Abstract
IMPORTANCE
Home aeroallergen exposure is associated with increased asthma morbidity in children, yet little is known about the contribution of school aeroallergen exposures to such morbidity.
OBJECTIVE
To evaluate the effect of school-specific aeroallergen exposures on asthma morbidity among students, adjusting for home exposures.
DESIGN, SETTING, AND PARTICIPANTS
The School Inner-City Asthma Study was a prospective cohort study evaluating 284 students aged 4 to 13 years with asthma who were enrolled from 37 inner-city elementary schools in the northeastern United States between March 1, 2008, and August 31, 2013. Enrolled students underwent baseline clinical evaluations before the school year started and were then observed clinically for 1 year. During that same school year, classroom and home dust samples linked to the students were collected and analyzed for common indoor aeroallergens. Associations between school aeroallergen exposure and asthma outcomes during the school year were assessed, adjusting for home exposures.
EXPOSURES
Indoor aeroallergens, including rat, mouse, cockroach, cat, dog, and dust mites, measured in dust samples collected from inner-city schools.
MAIN OUTCOMES AND MEASURES
The primary outcome was maximum days in the past 2 weeks with asthma symptoms. Secondary outcomes included well-established markers of asthma morbidity, including asthma-associated health care use and lung function, measured by forced expiratory volume in 1 second.
RESULTS
Among 284 students (median age, 8 years [interquartile range, 6–9 years]; 148 boys and 136 girls), exposure to mouse allergen was detected in 441 (99.5%) of 443 school dust samples, cat allergen in 420 samples (94.8%), and dog allergen in 366 samples (82.6%). Levels of mouse allergen in schools were significantly higher than in students’ homes (median settled dust level, 0.90 vs 0.14 μg/g; P < .001). Exposure to higher levels of mouse allergen in school (comparing 75th with 25th percentile) was associated with increased odds of having an asthma symptom day (odds ratio, 1.27; 95%CI, 1.05–1.54; P = .02) and 4.0 percentage points lower predicted forced expiratory volume in 1 second (95%CI, −6.6 to −1.5; P = .002). This effect was independent of allergic sensitization. None of the other indoor aeroallergens were associated with worsening asthma outcomes.
CONCLUSIONS AND RELEVANCE
In this study of inner-city students with asthma, exposure to mouse allergen in schools was associated with increased asthma symptoms and decreased lung function. These findings demonstrate that the school environment is an important contributor to childhood asthma morbidity. Future school-based environmental interventions may be beneficial for this important public health problem.
Asthma affects a large proportion of children in the United States, accounting for more than 14 million missed school days per year1 and costing billions of dollars in health care use.2 Furthermore, asthma morbidity disproportionately affects minorities and low-income groups in inner-city neighborhoods.3 Previous studies have identified unique allergen exposures in inner-city homes as important risk factors for asthma morbidity4 and demonstrated that interventions to reduce home exposure to these allergens improve asthma outcomes.5 There are some published data on the presence of allergens in schools6–12;however, to our knowledge, there are no comprehensive studies evaluating asthma outcomes resulting from allergen exposures in schools, where children spend most of their day.13 The primary purpose of the School Inner-City Asthma Study was to comprehensively evaluate the role of school-specific indoor allergen exposures on asthma morbidity, adjusting for home allergen exposures.
Methods
Study Population and Overall Design
The study population consisted of children aged 4 to 13 years with asthma who were attending inner-city public elementary schools in the northeastern United States from March 1, 2008, to August 31, 2013. Each year of the study, approximately 70 students were recruited from approximately 7 elementary schools. Enrolled students were observed clinically for 1 school year; their school and home environments were evaluated during that same year. With each subsequent year of the study, a new set of schools was evaluated and a new set of students was enrolled. In total, 351 students from 38 elementary schools were evaluated for 1 year each during the 5-year study period (eFigure 1 in the Supplement).
Children with asthma attending these schools were recruited based on established criteria modeled from other inner-city asthma studies.14,15 Inclusion criteria included asthma diagnosed by a physician for at least 1 year and at least 1 of the following: current daily preventive asthma medication use, wheezing in the past year, or an unscheduled health care visit for asthma in the past year. Exclusion criteria included lung disease other than asthma and cardiovascular disease. Written informed consent was obtained from the participants’ legal guardian, and written assent was obtained from participants older than 7 years. The protocol was approved by the Boston Children’s Hospital institutional review board and the participating school system.
Study Recruitment, Baseline Study Visit, and Sensitization Testing
Every spring, validated screening questionnaires were distributed to the parents of all students attending participating elementary schools to determine possible eligibility.16 Enrolled students with asthma completed a baseline clinical assessment during the summer prior to the academic year, including spirometry with a Koko spirometer (Ferraris Respiratory) using guidelines from the American Thoracic Society17 and aeroallergen sensitization testing by allergy skin testing (MultiTest device; Lincoln Diagnostics) and/or serum-specific IgE testing (ImmunoCAP; Phadia AB). Sensitization was defined by a wheal 3 mm or larger than that induced by the negative saline control on prick testing or a specific IgE level of 0.35 kU/L or greater. The tested allergens included tree pollen, grass, ragweed, dust mites, cat, dog, mouse, rat, cockroach, and molds (Greer).
Follow-up Questionnaires and Follow-up Spirometry
Follow-up surveys evaluating asthma symptoms, health care use, and effect on parent or caregiver were performed during telephone interviews at 3, 6, 9, and 12 months. For example, the first follow-up questionnaire occurred during the fall season, 3 months after the summer baseline visit. Follow-up spirometry was performed twice during the academic year, approximately 6 months apart.
Exposure Assessment
Classroom-settled dust samples were collected twice during the academic year (eFigure 1 in the Supplement). School dust samples were obtained by research personnel using an Oreck XL (model BB870-AD) handheld vacuum with a special dust collector (DACI laboratory, Johns Hopkins) fitted into the inlet hose of the vacuum using a standardized protocol.15 Vacuum sampling was performed for a total of 6 minutes per sample: 3 minutes on the floor and 3 minutes on desk and chair surfaces.18 One settled dust sample was collected in the participant’s bedroom, established as the most clinically relevant home exposure location, using a standardized protocol.19
Dust samples were analyzed using a multiplex array for indoor allergens (MARIA; Indoor Biotechnologies)20 that measured the following indoor aeroallergens simultaneously: cockroach (Bla g 2), cat (Fel d 1), dog (Can f 1), mouse (Mus m 1), dust mite (Der p 1 and Der f 1 and group 2), and rat (Rat n 1). The lower limits of detection were 0.196 μg/g of dust for cockroach, 0.004 μg/g for cat and rat, 0.002 μg/g for mouse, and 0.012 μg/g for dust mites and dog. For samples with an undetectable allergen level, the value was set to the lower limit of detection.
Outcome Measures
A priori, the primary outcome was days with asthma symptoms, as used in prior inner-city home-based studies.4,5 To define this outcome, the following 3 variables of symptoms in the 2 weeks prior to each survey were evaluated: number of days with wheezing, chest tightness, or cough; number of days on which the child had to slow down or discontinue play activities owing to wheezing, chest tightness, or cough; or number of nights with wheezing, chest tightness, or cough leading to disturbed sleep. The greatest result of these 3 variables was used as the outcome of days wth asthma symptoms. As such, this outcome was a score ranging from 0 to 14 days.
Secondary outcome measures included the following: number of days the child missed school owing to asthma; health care use, defined as the number of hospitalizations and unscheduled health care visits for asthma; number of days the caregiver changed plans because of the child’s asthma; number of nights the caregiver lost sleep because of the child’s asthma; poor asthma control as identified by any of following in the past 4 weeks: shortness of breath more than twice weekly, nighttime awakenings owing to asthma at least once, limitation in activity level, or use of rescue asthma medication 2 or more times weekly; and lung function based on percentage of predicted forced expiratory volume in 1 second (FEV1) before the child used a bronchodilator.
Statistical Analysis
Only allergens that were detected in 50% or more of school samples were analyzed for associations with asthma outcomes. Asthma morbidity outcomes were linked with the temporally closest allergen exposure. Only outcome measures obtained during the school year were included in the analysis, with children having between 1 and 4 outcome measures across the school year.
We first explored unadjusted patterns of allergen exposure and response across quintiles of exposure to evaluate if a dose-response association existed between exposure to school allergens and the primary outcome. We then performed the primary analysis of the study, which evaluated the association between allergen exposure and outcome using exposure as a continuous variable while adjusting for confounders using generalized estimating equations with an exchangeable correlation structure, robust variance estimates, and clustering at the participant level. We considered clustering at the school level in addition to the participant level within a multilevel random effects model containing both child and school random effects, but it was deemed unnecessary because there was little to no between-school variability in all outcomes (intraclass correlations between 0.00 and 0.04). In this analysis, allergen levels were log transformed to minimize the effect of highly influential points arising from the skewed distribution of exposure. Binomial family generalized estimating equations with a logit link and an overdispersion parameter were used for 2-week outcomes (ie, 2-week outcomes were modeled as the sum of 14 binomial successes) and poor asthma control, negative binomial family generalized estimating equations and log link were used for health care use and school absences, and FEV1 was modeled using gaussian family and identity link.
To investigate the role of allergic sensitization in modifying the exposure-response association for each allergen, we first tested the interaction effect of school allergen exposure and sensitization to that particular allergen. If the interaction effect was not significant (P ≥ .20), then the interaction effect was removed and the main effect of allergen exposure was reported. To limit multiple comparisons, secondary outcomes were analyzed only if a significant association was found between an allergen and the primary outcome. A priori, we decided to adjust for the following in all models: age, sex, race/ethnicity, use of medication to control asthma, home allergen exposure, linked school endotoxin exposure, and season. Season was defined as a continuous measure of the number of days since school started and was modeled with linear and quadratic terms (based on its observed association with maximum symptom days). The home allergen exposures of the 18 participants missing these data were set to the mean of the sample, and an indicator variable identifying these participants was included in all models.
Statistical computations were performed using STATA software, version 13.1 (StataCorp). All tests were 2-tailed, and P < .05 was considered significant.
Results
A total of 351 students with asthma from 38 schools participated in the baseline study visit. Participants were excluded from this analysis if they were lacking outcome measures during the school year, lacking sensitization testing, or lacking collection of classroom dust exposures (eFigure 2 in the Supplement). As a result, 284 participants (median age, 8 years [interquartile range, 6–9 years]; 148 boys and 136 girls) from 37 schools were included in this analysis. There were a total of 714 follow-up observations, including 40 participants (14.1%) with 1 follow-up, 82(28.9%)with 2 follow-ups, 138 (48.6%)with 3 follow-ups, and 24 (8.5%) with 4 follow-ups. The baseline characteristics of the study population are detailed in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Valuea |
|---|---|
| Male | 148 (52.1) |
| Age, median (IQR), y | 8 (6–9) |
| Race | |
| White | 13 (4.6) |
| Black | 99 (34.9) |
| Hispanic | 103 (36.3) |
| Other | 69 (24.3) |
| Annual household income, $b | |
| <15 000 | 61 (25.5) |
| <45 000 | 172 (72.0) |
| Family history of asthma | 228 (80.3) |
| Household smoke exposure | 90 (31.7) |
| Body mass indexc | |
| Normal | 141 (50.0) |
| Overweight or obese (>85th percentile) | 141 (50.0) |
| Asthma medications | |
| SABA only | 128 (45.1) |
| ICS and/or montelukast | 156 (54.9) |
| Allergen sensitization rates | |
| Any sensitization | 196 (69.0) |
| Cat | 104 (36.6) |
| Dust mites | 96 (33.8) |
| Tree pollen | 87 (30.6) |
| Mouse | 86 (30.3) |
| Grass pollen | 72 (25.4) |
| Cockroach | 63 (22.2) |
| Ragweedd | 58 (20.5) |
| Rat | 58 (20.4) |
| Mold | 53 (18.7) |
| Dog | 30 (10.6) |
| Maximum days of symptoms in past 2 wk | |
| 0–1 | 150 (52.8) |
| 2–3 | 56 (19.7) |
| 4–9 | 48 (16.9) |
| 10–14 | 30 (10.6) |
| Median (IQR), d | 1 (0–4) |
| Hospitalization or urgent care visit for asthma in past y | 119 (41.9) |
| Predicted FEV1, median (IQR), %e | 100 (91–113) |
Abbreviations: FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; IQR, interquartile range; SABA, inhaled short-acting β-agonist.
Data are presented as number (percentage) of children unless otherwise indicated.
n = 239. Not all income levels are reported in the table.
n = 282.
n = 283.
n = 281.
Mouse allergen was the most commonly detected allergen, with rates of detection of 99.5% in schools and 96.0% in homes (Table 2). The school mouse allergen levels were high, with the median and 90th percentile levels of Mus m 1 at 0.90 and 10.95 μg/g, respectively. Mouse allergen levels in schools were significantly higher than in homes (median, 0.90 vs 0.14 μg/g; P < .001, Wilcoxon rank sum test). Cat and dog allergen were commonly detected in schools (cat, 94.8%; dog, 82.6%) and homes (cat, 79.4%; dog, 49.8%). Dust mites were detected in 46.5% of school samples, and the absolute levels of dust mites were low (maximum, 1.64 μg/g). Cockroach and rat allergen were mostly undetectable in schools (cockroach, 0.7%; rat, 1.4%) and homes (cockroach, 3.1%; rat, 2.2%).
Table 2.
Allergen Levels in Schools and Homes of Students With Asthma
| Allergen by Location | Detectable, No. (%)a | Allergen Levels, μg/g | |||||
|---|---|---|---|---|---|---|---|
| 10th Percentile | 25th Percentile | Median | 75th Percentile | 90th Percentile | Maximum | ||
| Mouse (Mus m 1) | |||||||
| School | 441 (99.5) | 0.08 | 0.23 | 0.90 | 3.84 | 10.95 | 144.25 |
| Home | 308 (96.0) | 0.01 | 0.02 | 0.14 | 0.53 | 2.93 | 82.56 |
| Cat (Fel d 1) | |||||||
| School | 420 (94.8) | 0.02 | 0.07 | 0.23 | 0.54 | 1.47 | 285.78 |
| Home | 255 (79.4) | b | 0.01 | 0.06 | 0.64 | 10.84 | 235.23 |
| Dog (Can f 1) | |||||||
| School | 366 (82.6) | b | 0.03 | 0.11 | 0.27 | 0.52 | 99.42 |
| Home | 160 (49.8) | b | b | b | 0.07 | 2.18 | 140.15 |
| Cockroach (Bla g 2) | |||||||
| School | 3 (0.7) | b | b | b | b | b | 0.27 |
| Home | 10 (3.1) | b | b | b | b | b | 1.06 |
| Dust mite (Der f 1) | |||||||
| School | 206 (46.5) | b | b | b | 0.05 | 0.18 | 1.64 |
| Home | 189 (58.9) | b | b | 0.03 | 0.20 | 0.80 | 95.57 |
| Dust mite (Der p 1) | |||||||
| School | 50 (11.3) | b | b | b | b | 0.01 | 0.78 |
| Home | 71 (22.1) | b | b | b | b | 0.08 | 11.01 |
| Rat (Rat n 1) | |||||||
| School | 6 (1.4) | b | b | b | b | b | 0.27 |
| Home | 7 (2.2) | b | b | b | b | b | 0.02 |
School settled dust samples: 443 samples from 223 classrooms in 37 schools. Home settled dust samples: 321 samples.
Value below the limits of detection.
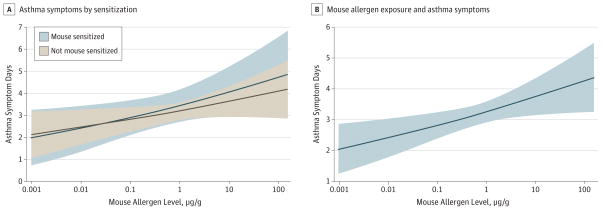
The crude data show an association between allergen exposure and the primary outcome only with mouse allergen. Participants in the highest quintile of mouse allergen exposure at school had 3.6 days with asthma symptoms per 2-week period compared with 2.9 days with symptoms for participants in the lowest quintile of exposure (eTable in the Supplement). In multivariable models, we found no evidence that sensitization to a specific allergen modified the association between allergen exposure level and days with asthma symptoms for the commonly detected allergens in schools (mouse, cat, and dog). The interaction effects were not significant for any of these allergens, with an odds ratio of 1.07 (95% CI, 0.78–1.46;P = .69) for mouse allergen (Figure 1A), 0.87 (95%CI,0.59–1.26; P = .45) for cat allergen, and 0.77 (95% CI, 0.35–1.69; P = .52) for dog allergen.
Figure 1. Association of Increasing Mouse Allergen Exposure in School and Asthma Symptoms.
A, Children sensitized to mouse allergen and not sensitized to mouse allergen (P = .69 for interaction effect). B, Exposure to mouse allergen in school and asthma symptoms regardless of sensitization (P = .02). All models adjusted for age, sex, race/ethnicity, use of medication to control asthma, linked mouse allergen exposure at home, linked endotoxin exposure at school, and time of year of allergen collection. Asthma symptom days: maximum number of days during the previous 2 weeks with daytime wheezing, chest tightness, or cough; days on which child had to slow down or discontinue play activities owing to wheezing, chest tightness, or cough; or nights with wheezing, chest tightness, or cough leading to disturbed sleep.
Independent of sensitization status, exposure to mouse allergen was significantly associated with increased number of days with asthma symptoms. The estimated odds ratio comparing the odds of a day with asthma symptoms associated with the 75th percentile (3.84 μg/g)of mouse allergen in school dust compared with exposure to the 25th percentile (0.23 μg/g) of school mouse allergen was 1.27 (95% CI, 1.05–1.54; P = .02) (Table 3 and Figure 1B). This finding indicates that children exposed to the 75th percentile of mouse allergen in school dust are expected to have 0.6 more days with asthma symptoms in a 2-week period when compared with children exposed to the 25th percentile of mouse allergen in school dust (3.54 vs 2.97 days with symptoms). Cat and dog allergen exposures in schools were not significantly associated with the primary outcome (Table 3).
Table 3.
Association of School Allergen Exposure on Asthma Symptom Days
| Allergen Exposurea | Exposure Difference, 25th to 75th Percentile, μg/gb | Days With Asthma Symptoms, Adjusted Odds Ratio (95% CI)c | P Value |
|---|---|---|---|
| Mouse (Mus m 1) | 3.61 | 1.27 (1.05–1.54) | .02 |
| Cat (Fel d 1) | 0.47 | 0.97 (0.83–1.14) | .71 |
| Dog (Can f 1) | 0.24 | 0.97 (0.79–1.19) | .80 |
Other allergen exposures (dust mites, cockroach, or rat) were not analyzed for asthma outcomes owing to low rates (<50%) of detectability in dust samples from school.
Difference in expected days with asthma symptoms between the 75th percentile of allergen exposure and the 25th percentile (714 observations [284 children]).
Adjusted for age, sex, race, use of medication to control asthma, exposure to allergen at home, linked endotoxin level at school, and time of year. Days with asthma symptoms is defined as the maximum number of days during the previous 2 weeks with daytime wheezing, chest tightness, or cough; days on which child had to slow down or discontinue play activities owing to wheezing, chest tightness, or cough; or nights with wheezing, chest tightness, or cough leading to disturbed sleep.
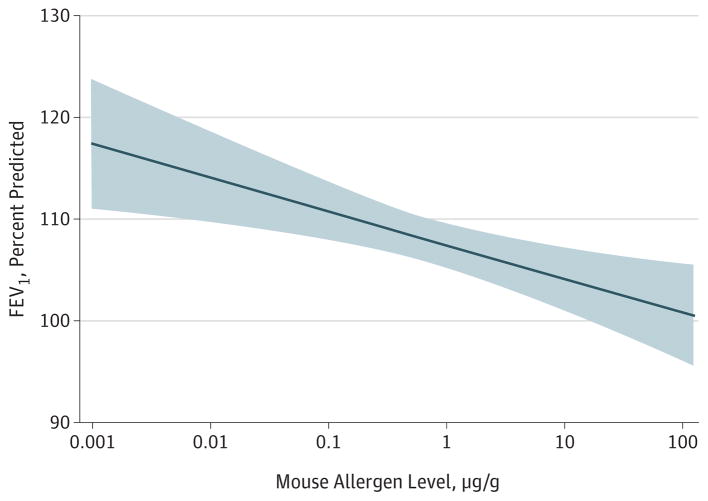
Given the significant association of mouse allergen exposure in school with the primary outcome of asthma symptoms, we analyzed this exposure relative to secondary outcomes. As seen in Figure 2, lung function was significantly associated with mouse exposure in school dust. Exposure to the 75th percentile of mouse allergen in school dust was associated with a 4.0 percentage points–lower predicted FEV1 (95%CI, −6.6 to −1.5; P = .002) relative to children exposed to the 25th percentile of mouse allergen in school dust, independent of sensitization status and other covariates. None of the other secondary outcomes was significantly associated with school mouse allergen exposure.
Figure 2. Association of Increasing Mouse Allergen Exposure in School and Decline in Lung Function.
Exposure to increasing levels of mouse allergen in schools was associated with a decrease in forced expiratory volume in 1 second (FEV1) (P = .002). Adjusted for age, sex, race/ethnicity, use of medication to control asthma, linked mouse allergen exposure at home, linked endotoxin exposure at school, and time of year of allergen collection.
Discussion
In our study of inner-city school-aged children with asthma, exposure to higher levels of school mouse allergen was associated with a higher number of days with asthma symptoms and decreased lung function, independent of home environmental exposure. This effect was seen in all children with asthma studied, regardless of whether they were sensitized to mouse allergen, and further underscores the public health relevance of school-associated allergen exposure as an important contributor to asthma morbidity in children. We acknowledge that other allergens in schools were mostly undetectable or detected at low levels, limiting our ability to assess those allergens; however, mouse allergen was present at high levels in schools. To our knowledge, this study is the first comprehensive inner-city school-based study to examine classroom allergen exposures and asthma morbidity in students, adjusting for home exposure.
In the inner-city schools in our study, mouse allergen was the predominant exposure, whereas levels of cockroach, pet, and dust mite allergens were undetectable or low. Other investigators have reported low levels of cockroach and dust mite allergens in similar northeastern US cities.4,21 In contrast, other cities with warmer climates and different building conditions have demonstrated high levels of school cockroach allergen.8,9 The low levels of dust mites and cockroach in our study are likely owing to the long, dry, and very cold winters in the studied region, as these pests require humidity and warmth to survive. Although cat and dog allergens were commonly detected in our schools, the absolute levels of these allergens were relatively low compared with those in studies from homes in the United States22,23 or schools in Europe.11,24 The pet allergen levels in the schools in our study were well below the standard threshold associated with asthma symptoms.25 This finding is likely owing to the low prevalence of household pets in our inner-city setting, with only 79 of 350 participants (22.6%) and 107 of 350 participants (30.6%) reporting having dogs or cats, respectively. Our findings are consistent with recent work suggesting that mouse allergen may be the most relevant allergen exposure in inner-city settings.26,27 Matsui et al28 reported increased asthma morbidity for children exposed to more than 0.5 μg/g of mouse allergen in settled dust in a bedroom. Our study found 266 of 443 school samples (60.0%) above this threshold.
The clinical significance of these findings is important. A child in a classroom with a mouse allergen level at the 25th percentile exposure of our study will have an estimated 0.6 fewer days of asthma symptoms in a 2-week period compared with a child in a classroom with mouse allergen exposure at the 75th percentile. This difference would translate into 12 fewer days of asthma symptoms during the school year (61 vs 73 days). These estimated differences in days with asthma symptoms between the groups with high and low mouse allergen exposure in school are consistent with findings of other important asthma intervention and treatment trials. For example, our effect size was similar to the effect size (0.7 fewer symptom days) seen in a home study of mouse allergen exposure.29 Similarly, an inner-city asthma study demonstrated a 0.8-day difference in symptom days per 2 weeks after a home-based environmental intervention5 that was cost-effective.30 This difference in days with asthma symptoms decreased to 0.6 days after the intervention was stopped.5 Finally, our absolute effect size was greater than the effect size (reduction of 0.48 days with asthma symptoms) from treatment with omalizumab in inner-city children.31 The findings of our school study highlight the potential for robust and clinically important improvements in students’ asthma with school-based environmental interventions.
Students who were both sensitized and exposed to elevated levels of mouse allergen did exhibit increased asthma morbidity; however, we were surprised to find an association irrespective of sensitization status. We considered endotoxin as a possible confounder based on prior reports that nonallergic symptomatic effect in mouse research workers occurs owing to airborne endotoxin exposure32; however, our findings remained even after adjusting for endotoxin levels. Rabito et al33 also reported similar findings of increased asthma morbidity independent of sensitization status for exposure to cockroach allergen in homes. A longitudinal study of apprentices exposed to laboratory animals also found that respiratory symptoms developed even in those who were not sensitized.34 It is possible that extremely high levels of mouse allergen, as we detected in our study, could have a direct irritant effect. Studies also have shown that multiple allergens can directly activate the innate immune system35,36; it is possible that increased inflammation results in asthma symptoms in nonsensitized individuals.
Further support for the significant association between mouse allergen exposure in school and asthma morbidity is the decline in percent predicted FEV1 associated with increased levels of mouse allergen in school. Although participants exposed to high concentrations of mouse allergen maintained an average FEV1 in the normal range, this finding offers objective physiological evidence to support our primary symptom-based results.
Limitations
Of the enrolled 351 study participants, 67 (19.1%) were not included in this analysis as they lacked exposure or outcome measures. We found no statistically significant differences in the demographics between the analyzed and excluded participants except for the rate of ragweed sensitization (20.5% vs 8.3%; P = .046), which is not expected to have influenced our findings. We likely had insufficient power to detect differences in certain secondary outcomes such as health care use, school absences, lost sleep, and change of plans that are relatively infrequent occurrences. Furthermore, we acknowledge that there was not a differentiation of participants by asthma severity, which may have affected results; however, we did adjust for the use of medication to control asthma. Finally, our results may not be generalizable to other cities that may have different allergens predominating in schools owing to differing climate and sociodemographic conditions. The exact problematic allergen is not as important as the demonstration that schools can be a source of allergen exposure associated with asthma morbidity.
Conclusions
To our knowledge, our findings are the first to provide substantial evidence that exposure to high levels of an aeroallergen in school plays an important role in asthma morbidity in inner-city children. The association of mouse allergen exposure in school with the primary outcome was seen in students with asthma regardless of allergic sensitization status. These findings suggest that exposure reduction strategies in the school setting may effectively and efficiently benefit all children with asthma. Future school-based environmental intervention studies may be warranted.
Supplementary Material
Key Points.
Question
What is the effect of school-specific aeroallergen exposures on students’ asthma morbidity?
Findings
In this cohort study evaluating students with asthma, higher mouse allergen exposure at school was significantly associated with both increased asthma symptoms and lower lung function, independent of allergic sensitization and allergen exposure in the home.
Meaning
The school environment is an important contributor to childhood asthma morbidity, and future school-based environmental interventions may benefit all children with asthma.
Acknowledgments
Funding/Support: This study was supported by grants R01AI073964, R01AI073964-02S1, K24AI106822, K23AI106945, K23ES023700, K23AI104780, U10HL098102, U01AI110397, and ES-000002 from the National Institutes of Health. This work was conducted with support from Harvard Catalyst and The Harvard Clinical and Translational Science Center, which received award 8UL1TR000170 from the National Institutes of Health, and financial contributions from Harvard University and its affiliated academic health care centers. This work was also supported in part by: the American College of Allergy, Asthma, and Immunology Young Faculty Award; Boston Children’s Hospital, Division of Immunology Clinical Research Advisory Group Research Grant; the American Lung Association and American Academy of Allergy, Asthma, and Immunology Respiratory Diseases Faculty Award; and the Deborah Munroe Noonan Memorial Award.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers; the National Center for Research Resources; or the National Institutes of Health Clinical and Translational Study Unit.
Author Contributions: Dr Phipatanakul had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Sheehan and Permaul contributed equally to this work.
Study concept and design: Sheehan, Permaul, Baxi, Gaffin, Gold, Phipatanakul.
Acquisition, analysis, or interpretation of data: Sheehan, Permaul, Petty, Coull, Gaffin, Lai, Gold, Phipatanakul.
Drafting of the manuscript: Sheehan, Permaul, Baxi, Lai, Phipatanakul.
Critical revision of the manuscript for important intellectual content: Sheehan, Permaul, Petty, Coull, Gaffin, Lai, Gold, Phipatanakul.
Statistical analysis: Sheehan, Petty, Coull, Gaffin, Lai, Phipatanakul.
Obtained funding: Sheehan, Gold, Phipatanakul.
Administrative, technical, or material support: Permaul, Phipatanakul.
Study supervision: Sheehan, Baxi, Gaffin, Gold, Phipatanakul.
Additional Contributions: Ann Bailey, BA, Boston Children’s Hospital, served as the research coordinator of the study. She was not compensated for her contribution. We thank the community of parents, nurses, research staff, teachers, school administrators, and children who have contributed to this work. Lincoln Diagnostics Inc donated Multi-Test II devices, and Greer Laboratories Inc donated allergenic extracts for skin testing.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–552. [PubMed] [Google Scholar]
- 2.Hasegawa K, Tsugawa Y, Brown DF, Camargo CA., Jr Childhood asthma hospitalizations in the United States, 2000–2009. J Pediatr. 2013;163(4):1127–1133. e3. doi: 10.1016/j.jpeds.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431–439. doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 5.Morgan WJ, Crain EF, Gruchalla RS, et al. Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 6.Permaul P, Hoffman E, Fu C, et al. Allergens in urban schools and homes of children with asthma. Pediatr Allergy Immunol. 2012;23(6):543–549. doi: 10.1111/j.1399-3038.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheehan WJ, Rangsithienchai PA, Muilenberg ML, et al. Mouse allergens in urban elementary schools and homes of children with asthma. Ann Allergy Asthma Immunol. 2009;102(2):125–130. doi: 10.1016/S1081-1206(10)60242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramson SL, Turner-Henson A, Anderson L, et al. Allergens in school settings: results of environmental assessments in 3 city school systems. J Sch Health. 2006;76(6):246–249. doi: 10.1111/j.1746-1561.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew GL, Correa JC, Perzanowski MS. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor Air. 2005;15(4):228–234. doi: 10.1111/j.1600-0668.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Berge M, Munir AK, Dreborg S. Concentrations of cat (Fel d1), dog (Can f1) and mite (Der f1 and Der p1) allergens in the clothing and school environment of Swedish schoolchildren with and without pets at home. Pediatr Allergy Immunol. 1998;9(1):25–30. doi: 10.1111/j.1399-3038.1998.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 11.Munir AK, Einarsson R, Schou C, Dreborg SK. Allergens in school dust, I: the amount of the major cat (Fel d I) and dog (Can f I) allergens in dust from Swedish schools is high enough to probably cause perennial symptoms in most children with asthma who are sensitized to cat and dog. J Allergy Clin Immunol. 1993;91(5):1067–1074. doi: 10.1016/0091-6749(93)90221-z. [DOI] [PubMed] [Google Scholar]
- 12.Almqvist C, Wickman M, Perfetti L, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. Am J Respir Crit Care Med. 2001;163(3 pt 1):694–698. doi: 10.1164/ajrccm.163.3.2006114. [DOI] [PubMed] [Google Scholar]
- 13.Salo PM, Sever ML, Zeldin DC. Indoor allergens in school and day care environments. J Allergy Clin Immunol. 2009;124(2):185–194. e181–e189. doi: 10.1016/j.jaci.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipatanakul W, Bailey A, Hoffman EB, et al. The School Inner-City Asthma Study: design, methods, and lessons learned. J Asthma. 2011;48(10):1007–1014. doi: 10.3109/02770903.2011.624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell H, Senturia Y, Gergen P, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24(4):237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Redline S, Gruchalla RS, Wolf RL, et al. Development and validation of school-based asthma and allergy screening questionnaires in a 4-city study. Ann Allergy Asthma Immunol. 2004;93(1):36–48. doi: 10.1016/S1081-1206(10)61445-7. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Smedje G, Norbäck D, Edling C. Asthma among secondary schoolchildren in relation to the school environment. Clin Exp Allergy. 1997;27(11):1270–1278. [PubMed] [Google Scholar]
- 19.Arbes SJ, Jr, Sever M, Vaughn B, et al. Feasibility of using subject-collected dust samples in epidemiologic and clinical studies of indoor allergens. Environ Health Perspect. 2005;113(6):665–669. doi: 10.1289/ehp.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King EM, Filep S, Smith B, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods. 2013;387(1–2):89–95. doi: 10.1016/j.jim.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288(8):963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 23.Ingram JM, Sporik R, Rose G, Honsinger R, Chapman MD, Platts-Mills TA. Quantitative assessment of exposure to dog (Can f 1) and cat (Fel d 1) allergens: relation to sensitization and asthma among children living in Los Alamos, New Mexico. J Allergy Clin Immunol. 1995;96(4):449–456. doi: 10.1016/s0091-6749(95)70286-5. [DOI] [PubMed] [Google Scholar]
- 24.Perzanowski MS, Rönmark E, Nold B, Lundbäck B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103(6):1018–1024. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 25.Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts-Mills TA. Mite, cat, and cockroach exposure, allergen sensitisation, and asthma in children: a case-control study of three schools. Thorax. 1999;54(8):675–680. doi: 10.1136/thx.54.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ownby DR. Will the real inner-city allergen please stand up? J Allergy Clin Immunol. 2013;132(4):836–837. doi: 10.1016/j.jaci.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia SK, Peng RD, Breysse PN, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132(4):830–835. e1–2. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 29.Pongracic JA, Visness CM, Gruchalla RS, Evans R, III, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101(1):35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 30.Kattan M, Stearns SC, Crain EF, et al. Cost-effectiveness of a home-based environmental intervention for inner-city children with asthma. J Allergy Clin Immunol. 2005;116(5):1058–1063. doi: 10.1016/j.jaci.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacheco KA, McCammon C, Liu AH, et al. Airborne endotoxin predicts symptoms in non–mouse-sensitized technicians and research scientists exposed to laboratory mice. Am J Respir Crit Care Med. 2003;167(7):983–990. doi: 10.1164/rccm.2112062. [DOI] [PubMed] [Google Scholar]
- 33.Rabito FA, Carlson J, Holt EW, Iqbal S, James MA. Cockroach exposure independent of sensitization status and association with hospitalizations for asthma in inner-city children. Ann Allergy Asthma Immunol. 2011;106(2):103–109. doi: 10.1016/j.anai.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Gautrin D, Ghezzo H, Infante-Rivard C, Malo JL. Natural history of sensitization, symptoms and occupational diseases in apprentices exposed to laboratory animals. Eur Respir J. 2001;17(5):904–908. doi: 10.1183/09031936.01.17509040. [DOI] [PubMed] [Google Scholar]
- 35.Trompette A, Divanovic S, Visintin A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herre J, Grönlund H, Brooks H, et al. Allergens as immunomodulatory proteins: the cat dander protein Fel d 1 enhances TLR activation by lipid ligands. J Immunol. 2013;191(4):1529–1535. doi: 10.4049/jimmunol.1300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.