Abstract
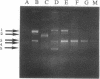
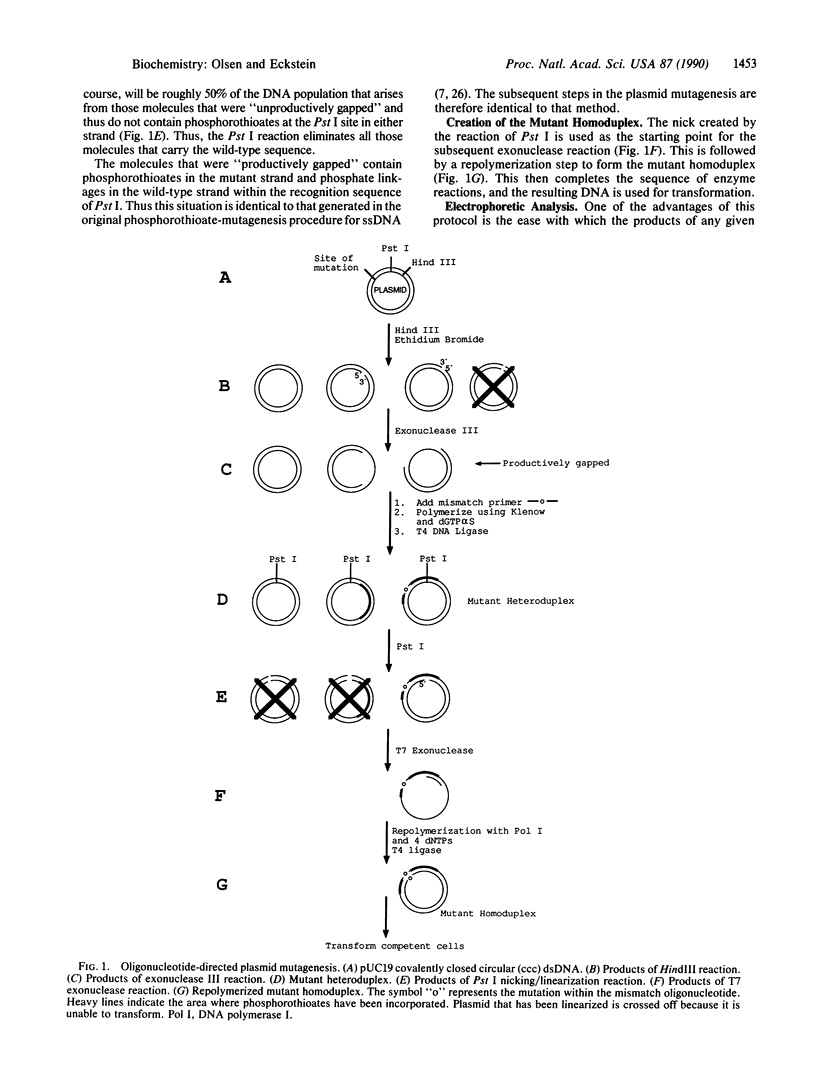
A number of single- and double-base substitutions have been introduced into either the polylinker region or the lacZ gene in the plasmid vector pUC19. The efficiencies of these changes upon transfection of TG-1 bacterial cells were generally 70-80%. A strategy has been devised by which the wild-type DNA can be selectively destroyed. It is primarily based on the resistance of phosphorothioate internucleotide linkages to some restriction enzymes. A mismatch oligonucleotide is introduced into a gapped region and the gap is filled using three deoxynucleoside 5'-triphosphates and one deoxynucleoside 5'-[alpha-thio]triphosphate. Reaction with a restriction enzyme that is unable to hydrolyze phosphorothioates ensures that the DNA containing the mismatch oligonucleotide is only nicked. Concomitantly, the DNA that does not contain the desired mutation is linearized. Subsequent reactions with an exonuclease and DNA polymerase I yield mutant homoduplex DNA for transfection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellini A. V., de Ferra F., Grandi G. A rapid and versatile site-directed method of mutagenesis for double-stranded plasmid DNA. Gene. 1988 Sep 30;69(2):325–330. doi: 10.1016/0378-1119(88)90442-8. [DOI] [PubMed] [Google Scholar]
- Carter P. Improved oligonucleotide-directed mutagenesis using M13 vectors. Methods Enzymol. 1987;154:382–403. doi: 10.1016/0076-6879(87)54086-1. [DOI] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulau L., Cheyrou A., Dubourdieu D., Aigle M. Directed mutagenesis using PCR. Nucleic Acids Res. 1989 Apr 11;17(7):2873–2873. doi: 10.1093/nar/17.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss K., McClain W. H. Rapid site-specific mutagenesis in plasmids. Gene. 1987;59(2-3):285–290. doi: 10.1016/0378-1119(87)90336-2. [DOI] [PubMed] [Google Scholar]
- Hemsley A., Arnheim N., Toney M. D., Cortopassi G., Galas D. J. A simple method for site-directed mutagenesis using the polymerase chain reaction. Nucleic Acids Res. 1989 Aug 25;17(16):6545–6551. doi: 10.1093/nar/17.16.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Joannes M., Saucier J. M., Jacquemin-Sablon A. DNA filter retention assay for exonuclease activities. Application to the analysis of processivity of phage T5 induced 5'-exonuclease. Biochemistry. 1985 Dec 31;24(27):8043–8049. doi: 10.1021/bi00348a031. [DOI] [PubMed] [Google Scholar]
- Kadowaki H., Kadowaki T., Wondisford F. E., Taylor S. I. Use of polymerase chain reaction catalyzed by Taq DNA polymerase for site-specific mutagenesis. Gene. 1989 Mar 15;76(1):161–166. doi: 10.1016/0378-1119(89)90018-8. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Tinkering with enzymes: what are we learning? Science. 1987 Jun 5;236(4806):1252–1258. doi: 10.1126/science.3296192. [DOI] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller H. Practical aspects of preparing phage and plasmid DNA: growth, maintenance, and storage of bacteria and bacteriophage. Methods Enzymol. 1987;152:145–170. doi: 10.1016/0076-6879(87)52016-x. [DOI] [PubMed] [Google Scholar]
- Osterlund M., Luthman H., Nilsson S. V., Magnusson G. Ethidium-bromide-inhibited restriction endonucleases cleave one strand of circular DNA. Gene. 1982 Nov;20(1):121–125. doi: 10.1016/0378-1119(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Muzyczka N. Construction of a specific amber codon in the simian virus 40 T-antigen gene by site-directed mutagenesis. J Virol. 1980 Nov;36(2):611–616. doi: 10.1128/jvi.36.2.611-616.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Olsen D. B., Eckstein F. Inhibition of restriction endonuclease hydrolysis by phosphorothioate-containing DNA. Nucleic Acids Res. 1989 Nov 25;17(22):9495–9495. doi: 10.1093/nar/17.22.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Wendler A., Eckstein F. Strand specific cleavage of phosphorothioate-containing DNA by reaction with restriction endonucleases in the presence of ethidium bromide. Nucleic Acids Res. 1988 Feb 11;16(3):803–814. doi: 10.1093/nar/16.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Protein engineering. The design, synthesis and characterization of factitious proteins. Biochem J. 1987 Aug 15;246(1):1–17. doi: 10.1042/bj2460001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Dovey S., O'Rourke D. M. A rapid method for site directed mutagenesis of plasmid DNA. Biotechniques. 1988 Jun;6(6):511-2, 517-8. [PubMed] [Google Scholar]
- Sugimoto M., Esaki N., Tanaka H., Soda K. A simple and efficient method for the oligonucleotide-directed mutagenesis using plasmid DNA template and phosphorothioate-modified nucleotide. Anal Biochem. 1989 Jun;179(2):309–311. doi: 10.1016/0003-2697(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Vallette F., Mege E., Reiss A., Adesnik M. Construction of mutant and chimeric genes using the polymerase chain reaction. Nucleic Acids Res. 1989 Jan 25;17(2):723–733. doi: 10.1093/nar/17.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Woodhead J. L., Bhave N., Malcolm A. D. Cation dependence of restriction endonuclease EcoRI activity. Eur J Biochem. 1981 Apr;115(2):293–296. doi: 10.1111/j.1432-1033.1981.tb05237.x. [DOI] [PubMed] [Google Scholar]