Abstract
Objective
Netrin-1 is a chemorepulsant and matrix protein expressed during and required for osteoclast differentiation, which also plays a role in inflammation by preventing macrophage egress. Because wear particle-induced osteolysis requires osteoclast-mediated destruction of bone, we hypothesized that blockade of Netrin-1 or Unc5b, a receptor for Netrin-1, may diminish this pathological condition.
Methods
6–8-wk-old C57BL/6 mice had 3mg of ultrahigh-molecular-weight-polyethylene particles (UHMWPE) implanted over the calvaria and then received 10μg of monoclonal antibodies for Netrin-1 or its receptors, Unc5b and DCC injected IP on a weekly basis. After 2 weeks, microCT and histology analysis were performed. Netrin-1 expression was analyzed in human tissue obtained following primary prosthesis implantation or after prosthesis revision for peri-implant osteolysis and aseptic implant loosening.
Results
Weekly injection of anti-Netrin-1 or anti-Unc5b-antibodies significantly reduced particle-induced bone pitting in calvaria-exposed to wear particles (46±4 and 49±3% of control bone pitting respectively, p<0.001) but anti-DCC-antibody did not affect inflammatory osteolysis (80±7% of control bone pitting, p=ns). Anti-Netrin-1 or anti-Unc5b, but not anti-DCC, antibody-treatment markedly reduced the inflammatory infiltrate and the number of TRAP-positive osteoclasts (7±1, 4±1 and 14±1 cells/high power field (hpf) respectively vs. 12±1 cells/hpf for control, p<0.001), with no significant changes in Alkaline Phosphatase-positive osteoblasts on bone forming surfaces in any antibody-treated-group. Netrin-1 immunostaining colocalized with CD68 staining for macrophages. The peri-implant tissues of patients undergoing prosthesis revision surgery showed an increase in Netrin-1 expression whereas there was little Netrin-1 expression in soft tissues removed at the time of primary joint replacement.
Conclusion
These results demonstrate a unique role for Netrin-1 in osteoclast biology and inflammation and may be a novel target for prevention/treatment of inflammatory osteolysis.
Keywords: Netrin-1, Unc5b, antibodies, inflammation, osteolysis
INTRODUCTION
Total hip and knee replacements are successful surgical interventions with success rates close to 90% at 10–25 years after surgery in terms of reducing pain, improving function, and enhancing quality of life in patients.[1] However, despite improvements in surgical techniques, material and implant designs, nearly 40,000 hip arthroplasties have to be revised each year in the US[1], and it is expected that the rates of revision will increase by 137% for total hip and 601% for total knee revisions over the next 25 years.[2] Peri-prosthetic inflammatory bone destruction due to wear particles is the most common cause of prosthesis failure requiring revision.[3]
Wear particles consist of debris shed from joint prostheses, whether polymeric, metallic or ceramic, and these particles are phagocytosed by macrophages and other local cells, which become activated.[4, 5] Further recruitment of both macrophages and osteoclasts (bone-resorbing cells) to the site[6, 7] leads to up-regulation of pathways leading to bone destruction, and down-regulation of bone formation.[8, 9] To date, no biological approaches to limiting the chronic inflammatory reaction associated with wear particles have proved clinically successful.[10]
Netrin-1, a secreted laminin-related protein, is a member of the axonal guidance protein family that inhibits migration of monocytes, neutrophils and lymphocytes by activation of its receptors A2BR (adenosine A2B receptor) and Unc5.[11–13] Acting through Unc5b receptor, Netrin-1 reduces renal ischemia–reperfusion injury and its associated renal inflammation.[14] Netrin-1 is expressed on vascular endothelium, where it is regulated by infection and inflammatory cytokines, and inhibits inflammatory cell migration into tissues and its down-regulation at the onset of sepsis/inflammation may facilitate leukocyte recruitment[11]. Netrin-1 also promotes chronic inflammation in atherogenesis and diet-induced obesity; tissue macrophages in atherosclerotic plaques and obese adipose tissue increase their expression of Netrin-1,[15, 16] which promotes macrophage retention and survival[17] further increasing chronic inflammation. Recently we have demonstrated that Netrin-1 is an autocrine and paracrine regulator of osteoclast differentiation.[18] In osteoclast precursors, the binding of Netrin-1 to its receptor Unc5b, but not to its alternative receptor DCC, triggers the signaling cascade involved in the activation of the small GTPase RhoA by a mechanism that has not previously been observed in osteoclast precursors. Activation of RhoA via LARG and RGMa leads to the cytoskeletal rearrangements required for osteoclast fusion and differentiation.[18]
Here we tested the hypothesis that blockade of Netrin-1 or Unc5b might be a novel approach to the prevention of osteoclast-mediated bone resorption and inflammation at the site of prosthesis shedding of wear particles.
METHODS
Wear particle preparation
Ultrahigh molecular weight polyethylene particles (UHMWPE) particles, a gift of P. H. Wooley (Via Christi Regional Medical Center), had a mean particle size (equivalent circle diameter) of 1.74±1.43 μm (range 0.05–11.06), with more than 34% of the particles smaller than 1 μm. For decontamination from endotoxins, the particles were washed twice in 70% ethanol for 24 h at room temperature. The particles were washed in phosphate-buffered saline (PBS) and dried in a desiccator.
Surgical procedure
All protocols followed internationally recognized guidelines and were approved by the NYU SoM Institutional Animal Care and Use Committee. Osteolysis was induced by implantation of UHMWPE particles over the mouse calvaria, as we have previously described.[19] Briefly, 6–8-wk-old male C57BL/6 mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine, and a 1-cm midline sagittal incision was made over the calvaria anterior to the line connecting both ears as previously described.[19] 5 mice received no particles (sham, untreated mice) and the incision was closed without any further intervention. The remaining animals (n=40) received 3mg of dried UHMWPE particles. Of these animals, 10 mice received intraperitoneally (IP) 100μl of 0.9% saline (control) and the rest received 10μg (in a final volume of 100μl) of mouse monoclonal Netrin-1, Unc5b or DCC antibodies (n=10 each), beginning immediately before surgery and continuing on a weekly basis until sacrifice (2 weeks later). Water and food were given ad libitum until sacrifice. Animals were sacrificed after 14 days in a CO2 chamber and the calvaria were removed, fixed, and prepared for microCT, histological staining, and cytokine measurements.
Histomorphometry
Micro-X-Ray Computed Tomography (micro-CT) analysis
After sacrifice, 5 calvaria per treatment group were fixed in 70% ethanol and prepared for high-resolution microCT as previously described to perform qualitative and quantitative analyses of resorbing areas in murine calvarial bone.[19] Analyses were performed in the NYU College of Dentistry microCT core with a Skyscan 1172 microCT (Bruker, WI, USA) using the previously described parameters[20]: 60kV, 167uA, 9.7 micron pixel size, 2000×1332 matrix, 0.3 degree rotation steps, 6 averages, movement correction of 10, 0.5mm Al filter, 2 segments scanned per sample (56 minutes/segment). Images were reconstructed using the Skyscan NRECON software (histogram range 0–0.065, beam hardening correction of 35, Gaussian smoothing (factor 1), ring artifact correction of 7). For quantitative analysis of UHMWPE particle-induced osteolysis, a square-shaped region of interest across the parietal bone of approximately 4 mm right and left of the midline suture of the skull was placed in one of the 2D-reconstructed slices, as described previously,[19, 21] and a Matlab software application was utilized to analyze calvarial bone resorption, as previously described.[19]
Histological studies
Calvariae were fixed in 4% paraformaldehyde for 48h, followed by decalcification in 10% EDTA for four weeks and paraffin embedding (n=5 per treatment). Sections (5 μm) were cut and H&E staining was performed. Inflammatory infiltration in midsaggital suture areas was quantified from 5 images per animal using Sigma Scan Pro Image 5.0.0 software.
TRAP staining was carried out in a TRAP buffer (0.1M acetate buffer, 0.3M Sodium Tartrate 10mg/ml Naphtol AS-MX phosphate, 0.1% Triton X-100, 0.3mg/ml Fast Red Violet LB (Sigma-Aldrich, MO, USA)). After deparaffinization and acetate buffer incubation, samples were incubated in TRAP buffer for 30 minutes and counterstained with Fast Green.
Immunohistochemistry analysis for Netrin-1, Unc5b, DCC, Alkaline Phosphatase (ALP), Cathepsin K and CD68 were carried out as previously described[19] (See online supplementary text).
Images were observed in a Leica microscope equipped with SlidePath Digital Image Hub Version 3.0 software, or under light microscope (Nikon) equipped with Nis Elements F3.0 SP7 software.
Netrin-1 expression in implant biopsies
Netrin-1 immunostaining was performed in human tissue obtained from donors following primary prosthesis implantation or after prosthesis revision for peri-implant osteolysis and aseptic implant loosening (Hospital for Special Surgery, New York NY, USA).[22] Tissue was associated with reactions to orthopedic implant wear debris. Specimens of soft tissue and bone were collected from regions of bone resorption during joint revision surgery. The specimens were fixed in freshly prepared 4% paraformadehyde, followed by demineralization with 14% EDTA, processed and embedded in paraffin and 5 μm sections were prepared. Sections of bone and soft tissue taken at the time of primary hip implant were obtained from fixed, paraffin-embedded tissue. Paraffin embedded sections (n=5 each) were deparaffinized and after rehydration, Netrin-1 immunostaining was performed following the protocol described above. TRAP staining was also performed as described above. All procedures were approved by the Institutional Review Boards of the Hospital for Special Surgery and NYU School of Medicine.
Measurement of inflammatory cytokines
Statistical analysis
Statistical significance for differences between groups was determined by use of one-way ANOVA and Bonferroni post-hoc test or Student’s t test, as appropriate. All statistics were calculated using GraphPad® software (GraphPad, San Diego, CA, USA).
RESULTS
Netrin-1 is highly expressed in human periarticular soft tissue removed during revision
We have recently reported that during osteoclast maturation, expression of Netrin-1 and its receptor Unc5b is increased in osteoclast precursors, and required for osteoclast differentiation.[18] In parallel, activated tissue macrophages were reported to induce Netrin-1 expression in atherosclerotic plaques and diet-induced obesity[15, 17] where Netrin-1 contributes to inflammation.
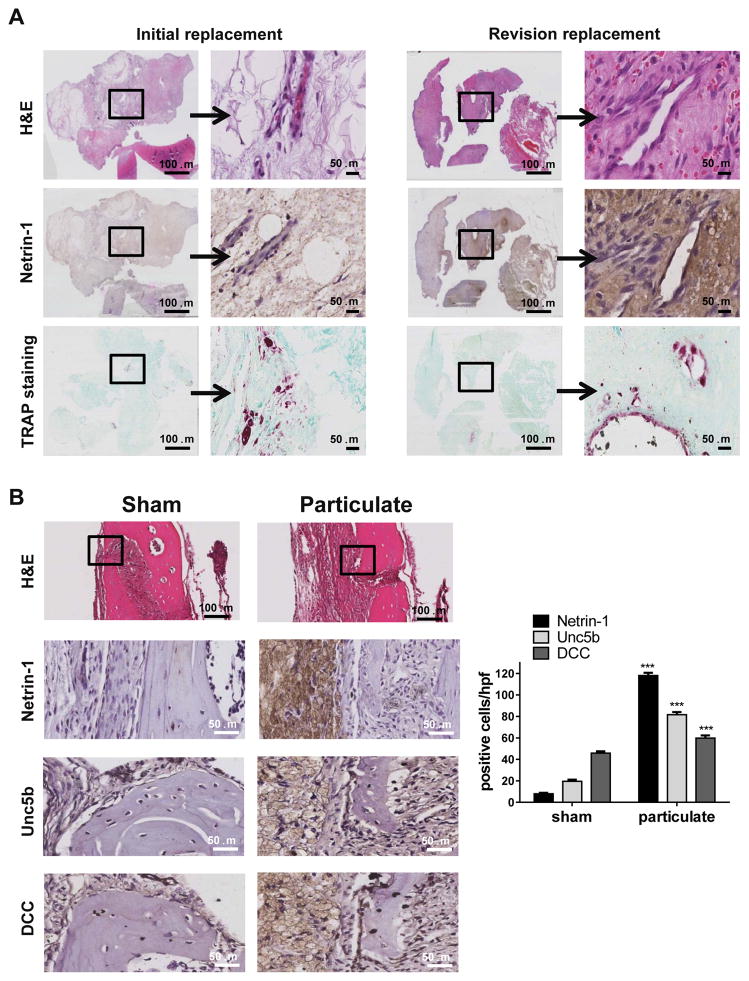
To determine if these findings are relevant to orthopaedic wear-particle-induced osteolysis in humans, we studied the expression of Netrin-1 in peri-implant tissues retrieved from patients undergoing revision surgery for peri-implant osteolysis and aseptic implant loosening. As seen in Figure 1A there is little expression of Netrin-1 in cells populating the soft tissues removed at the time of primary joint replacement but high levels of Netrin-1 expression in the matrix and the cells populating the peri-implant tissues from patients undergoing revision surgery. TRAP staining revealed the presence of osteoclasts in patches on the bone surfaces in both specimens from initial joint replacement and revision implant loosening samples.
Figure 1. Netrin-1 is highly expressed in inflammatory infiltrates in wear particle-induced osteolysis.
A) The figures show representative images for H&E, Netrin-1 immunostaining and TRAP staining in human tissue biopsies from implant revision and primary implants (n=5 each). B) The figures show representative images for Netrin-1, Unc5b and DCC immunostaining in a murine UHMWPE particle-induced osteolysis model (n=5 mice per group). Images were taken at 100X and 400X magnification. Scale bar indicates 50 and 100 μm. ***p<0.001, compared to untreated (Sham) (ANOVA).
Netrin-1 and Unc5b are induced in the inflammatory infiltrate after wear particle exposure in mice
The results described above suggested that Netrin-1 might play a critical role in inflammatory osteolysis and contribute to the progression of inflammatory osteolysis. To test this hypothesis we employed a model of orthopaedic wear particle-induced osteolysis[19] in which UHMWPE particles are placed under the periosteum over the calvaria. In sham-operated animals we observed negligible levels of Netrin-1-, Unc5b- and DCC-positive cells in the bone and soft tissue at the surgical site (Figure 1B). In contrast, in the calvaria of mice exposed to UHMWPE particles there was a marked increase in Netrin-1 and Unc5b expression (118±3 Netrin-1 positive cells/hpf (high power field) and 60±3 Unc5b positive cells/hpf vs. 8±1 Netrin-1 positive cells/hpf and 20±2 Unc5b positive cells/hpf for Sham-operated, p<0.001, n=5), primarily in the cells of the inflammatory infiltrate which was composed almost entirely of macrophages, as previously described.[19] In addition, we observed a modest increase in DCC-positive cells (59±2 DCC positive cells/hpf vs. 45±2 positive cells/hpf for Sham, p<0.001, n = 5) in the inflammatory infiltrate of mice exposed to UHMWPE particles (Figure 1B).
Netrin-1 and Unc5b are required for wear particle-induced osteolysis
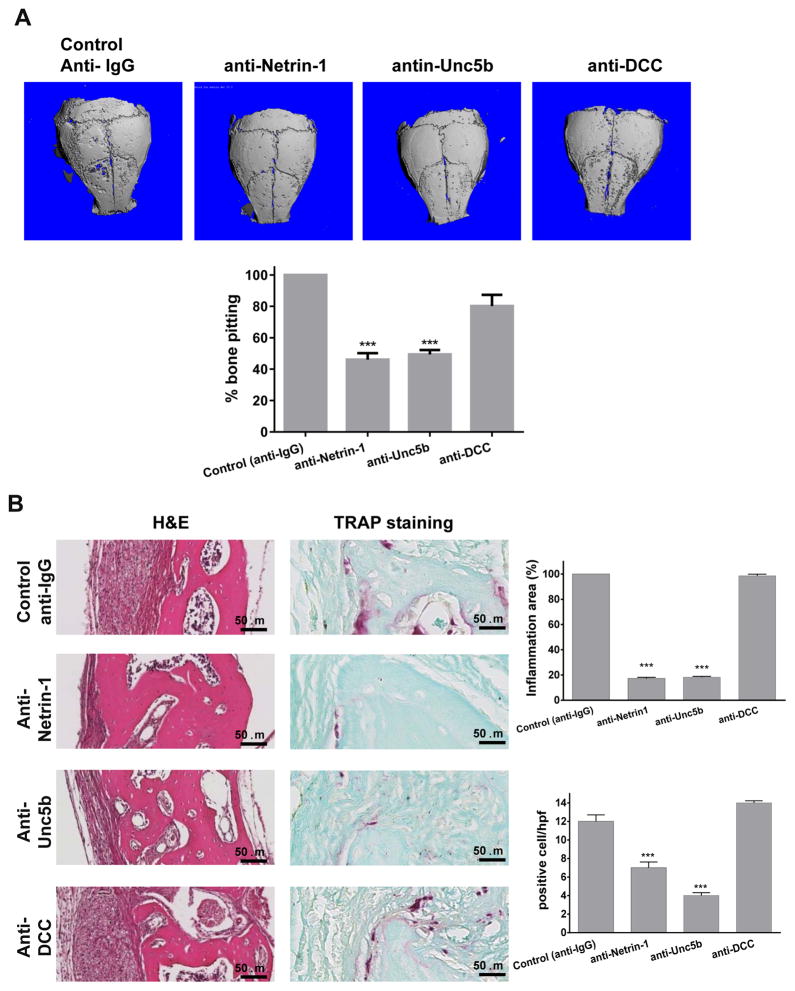
Since Netrin-1 is highly expressed in inflammatory infiltrates in mice and in peri-implant joint tissue in humans, we determined if targeting Netrin-1 or its receptors blocked progression of or reversed inflammatory osteolysis in vivo. 10 μg murine monoclonal anti-Netrin-1, anti-Unc5b or anti-DCC antibodies were injected IP on a weekly basis in the murine model of inflammatory osteolysis. Weekly injections of anti-Netrin-1 or anti-Unc5b antibodies significantly reduced the area of particle-induced bone pitting in calvaria exposed to UHMWPE (46±4 and 49±3% of control bone pitting, respectively, p<0.001, n=5, Figure 2A) but anti-DCC receptor antibody did not affect UHMWPE-induced pitting and resorption (80±7% bone pitting, p=ns vs control, Figure 2A). MicroCT also revealed a significant increase in bone volume (BV) and bone volume/total volume ratio (BV/TV) in both anti-Netrin-1 and -Unc5b treated mice (Figure 2A, Table 1).
Figure 2. Blocking Netrin-1 using monoclonal antibody injections decreases bone pitting, inflammation and osteoclasts in mouse calvariae after exposure to wear particles.
A) The figures show representative microCT images of calvaria of mice treated with UHMWPE wear particles combined with anti-IgG (Control), anti-Netrin-1, anti-Unc5b or anti-DCC blocking antibodies at 10μg (n=5 mice per group). B) Calvariae were stained with hematoxylin & eosin to determine the presence of inflammation on the outer bone surface. The area of inflammatory infiltrate was quantified and expressed as a percentage of the area of the control particle-exposed mice (n = 5 mice per group). Shown are representative images for TRAP staining for osteoclasts in mice calvariae and the mean (±SEM, n=5 mice per group) number of osteoclasts/hpf. Images were taken at 400X magnification. Scale bar indicates 50 μm. Data were expressed as mean±SEM (n=5 per group). * p<0.05, **p<0.001, ***p<0.001 compared to control (ANOVA).
Table 1. microCT analysis of mouse calvariae after treatment.
Digital morphometric analysis of microCT images from wild-type mice treated with saline or murine monoclonal antibodies against Netrin-1, Unc5b or DCC. Data are reported as means±SEM.
| Treatment | Bone volume (BV) (mm3) | Total volume (TV) (mm3) | BV/TV | Bone mineral density (BMD) (mg) |
|---|---|---|---|---|
| Particulate + saline | 1.98 ± 0.02 | 1.37 ± 0.01 | 0.17 ± 0.003 | 809.99 ± 1.20 |
| Particulate + anti-Netrin-1 | 2.31 ± 0.02 *** | 1.48 ± 0.06 | 0.19 ± 0.004 * | 819.94 ± 2.06 ** |
| Particulate + anti-Unc5b | 2.28 ± 0.04 *** | 1.46 ± 0.05 | 0.18 ± 0.002 * | 819.39 ± 1.39 ** |
| Particulate + anti-DCC | 1.95 ± 0.08 | 1.43 ± 0.03 | 0.17 ± 0.002 | 811.43 ± 1.40 |
P<0.05,
P < 0.01,
P<0.005, compared to saline (ANOVA).
MicroCT results were confirmed by histomorphometry (see online supplementary text).
We also observed a marked reduction in the inflammatory infiltrate in calvaria from mice treated with anti-Netrin-1 and -Unc5b antibodies, whereas anti-DCC antibody treatment did not affect the inflammatory infiltrate (83±2% reduction for anti-Netrin-1, 81±1% reduction for anti-Unc5b and 2±2% reduction for anti-DCC, p<0.001 and p=ns respectively, n=5) (Figure 2B). Anti-Netrin-1 and anti-Unc5b treatment, but not anti-DCC treatment, markedly reduced the number of TRAP-positive osteoclasts in affected bone (7±1 TRAP-positive cells/hpf for anti-Netrin-1, 4±1 TRAP-positive cells/hpf for anti-Unc5b and 14±1 TRAP-positive cells/hpf for anti-DCC compared to 12±2 TRAP-positive cells/hpf for control, p<0.001 and p=ns respectively, n=5) (Figure 2B).
Inflammatory cytokines also contribute to inflammatory osteolysis and anti-Netrin-1 and –Unc5b, but not –DCC, antibody treatment abrogated wear particle-induced increases in IL-1β and TNFα (see online supplementary text).
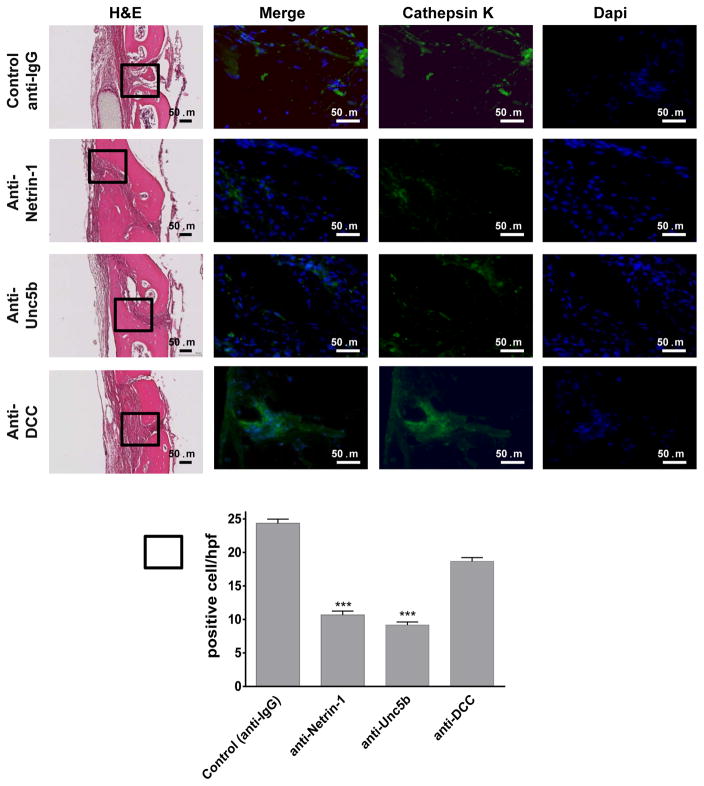
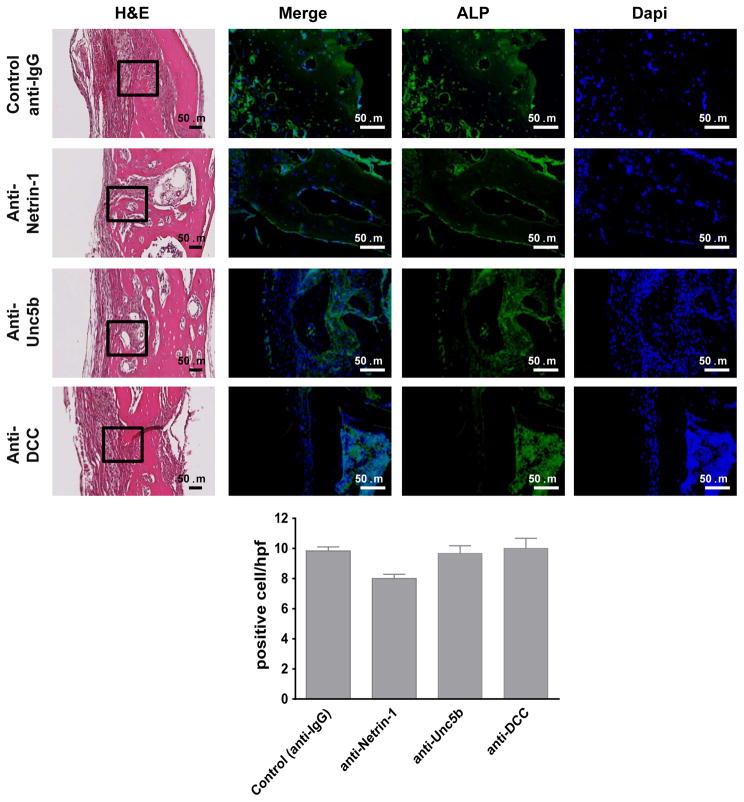
To further confirm that Netrin-1 and Unc5b are key factors in bone resorption in vivo, immunohistologic examination was performed. When osteoclasts were identified by immunohistologic staining for Cathepsin K, similar results as those observed for TRAP staining were found (Figure 3). Treatment with anti-Netrin-1 or anti-Unc5b antibody markedly reduced the number of Cathepsin K positive cells, whereas treatment with anti-DCC antibody did not significantly change the number of Cathepsin K-positive cells (10±2 cells/hpf for anti-Netrin-1, 9±1 cells/hpf for anti-Unc5b and 19±3 cells/hpf for anti-DCC compared to 24±2 cells/hpf for control, p<0.001 and p=ns respectively, n=5) (Figure 3). In contrast, there were no significant changes in Alkaline Phosphatase positive osteoblasts on bone forming surfaces in any antibody-treated group compared to control (8±1 cells/hpf for anti-Netrin-1, 10±1 cells/hpf for anti-Unc5b and 10±2 cells/hpf for anti-DCC compared to 9±2 cells/hpf for control, p=ns, n=5) (Figure 4).
Figure 3. Immunohistochemistry for markers of osteoclasts.
Calvaria were processed and immunohistologic staining carried out. Shown are representative H&E sections of calvariae (from n=5 mice per group) stained for Cathepsin K (green). Nuclei are shown in blue (Dapi). Quantification of the number of positive cellst/hpf was done. Data are means±SEM (n=5 mice per group). All images were taken at the same magnification 200X and 400X. Scale bar indicates 50 μm. ***p<0.001 (ANOVA).
Figure 4. Immunohistochemistry for markers of osteoblasts.
Calvariae were processed and immunohistologic staining carried out. Shown are representative representative H&E sections of calvaria (from n=5 mice per group) stained for Alkaline Phosphatase (green). Nuclei are shown in blue (Dapi). Quantification of the number of positive cellst/hpf was done. Data are means±SEM (n=5 mice per group). All images were taken at the same magnification 200X and 400X. Scale bar indicates 50 μm. ***p<0.001 (ANOVA).
As previously described,[18] the only cells that express Netrin-1 in bone are osteoclasts and osteoclast precursors. This is confirmed in vivo as Netrin-1 stain co-localized with CD68 immunostaining (Supplemental Figure 2). Both anti-Netrin-1 and -Unc5b treatments markedly reduced the number of CD68-positive macrophages and, consequently, the immunostaining for Netrin-1, whereas anti-DCC treatment did not change the number of CD68-positive macrophages when compared to control (6±1 cells/hpf for anti-Netrin-1, 8±2 cells/hpf for anti-Unc5b and 24±3 cells/hpf for anti-DCC compared to 29±4 cells/hpf for control, p<0.001 and p=ns respectively, n=5) (Supplemental Figure 2).
Because of their effect on osteoclast differentiation we tested the effect of monoclonal anti-Netrin-1 and –Unc5b antibodies on bone density and found that treatment with these antibodies directly increased bone density in mice (see online supplementary text and Table 2).
DISCUSSION
Here we report that Netrin-1 is highly expressed by macrophages at sites of wear particle-induced osteolysis in the inflamed peri-implant soft tissue from patients undergoing implant revision and in macrophages and osteoclasts in a murine model of wear particle-induced bone destruction. Moreover, in vivo blockade of Netrin-1 and its receptor Unc5b by murine monoclonal antibodies prevents wear particle-induced bone destruction. These results indicate that Netrin-1 plays a critical role in inflammatory osteolysis.
Multiple studies have shown that orthopaedic wear products are potent inducers of proinflammatory mediators, including IL-1β and TNFα, and other soluble stimuli for osteoclast formation and bone resorption.[19] Here we demonstrated that treatment with monoclonal antibodies in both ex vivo cultures of calvaria exposed to wear particles, and in the RAW264.7 cell line, diminished both IL-1β and TNFα secretion. Macrophages represent a major component of the cellular infiltrate associated with wear particle-induced inflammation. Based on the findings in atherosclerotic plaques it is reasonable to hypothesize that Netrin-1 may contribute to the recruitment and retention of macrophages at the site of wear particle deposition and that signaling by Netrin-1 may further contribute to the osteolytic process via enhancement of osteoclast differentiation and activity. Prevention of wear particle-induced bone destruction by blockade of Netrin-1 and Unc5b by murine monoclonal antibodies indicates that Netrin-1 is acting in both an autocrine and paracrine fashion to promote bone destruction, as described in vitro.[18] The demonstration that blockade of DCC had no effect on bone destruction provides strong evidence that the effects of the anti-Netrin-1 and anti-Unc5b antibodies on osteolysis are specific, since DCC is also expressed by osteoclasts and macrophages.[18]
Because of its chemorepulsant activities, administration of Netrin-1 has been described as an anti-inflammatory treatment in a number of settings, including inflammatory bowel disease, pancreatitis, peritonitis and pulmonary inflammation.[11, 12, 14, 23–29] Neuronal guidance proteins present in peripheral tissues contribute to the local control of leukocyte migration and inflammation.[30, 31] Netrin-1 administration was found to direct leukocyte traffic during acute inflammation in peripheral organs,[23, 27] to reduce local injury and inflammatory responses,[27, 31] and to stimulate resolution mechanisms and production of resolvins.[32] In accordance, Netrin-1 administration regulates inflammation and infiltration of monocytes and ameliorates ischemia reperfusion-induced kidney injury,[14, 33] by inducing overexpression of macrophage a M2-like phenotype and suppression of expression of M1 markers,[34] and regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 and thromboxane A2 production.[33] Netrin-1 is also generated within the intestinal epithelium to dampen neutrophil recruitment in acute colitis and may have a beneficial function in chronic models of intestinal inflammation.[35]
However, the anti-inflammatory effects of Netrin-1 do not accord with the demonstration that Netrin-1 plays a critical role in promoting atherogenesis by inhibiting macrophage emigration from plaque,[15, 17] and other findings that identify Netrin-1 as a macrophage retention signal in adipose tissue of obese mice that promotes chronic inflammation and insulin resistance. Recently, the molecular mechanism linking plaque hypoxia and macrophage infiltration has been demonstrated in mouse and human plaques.[17] It involves hypoxia-inducible factor 1α–mediated up-regulation of Netrin-1 and Unc5b in human and mouse atherosclerotic plaques and subsequent macrophage chemostasis and protection from apoptosis.[17]
Nonetheless, the observation that there is marked overexpression of Netrin-1 in cells at sites of inflammatory bone destruction both in mice and in humans suggests that Netrin-1 plays an important role in inflammatory osteolysis. Our in vitro data suggest that this may be in part attributable to the effects of Netrin-1 on enhancing osteoclast differentiation, attachment and adhesion to the bone surfaces, which are known to play a role in bone resorption.[36] Moreover, histomorphometry analysis in long bones treated with monoclonal antibodies for two weeks, suggested a trend to increased trabecular bone formation with changes in BV/TV and BMD and a decrease in osteoclast number. Although the change in bone formation detected by the calcein/declomycin double-labeling technique was not significant there was a tendency to increased bone formation. In some prior studies Netrin-1 was reported to mediate its effects on myeloid cells and inflammation by a mechanism that involves adenosine A2B receptors.[13, 23, 27, 37, 38] Nonetheless, it is unlikely that adenosine A2B receptors are involved in the effects of Netrin-1 on osteoclast differentiation and function since He and colleagues recently reported that stimulation of adenosine A2B receptors inhibits osteoclast differentiation.[39] Thus, Netrin-1 most likely plays a direct role in inflammatory bone destruction by increasing or permitting osteoclast differentiation and function.
In conclusion, our results indicate that Netrin-1 is an autocrine factor produced by osteoclast precursors that enhances osteoclast differentiation and function. The demonstration of high levels of Netrin-1 expression in cells at sites of inflammatory bone resorption, furthermore suggests a contributory role in the enhanced bone resorption associated with inflammatory processes. Thus, Netrin-1 may be a novel therapeutic target for the reduction of osteoclast-mediated bone resorption and other forms of inflammatory bone destruction.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR56672, AR54897, AR046121, RC1HL100815, K99 HL125667), the NYU-HHC Clinical and Translational Science Institute (UL1TR000038), the NYUCI Center Support Grant, 9NIH/NCI 5 P30CA16087-310 and grants from Celgene and Gilead Pharmaceuticals. We want to ackowledge Dr. Giogio Perino for his help with human histological sections. We want to thank L. Mazinski and S. Ahmed from the NYU Histopathology Core (supported by Cancer Center Support Grant P30CA016087 at the Laura and Isaac Perlmutter Cancer Center) for technical assistance and Drs. N. Dyment and D. Rowe at the University of Connecticut Health Center for advice on tissue preparation and sectioning.
Footnotes
DISCLOSURES:
A.M. and B.N.C. have filed a patent on use of adenosine A2AR agonists to prevent prosthesis loosening (pending). A.M. B.N.C., B.R, K.J.M., have filled a patent on the use of Antibodies against Netrin-1 for the treatment of bone diseases. T.W., E.P. S.G. Z.D. C.L. do not have any disclosures. B.N.C. holds patents numbers 5,932,558; 6,020,321; 6,555,545; 7,795,427; adenosine A1R and A2BR antagonists to treat fatty liver (pending); adenosine A2AR agonists to prevent prosthesis loosening (pending). B.N.C. is a consultant for Bristol-Myers Squibb, AstraZeneca, Novartis, CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, DSMB), Endocyte, Protalex, Allos, Inc., Savient, Gismo Therapeutics, Antares Pharmaceutical, Medivector, King Pharmaceutical, Celizome, Tap Pharmaceuticals, Prometheus Laboratories, Sepracor, Amgen, Combinatorx, Kyowa Hakka, Hoffman-LaRoche and Avidimer Therapeutics. BNC has stock in CanFite Biopharmaceuticlas.
Author contribution:
BC and KM have designed the experiments, wrote and revised the manuscript. AM designed the experiments and has been the primary person responsible for carrying out all experimental procedures and writing the manuscript. TW helped on surgery and animal treatments and revised the manuscript. BR performed the immunoshitochemistries and revised the manuscript. EP and SG carried out human subjects experiments and revised the manuscript. Z.D. and C.L performed undercalcified bone preparation, sectioning, staining and scanning.
References
- 1.Berry DJ, et al. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84-A(2):171–7. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Wedemeyer C, et al. Particle-induced osteolysis in three-dimensional micro-computed tomography. Calcif Tissue Int. 2007;81(5):394–402. doi: 10.1007/s00223-007-9077-2. [DOI] [PubMed] [Google Scholar]
- 4.Nich C, et al. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101(10):3033–45. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271–86. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz EM, et al. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000;18(3):472–80. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 7.Silva MJ, Sandell LJ. What’s new in orthopaedic research. J Bone Joint Surg Am. 2002;84-A(8):1490–6. doi: 10.2106/00004623-200208000-00040. [DOI] [PubMed] [Google Scholar]
- 8.Goodman SB, et al. Effects of orthopaedic wear particles on osteoprogenitor cells. Biomaterials. 2006;27(36):6096–101. doi: 10.1016/j.biomaterials.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Chiu R, et al. Polymethylmethacrylate particles inhibit osteoblastic differentiation of bone marrow osteoprogenitor cells. J Biomed Mater Res A. 2006;77(4):850–6. doi: 10.1002/jbm.a.30697. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz EM G. Implant Wear Symposium Biologic Work. What potential biologic treatments are available for osteolysis? J Am Acad Orthop Surg. 2008;16(Suppl 1):S72–5. doi: 10.5435/00124635-200800001-00015. [DOI] [PubMed] [Google Scholar]
- 11.Ly NP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(41):14729–34. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirakaj V, et al. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med. 2010;181(8):815–24. doi: 10.1164/rccm.200905-0717OC. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10(2):195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 14.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–8. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 15.van Gils JM, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13(2):136–43. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramkhelawon B, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014 doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramkhelawon B, et al. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol. 2013;33(6):1180–8. doi: 10.1161/ATVBAHA.112.301008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mediero A, et al. Netrin-1 is a critical autocrine/paracrine factor for osteoclast differentiation. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mediero A, et al. Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci Transl Med. 2012;4(135):135ra65. doi: 10.1126/scitranslmed.3003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mediero A, et al. Brief Report: Methotrexate Prevents Wear Particle-Induced Inflammatory Osteolysis in Mice Via Activation of Adenosine A2A Receptor. Arthritis Rheumatol. 2015;67(3):849–55. doi: 10.1002/art.38971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perilli E, Baruffaldi F. Proposal for shared collections of X-ray micro CT datasets of bone specimens. International Conference of Computional Bioengineering; Zaragoza, Spain. 2003. [Google Scholar]
- 22.Shen Z, et al. The role played by cell-substrate interactions in the pathogenesis of osteoclast-mediated peri-implant osteolysis. Arthritis Res Ther. 2006;8(3):R70. doi: 10.1186/ar1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aherne CM, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61(5):695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, et al. Netrin-1 Protects against L-Arginine-Induced Acute Pancreatitis in Mice. PLoS One. 2012;7(9):e46201. doi: 10.1371/journal.pone.0046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grenz A, et al. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One. 2011;6(5):e14812. doi: 10.1371/journal.pone.0014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, et al. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci. 2012;53(3):1285–95. doi: 10.1167/iovs.11-8722. [DOI] [PubMed] [Google Scholar]
- 27.Mirakaj V, et al. Netrin-1 signaling dampens inflammatory peritonitis. J Immunol. 2011;186(1):549–55. doi: 10.4049/jimmunol.1002671. [DOI] [PubMed] [Google Scholar]
- 28.Schubert T, et al. Role of the netrin system of repellent factors on synovial fibroblasts in rheumatoid arthritis and osteoarthritis. Int J Immunopathol Pharmacol. 2009;22(3):715–22. doi: 10.1177/039463200902200317. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2008;294(4):F739–47. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao Y, et al. Neuronal migration and molecular conservation with leukocyte chemotaxis. Genes Dev. 2002;16(23):2973–84. doi: 10.1101/gad.1005802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirakaj V, et al. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc Natl Acad Sci U S A. 2011;108(16):6555–60. doi: 10.1073/pnas.1015605108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirakaj V, et al. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211(6):1037–48. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganathan PV, et al. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int. 2013;83(6):1087–98. doi: 10.1038/ki.2012.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am J Physiol Renal Physiol. 2013;304(7):F948–57. doi: 10.1152/ajprenal.00580.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aherne CM, Collins CB, Eltzschig HK. Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue Barriers. 2013;1(2):e24957. doi: 10.4161/tisb.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuta J, et al. Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest. 2013;123(2):866–73. doi: 10.1172/JCI65054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grenz A, Clambey E, Eltzschig HK. Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care. 2012;18(2):178–85. doi: 10.1097/MCC.0b013e3283514bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKenna WL, et al. Netrin-1-independent adenosine A2b receptor activation regulates the response of axons to netrin-1 by controlling cell surface levels of UNC5A receptors. J Neurochem. 2008;104(4):1081–90. doi: 10.1111/j.1471-4159.2007.05040.x. [DOI] [PubMed] [Google Scholar]
- 39.He W, et al. Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. Faseb J. 2013 doi: 10.1096/fj.13-231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.