Abstract
Objective:
To assess the feasibility of conducting a randomised controlled trial of a home-based virtual reality system for rehabilitation of the arm following stroke.
Design:
Two group feasibility randomised controlled trial of intervention versus usual care.
Setting:
Patients’ homes.
Participants:
Patients aged 18 or over, with residual arm dysfunction following stroke and no longer receiving any other intensive rehabilitation.
Interventions:
Eight weeks’ use of a low cost home-based virtual reality system employing infra-red capture to translate the position of the hand into game play or usual care.
Main measures:
The primary objective was to collect information on the feasibility of a trial, including recruitment, collection of outcome measures and staff support required. Patients were assessed at three time points using the Wolf Motor Function Test, Nine-Hole Peg Test, Motor Activity Log and Nottingham Extended Activities of Daily Living.
Results:
Over 15 months only 47 people were referred to the team. Twenty seven were randomised and 18 (67%) of those completed final outcome measures. Sample size calculation based on data from the Wolf Motor Function Test indicated a requirement for 38 per group. There was a significantly greater change from baseline in the intervention group on midpoint Wolf Grip strength and two subscales of the final Motor Activity Log. Training in the use of the equipment took a median of 230 minutes per patient.
Conclusions:
To achieve the required sample size, a definitive home-based trial would require additional strategies to boost recruitment rates and adequate resources for patient support.
Keywords: Rehabilitation, stroke, feasibility, virtual reality
Introduction
Approximately 70% of patients experience impaired arm function after a stroke, and it is estimated that 40% of survivors are left with reduced functioning in the affected arm.1 There is now strong evidence from high-quality trials to support intensive repetitive task-oriented training for recovery after stroke.2 Recent studies3 have found improvements in patients as much as 6 months post stroke, long after they have been discharged from any formal rehabilitation. Consequently there is a need to find the best way to support survivors once they stop accessing formal services.4
One route through which this may be achieved is the adoption of virtual reality and interactive video gaming which have emerged as new treatment approaches in stroke rehabilitation.5,6 The emergence of commercial gaming consoles has led to their adoption by therapists in clinical settings.7,8 These consoles have the advantages of mass acceptability, easily perceived feedback and most importantly, they are affordable. A disadvantage however, is that the games are not specifically designed for therapeutic use and while the games encourage movements of the arm, none capture sufficient information about the position of the fingers to be useful in the rehabilitation of the hand.
We developed a low cost home-based system for rehabilitation of the arm and hand designed to be flexible and motivating in order to improve adherence. Given the home-based, self-directed nature of the intervention and the introduction of new technology, a feasibility randomised controlled study was carried out in line with the MRC Framework for Complex Interventions.9 In preparation for an evaluation of the effectiveness of the intervention, the feasibility randomised controlled study aimed to answer the following questions:
Can we recruit patients?
Can we collect outcome measures?
What sample size is indicated by the outcome measures collected?
How much researcher and therapist support was required?
Methods
Ethics approval was obtained from the local NHS Research Ethics Committee: Nottingham Research Ethics Committee 1 (reference number 10/H0403/72). The trial was registered with the National Institute of Health ClinicalTrials.gov protocol registration and results system: registration number NCT02637791.
The design adopted was a two group feasibility randomised controlled trial comparing the intervention with usual care.
Patients were recruited who were aged 18 or over, with a confirmed diagnosis of stroke, no longer receiving any other intensive rehabilitation (intermediate care, early supported discharge) and who still had residual impairment of their arm. Patients were excluded if they had no detectable movement in the arm; premorbid disability in arm function; severe symptomatic arm or shoulder pain; severe visual impairments; other neurological conditions such as head injury or multiple sclerosis; an unstable medical condition; psychiatric illness; epi.tify triggered by screen images; cardiac pacemaker; were unable to tolerate sitting in a chair for 30 minutes; unable to follow a two stage command or were living in a care home.
The initial recruitment plan was to identify patients from the inpatient stroke unit and outpatient rehabilitation service. They were receiving on average a combined number of between 80 and 100 new referrals per month during this period. The therapists on the wards were briefed on inclusion and exclusion criteria and provided with demonstrations of any assessment procedures if required. For example, in order to determine if there was detectable movement in the arm the Medical Research Council power scale10 was used. Additionally, therapists were briefed on the procedures for gaining the patients’ permission for the research team to contact them.
However, in spite of weekly visits by a member of the research team to the wards, only one potential patient was referred to the research team. Consequently, efforts were focused on the three regional community services who worked with patients post discharge: the Stroke Outreach Service, the Early Supported Discharge and the Community Stroke Teams.
For those who met the inclusion criteria, informed consent was obtained and baseline assessments were collected during a home visit prior to randomising the patient to the intervention or control group. Randomisation was managed by a research administrator who held a web generated list for a two group randomisation sequence which was concealed from the researchers. The researcher who had collected baseline data phoned the administrator to discover the next unallocated number on the list to determine whether the patient would be allocated to the intervention or control group.
The intervention (the virtual glove, see Supplementary Figure) consisted of a hand-mounted power unit, with four infra-red light emitting diodes mounted on the user’s finger tips. The diodes were tracked using one or two Nintendo Wiimotes mounted by the monitor on which the games were displayed to translate the location of the user’s hand, fingers and thumb in three dimensional space. The intervention was developed based on motor learning theory and aimed to increase the number of repetitions of functional movements, whilst providing games that were challenging with feedback on performance. This is because increasing repetitions alone is not sufficient to drive neuroplasticity,11 with shaping (small s.tif of increasing difficulty with immediate feedback on performance) also known to improve recovery.12
Three games were produced specifically for the project with the help of therapists and stroke patients.13 In order to play them, users had to perform the movements of reach to grasp, grasp and release, pronation and supination that are necessary fort many activities of daily living. Spacerace required pronation and supination of the hand to guide a space craft through obstacles. Spongeball required the user to open their fist and extend their fingers in order to release a ball to hit a target. Balloonpop required a balloon to be grasped and popped by moving it to a pin protruding from the virtual floor. They were designed to be constantly challenging, with increasing levels of difficulty dependent on ability. This was in order to maximise motor learning and to keep the patients motivated to continue to use the system while ensuring they could achieve some success. Immediate feedback was given by scores displayed on the screen at the end of a game and a permanent visual display of scores and levels played. Difficulty was increased by greater movement being required to complete a task, an increase in the speed at which events occur and with which responses are required, or an increase in the precision required to complete a task. A log of when the system was in use was stored on the computer, as well as what games were played and what scores the user obtained.
Patients in the intervention group had the virtual glove in their homes for a period of eight weeks and were advised to try to build up to using the system for a maximum of twenty minutes, three times a day, for eight weeks. As the system worked on detecting position of the fingers in the glove and not the movement of the wrist, elbow or shoulder or sitting posture, it was important that a therapist provided initial instruction and subsequent ongoing support. The intention of this was to maximise use of the intervention and to reduce unwanted compensatory movements. Patients in the control group received only the visits to collect outcome measures.
After four weeks, all patients were visited at home for completion of the midpoint outcome measures. It was not always possible to ensure the researcher was blind to the allocation of the patient at this time as the equipment was sometimes visible and the patient might make reference to it. All patients still in the study completed final outcome measures at eight weeks post randomisation with a blinded assessor, once the equipment had been removed from the patient’s home. In order to minimise variability between raters, they underwent joint training sessions to gain an agreed level of competence in the procedure and administration of the outcome measures. This included practicing the administration on the patient representatives on the project steering committee.
The following outcome measures were collected at baseline, four weeks (midpoint) and eight weeks (final).
Wolf Motor Function Test.14–17 This produces an average time in seconds for a number of timed functional arm movements plus grip strength in kilograms.
Motor Activity Log. 21–23 Individuals are asked to rate Quality of Movement (QOM) and Amount of Movement (AOM) during 30 daily functional tasks. Items are scored on a 6-point ordinal scale. For this study, the number of tasks attempted were also recorded.
Nottingham Extended Activities of Daily Living Scale.24–26
For the intervention group, the frequency of use of the glove was collected by the software. These data are reported elsewhere.27
For those assigned to the intervention group, three procedures were put in place to encourage the use of the equipment at the recommended duration and frequency.
First, considerable face to face support was provided. The physiotherapist or occupational therapist from the research team delivered and set up the equipment. Based on the patient’s ability, the therapists drew up a sheet for each individual advising them what games to start with and at what level. The glove and games were demonstrated to the patient and their carer and they were then trained on how to use the equipment independently. The researcher then arranged to return to repeat this demonstration until they felt that the patient had understood how to use the glove or that there was a carer who understood how to use it. The researchers also provided phone support to check the patient had been able to use the equipment and to offer further visits to clarify any queries. After the initial setup and training period, a member of the team visited either weekly or fortnightly, depending on the level of support required, to check progress and retrieve data. There were no limits on the number of visits per patient.
Second, patients were provided with a phone number on which a member of the research team could be contacted during working hours if they needed any advice or if the equipment failed. Third, they were provided with an instruction manual that included Frequently Asked Questions and troubleshooting tips.
Total scores from the four outcome measures were analysed using IBM SPSS Statistics.
Results
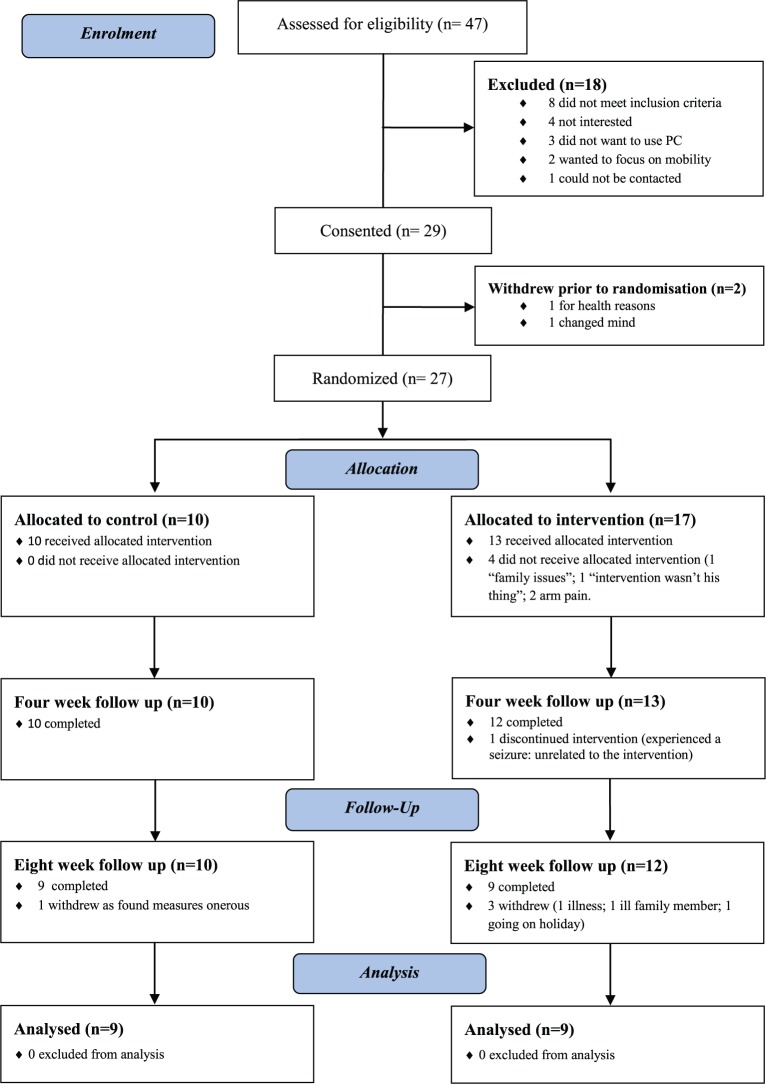
Over a 15 month period, only 47 patients were referred to the research team. During the recruitment period, combining the numbers for the three community services indicated that of new referrals an average of 49 patients a month was being referred to the three community services which became the sites for recruitment. Using a conservative estimate of 40%1 with persistent impairment in the affected arm, the potential pool of patients would have been approximately 274. This represents a referral rate of approximately 17%. Numbers followed up and reasons for dropouts are shown in Figure 1.
Figure 1.
CONSORT diagram.
Consent was obtained from 29 (62%) (see Figure 1) of whom 12 were referred by the Stroke Outreach Service, 16 from the Community Support Team and Early Supported Discharge and 1 from the outpatient rehabilitation service. Two withdrew prior to randomisation. The characteristics of those randomised are shown in Table 1. For those in the intervention group (n = 17) a significantly longer time had elapsed since their stroke than for those in the control group (n = 10). There was no significant difference between the groups on any of the outcome measures at randomisation.
Table 1.
Characteristics of randomised patients.
| Intervention (n=17) | Control (n=10) | |
|---|---|---|
| Mean age (SD) | 59 (12.03) | 63 (14.06) |
| Male/Female | 8/9 | 8/2 |
| Median time since stroke * (25th, 75th Percentiles) | 22 Weeks (16.00, 59.50) | 12 Weeks (7.75, 20.25) |
| Dominant side affected | 13 | 7 |
| Median wolf motor function test (seconds) at baseline (pre randomisation) (25th, 75th Percentiles) | 2.60 (1.65, 6.00) | 3.34 (1.90, 4.92) |
Mann Whitney indicated a difference significant at P<0.05.
Summarised data for outcome measures for those patients who went on to complete midpoint outcome measures are shown in Table 2. For each outcome measure, results are shown for two groups of patients: first those who completed outcome measures at all three time points and second for those who only completed baseline and midpoint outcome measures. Scores from the outcome measures were not always normally distributed, so the intervention group was compared with the control group on change from baseline to midpoint and final using the Mann Whitney test. Effect sizes have been estimated using r for nonparametric small samples. The only measures to show a significant difference were Wolf Grip strength at midpoint, Motor Activity Log Amount of Use at final and Motor Activity Log number of activities attempted at final with a greater improvement from baseline in the intervention group.
Table 2.
Outcome measures (median, minimum, maximum) for intervention and control at each time point. *Mann Whitney indicated significantly larger improvement in the intervention group than the control at P<0.05, ** significant at P<0.01. For full explanation see text.
| Outcome measures |
Baseline
|
Midpoint
|
Effect size (r) of baseline/ midpoint comparison |
Final
|
Effect size (r) of baseline/ final comparison | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||||
| Wolf MFT (secs) | Patients completing final outcome measures | 2.00 (1.49, 16.15) N = 9 |
2.72 (1.19, 8.84) N = 9 |
2.22 (1.00, 8.03) N = 9 |
2.28 (1.18, 6.22) N = 9 |
2.47 (1.06, 6.58) N = 9 |
2.19 (1.43, 5.62) N = 9 |
0.40 N = 18 |
|
| Patients who did not complete final outcome measures | 2.21 (1.49, 16.15) N = 12 |
2.60 (1.19, 8.84) N = 10 |
2.44 (1.00, 10.25) N = 12 |
2.17 (1.18, 6.22) N = 10 |
0.17 N = 22 |
||||
| Wolf Grip | Patients completing final outcome measures | 14.55 (6.33, 35.10) N = 8 |
12.77 (2.20, 47.27) N = 9 |
20.17 (5.83, 37.5) N = 9 |
12.23 (3.97, 34.70) N = 9 |
12.80 (2.30, 37.47) N = 9 |
12.53 (5.03, 29.40) N = 9 |
1.37 N = 18 |
|
| Patients who did not complete final outcome measures | 12.93 (1.93, 35.10) N = 11 |
14.25 (2.20, 47.27) N = 10 |
15.50 (2.23, 37.50) N = 12 |
13.50 (3.97, 34.70) N = 10 |
0.51* N = 21 |
||||
| NHPT (secs) Affected arm | Patients completing final outcome measures | 45.17 (40.16, 292.52) N = 8 |
45.66 (31.50, 136.41) N = 7 |
55.45 (31.28, 270.18) N = 8) |
43.81 (31.27, 109.60) N = 9 |
53.34 (36.93, 311.92) N = 8 |
37.39 (27.79, 120.85) N = 7 |
1.34 N = 15 |
|
| Patients who did not complete final outcome measures | 53.06 (26.68, 292.52) N = 11 |
46.44 (31.50, 136.41) N = 8 |
53.65 (25.45, 270.18) N = 11 |
43.12 (31.27, 109.60) N = 8 |
0.04 N = 19 |
||||
| MAL amount of use | Patients completing final outcome measures | 66.00 (21.00, 113.00) N = 9 |
69.00 (24.00, 150.00) N = 9 |
74.00 (34.00, 130.00) N = 9 |
83.00 (38.00, 145.00)) N = 9 |
76.00 (22.00, 135.00) N = 9 |
56.00 (18.00, 125.00) N = 9 |
2.26* N = 18 |
|
| Patients who did not complete final outcome measures | 69.00 (21.00, 140.00) N = 12 |
69.50 (24.00, 150.00) N = 10 |
81.50 (34.00, 149.00) N = 12 |
81.00 (38.00, 145.00) N = 10 |
−0.01 N = 22 |
||||
| MAL quality of movement | Patients completing final outcome measures | 54.00 (10.00, 86.00) N = 9 |
53.00 (16.00, 133.00) N = 9 |
61.00 (32.00, 117.00) N = 9 |
67.00 (27.00, 132.00) N = 9 |
74.00 (25.00, 125.00) N = 9 |
51.00 (9.00, 122.00) N = 9 |
1.68 N = 18 |
|
| Patients who did not complete final outcome measures | 60.00 (10.00, 130.00) N = 12 |
56.00 (16.00, 133.00) N = 10 |
69.00 (32.00, 145.00) N = 12 |
65.00 (27.00, 132.00) N = 10 |
0.20 N = 22 |
||||
| MAL activities attempted | Patients completing final outcome measures | 17.00 (8.00, 23.00) N = 9 |
16.00 (7.00, 30.00) N = 9 |
18.00 (13, 26) N = 9 |
19.00 (10.00, 29.00) N =9 |
20.00 (9.00, 27.00) N = 9 |
17.00 (6.00, 29.00) N = 9 |
2.50** N = 18 |
|
| Patients who did not complete final outcome measures | 18.00 (8.00, 28.00) N = 12 |
16.00 (7.00, 30.00) N = 10 |
20.00 (13.00, 30.00) N = 12 |
19.00 (10.00, 29.00) N = 10 |
−0.04 N = 22 |
||||
| NEADL | Patients completing final outcome measures | 38.00 (22.00, 59.00) N = 9 |
39.00 (15.00, 63.00) N = 9 |
41.00 (20.00, 57.00) N = 9 |
50 (13.00, 62.00) N = 9 |
39.00 (28.00, 57.00) N = 9 |
46.00 (11.00, 63.00) N = 9 |
1.06 N = 18 |
|
| Patients who did not complete final outcome measures | 39.00 (12.00, 63.00) N = 12 |
41.00 (15.00, 63.00) N = 10 |
42.00 (13.00, 63.00) N = 12 |
48.50 (13.00, 62.00) N = 10 |
0.09 N = 22 |
||||
Wolf MFT: Wolf Motor Function Test, NHPT: Nine Hole Peg Test, MAL: Motor Activity Log, NEADL: Nottingham Extended Activities of Daily Living.
Taking the Wolf Motor Function Test as the primary outcome measure, it is possible to calculate sample size for a comparison between the intervention and control groups on final outcome measures. To detect a difference of 1 second (the published Minimal Detectable Change based on a 95% confidence interval being 0.7 seconds)16 with a probability of 0.05 and 80% power (effect size 0.314) using a two tailed Mann Whitney test, 38 patients per group would be required. Taking into account the time it took to recruit 27 and a 67% retention rate at the collection of final outcome measures, 114 would need to be randomised. If it took 15 months to recruit 27, this would take approximately 63 months if recruited at the same rate.
Those in the intervention group received a total of 78 (median per patient = 4.0; minimum = 3.0, maximum = 14) visits from the research team in addition to those that were solely to collect outcome measures. Two patients (04, 13) received more than 10 visits in addition to those solely to collect outcome measures, but this could be explained by the need to resolve technical problems. A total of 92 hours 45 minutes (median per participant = 6 hours 10 minutes; minimum = 1 hour 20 minutes; maximum = 18 hours 10 minutes) contact time from the researchers was spent on delivering the intervention to patients. Table 3 breaks this down into different categories of researcher activity. Training in the correct rehabilitative use of the equipment and resolving technical issues accounted for a considerable proportion of the time spent in homes by the researchers.
Table 3.
Median (minimum and maximum) time in minutes per patient spent in different activities during home visits to the intervention group only. Other research (eg checking data log), other communications (eg giving advice on general rehabilitation).
|
Activities
|
||||
|---|---|---|---|---|
| Rehabilitation and training | Technical issues | Other research | Other communication | |
| Median | 230 | 45 | 30 | 65 |
| Minimum | 50 | 0 | 0 | 0 |
| Maximum | 540 | 430 | 50 | 135 |
Discussion
In terms of the first and third aims of the study, recruitment rates were so low that an impractically long recruitment period would be required to achieve the sample size indicated by the outcome measures. This was in spite of broad inclusion criteria and working closely with staff at the stroke unit and from three community teams. Approaching patients before discharge from hospital does give access to a larger group before they disperse to a wide range of services. However, at this early stage of their recovery it was difficult to determine which patients would recover enough movement to play the easiest level of the games and for staff to know which patients would meet inclusion criteria in terms of having no further intensive rehabilitation. Another challenge is that, on stroke units, rehabilitation studies compete with acute medical studies for recruitment.
An advantage of recruiting from the stroke unit is that there were dedicated staff to promote recruitment to studies. This was not the case for the community teams so more time was required from the research team for recruitment especially to make sure that referrals to the team did not exclude potential participants. Once data collection had started, time for liaising with the community teams was restricted.
There was a higher level of drop outs from the intervention group than in the control group where only one of those randomised was lost after completing midpoint outcome measures. Reasons given concerned factors that would have affected the use of the intervention but were not necessarily specific to this particular form of intervention. For example, physical ill health prevented its use or the patient was absent from home. Interviews with the intervention group after final outcome measures were collected27 also highlighted the role of ill health as well as competing commitments in using the intervention to the recommended level. However, both of these factors would affect any unsupervised, home based intervention for arm rehabilitation. Analysis of the interviews also suggested the possibility that the patients recruited were those who were more likely to be trying to return to their prestroke life, and attempts to return to work or other activities away from the home precluded the recommended level of use of the intervention. Obviously the reasons given to the research team may not have been the true reasons for dropping out and it is possible that the intervention itself may have been the reason for the high loss to the intervention group.
With such a small sample and considerable variation in outcome measures, it was only possible to detect differences on three outcome measures but these results give no reason to drop any of the outcome measures if planning a definitive trial. The variation is unsurprising given the deliberately wide inclusion criteria as during the development of the equipment13 it was only possible to gain a limited indication of which patients would benefit from the intervention. Although a small number of patients were unable to complete the Wolf Grip strength test and Nine Hole Peg Test at baseline this inability did not stop those in the intervention group from continuing with the intervention for the full eight weeks and one was able to complete the Wolf Grip strength test at midpoint data collection. However, the between group differences in change from baseline at final data collection detected by the Motor Activity Log suggest this is a useful addition to the battery of outcome measures and may indicate improvement before any improvements on tests of functional ability. Future research may indicate whether the Motor Activity Log might be mediating any improvement in functional ability in a home based self-directed intervention.
The intervention did require a considerable level of support from the research team. However, this is predictable given the complexities of delivering a novel intervention in a community setting and the fact that some of the patients had complex stroke pathology (cognitive issues, sensory disturbance) and thus demanded more support. The intervention was at prototype stage and the support required to deal with technical issues will diminish as the technology evolves. The team included three experienced members of staff supporting patients but in future, costs could be reduced if the initial assessment is carried out by experienced staff with trained support staff providing ongoing support.
The study suffered from limitations in terms of sample size, the difficulty ensuring that midpoint measures were carried out blind and in the low use of the intervention by some patients.27 However, the evaluation of home-based technology for rehabilitation post stroke poses challenges not seen in other evaluations. These need to be considered in the design of future studies.
In conclusion, this feasibility study found that recruitment rates were so low that an impractically long recruitment period would be required to achieve the sample size indicated by the outcome measures. In spite of considerable variation in outcome measures, a significantly greater change from baseline in the intervention group was found on the Wolf Grip strength at midpoint and two subscales of the final Motor Activity Log. The median number of visits from the research team to those in the intervention group was four with training in the correct rehabilitative use of the equipment and resolving technical issues accounting for the majority of the time spent in homes by the researchers. To achieve the required sample size indicated a definitive home-based trial would require additional strategies to boost recruitment rates and would have to include adequate resources for patient support.
Clinical messages.
To ensure satisfactory recruitment to community based trials, criteria given to those who are referring patients need to be very broad with the research team carrying out the final selection at the appropriate time .
Therapist support should be factored in to any home based, self-directed technology intervention.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Institute for Health Research
References
- 1. Intercollegiate Stroke Working Party. National clinical guideline for stroke, 4th edition. London: Royal College of Physicians, 2012. [Google Scholar]
- 2. Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? a systematic review and meta-analysis. PLoS One. 2014;9:e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of Robotics, Functional Electrical Stimulation, and Motor Learning Methods for Treatment of Persistent Upper Extremity Dysfunction After Stroke: A Randomized Controlled Trial Archives of Physical Medicine and Rehabilitation. 2015; 96:981–990. [DOI] [PubMed] [Google Scholar]
- 4. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. [DOI] [PubMed] [Google Scholar]
- 5. Laver K, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation: an abridged version of a Cochrane review. European journal of physical and rehabilitation medicine. European Journal of Physical and Rehabilitation Medicine 2015; 51:497–506. [PubMed] [Google Scholar]
- 6. Pietrzak E, Cotea C, Pullman S. Using Commercial Video Games for Upper Limb Stroke Rehabilitation: Is This the Way of the Future? Topics in Stroke Rehabilitation 2014;21:152–162. [DOI] [PubMed] [Google Scholar]
- 7. Saposnik G, Teasell R, Mamdani M, Hall J, McIlroy W, Cheung D, Thorpe KE, et al. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation: A pilot randomized clinical trial and proof of principle. Stroke 2010;41:1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adie K, Schofield C, Berrow M, Wingham J, Freeman J, Humfryes J, et al. Does the use of Nintendo Wii Sports™ improve arm function and is it acceptable to patients after stroke? Publication of the Protocol of the Trial of Wii™ in Stroke – TWIST International Journal of General Medicine 2014;7:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medical Research Council. A framework for development and evaluation of RCTs for complex interventions to improve health. Available at: www.mrc.ac.uk/documents/pdf/rcts-for-complex-interventions-to-improve-health/ (2000, accessed 18 February 2016).
- 10. Medical Research Council. Aids to the investigation of peripheral nerve injuries. London: HMSO, 1975. [Google Scholar]
- 11. Nudo RJ. Recovery after brain injury: mechanisms and principles. Frontiers in Human Neuroscience 2013; 7: Article ID 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woldag H, Stupka K, Hummelsheim H. Repetitive training of complex hand and arm movements with shaping is beneficial for motor improvement in patients after stroke. Journal of Rehabilitation Medicine 2010; 42:582–587. [DOI] [PubMed] [Google Scholar]
- 13. Standen PJ, Brown DJ, Battersby S, Walker M, Connell L, Richardson A, et al. A Study to evaluate a low cost virtual reality system for home based rehabilitation of the upper limb following stroke. In: Proceedings of the eighth International Conference on Disability, Virtual Reality and Associated Technologies (eds Sharkey PM, Sánchez J.), Viña del Mar/Valparaiso, Chile, 31 August – 2 September 2010, pp 139–146. [Google Scholar]
- 14. Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned non-use among chronic stroke and head-injured patients. Experimental Neurology 1989;104:125–132. [DOI] [PubMed] [Google Scholar]
- 15. Morris DM, Uswatte G, Crago JE, Cook EW, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Archives of Physical Medicine and Rehabilitation 2001;82: 750–755. [DOI] [PubMed] [Google Scholar]
- 16. Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL. Minimal Detectable Change Scores for the Wolf Motor Function Test. Neurorehabilitation and Neural Repair 2009; 23: 662–667 [DOI] [PubMed] [Google Scholar]
- 17. Whitall J, Savin DN, Harris-Love M, McCombe Waller S. Psychometric properties of a modified Wolf Motor Function test for people with mild and moderate upper-extremity hemiparesis. Archives of Physical Medicine and Rehabilitation 2006;87: 656–660. [DOI] [PubMed] [Google Scholar]
- 18. Kellor M, Frost J, Silberberg N, Iversent I, Cummings R. Hand strength and dexterity. American Journal of Occupational Therapy 1971;25:77–83. [PubMed] [Google Scholar]
- 19. Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabilitation and Neural Repair 2009; 23: 435–440. [DOI] [PubMed] [Google Scholar]
- 20. Parker VM, Wade DT, Langton Hewer R. Loss of arm function after stroke: measurement, frequency, and recovery. International Rehabilitation Medicine 1986; 8: 69–73. [DOI] [PubMed] [Google Scholar]
- 21. Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, et al. Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation 1993;74:347–354. [PubMed] [Google Scholar]
- 22. Van der Lee J, Beckerman H, Knol DL, de Vet HCW, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke 2004; 35: 1410–1414. [DOI] [PubMed] [Google Scholar]
- 23. Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke 2005; 36: 2493–2496. [DOI] [PubMed] [Google Scholar]
- 24. Nouri FM, Lincoln N. An extended activities of daily living scale for stroke patients. Clinical Rehabilitation 1987;1:301–305. [Google Scholar]
- 25. Wu C-Y, Chuang L-L, Lin K-C, Lee S-D, Hong W-H. Responsiveness, minimal detectable change, and minimal clinically important difference of the Nottingham Extended Activities of Daily Living scale in patients with improved performance after stroke rehabilitation. Archives of Physical Medicine and Rehabilitation 2011; 92:1281–1287. [DOI] [PubMed] [Google Scholar]
- 26. Wu CY, Chuang LL, Lin KC, Hong WH. Responsiveness and validity of two outcome measures of instrumental activities of daily living. Clinical Rehabilitation 2011; 26: 175–183. [DOI] [PubMed] [Google Scholar]
- 27. Standen PJ, Threapleton K, Connell L, Richardson A, Brown DJ, Battersby S, et al. Patients’ use of a home-based virtual reality system to provide rehabilitation of the upper limb following stroke. Physical Therapy 2015; 95:350–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.