell T, Koethe D, Krasnianski A, Gairing S, Schnell K, Daumann J, Gouzoulis-Mayfrank E. Ziprasidone versus clozapine in the treatment of dually diagnosed (DD) patients with schizophrenia and cannabis use disorders: A randomized study. The American Journal on Addictions. 2014;23(3):308–312. doi: 10.1111/j.1521-0391.2014.12126.x. http://doi.org/10.1111/j.1521-0391.2014.12126.x. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Vermani A, Greenstein DK, Bobb AJ, Spencer EP, Clasen LS, …Gogtay N. Clozapine treatment of childhood-onset schizophrenia: Evaluation of effectiveness, adverse effects, and long-term outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(10):1349–1356. doi: 10.1097/chi.0b013e31812eed10. http://doi.org/10.1097/chi.0b013e31812eed10. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophrenia Research. 2009;110(1):1–23. doi: 10.1016/j.schres.2009.03.005. http://doi.org/10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Van Dijk D, Koeter MWJ, Hijman R, Kahn RS, Van den Brink W. Effect of cannabis use on the course of schizophrenia in male patients: A prospective cohort study. Schizophrenia Research. 2012;137(1–3):50–57. doi: 10.1016/j.schres.2012.01.016. http://doi.org/10.1016/j.schres.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of Clozapine’s Effectiveness in Schizophrenia: A Systematic Review and Meta-Analysis of Randomized Trials. American Journal of Psychiatry. 1999:990–999. doi: 10.1176/ajp.156.7.990. http://ajp.psychiatryonline.org/doi/abs/10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Kennedy SH, Schaffer A, Parikh SV, Beaulieu S, O’Donovan C, … Young LT. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: Update 2013. Bipolar disorders. 2013;15:1–44. doi: 10.1111/bdi.12025. [DOI] [PubMed] [Google Scholar]
- Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: A retrospective survey. Journal of Clinical Psychopharmacology. 2000;20(1):94–98. doi: 10.1097/00004714-200002000-00016. [DOI] [PubMed] [Google Scholar]