FcpA from Leptospira interrogans, which is essential for pathogenesis, was crystallized but proved to be recalcitrant to X-ray diffraction studies. The orthologue from the saprophyte L. biflexa allowed three different crystal forms to be obtained that were suitable for crystallographic studies.
Keywords: spirochetes, motility, flagella, SAD phasing, Leptospira interrogans, Leptospira biflexa, FcpA, leptospirosis
Abstract
The protein FcpA is a unique component of the flagellar filament of spirochete bacteria belonging to the genus Leptospira. Although it plays an essential role in translational motility and pathogenicity, no structures of FcpA homologues are currently available in the PDB. Its three-dimensional structure will unveil the novel motility mechanisms that render pathogenic Leptospira particularly efficient at invading and disseminating within their hosts, causing leptospirosis in humans and animals. FcpA from L. interrogans was purified and crystallized, but despite laborious attempts no useful X ray diffraction data could be obtained. This challenge was solved by expressing a close orthologue from the related saprophytic species L. biflexa. Three different crystal forms were obtained: a primitive and a centred monoclinic form, as well as a hexagonal variant. All forms diffracted X-rays to suitable resolutions for crystallographic analyses, with the hexagonal type typically reaching the highest limits of 2.0 Å and better. A variation of the quick-soaking procedure resulted in an iodide derivative that was instrumental for single-wavelength anomalous diffraction methods.
1. Introduction
There are estimated to be more than 1 000 000 human cases of leptospirosis per year (Costa et al., 2015 ▸; Torgerson et al., 2015 ▸), making it one of the leading zoonoses worldwide. Leptospirosis is caused by pathogenic species of eubacteria from the genus Leptospira (Ko et al., 2009 ▸), a spirochete with a characteristic spiral body shape and hooked ends. Strikingly, spirochetes possess periplasmic endoflagella (Goldstein & Charon, 1988 ▸) that are essential for swimming motility. This translational motility, which is conferred by the coordinated rotation of two periplasmic flagella at opposite poles of the cell (Goldstein & Charon, 1990 ▸), is also necessary for the virulence of pathogenic Leptospira (Wunder et al., 2016 ▸). Despite their biological importance, information about the molecular structure of spirochetal endoflagella is extremely scarce, also obscuring the mechanism of cell translation. Bacterial flagella are organized into three main components: a basal body that acts as a rotary motor and a curved hook that works as a universal joint connecting to a relatively long filament. The protein composition of the Leptospira flagellar filament is among the most complex described to date. The filament core comprises four isoforms of FlaB (Malmström et al., 2009 ▸), which is homologous to flagellin, the single-filament component from Salmonella (Samatey et al., 2001 ▸). Several additional constituents have been identified, such as FlaA1 and FlaA2 (Lambert et al., 2012 ▸), as well as FcpA (flagellar coiling protein A), a novel flagellar protein unique to Leptospira (Fouts et al., 2016 ▸; Wunder et al., 2016 ▸). One of the most abundant flagellar components (Malmström et al., 2009 ▸), FcpA is essential for motility and for virulence in vivo (Wunder et al., 2016 ▸). As it is not similar to any protein in the current Protein Data Bank, the three-dimensional structure of FcpA will contribute to uncovering novel motility mechanisms in spirochetes.
We now report the crystallization of FcpA from the pathogen L. interrogans. Such crystals proved recalcitrant to X-ray diffraction studies. A close orthologue from the related saprophytic L. biflexa was purified and three crystal forms were grown that diffracted X-rays to suitable resolutions. A variation of the quick-soaking approach, which was otherwise deleterious, led to a successful iodine derivatization, providing anomalous diffraction signal for future experimental phasing.
2. Materials and methods
2.1. Macromolecule production
The genes encoding FcpA from L. interrogans (UniProt Q72MM7) and from L. biflexa (UniProt B0STJ8), excluding their 5′ signal peptide-encoding sequences, were cloned from genomic DNA (Table 1 ▸) using restriction-free (RF) cloning methods (van den Ent & Löwe, 2006 ▸). The first PCR reactions were performed with the primers listed in Table 1 ▸. The resulting products were purified from agarose gels and used as megaprimers for a second PCR (primer extension) using an expression plasmid derived from pQE80 (Qiagen) as a template (Trajtenberg et al., 2014 ▸), which includes an engineered Tobacco etch virus (TEV) protease cleavage site (sequence ENLYFQG) between the plasmid-encoded N-terminal 6×His tag and the FcpA target sequences. The sequence of the resulting plasmids, named pQE80_LinfcpA and pQE80_LbifcpA for the L. interrogans and L. biflexa targets, respectively, was confirmed by Sanger sequencing. Starting from pQE80_LinfcpA, a derived construct was eventually obtained by RF cloning to express the double mutant LinFcpA-C221T+C241A. These expression constructs (pQE80_LinfcpA, pQE80_LinfcpA-C221T+C241A and pQE80_LbifcpA) were used to transform Escherichia coli cells; small-scale induction experiments led to the choice of E. coli strain TOP10F′ (Thermo Fisher Scientific) to express Lin_FcpA and its double point mutant, whereas E. coli Rosetta-gami 2 (DE3) (Novagen) cells proved to be better for expressing Lbi_FcpA. Transformed E. coli cells were grown at 37°C in 4 l 2×YT (2× yeast extract and tryptone) medium containing chloramphenicol (30 mg l−1), ampicillin (100 mg l−1) and MgSO4 (2 mM). When the absorbance at 600 nm reached 0.8, overexpression was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside for 4 h at 30°C. The cells were harvested by centrifugation at 5000g for 20 min and the pellet was resuspended in lysis buffer [50 mM Tris–HCl pH 8, 0.1 M NaCl and EDTA-free protease-inhibitor cocktail (Roche)]. To disrupt the cells, hen egg-white lysozyme (1 mg ml−1) and DNAse (100 µg ml−1) were added, followed by four cycles of sonication (Branson Digital) using 30 s pulses at 30% amplitude, with resting periods of 45 s in between. The lysed suspension was clarified by centrifugation at 9000g for 45 min at 4°C. The supernatant was loaded onto an Ni–NTA affinity chromatography column (5 ml HisTrap, GE Healthcare) pre-equilibrated with loading buffer (50 mM Tris–HCl pH 8, 0.5 M NaCl, 20 mM imidazole). Elution was achieved with a linear gradient of elution buffer (50 mM Tris–HCl pH 8, 0.5 M NaCl, 500 mM imidazole) in 20 column volumes. The eluted peak was pooled and treated with 50 µg ml−1 6×His-TEV protease during overnight dialysis against five volumes of buffer consisting of 50 mM Tris–HCl pH 8, 0.2 M NaCl. A second Ni–NTA purification step allowed removal of the 6×His tag; the flowthrough fraction was concentrated by ultrafiltration (Vivaspin 20 device, 10 kDa molecular-weight cutoff, Sartorius) and 10 ml was injected onto a Superdex S75 HR 26/60 column (GE Healthcare) pre-equilibrated either with 20 mM HEPES pH 7, 200 mM NaCl or with 20 mM Tris–HCl pH 8, 300 mM NaCl (Table 2 ▸). The size-exclusion chromatography peak fractions were pooled and concentrated to approximately 20 mg ml−1 by ultrafiltration. Protein purification was assessed by SDS–PAGE with Coomassie Blue staining.
Table 1. Macromolecule-production information.
| Source organism | L. interrogans | L. biflexa |
|---|---|---|
| DNA source | NCBI locus tag LIC_RS00005 | NCBI locus tag LEPBI_I0267 |
| Forward primer | 5′-CCTGTATTTTCAGGGATCCGGTTCACAGCAAAACAATCAGGGCGGTAATC-3′ | 5′-CCTGTATTTTCAGGGATCCGCTAAAGACCAAGTCGACGAAC-3′ |
| Reverse primer | 5′-CTGCAGGTCGACGCCAAGATCCTTTTTTAAGGTCTAACCGAAATCACTTCGTC-3′ | 5′-GCTGCAGGTCGACGCCAAGATCCTTTTTTATTGTGCTACCGAAATAACCTC-3′ |
| Cloning vector | pQE80† | pQE80† |
| Expression vector | pQE80† | pQE80† |
| Expression host | E. coli strain TOP10F′ | E. coli strain Rosetta-gami 2 (DE3) |
| Complete amino-acid sequence of the construct produced‡ | MRGSHHHHHHGSGSENLYFQG SGSQQNNQGGNQQANESVEKIDELLKGELVPEDDDKNLTEEQKRRKKAIQEQEALWKNPDFKGYDKNFQELHQLSKAFANNKFRLALSNYQSGVNTILKMREAIEQYRKEEAEKKRLDEKWYWQKVDRKAREDRVVSRDKLVAKQQALNYFTKAINHLDEIKNPDLRERPEFKRLLSDTYRSWILTEYDLQNLPQCIPILELYIEIDENEKEYPAHKYLASCYAFEENMIKKNGGASEDQMFKYRYKKNVHLLRATELKYGKDSPEYKHIVNLVNKDEVISVRP | MRGSHHHHHHGSGSENLYFQG SAKDQVDELLKGELVPENDDAELTEDQKKKKKEIMEQESLWKNPDFKGYNKTFQELHQLSKTFANNQFRLALSNYQSGVNTIMKNRDWVEQYRKEEAEKKRLDEKWYWQKVDRKAREERVVYREKMKAKQDALNYFSKAINHLDEIKNPDLRERPEFKRLLSDVYRSWIMAEYDLQNLPQTIPILELYIEIDDNEKEYPAHKYLASAYSFEENMIKKTKGPDDMLFKYRYKKNVHLLRATELKYGKDSPEYKHIVNVINRDEVISVAQ |
Modified as described in Trajtenberg et al. (2014 ▸).
Cloning artefacts are underlined.
Table 2. Crystallization.
| Crystal form | 1 (centred monoclinic) | 2 (primitive monoclinic) | 3 (hexagonal) |
|---|---|---|---|
| Method | Hanging-drop vapour diffusion | Hanging-drop vapour diffusion | Sitting-drop vapour diffusion |
| Plate type | VDX 24-well | VDX 24-well | 96-well CrystalQuick |
| Temperature (K) | 293 | 293 | 293 |
| Protein concentration (mg ml−1) | 20 | 20 | 20 |
| Buffer composition of protein solution | 20 mM Tris–HCl pH 8.0, 300 mM NaCl | 20 mM Tris–HCl pH 8.0, 300 mM NaCl | 20 mM HEPES pH 7.0, 200 mM NaCl |
| Composition of reservoir solution | 20–25%(v/v) PEG 400, 100 mM Tris–HCl pH 7.5–8.5 | 3–5%(w/v) PEG 4000, 100 mM Tris–HCl pH 7.5–8.0 | 12%(w/v) PEG 8000, 0.1 M Na/K phosphate pH 6.2, 0.2 M NaCl |
| Volume and ratio of drop | 4 µl (1:1) | 4 µl (1:1) | 0.6 µl (1:1) |
| Volume of reservoir (ml) | 1 | 1 | 0.1 |
Seeking to improve the diffraction quality of the Lin_FcpA crystals, controlled proteolysis of the recombinant protein was assayed using different proteases. The best results were obtained by incubating Lin_FcpA at 1 mg ml−1 with proteinase K at room temperature at a 1:10 protease:Lin_FcpA molar ratio. Aliquots were drawn at different time points, stopping the reaction by the addition of a protease-inhibitor cocktail (EDTA-free, Roche) and incubation for 5 min at 100°C. The reaction products were analysed by SDS–PAGE with Coomassie Blue staining. Two major species were selected as protease-resistant. The proteolytic reaction was scaled up and purified using a 1 ml Mono S column (GE Healthcare) with 20 mM MES pH 6, 100 mM NaCl as the binding buffer and eluted with a 20 ml linear gradient to 20 mM MES pH 6, 1 M NaCl. Two major peaks were separated and were then analysed by MALDI-TOF mass spectrometry, identifying two cleavage sites at the N-terminal end. Taking this information into consideration, two truncated constructs of LinfcpA were generated by RF cloning, pQE80_LinfcpA_Δ31 and pQE80_LinfcpA_Δ42, encoding proteins starting at residues Ala31 and Leu42, respectively.
2.2. Crystallization
Initial crystallization screening was performed using the sparse-matrix kits The JCSG Core I–IV Suites (Qiagen), The PEGs Suite (Qiagen) and Classics Lite (Natrix) in 96-well CrystalQuick plates (Greiner), resulting in 576 different conditions. Sitting drops were dispensed using a robotic platform (Honeybee 963, Isogen Life Science) by mixing 300 nl protein solution with 300 nl reservoir solution. FcpA from L. interrogans (28.5 mg ml−1 in buffer consisting of 20 mM HEPES pH 7.0, 0.2 M NaCl) gave a single hit, which after manual optimization defined a final reservoir composition consisting of 0.1 M bicine pH 9.0, 2.4 M ammonium sulfate.
FcpA from L. biflexa (at 20 mg ml−1 in the buffers described in Table 2 ▸) resulted in definite crystalline material in nine different conditions. Some of these initial hits could be manually optimized using a hanging-drop setup in VDX 24-well crystallization plates (Hampton Research). Grid screens varying the precipitant concentration and pH were set up using solutions prepared with an Alchemist liquid-handling system (Rigaku). Three crystal forms were ultimately selected on the basis of better X-ray diffraction, as further described in Table 2 ▸. Eventually, crystals belonging to form 1 (with an ab-centred monoclinic cell) were also grown in the same mother liquor but with an additional 300 mM NaI, which was instrumental in obtaining an iodine derivative with an anomalous diffraction signal. Attempts to achieve this derivatization using more standard quick-soaking approaches (Dauter et al., 2000 ▸) proved deleterious to the crystals, abolishing X-ray diffraction altogether.
2.3. Data collection and processing
Crystals were mounted in nylon loops and flash-cooled in liquid nitrogen after quick soaking in a cryoprotectant solution composed of mother liquor with 25%(v/v) glycerol. Single-wavelength anomalous diffraction (SAD) experiments with the iodine-derivatized crystals (Table 3 ▸) were performed in-house with a rotating copper-anode instrument (MicroMax-007 HF, Rigaku). All other X-ray diffraction data sets (Table 3 ▸) were collected on beamlines PROXIMA 1 and 2A at the SOLEIL synchrotron. Data sets were indexed and integrated with iMosflm (Battye et al., 2011 ▸) or XDS (Kabsch, 2010 ▸) and were scaled and reduced with AIMLESS (Evans & Murshudov, 2013 ▸).
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Form 1: centred monoclinic | ||||
|---|---|---|---|---|
| Crystal form | Iodine derivative | Native | Form 2: primitive monoclinic | Form 3: hexagonal |
| Diffraction source | Rotating copper anode (MicroMax-007 HF, Rigaku) | PROXIMA 1, SOLEIL | PROXIMA 1, SOLEIL | PROXIMA 2A, SOLEIL |
| Wavelength (Å) | 1.5418 | 0.97857 | 0.97857 | 0.97910 |
| Temperature (K) | 110 | 100 | 100 | 100 |
| Detector | Image plate (MAR345, MAR Research) | Hybrid photon counting (PILATUS 6M, Dectris) | Hybrid photon counting (PILATUS 6M, Dectris) | Area detector (Q315r CCD, ADSC) |
| Crystal-to-detector distance (mm) | 285.00 | 484.81 | 485.34 | 274.35 |
| Rotation range per image (°) | 1 | 0.2 | 0.2 | 0.5 |
| Total rotation range (°) | 193 | 180 | 180 | 100 |
| Exposure time per image (s) | 1200 | 0.2 | 0.2 | 0.5 |
| Space group | C2 | C2 | P21 | P622 |
| a, b, c (Å) | 82.0, 99.3, 106.0 | 82.3, 99.6, 106.7 | 85.5, 96.5, 121.1 | 132.7, 132.7, 67.6 |
| α, β, γ (°) | 90, 92.96, 90 | 90, 91.95, 90 | 90, 105.2, 90 | 90, 90, 120 |
| Mosaicity (°) | 0.29 | 0.24 | 0.15 | 0.20 |
| Resolution range (Å) | 44.94–2.92 (3.10–2.92) | 45.12–2.50 (2.60–2.50) | 48.23–2.95 (3.07–2.95) | 43.76–2.00 (2.11–2.00) |
| Total No. of reflections | 74051 (11628) | 99658 (10749) | 137503 (15749) | 286178 (40385) |
| No. of unique reflections | 18451 (2931) | 29300 (3278) | 39975 (4485) | 24246 (3460) |
| Completeness (%) | 99.6 (98.7) | 98.4 (97.9) | 99.5 (99.7) | 99.9 (99.6) |
| Mulitplicity | 4.0 (4.0) | 3.4 (3.3) | 3.4 (3.5) | 11.8 (11.7) |
| 〈I/σ(I)〉 | 15.1 (2.0) | 18.2 (2.3) | 17.2 (2.0) | 17.5 (2.5) |
| R meas (%) | 10 (91) | 4 (57) | 6 (76) | 11 (119) |
| Overall B factor from Wilson plot (Å2) | 72.5 | 61.3 | 75.6 | 30.5 |
3. Results and discussion
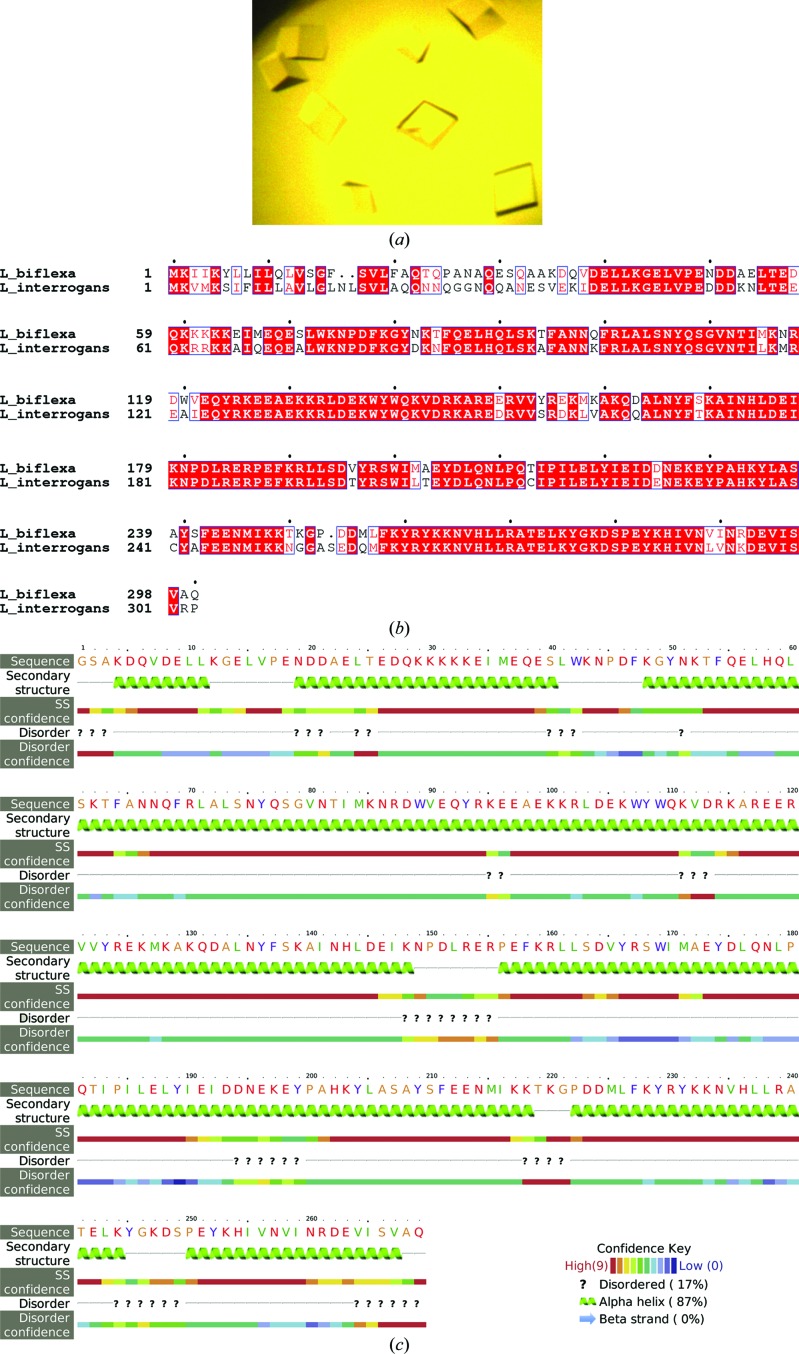
The amino-acid sequence of L. interrogans FcpA predicts an N-terminal leader peptide of 22 residues, anticipating the secretion of FcpA to the periplasm via the Sec pathway. The fcpA gene was cloned excluding this signal peptide sequence and introducing an N-terminal 6×His tag instead. Soluble recombinant protein was produced and straightforwardly purified using a standard two-step metal-affinity and size-exclusion chromatographic approach (data not shown). Crystallization conditions were screened, eventually obtaining a single hit from 576 different conditions. Initial crystals were further optimized, ultimately growing to well faceted forms measuring 0.2 × 0.2 × 0.3 mm (Fig. 1 ▸ a). Despite its external quality, this unique crystal form proved to be mediocre, diffracting X-rays to worse than 10 Å resolution in the best of cases. Extensive efforts were made to improve the crystal quality in addition to straightforward refinement of the initial conditions by variation of protein concentration, the mother-liquor composition and the temperature. Two N-terminally truncated versions were also assayed, as indicated by limited proteolysis experiments, anticipating a species with fewer flexible loops exposed and hence tighter crystalline packing. Moreover, a double point-mutant form of Lin_FcpA was also tested with the native Cys residues 215 and 241 substituted by the corresponding residues in Lbi_FcpA (Cys215Thr + Cys241Ala). This construct was generated with the rationale of avoiding a suspected heterogeneous oxidation phenomenon. Despite all of these efforts, L. interrogans FcpA proved recalcitrant to X-ray diffraction studies.
Figure 1.
(a) Crystals of FcpA from L. interrogans obtained with the full-length mature form of the protein (starting at residue Gln21). Despite extensive optimization efforts these crystals diffracted X-rays to worse than 10 Å resolution (see main text). (b) Sequence alignment of FcpA from L. interrogans (a pathogen) and L. biflexa (a saprophyte). Similar amino acids are boxed, with identities highlighted by a red background. (c) L. biflexa FcpA secondary-structure prediction calculated with the Protein Homology/analogy Recognition Engine v.2.0 (Phyre2) server. The leading Gly-Ser is part of the plasmid-encoded tag; the native protein starts at the following Ala, which corresponds to Ala32 of the full-length sequence numbering as depicted in (b).
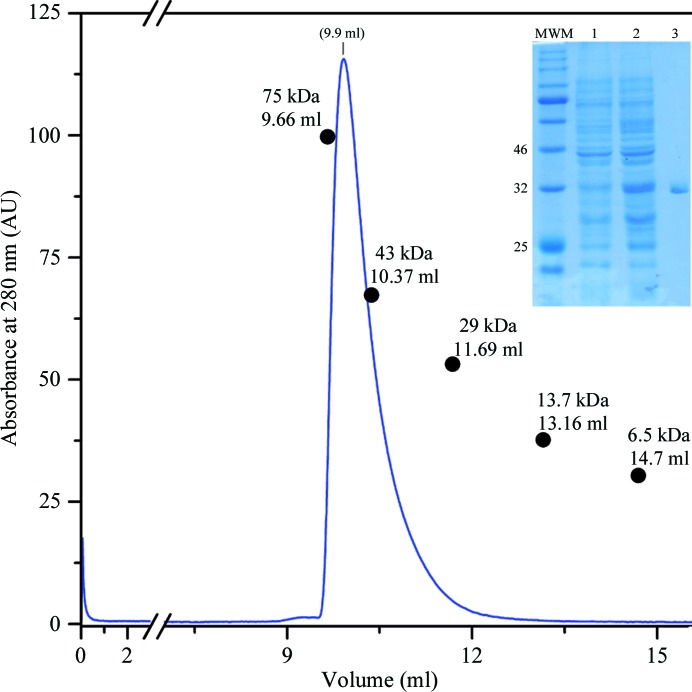
Looking for potential orthologues, sequence alignments revealed a closely related target encoded in the genome of the saprophytic species L. biflexa (Fig. 1 ▸ b). The FcpA orthologues are 77% identical, with almost no gaps except for two residues within the predicted signal peptides and one more amino acid towards the final portion of the mature protein, making L. biflexa FcpA three residues shorter overall. We extended the sequence analysis of the target from the saprophyte, anticipating an all-helical secondary-structure organization (Fig. 1 ▸ c) with virtually no predicted disordered regions (Kelley et al., 2015 ▸), and hence with an equally reasonable propensity to crystallize; potentially improving the quality of the crystals compared with those of L. interrogans FcpA. Substantial amounts of soluble L. biflexa FcpA were obtained after overexpressing a 6×His-tagged construct lacking the leader peptide in E. coli Rosetta-gami 2 cells. The protein was purified to homogeneity using the same two-step chromatographic approach as used for L. interrogans FcpA. Size-exclusion chromatography profiles suggested that L. biflexa FcpA is dimeric (Fig. 2 ▸), with the expected molecular weight of the protomer being 32.3 kDa.
Figure 2.
Isocratic elution of a size-exclusion chromatography (SEC) column to separate recombinant FcpA from L. biflexa as obtained after Ni2+-affinity purification. Elution volumes of standard globular proteins are overlaid with their molecular weights indicated to estimate the apparent molecular mass. Inset, 12% polyacrylamide electrophoresis gel run under denaturing conditions (SDS–PAGE) stained with Coomassie Blue. Molecular-weight markers (MWM) were separated in the leftmost lane; three of them are labelled (in kDa). A soluble protein extract from non-induced E. coli Rosetta-gami 2 (DE3) cells transformed with the pQE80_LbifcpA expression plasmid was separated in lane 1. Lane 2 is similar but after IPTG induction. Lane 3 shows the primary SEC peak.
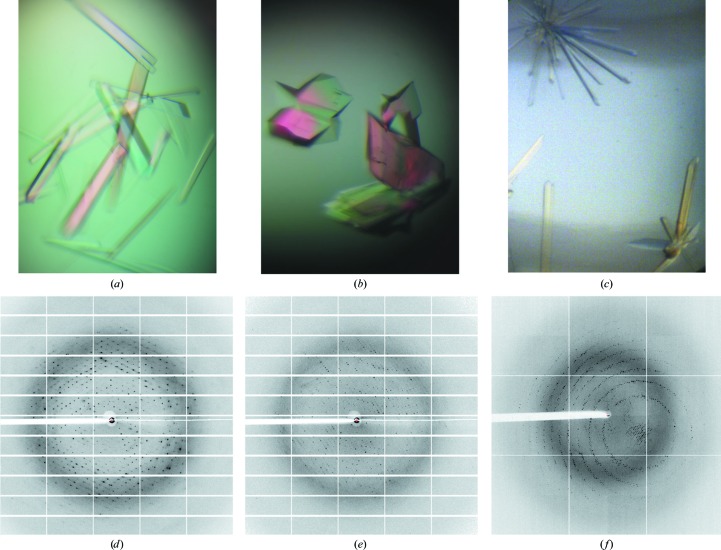
FcpA from L. biflexa allowed crystals to be grown in three different forms, all of which diffracted to a high resolution (Fig. 3 ▸). X-ray diffraction data were collected from the three crystal forms using synchrotron radiation. Data-collection and processing statistics are summarized in Table 3 ▸. Of note, the hexagonal crystals (Fig. 3 ▸ c) were directly harvested from crystallization screening plates and diffracted to the highest resolution (Fig. 3 ▸ f), better than those of the two monoclinic forms (Figs. 3 ▸ a and 3 ▸ b), for which the growing conditions were carefully optimized.
Figure 3.
L. biflexa FcpA crystals and representative X-ray diffraction images. (a, d) Crystal form 1 (centred monoclinic). (b, e) Crystal form 2 (primitive monoclinic). (c, f) Crystal form 3 (hexagonal).
To calculate experimental phases, data were collected from an iodine derivative of crystal form 1 at 1.54 Å wavelength, and after processing significant anomalous signal was detected to 4.1 Å resolution (where the correlation coefficient between random half data sets of the intensity differences of Bijvoet pairs fell below 0.3). Single-wavelength anomalous diffraction phasing followed by density-modification approaches are currently under way.
Acknowledgments
The Institut Pasteur International Network is acknowledged for support through the Integrative Microbiology of Zoonotic Agents (IMiZA) International Joint Unit. We thank the staff at synchrotron beamlines PROXIMA 1 and 2A at SOLEIL, France for assistance during X ray diffraction data collection.
References
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Costa, F., Hagan, J. E., Calcagno, J., Kane, M., Torgerson, P., Martinez-Silveira, M. S., Stein, C., Abela-Ridder, B. & Ko, A. I. (2015). PLoS Negl. Trop. Dis. 9, e0003898. [DOI] [PMC free article] [PubMed]
- Dauter, Z., Dauter, M. & Rajashankar, K. R. (2000). Acta Cryst. D56, 232–237. [DOI] [PubMed]
- Ent, F. van den & Löwe, J. (2006). J. Biochem. Biophys. Methods, 67, 67–74. [DOI] [PubMed]
- Evans, P. R. & Murshudov, G. N. (2013). Acta Cryst. D69, 1204–1214. [DOI] [PMC free article] [PubMed]
- Fouts, D. E. et al. (2016). PLoS Negl. Trop. Dis. 10, e0004403. [DOI] [PMC free article] [PubMed]
- Goldstein, S. F. & Charon, N. W. (1988). Cell Motil. Cytoskeleton, 9, 101–110. [DOI] [PubMed]
- Goldstein, S. F. & Charon, N. W. (1990). Proc. Natl Acad. Sci. USA, 87, 4895–4899. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. (2015). Nature Protoc. 10, 845–858. [DOI] [PMC free article] [PubMed]
- Ko, A. I., Goarant, C. & Picardeau, M. (2009). Nature Rev. Microbiol. 7, 736–747. [DOI] [PMC free article] [PubMed]
- Lambert, A., Picardeau, M., Haake, D. A., Sermswan, R. W., Srikram, A., Adler, B. & Murray, G. A. (2012). Infect. Immun. 80, 2019–2025. [DOI] [PMC free article] [PubMed]
- Malmström, J., Beck, M., Schmidt, A., Lange, V., Deutsch, E. W. & Aebersold, R. (2009). Nature (London), 460, 762–765. [DOI] [PMC free article] [PubMed]
- Samatey, F. A., Imada, K., Nagashima, S., Vonderviszt, F., Kumasaka, T., Yamamoto, M. & Namba, K. (2001). Nature (London), 410, 331–337. [DOI] [PubMed]
- Torgerson, P. R., Hagan, J. E., Costa, F., Calcagno, J., Kane, M., Martinez-Silveira, M. S., Goris, M. G., Stein, C., Ko, A. I. & Abela-Ridder, B. (2015). PLoS Negl. Trop. Dis. 9, e0004122. [DOI] [PMC free article] [PubMed]
- Trajtenberg, F., Albanesi, D., Ruétalo, N., Botti, H., Mechaly, A. E., Nieves, M., Aguilar, P. S., Cybulski, L., Larrieux, N., de Mendoza, D. & Buschiazzo, A. (2014). MBio, 5, e02105. [DOI] [PMC free article] [PubMed]
- Wunder, E. A. Jr, Figueira, C. P., Benaroudj, N., Hu, B., Tong, B. A., Trajtenberg, F., Liu, J., Reis, M. G., Charon, N. W., Buschiazzo, A., Picardeau, M. & Ko, A. I. (2016). Mol. Microbiol. 101, 457–470. [DOI] [PMC free article] [PubMed]