Abstract
Lipopolysaccharide (LPS) might affect the central nervous system by causing neuroinflammation, which subsequently leads to brain damage and dysfunction. In this study, we evaluated the role of nod-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome activation in long-term behavioral alterations of 8-week-old male C57BL/6 mice injected intraperitoneally with LPS (5 mg/kg). At different time points after injection, we assessed locomotor function with a 24-point neurologic deficit scoring system and the rotarod test; assessed recognition memory with the novel object recognition test; and assessed emotional abnormality (anhedonia and behavioral despair) with the tail suspension test, forced swim test, and sucrose preference test. We also assessed protein expression of NLRP3, apoptosis-associated speck-like protein (ASC), and caspase-1 p10 in hippocampus by Western blotting; measured levels of interleukin (IL)-1β, IL-18, tumor necrosis factor α (TNFα), and IL-10 in hippocampus; measured TNFα and IL-1β in serum by ELISA; and evaluated microglial activity in hippocampus by Iba1 immunofluorescence. We found that LPS-injected mice displayed long-term depression-like behaviors and recognition memory deficit; elevated expression of NLRP3, ASC, and caspase-1 p10; increased levels of IL-1β, IL-18, and TNFα; decreased levels of IL-10; and increased microglial activation. These effects were blocked by the NLRP3 inflammasome inhibitor Ac-Tyr-Val-Ala-Asp-chloromethylketone. The results demonstrate proof of concept that NLRP3 inflammasome activation contributes to long-term behavioral alterations in LPS-exposed mice, probably through enhanced inflammation, and that NLRP3 inflammasome inhibition might alleviate peripheral and brain inflammation and thereby ameliorate long-term behavioral alterations in LPS-exposed mice.
Keywords: depression, NLRP3 inflammasome, recognition memory, LPS, tail suspension test, forced swim test
INTRODUCTION
Intraperitoneal administration of bacterial lipopolysaccharide (LPS) is known to cause both local and systemic inflammatory responses (Kluger, 1991). LPS might affect the central nervous system by causing neuroinflammation, which subsequently leads to brain damage and dysfunction (He et al., 2016). In one study, authors reported that LPS produced long-lasting increases in depressive- and anxiety-like behaviors in mice (Anderson et al., 2015). Another study showed that 2-day-old male mice injected with LPS exhibited impaired object recognition memory (Comim et al., 2015). However, the underlying mechanisms of depression and recognition memory deficit after LPS injection are not clear.
Nod-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome is a cytoplasmic multiprotein complex of the innate immune system that can trigger a series of immune-inflammatory reactions (Shao et al., 2015). Once activated, NLRP3 recruits the adapter apoptosis-related speck-like protein (ASC), which contains a caspase recruitment domain and pro-caspase-1. The pro-caspase-1, in turn, proteolytically cleaves pro-interleukin (IL)-1β and pro-IL-18 into their active forms, thus aggravating the inflammatory reaction (Ozaki et al., 2015). Increasing evidence indicates that inflammation may play a crucial role in the pathophysiology of anxiety, major depression, and recognition memory deficit (Bayramgurler et al., 2013; Krugel et al., 2013; Comim et al., 2015; Li et al., 2016). Furthermore, several studies have suggested that NLRP3 inflammasome contributes to the development of depressive disorder and memory deficit (Zhang et al., 2014; Alcocer-Gomez et al., 2015; Li et al., 2016; Sui et al., 2016). However, whether NLRP3 inflammasome participates in long-term depressive disorder and recognition memory deficit in mice injected with LPS is not clear. In the current study, we investigated the effect of NLRP3 inflammasome inhibitor Ac-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK). Ac-YVAD-CMK, or YVAD for short, is an irreversible, cell-permeable, caspase-1-specific inhibitor that blocks NLRP3 inflammasome assembly by binding specifically to caspase-1 subunits (Rozman-Pungercar et al., 2003; Zhang et al., 2014). The goal of this study was to determine whether injection of LPS produces long-term brain alterations in mice and whether NLRP3 inflammasome activation contributes to the behavioral deficits.
EXPERIMENTAL PROCEDURES
Animals
Eight-week-old male C57BL/6 mice (20–25 g) were used for all experiments. This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee (protocol number: MO12M111). The mice were kept in the animal room at 22 ± 2 °C with a 12:12-h light–dark cycle, and were given standard food and water except during the experiments. In total, 121 mice were used.
Experimental groups
After 2 weeks of adaptation to the new environment, the mice were randomly assigned to control group, YVAD group, LPS group, and YVAD + LPS group based on our established protocol for group randomization (Chang et al., 2014; Wang et al., 2015b; Wu et al., 2015). The mice in the control group were injected intraperitoneally with phosphate-buffered saline (PBS) at 10 mL/kg body weight. The mice in the YVAD group were injected intraperitoneally with the NLRP3 inflammasome inhibitor Ac-YVAD-CMK (8 mg/kg body weight, Enzo Life Sciences, Inc., Farmingdale, NY, USA). The mice in the LPS group were injected intraperitoneally with LPS (5 mg/kg body weight, serotype 055:B5, Sigma, St. Louis, MO, USA) (Anderson et al., 2015). The mice in the YVAD + LPS group were administered Ac-YVAD-CMK (8 mg/kg body weight) 30 min before LPS (5 mg/kg body weight) injection. Both Ac-YVAD-CMK and LPS were freshly prepared on the treatment day and administered intraperitoneally in a final injection volume of 0.1–0.15 mL. Mice were killed on days 3 and 29 after LPS injection. Results were analyzed in a blinded manner. Based on published studies and our preliminary results, we chose days 3, 28, and 29 after LPS injection to evaluate biochemistry and behavioral parameters (Anderson et al., 2015) (Fig. 1).
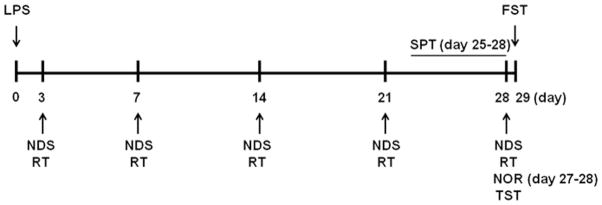
Fig. 1.
Time scheme of experimental procedures. Detailed timeline outlining the sequence of all behavioral tests and experimental procedures, beginning on the day of LPS injection (day 0). NDS, neurologic deficit score; RT, rotarod test; TST, tail suspension test; FST, forced swim test; NOR, novel object recognition test (day 27 for adaptation to environment, day 28 for formal testing); SPT, sucrose preference test (experimental procedure from day 25 to day 28).
Behavioral measurements
All behavioral tests were conducted during the light cycle phase in enclosed behavior rooms and were evaluated and analyzed by an observer blinded to the study. The same mice were used for locomotor, memory, and emotional function assessments and therefore underwent multiple behavioral tests (Fig. 1).
An experimenter evaluated mice with a 24-point neurologic deficit scoring system on days 3, 7, 14, 21, and 28 after LPS injection according to the following six neurologic tests: body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling (Zhu et al., 2014). Each test was graded from 0 to 4, establishing a maximum deficit score of 24.
The rotarod test was used to evaluate motor coordination and balance in each mouse on days 3, 7, 14, 21, and 28 after LPS injection. An automated rotarod device was used (BW-YLS-4C, Shanghai, China). For habituation/training, mice were placed on a rotating horizontal rod and trained first at minimum speed for 5 min the day before the test day. After the mice were habituated, the speed was accelerated to 40 rounds/min, and the average latency to falling was documented (Wang et al., 2015a).
The novel object recognition test was performed as described previously (Bevins and Besheer, 2006; Zhu et al., 2014). The mice were put in a cage (47 × 26 × 20 cm) and allowed to explore the open field for 5 min on day 27 after LPS injection for adaptation to the environment. On day 28 after LPS injection, two identical novel objects (green cubes, 4 × 4 × 3 cm) were placed in the arena, and mice were allowed to explore them for 10 min. One hour later, the mice explored a novel object (white ball, 5 cm in diameter) and a familiar green cube for 5 min. A camera recorded the behaviors of each mouse during the test, and the total time spent exploring the new and old object was calculated. A discrimination index (total time spent with new object/total time devoted to exploration of objects) was calculated for each experimental group. Exploration of an object was defined as any direct contact with mouth, nose, or paw, or attention directed at the object with the nose <0.5 cm away. Exploration did not include contacts that were judged to be accidental, such as standing, sitting, or leaning on the object (Besheer and Bevins, 2003).
The tail suspension test was carried out on day 28 after LPS injection. According to the protocol described previously (Bai et al., 2001; Mineur et al., 2006; Can et al., 2012; Zhu et al., 2014), mice were suspended by their tails at the edge of a shelf. A camera recorded the movement of mice for a period of 6 min, and the duration of mobility determined. The total mobility time was subtracted from the 360 s of test time to provide the immobility time. A mouse was considered immobile only when it hung passively and completely motionless.
We tested mice in the forced swim test on day 29 after LPS injection to avoid influence from the tail suspension test. As described previously (Hirani, 2002; Cervo et al., 2005; Can et al., 2012; Zhu et al., 2014), mice were placed individually in cylindrical tanks containing 10 cm of water at 24 ± 1 °C. A camera recorded the movement of mice for 6 min, and the duration of mobility during the last 4 min was determined. The total mobility time was subtracted from the 240 s of test time to provide the immobility time. A mouse was judged to be immobile when it remained floating in an upright position, making only small movements to keep its head above water (Renard et al., 2004).
The sucrose preference test was used to determine anhedonia (Wang et al., 2008). On day 25 after LPS injection, each mouse was housed alone in a cage and given access to two tubes, one containing water and the other containing a 1% sucrose solution. The tubes were weighed on day 25, and their positions in the cage were switched every day. The tubes were weighed again on day 28, and the amount of water and sucrose solution drunk during the 3-day period was calculated as follows: sucrose preference = sucrose consumption (g)/[(water consumption (g) + sucrose consumption (g)]. A reduction in sucrose intake was considered an index of anhedonia (Wang et al., 2008; De Bundel et al., 2013).
Western blot analysis
As published previously (Zhang et al., 2014; Li et al., 2015; Zhao et al., 2015), brain tissue from hippocampus was collected on day 3, homogenized in 20 mM phosphate buffer (pH 7.4) containing 0.5 mM butylated hydroxytoluene, and centrifuged at 1,500g for 15 min at 4 °C. Cytoplasmic proteins in the tissue homogenate were extracted with cytoplasmic extraction reagents according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL, USA). The BCA protein assay kit was used to determine protein concentration. Subsequently, the extracted proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was incubated with blocking solution containing 5% skim milk for more than 1 h at room temperature and probed by primary antibodies against NLRP3, ASC, and caspase-1 p10. The primary antibodies and dilutions used were as follows: NLRP3 (1:500; NBP1-77080, Novus, Littleton, CO, USA), ASC (1:500; 04–147, Millipore, Temecula, CA, USA), caspase-1 p10 (1:200; Santa Cruz Biotechnology, Dallas, TX, USA), and β-actin (1:1000; MAB1445, MultiSciences Biotech, Hangzhou, China). After being washed, the membrane was incubated with secondary goat anti-rabbit antibody conjugated to horseradish peroxidase for 1 h at room temperature. Immunoreactive proteins were visualized by chemiluminescence with the Western blot detection system (Amersham Biosciences, Piscataway, NJ, USA).
Enzyme-linked immunosorbent assay (ELISA)
Brain tissues from hippocampus and serum were obtained on day 3 and centrifuged. We used ELISA kits to measure mouse tumor necrosis factor α (TNFα) (CUSABIO, China), IL-10 (CUSABIO), IL-18 (Bender Medsystems, San Diego, CA, USA), and IL-1β (R&D Systems, Minneapolis, MN, USA). Every sample was assayed in duplicate, and assays were performed as recommended by the manufacturers (Chang et al., 2014).
Immunofluorescence staining
Immunofluorescence staining was carried out as described elsewhere (Wang and Tsirka, 2005; Wu et al., 2011). The expression of Iba1 in hippocampus on day 3 was measured in brain sections from a cohort of mice (n = 6/group). The primary antibody used was rabbit anti-Iba1 (microglia marker; 1:500; Wako Chemicals, Richmond, VA, USA). Sections were then incubated with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (1:1000; Molecular Probes, Eugene, OR, USA). Stained sections were examined under a fluorescence microscope (Eclipse TE2000-E, Nikon, Tokyo, Japan). The numbers of immunoreactive cells over a 20× microscopic field from 12 locations per mouse (4 fields per section × 3 sections per mouse) were averaged and expressed as positive cells per square millimeter. Sections were analyzed by an observer blinded to the experimental cohort.
Statistical analysis
All data are expressed as mean ± SD. Data were analyzed by one-way ANOVA with a Newman–Keuls post hoc analysis to correct for multiple comparisons. Group differences were considered statistically significant at p < 0.05.
RESULTS
The mortality of mice was 5 of 33 (15.2%) in the LPS group and 4 of 32 (12.5%) in the YVAD + LPS group. No animals died in the control group or YVAD group (0 of 56).
Ac-YVAD-CMK improves LPS-induced long-term behavioral alterations in mice
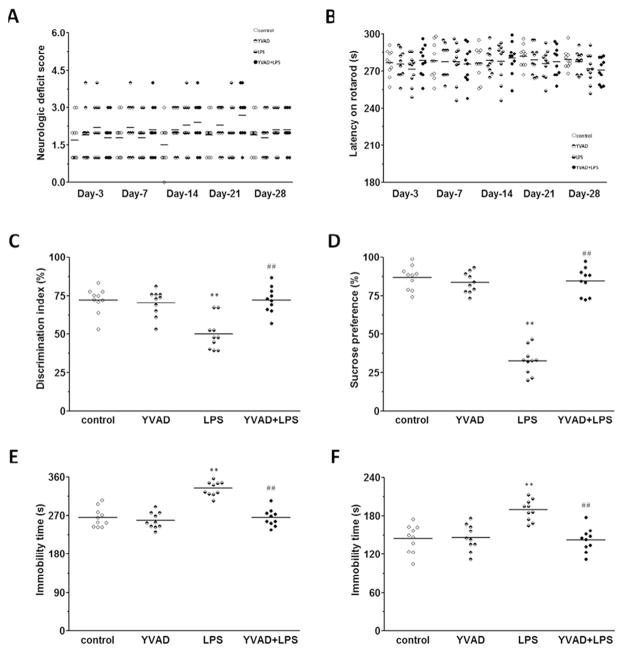
Locomotor deficits were measured by a 24-point neurologic scoring system and rotarod test on days 3, 7, 14, 21, and 28 after LPS injection. No differences were noted among control, YVAD, LPS, and YVAD +LPS groups at any time point (n = 10 mice/group, p>0.05; Fig. 2A, B).
Fig. 2.
NLRP3 inflammasome inhibitor Ac-YVAD-CMK attenuates LPS-induced long-term depressive-like behaviors and memory deficit. (A, B) Results of the 24-point neurologic scoring system and rotarod test did not differ significantly among the control, YVAD, LPS, and YVAD + LPS groups at any time point (n = 10 mice/group, p>0.05). (C) In the novel object recognition test, mice in the LPS group had a significantly lower discrimination index than did animals in the control and YVAD groups, but in YVAD + LPS mice, the discrimination index was increased to the level of control (n = 10 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group). (D) Mice in the LPS group had significantly less sucrose preference than did animals in the control and YVAD groups, but mice in the YVAD + LPS group had significantly greater sucrose preference than did animals in the LPS group (n = 10 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group). (E–F) Mice in the LPS group had significantly longer immobility times than did animals in the control and YVAD groups in the tail suspension test (E) and forced swim test (F). However, in both tests, mice in the YVAD + LPS group had significantly shorter immobility time than did animals in the LPS group (n = 10 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group).
On day 28, during the novel object recognition test, mice in the LPS group spent significantly less time with the new object than did animals in the control and YVAD groups, and the discrimination index was significantly smaller in the LPS group than in the control and YVAD groups (n = 10 mice/group, p < 0.01; Fig. 2C).
On days 25–28, mice in the LPS group showed significantly less preference for sucrose than did animals in the control and YVAD groups (n = 10 mice/group, p < 0.01; Fig. 2D). Similarly mice in the LPS group had significantly longer immobility times in the tail suspension test and forced swim test than did animals in the control and YVAD groups (n = 10 mice/group, p < 0.01; Fig. 2E–F).
However, LPS mice treated with Ac-YVAD-CMK had a significantly greater discrimination index and sucrose preference and shorter immobility times than did mice in the LPS group (all p < 0.01; Fig. 2C–F).
Ac-YVAD-CMK inhibits expression of the NLRP3 inflammasome
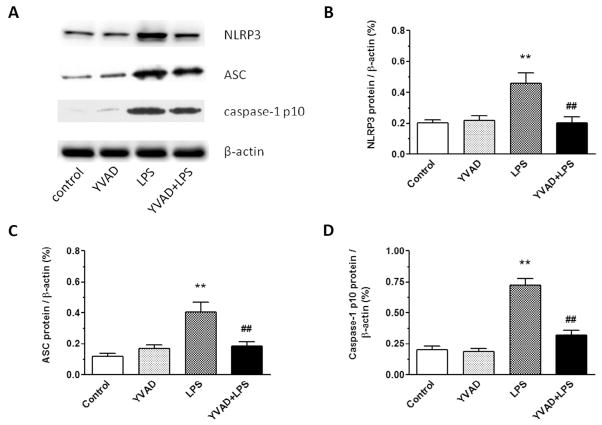
On day 3, the expression levels of NLRP3, ASC, and caspase-1 p10 were significantly higher in hippocampus of the LPS group than in that of the control group and YVAD group. Ac-YVAD-CMK treatment inhibited expression of NLRP3, ASC, and caspase-1 p10 in hippocampus (n = 6 mice/group, p < 0.01; Fig. 3A–D).
Fig. 3.
NLRP3 inflammasome inhibitor Ac-YVAD-CMK reduces expression of NLRP3 inflammasome in mice on day 3 after LPS injection. (A) Western blotting was used to determine hippocampal protein levels of NLRP3, ASC, and caspase-1 p10. (B–D) Protein expression levels of NLRP3, ASC, and caspase-1 p10 increased significantly in the hippocampus of LPS-exposed mice, but were reduced to near baseline in the LPS mice treated with Ac-YVAD-CMK (n = 6 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group). Data are presented as mean ± SD.
Ac-YVAD-CMK blocks the effect of LPS on IL-1β, IL-18, and TNFα in hippocampus and IL-1β and TNFα in serum
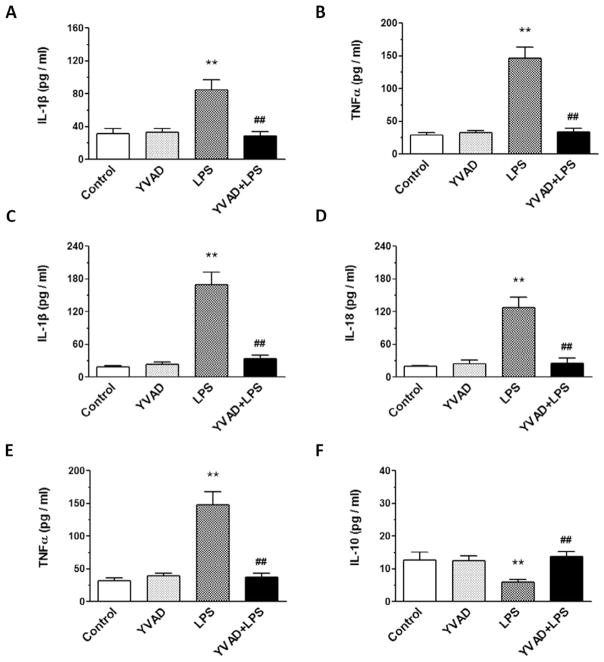
On day 3, the levels of IL-1β, IL-18, and TNFα in hippocampus and the levels of IL-1β and TNFα in serum were significantly greater in the LPS group than in the control and YVAD groups, but the level of IL-10 in hippocampus was significantly lower in the LPS group than in the control and YVAD groups. Treatment with Ac-YVAD-CMK prevented the increase in serum levels of IL-1β and TNFα, the increase in hippocampal levels of IL-1β, IL-18, and TNFα, and the decrease in hippocampal IL-10 in the YVAD + LPS group (n = 6 mice/group, p < 0.01; Fig. 4A–F).
Fig. 4.
NLRP3 inflammasome inhibitor Ac-YVAD-CMK prevents LPS-induced changes in IL-1β, TNFα, IL-18, TNFα, and IL-10. (A–E) ELISA was used to determine the levels of IL-1β and TNFα in serum (A, B) and IL-1β, IL-18, and TNFα in hippocampus (C–E) on day 3 after LPS injection. IL-1β, IL-18, and TNFα levels were significantly elevated in the LPS group, but not in LPS mice pretreated with Ac-YVAD-CMK (n = 6 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group). (F) ELISA showed that the level of IL-10 in hippocampus on day 3 after LPS injection was significantly decreased in the LPS group, but reversed in the LPS mice pretreated with Ac-YVAD-CMK (n = 6 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group). Data are presented as mean ± SD.
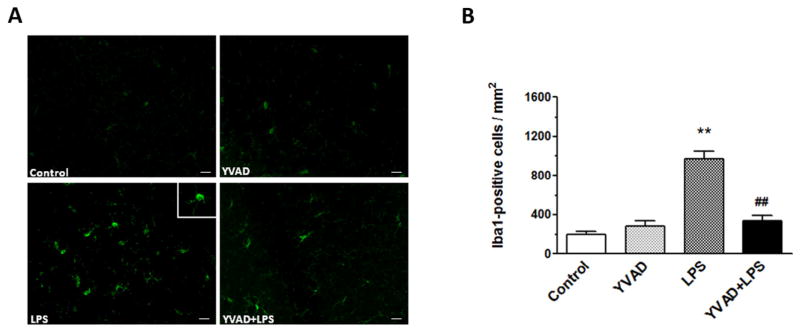
Ac-YVAD-CMK inhibits microglial activation in hippocampus
Microglial activation plays an important role in the pathogenesis of brain injury (Deng et al., 2013). On day 3, activated microglia/macrophages were evident in the hippocampus of the LPS group (Fig. 5A). Morphologic criteria and a cell body diameter cutoff of 7.5 μm were used to define microglia/macrophage activation (Wu et al., 2010b). Quantification analysis confirmed that significantly more activated microglia/macrophages were present in the hippocampus of the LPS group than in that of mice in control and YVAD groups. Additionally, significantly fewer Iba1-positive cells were present in the hippocampus of theYVAD + LPS group than in hippocampus of the LPS group (n = 6 mice/group, p < 0.01; Fig. 5B).
Fig. 5.
NLRP3 inflammasome inhibitor Ac-YVAD-CMK decreases microglial activation. (A) Fluorescent image shows Iba1-positive cells in the hippocampus. (B) Quantification analysis showed that LPS mice had significantly more Iba1-positive microglia in the hippocampus than did the control and YVAD groups. The number of activated, Iba1-positive microglia was significantly less in the Ac-YVAD-CMK-treated LPS mice than in the LPS-only mice (n = 6 mice/group, **p < 0.01 vs. control and YVAD groups, ##p < 0.01 vs. LPS group). Data are presented as mean ± SD. Scale bar = 40 μm.
DISCUSSION
Survivors of severe infection have a high prevalence of substantial depressive symptoms and memory deficit, but beginning interventions before hospital discharge may improve functional outcomes (Davydow et al., 2013; Calsavara et al., 2015). In the present study, we found that LPS can cause long-term depressive-like behaviors and memory deficit in mice, as evidenced by results from the tail suspension test, the forced swim test, the sucrose preference test, and the novel object recognition test. However, no difference was present in neurologic score or rotarod performance among control, YVAD, LPS, and YVAD + LPS groups. Additionally, expression levels of NLRP3 inflammasome, ASC, and caspase-1 p10 in the hippocampus, levels of IL-1β and TNFα in serum, and levels of IL-1β, IL-18, and TNFα in the hippocampus were all significantly elevated in the mice on day 3 after LPS injection, whereas hippocampal IL-10 was significantly decreased at that time point. Microglial activation was observed in the same brain regions on day 3. We found that NLRP3 inflammasome inhibitor Ac-YVAD-CMK reduced LPS-induced long-term depressive-like behaviors and memory deficit while normalizing the expression of NLRP3 inflammasome, ASC, caspase-1 p10, IL-1β, IL-18, and TNFα and inhibiting the activation of microglia.
Inflammasomes are typical pattern recognition receptors and play a crucial role in inflammation (Shao et al., 2015). One study found that LPS engaged the toll-like receptor 4 (TLR4) pathway to enhance NLRP3 inflammasome and pro-IL-1β expression in human dental pulp fibroblasts (Zhang et al., 2015). NLRP3 inflammasome, a representative inflammasome, is a multi-protein platform that is characterized by its ability to activate pro-caspase-1, which in turn proteolytically cleaves pro-IL-1β and pro-IL-18 into their active forms (Schroder and Tschopp, 2010; Chen and Nunez, 2011; Tong et al., 2015). IL-1β and IL-18 can activate immunerelated cells, such as monocytes, to produce the corresponding immune effects, which in turn release a large number of inflammatory cytokines and aggravate the inflammatory response (Ali et al., 2015; Inoue et al., 2015). The results of a meta-analysis showed that depression is accompanied by activation of the inflammatory response (Dowlati et al., 2010), and a review reported that the inflammatory response is one of the key factors in development of depression (Maes, 2011). Another study revealed a close relation between memory deficit and inflammation (Imamura et al., 2011; Jin et al., 2016). A few experimental studies have indicated that NLRP3 inflammasome activation in mononuclear blood cells is associated with depression, and could be a new target for depressive disorders (Alcocer-Gómez et al., 2014; Alcocer-Gomez and Cordero, 2014; Zhang et al., 2014). In our study, the mice injected with LPS exhibited elevated serum levels of IL-1β and TNFα, which may have contributed to the long-term behavioral alterations. However, we cannot be certain whether the NLRP3 inflammasome contributed to these long-term behavioral alterations. One prior study reported that NLRP3 inflammasome in brain was involved in LPS-induced depressive-like behaviors in mice, and another showed that resveratrol improved the spatial memory of mice with cecal ligation and puncture and inhibited the NLRP3/IL-1β axis in microglia (Zhang et al., 2014; Sui et al., 2016). In our study, we showed that LPS can cause long-term depressive-like behaviors and memory deficit in mice, results that are consistent with those of previous studies (Anderson et al., 2015; Sui et al., 2016). Additionally, we found that in brain tissue, the expression of NLRP3 inflammasome and the levels of IL-1β, IL-18, and TNFα were significantly increased in mice on day 3 after LPS injection, indicating that NLRP3 inflammasome was activated. The increases in IL-1β, IL-18, and TNFα and the decrease in IL-10 could aggravate the inflammatory response, as IL-10 is an anti-inflammatory cytokine that can inhibit the production of inflammatory cytokines. Lynch et al. (2004) showed that administration of recombinant IL-10 to rats before peripheral injection of LPS prevented the LPS-induced depression-like behavior. All of these results suggest that an imbalance in pro- and anti-inflammatory responses may contribute to the development of depressive-like behaviors and memory deficit in mice administered LPS.
According to a recent study, some forms of depression can be considered a microglial disease (Yirmiya et al., 2015). Microglial activation and brain inflammatory cytokines participate in depression and memory deficit (Miller et al., 2009; Kreisel et al., 2014; Sui et al., 2016). In a study by Anderson et al. (2015), the authors report that mice injected with LPS exhibited long-term depressive-like behaviors and that microglial activation might have been a contributory factor. Another study reported that microglia were the primary contributor to chronic, unpredictable, mild, stress-induced prefrontal cortex NLRP3 inflammasome activation in rats (Pan et al., 2014). Others have shown that inhibition of the NLRP3/IL-1β axis in microglia improves spatial memory in mice that have undergone cecal ligation and puncture (Sui et al., 2016). In our study, we also found that microglia were activated in the hippocampus of mice on day 3 after LPS injection. These results indicate that NLRP3 inflammasome activation, together with increased inflammatory markers and microglial activation, might contribute synergistically to the development of long-term depressive-like behaviors and memory deficit.
To further investigate the potential role of NLRP3 inflammasome activation in this process, we used Ac-YVAD-CMK (a known selective caspase-1 inhibitor) as a specific and potent inflammasome inhibitor. LPS enhances peripheral NLRP3 inflammasome expression, which, in turn, aggravates the peripheral inflammatory response (Inoue et al., 2015; Zhang et al., 2015). LPS alone or peripheral inflammatory factors induced by LPS can trigger NLRP3 inflammasome activation in the central nervous system and may cause long-term behavioral alterations (Maes, 2011; Jin et al., 2016; Liu et al., 2016; Shi et al., 2016; Wang et al., 2016). Inflammasome activation is an important step in the development of systemic and brain inflammation. It has been reported that administration of Ac-YVAD-CMK before LPS will inhibit the systemic inflammatory response (Guenther et al., 2000; Wu et al., 2010a; Fang et al., 2016; Zhang et al., 2014). In our study, wefound that Ac-YVAD-CMK inhibited NLRP3 inflammasome activation, alleviated peripheral and brain inflammation, and ameliorated long-term behavioral alterations in LPS-injected mice.
Our study does have limitations. We did not test female mice because male and female animals may differ in performance of behavioral tests relevant to depression and memory. Wewill confirm the findings of this study in female mice in the future.
CONCLUSIONS
We demonstrated proof of concept that mice subjected to LPS display long-term depressive-like behaviors and memory deficit, which might result from neuroinflammation, and NLRP3 inflammasome activation in particular, and that these negative outcomes can be attenuated by inhibition of NLRP3 inflammasome activation.
Acknowledgments
We thank Da-Qian Zhan for help with brain slicing and Jie Chen for help with blind analysis of immunofluorescence and behavioral tests. This study was supported in part by National Institutes of Health grants R01NS078026 and R01AT007317 and by a “Stimulating and Advancing ACCM Research (StAAR)” grant from the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medicine. We thank Claire Levine and Jiarui Wang for assistance with the manuscript.
Abbreviations
- ASC
apoptosis-associated speck-like protein
- ELISA
enzyme-linked immunosorbent assay
- IL
interleukin
- LPS
lipopolysaccharide
- NLRP3
nod-like receptor pyrin domain-containing protein 3
- TNFα
tumor necrosis factor α
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Wei Zhu performed the experiments and wrote the manuscript. Feng-Sheng Cao and Qing Lu assisted in data acquisition, figure preparation, and manuscript writing. Hua-Weng Chen and Jie-Ru Wan assisted in data acquisition and analysis of behavioral tests. Jun Feng assisted in study design. Jian Wang conceived, designed, and supervised the project, and provided financial support.
References
- Alcocer-Gomez E, Cordero MD. NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci Ther. 2014;20:294–295. doi: 10.1111/cns.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, Cordero MD. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, Ryffel B, Apetoh L, Ghiringhelli F, Bullon P, et al. Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9408-7. [DOI] [PubMed] [Google Scholar]
- Ali A, Na M, Svensson MN, Magnusson M, Welin A, Schwarze JC, Mohammad M, Josefsson E, Pullerits R, Jin T. IL-1 receptor antagonist treatment aggravates staphylococcal septic arthritis and sepsis in mice. PLoS One. 2015;10:e0131645. doi: 10.1371/journal.pone.0131645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ST, Commins S, Moynagh PN, Coogan AN. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav Immun. 2015;43C:98–109. doi: 10.1016/j.bbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Bayramgurler D, Karson A, Ozer C, Utkan T. Effects of long-term etanercept treatment on anxiety- and depression-like neurobehaviors in rats. Physiol Behav. 2013;119:145–148. doi: 10.1016/j.physbeh.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Besheer J, Bevins RA. Impact of nicotine withdrawal on novelty reward and related behaviors. Behav Neurosci. 2003;117:327–340. doi: 10.1037/0735-7044.117.2.327. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Calsavara AC, Soriani FM, Vieira LQ, Costa PA, Rachid MA, Teixeira AL. TNFR1 absence protects against memory deficit induced by sepsis possibly through over-expression of hippocampal BDNF. Metab Brain Dis. 2015;30:669–678. doi: 10.1007/s11011-014-9610-8. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012;59:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Canetta A, Calcagno E, Burbassi S, Sacchetti G, Caccia S, Fracasso C, Albani D, Forloni G, Invernizzi RW. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Cho S, Wang J. (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol. 2014;1:258–271. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology. 2011;141:1986–1999. doi: 10.1053/j.gastro.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comim CM, Bussmann RM, Simao SR, Ventura L, Freiberger V, Patricio JJ, Palmas D, Mendonca BP, Cassol OJ, Jr, Quevedo J. Experimental neonatal sepsis causes long-term cognitive impairment. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9495-5. [DOI] [PubMed] [Google Scholar]
- Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatric Psychiatry. 2013;21:887–897. doi: 10.1016/j.jagp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bundel D, Gangarossa G, Biever A, Bonnefont X, Valjent E. Cognitive dysfunction, elevated anxiety, and reduced cocaine response in circadian clock-deficient cryptochrome knockout mice. Front Behav Neurosci. 2013;7:152. doi: 10.3389/fnbeh.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YY, Fang M, Zhu GF, Zhou Y, Zeng HK. Role of microglia in the pathogenesis of sepsis-associated encephalopathy. CNS Neurol Disord Drug Targets. 2013;12:720–725. doi: 10.2174/18715273113126660178. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Fang Z, Liang W, Jun JW, Peng FL, Xing TW, Zhao FX. The caspase-1 inhibitor AC-YVAD-CMK attenuates acute gastric injury in mice: involvement of silencing NLRP3 inflammasome activities. Sci Rep. 2016;6:24166. doi: 10.1038/srep24166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther M, Guido G, Thomas H, Thomas L, Cecilia CZ, Sebastian AB, Heribert B, Lewis FN, Arnulf HH. Caspase-1-inhibitor ac-YVAD-cmk reduces LPS-lethality in rats without affecting haematology or cytokine responses. Br J Pharmacol. 2000;131:383–386. doi: 10.1038/sj.bjp.0703629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Geng T, Chen P, Wang M, Hu J, Kang L, Song W, Tang H. NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci Rep. 2016;6:27711. doi: 10.1038/srep27711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani K. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3alpha-hydroxy-5alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Wang H, Matsumoto N, Muroya T, Shimazaki J, Ogura H, Shimazu T. Interleukin-1beta causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience. 2011;187:63–69. doi: 10.1016/j.neuroscience.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Inoue T, Aoyama-Ishikawa M, Kamoshida S, Nishino S, Sasano M, Oka N, Yamashita H, Kai M, Nakao A, Kotani J, et al. Endogenous interleukin 18 regulates testicular germ cell apoptosis during endotoxemia. Reproduction. 2015;150:105–114. doi: 10.1530/REP-14-0427. [DOI] [PubMed] [Google Scholar]
- Jin G, Yin S, Yang Z, Zou D, Zhang Z, Li X, Sun Y, Zhu Q. Silibinin rescues learning and memory deficits by attenuating microglia activation and preventing neuroinflammatory reactions in SAMP8 mice. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Krugel U, Fischer J, Radicke S, Sack U, Himmerich H. Antidepressant effects of TNF-alpha blockade in an animal model of depression. J Psychiatr Res. 2013;47:611–616. doi: 10.1016/j.jpsychires.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu XL, Zhao D, Pan LN, Huang CW, Guo LJ, Lu Q, Wang J. TLR3 ligand Poly IC attenuates reactive astrogliosis and improves recovery of rats after focal cerebral ischemia. CNS Neurosci Ther. 2015;21:905–913. doi: 10.1111/cns.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wang X, Qin T, Qu R, Ma S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1beta production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res. 2016;296:318–325. doi: 10.1016/j.bbr.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Liu X, Nemeth DP, Tarr AJ, Belevych N, Syed ZW, Wang Y, Ismail AS, Reed NS, Sheridan JF, Yajnik AR, et al. Euflammation attenuates peripheral inflammation-induced neuroinflammation and mitigates immune-to-brain signaling. Brain Behav Immun. 2016;54:140–148. doi: 10.1016/j.bbi.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10–a role for IL-1 beta? J Neurochem. 2004;88:635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflammation Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Renard CE, Dailly E, Nic Dhonnchadha BA, Hascoet M, Bourin M. Is dopamine a limiting factor of the antidepressant-like effect in the mouse forced swimming test? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1255–1259. doi: 10.1016/j.pnpbp.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Rozman-Pungercar J, Kopitar-Jerala N, Bogyo M, Turk D, Vasiljeva O, Stefe I, Vandenabeele P, Brömme D, Puizdar V, Fonović M, et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 2003;10:881–888. doi: 10.1038/sj.cdd.4401247. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Ren H, Huang Z, Peng Y, He B, Yao X, Yuan TF, Su H. Fish oil prevents lipopolysaccharide-induced depressive-like behavior by inhibiting neuroinflammation. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0212-9. [DOI] [PubMed] [Google Scholar]
- Sui DM, Xie Q, Yi WJ, Gupta S, Yu XY, Li JB, Wang J, Wang JF, Deng XM. Resveratrol protects against sepsis-associated encephalopathy and inhibits the NLRP3/IL-1beta axis in microglia. Mediators Inflamm. 2016;2016:1045657. doi: 10.1155/2016/1045657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Ding ZH, Zhan FX, Cai L, Yin X, Ling JL, Ye JJ, Hou SY, Lu Z, Wang ZH, et al. The NLRP3 inflammasome and stroke. Int J Clin Exp Med. 2015;8:4787–4794. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang Z, Guo Y, Zhou H, Teng G, Chen B. Anhedonia and activity deficits in rats: impact of post-stroke depression. J Psychopharmacol. 2008;23:295–304. doi: 10.1177/0269881108089814. [DOI] [PubMed] [Google Scholar]
- Wang BF, Cui ZW, Zhong ZH, Sun YH, Sun QF, Yang GY, Bian LG. Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol Sin. 2015a;36:939–948. doi: 10.1038/aps.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li H, Yu J, Hong M, Zhou J, Zhu L, Wang Y, Luo M, Xia Z, Yang ZJ, et al. Protective effects of Chinese herbal medicine rhizoma drynariae in rats after traumatic brain injury and identification of active compound. Mol Neurobiol. 2015b doi: 10.1007/s12035-015-9385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu Y, Sheng H, Ni X, Lu J. Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav Brain Res. 2016;307:18–24. doi: 10.1016/j.bbr.2016.03.044. [DOI] [PubMed] [Google Scholar]
- Wu B, Ma Q, Khatibi N, Chen WQ, Sozen T, Cheng O, Tang JP. Ac-YVAD-CMK decreases blood–brain barrier degradation by inhibiting caspase-1 activation of interleukin-1β in intracerebral hemorrhage mouse model. Transl Stroke Res. 2010a;1:57–64. doi: 10.1007/s12975-009-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2010b;31:1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wu H, Wang J. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation. 2011;8:22. doi: 10.1186/1742-2094-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W, Yang Q, Qi J, et al. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging. 2015;36:1439–1450. doi: 10.1016/j.neurobiolaging.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, Zhou JR, Sun DY, Huang AJ, Wang X, et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther. 2014;20:119–124. doi: 10.1111/cns.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Wang P, Ma X, Yin X, Li J, Wang H, Jiang W, Jia Q, Ni L. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol Immunol. 2015;66:253–262. doi: 10.1016/j.molimm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Hou Z, Maruyama T, Zhang J, et al. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun. 2015;46:293–310. doi: 10.1016/j.bbi.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One. 2014;9:e97423. doi: 10.1371/journal.pone.0097423. [DOI] [PMC free article] [PubMed] [Google Scholar]