In superficial layers of sensory cortex, only a small fraction of neurons fire most of the spontaneous and sensory evoked spikes. However, the functional relevance of such “sparse” activity remains unknown. We found that a “dense” population response is evoked by high-velocity micromotions applied to whiskers. Our results suggest that flashes of precisely timed population response on an almost silent background can provide a high capacity for coding of ecologically salient stimuli.
Keywords: sparse coding, postsynaptic potentials, Fano factor, two-photon imaging, somatosensory, AP threshold, whisker velocity
Abstract
Supragranular layers of sensory cortex are known to exhibit sparse firing. In rodent vibrissal cortex, a small fraction of neurons in layer 2 and 3 (L2/3) respond to whisker stimulation. In this study, we combined whole cell recording and two-photon imaging in anesthetized mice and quantified the synaptic response and spiking profile of L2/3 neurons. Previous literature has shown that neurons across layers of vibrissal cortex are tuned to the velocity of whisker movement. We therefore used a broad range of stimuli that included the standard range of velocities (0–1.2 deg/ms) and extended to a “sharp” high-velocity deflection (3.8 deg/ms). Consistent with previous literature, whole cell recording revealed a sparse response to the standard range of velocities: although all recorded cells showed tuning to velocity in their postsynaptic potentials, only a small fraction produced stimulus-evoked spikes. In contrast, the sharp stimulus evoked reliable spiking in the majority of neurons. The action potential threshold of spikes evoked by the sharp stimulus was significantly lower than that of the spontaneous spikes. Juxtacellular recordings confirmed that application of sharp stimulus to single or multiple whiskers produced temporally precise spiking with minimal trial-to-trial spike count variability (Fano factors equal or close to the theoretical minimum). Two-photon imaging further confirmed that most neurons that were not responsive to the standard deflections responded to the sharp stimulus. Altogether, our results indicate that sparseness in L2/3 cortex depends on the choice of stimulus: strong single- or multiwhisker stimulation can induce the transition from sparse to “dense” population response.
NEW & NOTEWORTHY In superficial layers of sensory cortex, only a small fraction of neurons fire most of the spontaneous and sensory evoked spikes. However, the functional relevance of such “sparse” activity remains unknown. We found that a “dense” population response is evoked by high-velocity micromotions applied to whiskers. Our results suggest that flashes of precisely timed population response on an almost silent background can provide a high capacity for coding of ecologically salient stimuli.
the mammalian cerebral cortex contains a fine laminar organization for processing sensory information. In rodents, the whisker-associated area of the somatosensory cortex, known as the vibrissal cortex, provides an example of efficient functionality and a well-described circuitry (Ahissar and Kleinfeld 2003; Brecht 2007; Diamond and Arabzadeh 2013; Feldmeyer et al. 2013). Recent advances in functional imaging combined with conventional electrophysiological techniques have revealed that sensory processing within local circuits of neocortex is layer and cell type dependent (Gentet et al. 2010; Gentet et al. 2012; Harris and Mrsic-Flogel 2013; O’Connor et al. 2010b; Petersen and Crochet 2013). Investigating the contribution of different cortical layers to sensory processing is thus a key step in understanding cortical computations.
It is well established that neurons in layer 2 and 3 (L2/3) of vibrissal cortex have low spontaneous rates of spiking and sparsely respond to sensory stimulation (Harris and Mrsic-Flogel 2013; Petersen and Crochet 2013). During object localization tasks, a small fraction (~10%) of L2/3 neurons discriminated object location (O’Connor et al. 2010b; Peron et al. 2015). This is not limited to the active touch; passive whisker deflection in awake or anesthetized mice also evoked sparse activity in L2/3 vibrissal cortex (Clancy et al. 2015; Peron et al. 2015). However, the choice of stimulus may critically determine the degree of sparse coding (Barth and Poulet 2012; Spanne and Jörntell 2015).
The mechanisms underlying sparseness of L2/3 neurons are not well understood. Neurons with distinct degrees of responsiveness exhibit similar intrinsic biophysical properties and morphology (Elstrott et al. 2014). The interactions of inhibitory and excitatory synaptic inputs determine the specific properties of whisker-evoked postsynaptic potentials, or PSPs (Petersen and Crochet 2013). Intracellular recordings in vivo have revealed that amplitude of whisker-evoked PSPs is dependent on the state of membrane potential immediately preceding the stimulation (Petersen et al. 2003; Sachdev et al. 2004). In a majority of L2/3 neurons, whisker stimulation produces maximum reversal potentials that are more hyperpolarized than the action potential (AP) threshold, precluding spiking response (Crochet 2012; Sachidhanandam et al. 2013). Overall, these observations combined with evidence from connectivity studies (Avermann et al. 2012; Xu and Callaway 2009) suggest that strong inhibition through local GABAergic circuitry counterbalances the excitatory input to L2/3 pyramidal cells, resulting in excess silence and sparse sensory response in this layer (Petersen and Crochet 2013).
A question that arises here is whether the majority of neurons in L2/3 vibrissal cortex are always silent or certain mechanisms allow them to contribute to sensory processing despite their dominant inhibitory input. Strong inhibition may not always prevent spiking for two reasons: 1) the AP threshold can depend on the stimulus preference (Carandini and Ferster 2000; Wilent and Contreras 2005a), and 2) the temporal dynamics of inhibition and excitation can provide a “window of opportunity” for cortical neurons to fire APs in response to the preferred stimulus (Wilent and Contreras 2005b). Such mechanisms can modulate both the reversal potential and the AP threshold of the less active L2/3 pyramidal cells, allowing them to respond to certain sensory features (Andermann and Moore 2006; Barlow 1972; Garion et al. 2014; Harris and Mrsic-Flogel 2013; Petersen and Crochet 2013).
Previous literature has shown that neurons across layers of vibrissal cortex are tuned to the velocity of whisker movement (Arabzadeh et al. 2003; Arabzadeh et al. 2004; Boloori et al. 2010; Gerdjikov et al. 2010; Ito 1981; Ito 1985; Pinto et al. 2000; Simons 1978; Wilent and Contreras 2004). A relatively low range of velocities (typically up to 1.3 deg/ms) are used in controlled experimental settings to reveal the dynamic range of neuronal response, but a broader range of whisker velocities are reported in behaving mice (Bale et al. 2015; O’Connor et al. 2010a) and rats (Carvell and Simons 1990; Jadhav et al. 2009; Ritt et al. 2008; Wolfe et al. 2008), including velocities as high as 10 deg/ms (Bale et al. 2015; O’Connor et al. 2010a). It is not clear to what extent the sparse activation of L2/3 neurons may maintain over this wider range of velocities. In the present study, we hypothesized that the population of L2/3 neurons might be preserved for coding of such high-velocity events. We used whole cell and cell-attached recording and two-photon calcium imaging in anesthetized mice to quantify the synaptic and spiking response of L2/3 neurons. Critically, the stimulus set includes a “sharp” deflection that falls in the range of high-velocity events that occur in object/texture palpation.
METHODS
Surgery.
Male ~6-wk-old C57BL6/J mice were used in this study. Light anesthesia was induced with a brief exposure to isoflurane (3.5% in oxygen) followed by intraperitoneal injection of 5 mg/kg chlorprothixene and 500 mg/kg urethane in Ringer solution. Corneal and paw reflexes were checked to be absent before the animal was mounted on a custom-built head fixation plate with ear bars and nose clamp. The skull was then exposed, and the coordinates of the vibrissal cortex were marked. A head bar was attached onto the skull using a thin layer of tissue adhesive (Vetbond; 3M, St Paul, MN), adhesive gel, and dental acrylic cement. Tissue adhesive was used all around the skull (except for the top of the barrel cortex) to seal the area and to keep drops of Ringer solution on top of the barrel cortex. A cranial window of 1–3 mm in diameter was drilled over the vibrissal cortex using vascular patterns and stereotaxic coordinates (center; 1–1.5 mm posterior to bregma and 3–4 mm lateral to the midline) or with intrinsic signal optical imaging (see below). Throughout the surgery, drops of artificial cerebrospinal fluid (ACSF) were applied to the exposed area. Dura mater was left intact.
Intrinsic signal optical imaging.
Intrinsic signal optical imaging (Grinvald et al. 1986) was carried out through the intact skull to map the S1 area representing whisker C2 (Ferezou et al. 2006). Ringer solution was applied to make the skull translucent, and a coverslip was used to trap the Ringer solution and prevent it from drying. Surface blood vessels were visualized by green light (527 nm; see Fig. 3B, top). Images were captured under red light (626 nm) using a complementary metal-oxide semiconductor (CMOS) camera (Photonfocus, Lachen, Switzerland) mounted on a Leica M80 stereomicroscope. In each trial, a sequence of 100 frames was acquired at 10 Hz with 70-ms exposure time. A sharp deflection (Fig. 1B, green) was applied to whisker C2 at the onset of each frame during the second half of the sequence (frames 51–100). This sequence was repeated 30 times, with 20-s intervals. In each sequence the first 50 frames (background signal) were averaged and subtracted from the second 50 frames (sensory stimulation). The difference matrix was averaged across trials to produce the localized intrinsic signal (see Fig. 3B, middle, dark spot) in the final image. This image was then adjusted, filtered, and merged with the vasculature image (see Fig. 3B, bottom) to guide surgery and recording. This imaging protocol was repeated if the vasculature pattern was obscured after craniotomy.
Fig. 3.
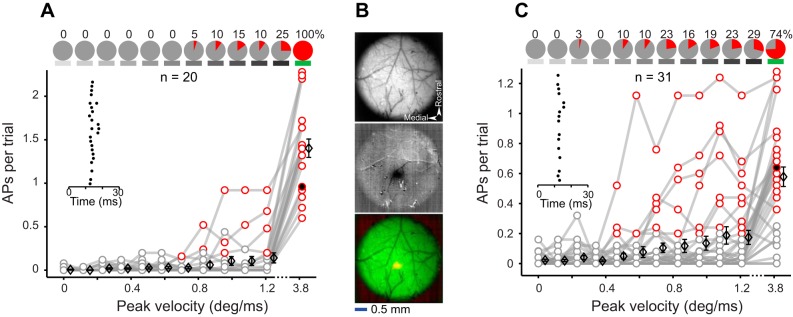
Spiking responses for juxtacellular recordings. A: spiking responses evoked by multiwhisker deflections (details as described in Fig. 2D). B: intrinsic signal optical imaging through intact skull. Top, vasculature pattern imaged using 527-nm light. Middle, intrinsic signal (dark spot) captured by whisker deflections under 626-nm light. Bottom, merged image of top (green) and middle (red) panels. C: spiking responses evoked by C2 whisker deflections (details as described in Fig. 2D).
Fig. 1.
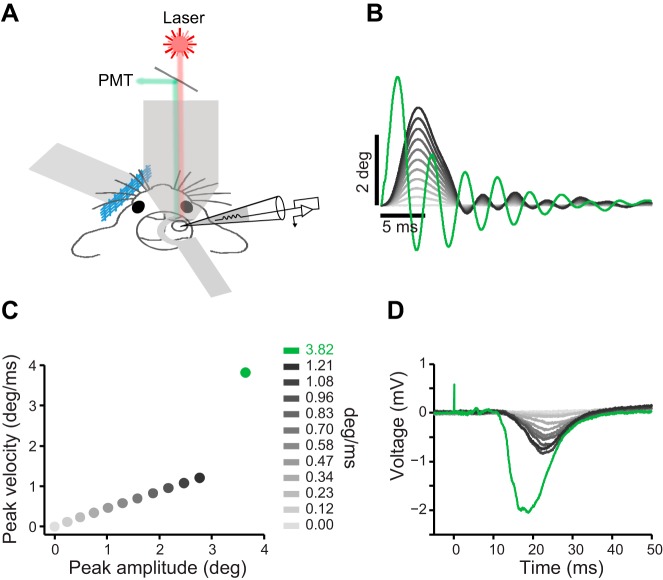
Experimental setup and stimulus properties. A: schematic illustration of the experimental setup. B: average position profile of the piezo movement (100 trials per each stimulus). This color convention (grayscale for the standard and green for the sharp stimulus) is used in all figures. C: peak velocity of the piezo movement plotted against peak amplitude. Every dot represents 1 stimulus. D: example evoked LFPs averaged across 25 trials per stimulus.
Calcium imaging.
The animal was transferred to a two-photon microscope system with a Chameleon (Coherent) Ti:Sapphire laser tuned at 810 nm and focused by a water-immersion Nikon objective (×16, 0.8 NA). Calcium florescent dye Cal-520 AM (AAT Bioquest) was dissolved in 20% Pluronic acid in DMSO and diluted in ACSF. Sulforhodamine (0.5 µl) was added to the solution such that the final concentrations of Cal-520 and sulforhodamine were 1.0 and 0.06 mM, respectively. This solution was pressure-injected (100–200 mmHg) at 100- to 300-µm depth using micropipettes with a 4- to 6-µm tip diameter. The injection was monitored with the two-photon microscope. Whisker-evoked local field potentials (LFPs; Fig. 1D) were monitored with a MultiClamp 700B amplifier (Molecular Devices) and AxoGraph software while the pipette was injecting the dye. Image acquisition began 30–60 min after the dye injection with recording of 100 frames of background activity in two channels: red for sulforhodamine, which labels astroglia (Nimmerjahn et al. 2004), and green for Cal-520. During the whisker stimulation protocol, only the green channel was recorded at 30 frames/s. Image stacks were corrected for drifts in the x-y plane using TurboReg in ImageJ (Thévenaz et al. 1998). Average images were then merged (see Fig. 4A) to differentiate neurons from glia.
Fig. 4.
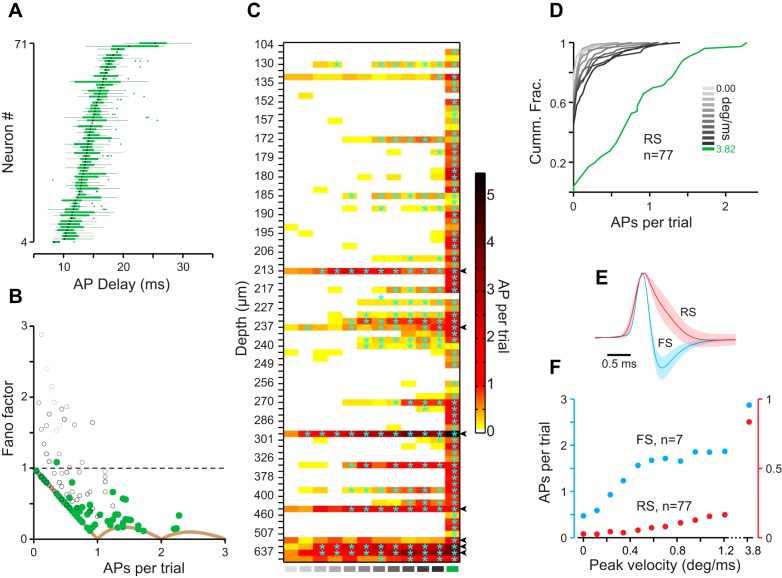
A: box and whisker plot representing spike time variation about the median (black lines) in response to the sharp stimulus. B: Fano factor vs. APs per trial (75 ms). Color coding of the circles is retained from Fig. 1B. Solid brown curve represents the theoretical minimum Fano factor. C: color plot representing APs per trial (color coded) vs. stimulus intensity (x-axis) vs. depth (y-axis) across 84 neurons, including all previous neurons from Figs. 2 and 3 (71 neurons), 7 fast-spiking (FS) neurons, and 6 deep neurons (>400 µm). Significant responses are marked with cyan asterisks. Arrowheads at right indicate FS neurons. D: cumulative distribution of response across all 77 regular-spiking (RS) neurons color coded for different stimuli as in Fig. 1B. E: juxtacellular spike waveforms of RS (red; n = 19) and FS (blue; n = 7) neurons (means ± SD). F: average response of FS (blue; left y-axis) and RS (red; right y-axis) neurons vs. stimulus intensity.
Electrophysiology.
Whole cell recordings were performed using 4- to 6-mΩ pipettes loaded with intracellular solution containing (in mM) 10 KCl, 130 K-gluconate, 10 HEPES, 4 MgATP, 0.3 Na2GTP, 10 Na2 phosphocreatine, and 0.047 Alexa 594 (pH ~7.25, osmolality ~280 mosM). The electrode passed the dura with ~200 mmHg of positive pressure. The pressure was then dropped to ~45 mmHg at a depth of ~100 µm, and pipette resistance and capacitance were corrected. When a gigaseal was established, membrane rupture was made with brief suctions applied to the pipette. The whole cell configuration was confirmed with a stable membrane potential in the range of −50 to −70 mV, the firing of overshooting action potentials, and responsivity to a current-step protocol (100-pA steps from −300 to 1,000 pA, 500-ms duration). Voltage was low-pass filtered at 10 kHz with a Bessel filter before being digitized at a 20-kHz sampling rate with either an ITC-18 (Instrutech) or a PCIe-6321 data acquisition board (National Instruments). Juxtacellular recordings were made by using patch pipettes filled with ACSF. Sulforhodamine (0.06 mM) was added to ACSF to visualize the pipette and allow targeting of Cal-520-loaded cells for juxtacellular recording combined with functional imaging (see Fig. 5, A and B).
Fig. 5.
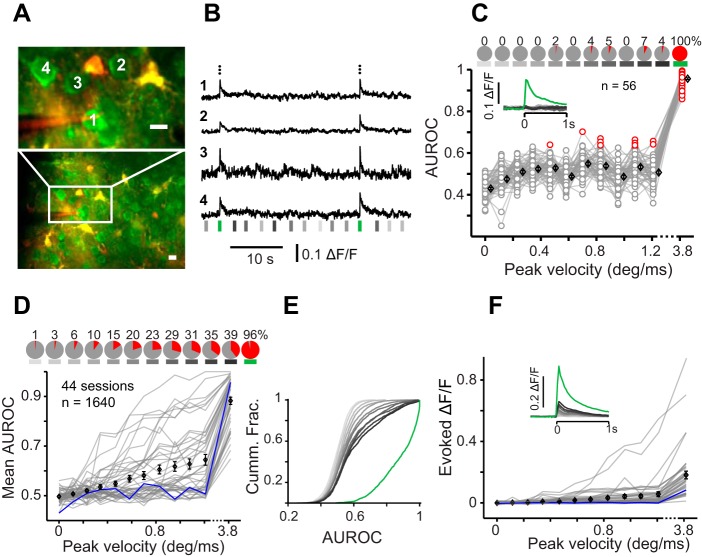
Calcium imaging from L2/3 vS1. A: imaging field with green and red channels overlaid. Top image is the area outlined in white at bottom, recorded with higher magnification (scale bars are 10 µm). Neuron 1 is targeted for simultaneous juxtacellular recording. B: example calcium-dependent change in fluorescence (ΔF/F) of 4 neurons labeled in A. Black dots at top represent juxtacellular spikes of neuron 1. Subsequent spikes occurring in the same frame are shown above the previous ones. Vertical lines represent stimuli, color coded as per convention. C: an example session where the area under the ROC curve (AUROC) is plotted vs. stimulus velocity (n = 56). Red circles represent significant detection performance (P < 0.05). Pie charts at top represent fraction of neurons (in percent) with significant response. Inset shows average peristimulus (at zero) ΔF/F over trials, over neurons. D: average AUROC for each session (gray plots, 44 sessions). The average AUROC of the example session in C is plotted in blue. Pie charts at top show the fraction of all imaged neurons with significant response. E: cumulative distribution of AUROCs across 1,640 imaged cells color coded for different stimuli as in Fig. 1B. Cumm. Frac., cumulative fraction. F: average of the evoked ΔF/F (baseline subtracted) for each session (gray plots). Inset shows peristimulus ΔF/F averaged over all sessions. Black diamonds in all panels represent means ± SE.
Whisker stimulation.
To stimulate a single whisker, a small pipette was attached to a piezoelectric ceramic (piezo). The pipette was positioned ~4 mm away from the base of the whisker. For the whole whisker pad stimulation, a rigid aluminum mesh was glued to the piezo and was positioned ~4 mm away from the base of the whiskers and tilted to engage as many whiskers as possible. Deflection commands were generated in MATLAB (The MathWorks) and sent to the analog output of the National Instruments board (20-kHz sampling rate) via a piezo amplifier (PiezoDrive, amplification gain of 20) to the piezo. The voltage signal had a Gaussian waveform, which produced a brief deflection (4-ms rise, 5-ms drop). Deflections were generated at 11 different amplitudes (0–2.8 deg; peak velocities of 0–1.21 deg/ms; Fig. 1B). The Gaussian function resulted in a smooth rise and drop in piezo’s movement trajectory with minimal ringing (~10%, second peak/first peak). In addition, a step voltage command to the piezo generated a sharp deflection with ~2-ms rise time, 3.6 deg amplitude, 3.82 deg/ms peak velocity, and ~40% ringing (Fig. 1C). We used a calibrated infrared optical sensor and obtained the actual movement trajectory of the vibrating mesh (Fig. 1B; average of 100 trials). Partial blockade of the infrared beam by the piezo deflections generated proportional changes in the sensor’s output voltage, which was then converted to actual position (in µm) by matching the voltage values to a voltage-position curve obtained from sensor calibration. The linear position was then converted to angular position to yield angular velocities (in deg/ms). Moment-by-moment velocity was calculated as the first derivative of the position profile, and the peak velocity was used to represent stimulus intensity. The movements were highly reproducible across trials: movement profiles were visually identical with a negligible variation at the peak velocity (across 100 trials, standard deviation = 0.0097 deg/ms for the sharp stimulus that had the highest variability). The 12 stimuli were repeated 25 times each with a pseudorandom order and with 1.5- to 2.5-s interstimulus intervals. One minute of spontaneous activity was recorded at the beginning and end of each recording session.
Data analysis.
We applied two procedures to isolate synaptic responses (PSPs) from action potentials (APs): 1) APs were truncated from the membrane potential (Vm) and substituted with a linear interpolation (Fig. 2, A and B). 2) As an alternative, we excluded trials that contained APs within 75 ms from the stimulus onset (Fig. 2C), which allowed direct quantification of the peak PSPs. Peak value of PSPs was measured at the interval of 10–75 ms after stimulus onset and was averaged over trials and over cells for each stimulus (Fig. 2C). To quantify spiking response, recordings were high-pass filtered (at 200 Hz) in MATLAB by using a Butterworth filter to magnify APs, which were then reliably isolated from artifacts. Spiking response was defined as the number of APs within 75 ms from the stimulus onset, averaged across 25 repetitions of each stimulus (Fig. 2D). AP waveforms were filtered using a 10-sample moving average implemented by the smooth function in MATLAB. AP threshold was then calculated as the positive peak of the second derivative of the AP waveform (Fig. 2, E and F). AP latency was calculated at 50-µs resolution within 35 ms from the stimulus onset (only 2 neurons fired a few outlier spikes beyond 35 ms). Response jitter was defined as the standard deviation of spike latency across trials. Fast-spiking (FS) neurons were identified on the basis of their characteristic narrow spike waveform and excluded from analyses (except Fig. 4C). Spike width was measured at 10% of the normalized spike peak (Fig. 4E). Initial analysis on calcium imaging data was performed using ImageJ software. After all recorded frames were averaged and merged with the red channel, free-hand regions of interest were drawn around each soma. Average fluorescence was then calculated across frames and converted into ΔF/F (change in fluorescence) and further analyzed in MATLAB (Fig. 4B). For receiver operating characteristic (ROC) analysis, the average ΔF/F of the ~200 ms (6 frames) before stimulus onset was used as the noise distribution and that of the six frames after stimulus (including the stimulus frame) as the signal distribution. These distributions were used to calculate ROC curves and the area under these curves (AUROCs). A random permutation test was performed to determine statistical significance of detection performance (P < 0.05).
Fig. 2.
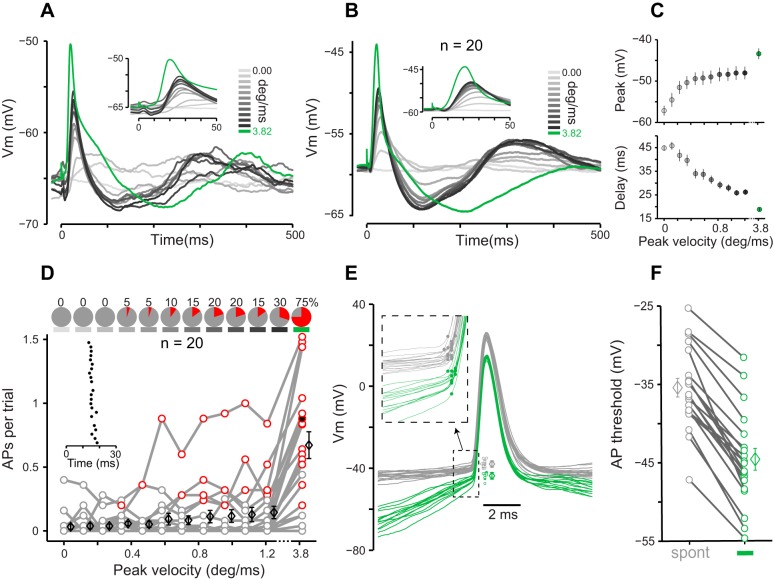
Synaptic responses in L2/3 neurons. A: average PSPs of an example neuron plotted vs. time from the stimulus onset (25 trials per stimulus). Inset shows an expanded view of the first 50 ms. B: average PSPs across 20 neurons. C, top: peak of PSP is averaged over trials and then over neurons (mean ± SE over neurons). Bottom, delay of the peak PSP averaged over trials and then over neurons (means ± SE over neurons). Note that in both panels (and subsequent figures), x-axis is broken beyond 1.2. D: APs per trial vs. stimulus peak velocity for whole cell recordings (circles). Red circles represent significant response based on ROC analysis. Average AP per trial across 20 cells is slightly shifted rightward and plotted as black diamonds (means ± SE). Inset raster plot represents spikes in response to the sharp stimulus for an example neuron-stimulus pair (25 trials). The red circle corresponding to the example neuron-stimulus pair is filled in black. Pie charts at top represent fraction (in percent) of significantly responsive neurons for each stimulus. E: spontaneous (gray) and sharp stimulus evoked (green) APs of an example neuron. Individual AP thresholds are shifted to the right for better visibility and are plotted along with their mean and SD. Inset represents an expanded view of the initial segment of the AP waveforms (dashed box). The dots on each expanded waveform represent the position of the peak of the second derivative, representing the AP threshold. F: AP thresholds across 20 neurons for sharp stimulus evoked (green) and spontaneous (gray) spikes. Each line represents a neuron, and diamonds are means ± SE.
RESULTS
To determine the response profile of L2/3 neurons to sensory stimulation, we performed whole cell recording (n = 20), juxtacellular recording (n = 20), and two-photon calcium imaging (n = 44 sessions; n = 1,640 cells) in the primary vibrissal somatosensory (vS1) cortex of urethane-anesthetized mice. We quantified the synaptic response and spiking profile of L2/3 neurons to 11 “standard” deflections (amplitudes, 0–2.8 deg; peak velocities, 0–1.21 deg/ms) as well as a “sharp” deflection (amplitude, 3.6 deg; peak velocity, 3.82 deg/ms) that falls in the range of high-velocity events that occur when whiskers interact with objects and textures. Figure 1A illustrates the experimental setup, the stimulus parameters, and the corresponding local field potentials (LFPs) measured on a dye-injecting pipette. The peak velocity of the standard stimuli linearly increased with the peak amplitude (Fig. 1C, gray). The sharp stimulus had a peak velocity more than three times higher than that of the fastest standard stimulus (Fig. 1B, green; Fig. 1C). The amplitude of the evoked LFPs increased systematically with deflection velocity. The sharp stimulus produced a prominent LFP with an average amplitude that was ~2.5 times larger than that evoked by the fastest standard stimulus (25 trials; Fig. 1D).
Synaptic response in L2/3 cells.
We recorded membrane potential (Vm) of 20 neurons in L2/3 vS1 cortex (104–256 µm below dura, 4 mice). The standard stimuli evoked a quick depolarization followed by a longer hyperpolarization and a second delayed depolarization (Fig. 2, A and B). The sharp stimulus evoked a considerably larger depolarization compared with the standard stimuli. We quantified maximum depolarization by the peak value of the evoked PSPs. Figure 2C plots the average peak PSP over trials and then over neurons. Because the lowest stimulus amplitude was set to zero, peak PSP for this stimulus represents the magnitude of spontaneous PSPs (3.8 mV above average Vm; Fig. 2C, lightest gray). The peak PSP increased with stimulus velocity and reached an average 9.1 mV above the spontaneous peaks for the fastest standard stimulus (Fig. 2C, top). The peak PSP for the sharp stimulus was 4.6 mV more depolarized than that of the fastest standard stimulus (Fig. 2C, green; P = 0.036, Wilcoxon rank sum test). The latency of the peak PSP systematically decreased as the stimulus velocity increased (Fig. 2C, bottom, gray): on average, the peak PSP evoked by the sharp stimulus (Fig. 2C, bottom, green) occurred 7.3 ms earlier than that of the fastest standard stimulus (P < 0.001, Wilcoxon rank sum test).
Spiking response in L2/3 cells.
The whole cell recordings revealed that the probability of spiking in response to the sharp stimulus was 0.67 AP per trial, which was ~5 times higher than that of the fastest standard stimulus (0.14 AP per trial). The sharp stimulus was reliably encoded in the spiking activity of 15 of 20 patched neurons (Fig. 2D; P < 0.05, ROC analysis random permutation test). APs exhibited a precise timing relative to the onset of the sharp stimulus: mean AP latency was 15.6 ms with 1.3 ms of jitter (standard deviation across trials) for the example neuron (Fig. 2D, inset). We also observed a consistently lower AP threshold for the spikes evoked by the sharp stimulus compared with the spontaneous spikes. Figure 2E shows an example recording where the threshold of the spikes evoked by the sharp stimulus was, on average, 5.7 mV more hyperpolarized than that of the spontaneous spikes (P < 0.001, Wilcoxon rank sum test). Across 20 neurons, the threshold of the spikes evoked by the sharp stimulus was, on average, 9.2 mV more hyperpolarized than that of the spontaneous APs (Fig. 2F; P < 0.001, Wilcoxon rank sum test). Despite the application of an inclusion criterion for whole cell recording based on resting membrane potential and overshooting APs (see methods), a small number of neurons showed action potential thresholds above −30 mV (Fig. 2F), which were higher than what is typically reported (Crochet et al. 2011). Excluding those cells did not affect the main finding: the sharp stimulus showed a lower AP threshold compared with the spontaneous spikes (P < 0.001, Wilcoxon rank sum test; n = 15). Figure 2E also revealed a systematic difference in the membrane potential preceding the APs, which was higher for the spontaneous spikes compared with those evoked by the sharp stimulus. To verify whether this finding generalized across neurons, we calculated the average membrane potential value during the 5- to 10-ms window preceding the AP threshold. This analysis revealed an average 19.4-mV difference, which was statistically significant across neurons (P < 0.001, Wilcoxon rank sum test; n = 20). This observation is consistent with state-dependent spiking previously reported in barrel cortex (Petersen et al. 2003; Sachdev et al. 2004).
In the next step, we recorded spiking activity of 20 L2/3 neurons (142–400 µm, 5 mice) without breaking into the cell (juxtacellular method). These recordings replicated the transition from a low to a high probability of spiking observed in the whole cell experiments; the average number of APs per trial was more than 10 times higher for the sharp stimulus compared with that for the fastest standard stimulus (1.5 vs. 0.14 AP per trial), and all of the recorded neurons were significantly responsive to the sharp stimulus (Fig. 3A). To test whether such high spiking probability was due to the simultaneous stimulation of multiple whiskers, we repeated the experiments with single-whisker deflections. Barrel C2 was localized using intrinsic signal optical imaging (Fig. 3B), and juxtacellular recordings were targeted to the center of the activated area. In this experiment, the average response to the sharp stimulus was 0.58 AP per trial, 3.4 times higher than that of the fastest standard stimulus (0.17 AP per trial), and the majority of neurons (74%) were significantly responsive to the sharp stimulus (n = 31, 152–400 µm below dura, 4 mice; Fig. 3C).
Next, we pooled the whole cell and juxtacellular recordings to characterize trial-to-trial variability of the spike times (box and whisker plots in Fig. 4A). For neurons that fired a minimum of 2 spikes (n = 66), the average interquartile range of spike times (boxes in Fig. 4A) was 2.6 ± 1.5 ms (mean ± SD). For the fastest standard stimulus, the interquartile range was 4.2 ± 3.1 ms (n = 28). Average spike latency was 14.6 ± 2.4 ms (median = 14.1 ms, n = 68), ranging from 9.0 to 20.3 ms (Fig. 4A). The standard deviation of spike times across trials provides another measure of spike time variability or jitter: for the sharp stimulus, this measure was 1.7 ± 0.7 ms (median = 1.7 ms, n = 66), ranging from 0.6 to 5.6 ms. To quantify variability in neuronal response across trials, we calculated the spike count Fano factors (Fig. 4B). The evoked response to the sharp stimulus produced Fano factors close to the theoretical minimum (brown curve in Fig. 4B; for the sharp stimulus, 58% of neurons were on this curve).
The preceding analyses only included regular-spiking (RS) neurons recorded from L2/3 cortex. Figure 4C plots the spontaneous and evoked spike rates for all L2/3 neurons (regular and fast spiking) as well as those recorded from deeper layers (>400 µm) after sorting neurons on the basis of their depth (n = 83). Figure 4D plots the cumulative distribution of the response of RS neurons, revealing reliable spiking to the sharp stimulus (green) at the population level. A total of seven fast-spiking (FS) neurons were identified by their narrow spikes in juxtacellular recordings (FS: 0.46 ± 0.032 ms, n = 7; RS: 1.23 ± 0.39 ms, n = 19; Fig. 4E). FS neurons exhibited higher spontaneous (FS: 0.47 ± 0.16 AP per trial; RS: 0.026 ± 0.085AP per trial; P < 0.001) and evoked spike rates (Fig. 4F, sharp stimulus; FS: 2.87 ± 1.42 AP per trial; RS: 0.83 ± 0.55AP per trial; P < 0.001, Wilcoxon rank sum). FS neurons were also highly responsive to the standard stimuli (Fig. 4F): e.g., a stimulus with peak velocity as low as 0.47 deg/ms evoked significant spiking response in 6 of 7 neurons (P < 0.05, ROC analysis followed by permutation test).
Finally, we employed two-photon calcium imaging to further establish the response profile of L2/3 neurons at the population level (Fig. 5, A and B). Consistent with the electrophysiological data, we found that across 44 imaging sessions from 8 mice, 1,574 of 1,640 neurons (96%) produced a significant response to the sharp stimulus (i.e., AUROC values that were significantly above 0.5; permutation test, P < 0.05, Fig. 5, C and D). The fastest standard stimulus, on the other hand, induced a statistically significant evoked activity in 647 of 1,640 neurons (39%; Fig. 5D). Figure 5E provides a criterion-free illustration of changes in sparseness by plotting the cumulative distribution of AUROC values generated in response to the sharp stimulus (green) and to all other stimuli (gray). Across sessions, average ΔF/F evoked by the sharp stimulus was more than three times higher than that of the fastest standard stimulus (0.1745 vs. 0.0553; P < 0.001, Wilcoxon rank sum test; Fig. 5F). Altogether, our electrophysiological and imaging data demonstrate a transition from sparse to “dense” L2/3 population activity with sharp, high-velocity vibrissal stimulation.
DISCUSSION
Sparse, distributed coding is a well-known property of the rodent sensory cortex (Barth and Poulet 2012; Harris and Mrsic-Flogel 2013; Petersen and Crochet 2013; Shoham et al. 2006). The sparse neuronal activation is well-documented in the supragranular layers (L2/3) of the vS1 cortex during both active and passive stimulation of the vibrissae in awake or anesthetized preparations (Brecht et al. 2003; Clancy et al. 2015; de Kock et al. 2007; Kerr et al. 2007; O’Connor et al. 2010b; Peron et al. 2015; Petersen and Crochet 2013; Ramirez et al. 2014). However, the functional relevance of sparse activity remains unknown (Barth and Poulet 2012; Spanne and Jörntell 2015). In this study, we found that the choice of stimulus affects the extent of sparseness in L2/3 neurons and that a dense population response is evoked by high-velocity micromotions applied to whiskers.
We measured synaptic and spiking responses in L2/3 vS1 cortex of urethane-anesthetized mice. All recorded neurons received the sensory signal: standard deflections evoked a PSP with an early, brief depolarization followed by a prolonged hyperpolarization and a delayed slow depolarization, as observed previously (Carvell and Simons 1988; Sachdev et al. 2004; Zhu and Connors 1999). As reported previously, synaptic responses increased with the peak angular velocity of the stimulus (Wilent and Contreras 2004). However, we found that synaptic responses tended to plateau at around 0.4 deg/ms for the standard range of stimulation and often did not evoke spiking activity (Fig. 2C). The sharp stimulus, on the other hand, evoked reliable spiking in the majority of neurons. These spikes had a significantly lower action potential threshold compared with those generated during the spontaneous activity (Fig. 2, E and F). The transition from sparse activation to high-probability spiking response was further confirmed in juxtacellular recordings, as well as in calcium imaging, despite its limitation in single spike detection (Clancy et al. 2015; Sato et al. 2007). Finally, we demonstrated that deflection of a single whisker with the sharp stimulus was sufficient to evoke reliable spiking in a majority of L2/3 neurons in the corresponding barrel (Fig. 3C).
Sparse coding might be due to the energy constraints over spiking activity (Lennie 2003) and is known to present several advantages for cortical computation (Barth and Poulet 2012; Harris and Mrsic-Flogel 2013; Olshausen and Field 2004). The term “sparseness” may refer to a number of different scenarios: 1) the overall rate of spiking (Shoham et al. 2006), 2) the number of spikes a single neuron fires in response to a stimulus (DeWeese et al. 2003; Jadhav et al. 2009; Ramirez et al. 2014), 3) the fraction of active cells in a neuronal population (Barth and Poulet 2012; Rolls and Tovee 1995; Wolfe et al. 2010), or 4) the selectivity of neurons to the stimulus space, i.e., being responsive only to the “grandmother” stimulus (Barlow 1972; Quiroga et al. 2008). In the present study, we quantified sparseness as the fraction of neurons that produced a statistically significant response to a certain stimulus based on an ROC analysis. Furthermore, the cumulative distributions of evoked responses provided a criterion-free demonstration of sparseness for both the electrophysiological (Fig. 4D) and the imaging data (Fig. 5E), which were comparable with those reported earlier (Clancy et al. 2015; O’Connor et al. 2010b).
Previous literature identified velocity as the effective stimulus feature in driving vS1 neurons (Arabzadeh et al. 2003; Arabzadeh et al. 2004; Boloori et al. 2010; Gerdjikov et al. 2010; Ito 1981; Ito 1985; Pinto et al. 2000; Simons 1978). Our hypothesis was that most L2/3 neurons may remain silent for the standard range of velocities and be preserved for coding of high-velocity events that occur in object/texture palpation. This scenario would increase the capacity to encode distinct high-velocity events and enhance the overall perceptual capacity by expanding the dynamic range of population responses (Elstrott et al. 2014; Pouille et al. 2009; Spanne and Jörntell 2015). Sparseness may also arise from selectivity of neurons to specific features of the stimulus space such as directional tuning (Andermann and Moore 2006; Lee and Simons 2004; Simons 1985). As another example, in behaving animals whisking and touch can be represented by distinct and spatially intermixed populations of neurons (Peron et al. 2015). It is therefore possible that strong selectivity of L2/3 neurons preserves them for encoding additional stimulus features or task variables in awake animals (Chen et al. 2013).
Behavioral studies have shown that whisker velocities can reach as high as 10 deg/ms (Bale et al. 2015; O’Connor et al. 2010a). However, neuronal and behavioral detection thresholds reveal that rats can detect stimuli with velocities much slower than the fastest standard stimulus used in the present study (Adibi et al. 2012; Lee et al. 2016; Ollerenshaw et al. 2012; Ollerenshaw et al. 2014). Such low detection thresholds are compatible with the motor strategy of the animals to gently palpate obstacles during exploration (Mitchinson et al. 2007). On the other hand, animals might use different whisking strategies depending on the behavioral task. For example, high-velocity micromotions are widely reported in texture discrimination tasks (Jadhav and Feldman 2010; Wolfe et al. 2008) and object palpation (Bale et al. 2015; O’Connor et al. 2010a). Although mice use a broad range of whisker velocities in object localization and palpation (Bale et al. 2015; O’Connor et al. 2010a), it is not clear whether the high-velocity slip events contribute to neuronal and behavioral performances in such tasks (O’Connor et al. 2010a, O’Connor et al. 2010b). In texture discrimination tasks, slip events with fast kinetics provide a neuronal signature to distinguish between various textures (Diamond et al. 2008; Jadhav et al. 2009; Jadhav and Feldman 2010; Ritt et al. 2008; Wolfe et al. 2008). The high-velocity events evoke precisely timed spikes in the first-order neurons in the trigeminal ganglion (Arabzadeh et al. 2005; Bale et al. 2015; Lichtenstein et al. 1990) and in the vibrissal cortex (Arabzadeh et al. 2006; Jadhav et al. 2009). Our data suggest that dense activation of L2/3 may contribute to coding of object surface properties by highlighting the slip events critical for such discriminations (Jadhav et al. 2009; Jadhav and Feldman 2010; Wolfe et al. 2008).
High-velocity whisker stimulations such as that of the sharp stimulus employed in the present study are not commonly used in anesthetized preparations (but see Arabzadeh et al. 2005 and Bale et al. 2015). However, a broad range of angular movements and velocities are found in behaving mice (Bale et al. 2015; O’Connor et al. 2010a) and rats (Carvell and Simons 1990; Jadhav et al. 2009; Ritt et al. 2008; Wolfe et al. 2008). Bale et al. (2015) found that during object palpation, the high-velocity events had a median of 6.6 deg/ms. They therefore delivered a high-velocity “ping stimulus” by a piezoelectric actuator to explore temporal precision of the spikes in trigeminal ganglion (Bale et al. 2015). Intense ringing is a characteristic of such ultrafast deflections (Bale et al. 2015), as is the case for our sharp, high-velocity deflection (Fig. 1B). The contribution of the resonation of the sharp stimulus to generation of the dense response of L2/3 cannot be ruled out in our study. However, whisker resonations following high-velocity events are observed in behaving mice (see supplementary Fig. 6 in O’Connor et al. 2010a) and rats (Jadhav et al. 2009; Lottem and Azouz 2009; Ritt et al. 2008). Although the velocity of the sharp stimulus presented in our study falls in the range of the salient high-velocity events observed in natural whisker object interactions, the mechanical properties are not necessarily the same. Future experiments could quantify the response of L2/3 neurons to the high-velocity slip events in behaving animals.
In our whole cell recordings, the AP threshold was significantly decreased by the sharp stimulus (Fig. 2, E and F). This is consistent with earlier studies reporting highly variable AP thresholds in vivo (Azouz and Gray 1999; Azouz and Gray 2000; Henze and Buzsáki 2001). This variability can provide a mechanism for stimulus selectivity (Anderson et al. 2000; Azouz and Gray 2003; Sachdev et al. 2004; Wilent and Contreras 2005a). In particular, the AP threshold correlates with the rate of rise in the membrane potential immediately before the threshold (Anderson et al. 2000; Azouz and Gray 2003; Poulet and Petersen 2008). Barrel cortex neurons are found to have a higher probability of spiking during the down state (Petersen et al. 2003; Sachdev et al. 2004). The effect of state on spiking was also present in our data, where APs evoked by the sharp stimulus tended to occur in the down state (Fig. 2E). On the other hand, the spontaneous spikes were less likely to occur during the down state; hence, they were preceded by a higher membrane potential corresponding to the up state (Fig. 2E). In the down state, the highly synchronous excitatory input (Azouz and Gray 2000) from L4 that is evoked by the sharp stimulus can raise the membrane potential at a high rate and thus produce spiking at the observed lower AP threshold. Although the contribution of the AP threshold modulation to cortical computation is not entirely clear (Yu et al. 2008), our results indicate that regulation of AP threshold along with the modulation of excitatory and inhibitory currents (Cohen-Kashi Malina et al. 2013; Wilent and Contreras 2005b) may play a role in selective spiking of L2/3 neurons. To confirm, this requires measurement and comparison of the membrane potential and synaptic currents in anesthetized, awake, and actively behaving animals, because the balance of the synaptic currents can vary with the brain state (Haider et al. 2013; Taub et al. 2013).
Another advantage of the synchronous excitatory input to a sparsely active network is reliability of response across trials. Accumulating evidence suggests that cortical neurons can process the sensory input with high trial-to-trial fidelity both in terms of spike count and spike timing (Baudot et al. 2013; DeWeese et al. 2003; Hires et al. 2015). The trial-to-trial variability can be stimulus dependent: in response to the sharp stimulus, we observed lower temporal variability compared with earlier recordings in the supragranular layers (L2/3) of the vibrissal cortex (Ahissar et al. 2001; Brumberg et al. 1999; Glazewski and Barth 2015). Furthermore, the high-velocity whisker stimulus excited L2/3 neurons with minimal spike count variability, suggesting a binary process as observed in L4 barrel (Hires et al. 2015), auditory (DeWeese et al. 2003), and visual cortex (Baudot et al. 2013). These findings indicate that the supragranular coding of the whisker stimuli can be robust and highly reliable. Strong inhibition in L2/3 of the vibrissal cortex (Crochet et al. 2011; Xu and Callaway 2009) seems to contribute to such reliable coding. The inhibition dampens the spontaneous fluctuations and the evoked response to the weak inputs (Pinto et al. 2003) to preserve the precisely timed spiking of silent neurons for the more prominent events during whisker-object interactions. On such a low background activity, a flash of driven response in a population of neurons is a salient signal that can reliably encode the time, frequency, and magnitude of events during sweeps of whiskers across surfaces.
The laminar organization of the vS1 suggests distinct information processing across cortical layers (Petersen and Crochet 2013). Unlike infragranular layers, which mainly project to the subcortical targets, L2/3 neurons make prominent connections with higher cortical areas such as the secondary vibrissal somatosensory cortex and the primary motor cortex (Chen et al. 2013). This positions the L2/3 neurons at the core of the whisker-mediated decision process (Kwon et al. 2016). It is therefore surprising that the majority of neurons in this layer remain silent during sensory processing (Clancy et al. 2015; O’Connor et al. 2010b; Peron et al. 2015; Petersen and Crochet 2013). Consistently (Crochet et al. 2011; Sachidhanandam et al. 2013), we found that L2/3 neurons elicit synaptic responses with a high sensitivity, reflecting a strong connection with L4, which is the primary recipient of the sensory signal in vS1. The overall profile of the PSPs (Fig. 4C, top, gray) also reflects the nonlinearities observed in stimulus-response functions in L4 vS1 (Adibi and Arabzadeh 2011) and may reflect the probabilistic nature of cortical activation (Gollnick et al. 2016). Despite a prominent functional connection with L4 (Lefort et al. 2009), rapid recruitment of inhibitory neurons precludes spiking response in a majority of excitatory L2/3 neurons (Petersen and Crochet 2013). In our data, we were able to distinguish a fraction of neurons as fast-spiking cells. Unlike regular-spiking neurons, these cells were highly responsive to the standard stimuli (Fig. 4F), suggesting that they may have a role in blocking action potential in L2/3 regular-spiking neurons (Crochet et al. 2011; Xu and Callaway 2009). The sharp stimulus, on the other hand, revealed a transition from sparse to dense activity in the population of L2/3 neurons. This transition could be attributed to a number of cellular and circuit mechanisms such as 1) the modulation of AP threshold, 2) the increased synchrony of the excitatory input from thalamus to L4 (Wang et al. 2010), which can propagate from L4 to L2/3 (Jadhav et al. 2009; Bruno 2011) due to the sharp event, and 3) the temporal modulation of inhibition/excitation. The interplay of these mechanisms may provide additional coding capabilities for L2/3 circuits involved in perception of ecologically relevant stimuli.
In summary, we found that sparse spiking response in L2/3 vibrissal cortex critically depends on sensory input and that a “dense” population response can be evoked by a sharp high-velocity whisker deflection. Given the low trial-to-trial variability, this strong signal can effectively convey sensory information to downstream targets of L2/3, such as the secondary somatosensory cortex or the motor and premotor areas. Moreover, the transition from sparse to dense spiking implies a high capacity in the population of L2/3 neurons for encoding a broad range of stimuli and may serve as an intrinsic mechanism to enhance stimulus discrimination rather than detection (Ollerenshaw et al. 2014; Waiblinger et al. 2015). These results motivate further investigation of sparse and dense coding in awake and behaving animals as well as an investigation of the cellular and circuit mechanisms involved in stimulus-dependent spike generation in L2/3 neurons.
GRANTS
This work was supported by the Australian Research Council (ARC) Discovery Project DP130101364, Future Fellowship FT20100357, and the ARC Centre of Excellence for Integrative Brain Function CE140100007.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.R.-S. and E.A. conceived and designed research; Y.R.-S. performed experiments; Y.R.-S. analyzed data; Y.R.-S. and E.A. interpreted results of experiments; Y.R.-S. prepared figures; Y.R.-S. drafted manuscript; Y.R.-S. and E.A. edited and revised manuscript; Y.R.-S. and E.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all members of the laboratory for valuable technical advice and comments.
REFERENCES
- Adibi M, Arabzadeh E. A comparison of neuronal and behavioral detection and discrimination performances in rat whisker system. J Neurophysiol 105: 356–365, 2011. doi: 10.1152/jn.00794.2010. [DOI] [PubMed] [Google Scholar]
- Adibi M, Diamond ME, Arabzadeh E. Behavioral study of whisker-mediated vibration sensation in rats. Proc Natl Acad Sci USA 109: 971–976, 2012. doi: 10.1073/pnas.1116726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar E, Kleinfeld D. Closed-loop neuronal computations: focus on vibrissa somatosensation in rat. Cereb Cortex 13: 53–62, 2003. doi: 10.1093/cercor/13.1.53. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol 86: 354–367, 2001. [DOI] [PubMed] [Google Scholar]
- Andermann ML, Moore CI. A somatotopic map of vibrissa motion direction within a barrel column. Nat Neurosci 9: 543–551, 2006. doi: 10.1038/nn1671. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lampl I, Gillespie DC, Ferster D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science 290: 1968–1972, 2000. doi: 10.1126/science.290.5498.1968. [DOI] [PubMed] [Google Scholar]
- Arabzadeh E, Panzeri S, Diamond ME. Whisker vibration information carried by rat barrel cortex neurons. J Neurosci 24: 6011–6020, 2004. doi: 10.1523/JNEUROSCI.1389-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Panzeri S, Diamond ME. Deciphering the spike train of a sensory neuron: counts and temporal patterns in the rat whisker pathway. J Neurosci 26: 9216–9226, 2006. doi: 10.1523/JNEUROSCI.1491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Petersen RS, Diamond ME. Encoding of whisker vibration by rat barrel cortex neurons: implications for texture discrimination. J Neurosci 23: 9146–9154, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh E, Zorzin E, Diamond ME. Neuronal encoding of texture in the whisker sensory pathway. PLoS Biol 3: e17, 2005. doi: 10.1371/journal.pbio.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avermann M, Tomm C, Mateo C, Gerstner W, Petersen CCH. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J Neurophysiol 107: 3116–3134, 2012. doi: 10.1152/jn.00917.2011. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J Neurosci 19: 2209–2223, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci USA 97: 8110–8115, 2000. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron 37: 513–523, 2003. doi: 10.1016/S0896-6273(02)01186-8. [DOI] [PubMed] [Google Scholar]
- Bale MR, Campagner D, Erskine A, Petersen RS. Microsecond-scale timing precision in rodent trigeminal primary afferents. J Neurosci 35: 5935–5940, 2015. doi: 10.1523/JNEUROSCI.3876-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. Single units and sensation: a neuron doctrine for perceptual psychology? Perception 1: 371–394, 1972. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci 35: 345–355, 2012. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Baudot P, Levy M, Marre O, Monier C, Pananceau M, Frégnac Y. Animation of natural scene by virtual eye-movements evokes high precision and low noise in V1 neurons. Front Neural Circuits 7: 206, 2013. doi: 10.3389/fncir.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boloori A-R, Jenks RA, Desbordes G, Stanley GB. Encoding and decoding cortical representations of tactile features in the vibrissa system. J Neurosci 30: 9990–10005, 2010. doi: 10.1523/JNEUROSCI.0807-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M. Barrel cortex and whisker-mediated behaviors. Curr Opin Neurobiol 17: 408–416, 2007. doi: 10.1016/j.conb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Brecht M, Roth A, Sakmann B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. J Physiol 553: 243–265, 2003. doi: 10.1113/jphysiol.2003.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Cortical columnar processing in the rat whisker-to-barrel system. J Neurophysiol 82: 1808–1817, 1999. [DOI] [PubMed] [Google Scholar]
- Bruno RM. Synchrony in sensation. Curr Opin Neurobiol 21: 701–708, 2011. doi: 10.1016/j.conb.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Ferster D. Membrane potential and firing rate in cat primary visual cortex. J Neurosci 20: 470–484, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Membrane potential changes in rat SmI cortical neurons evoked by controlled stimulation of mystacial vibrissae. Brain Res 448: 186–191, 1988. doi: 10.1016/0006-8993(88)91118-3. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 499: 336–340, 2013. doi: 10.1038/nature12236. [DOI] [PubMed] [Google Scholar]
- Clancy KB, Schnepel P, Rao AT, Feldman DE. Structure of a single whisker representation in layer 2 of mouse somatosensory cortex. J Neurosci 35: 3946–3958, 2015. doi: 10.1523/JNEUROSCI.3887-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kashi Malina K, Jubran M, Katz Y, Lampl I. Imbalance between excitation and inhibition in the somatosensory cortex produces postadaptation facilitation. J Neurosci 33: 8463–8471, 2013. doi: 10.1523/JNEUROSCI.4845-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S. Intracellular whole-cell patch-clamp recordings of cortical neurons in awake head-restrained mice. In: Neuronal Network Analysis. Concepts and Experimental Approaches, edited by Fellin T and Halassa M. New York: Humana, 2012, vol. 67, p. 219–235. doi: 10.1007/7657_2011_7. [DOI] [Google Scholar]
- Crochet S, Poulet JF, Kremer Y, Petersen CC. Synaptic mechanisms underlying sparse coding of active touch. Neuron 69: 1160–1175, 2011. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- de Kock CPJ, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol 581: 139–154, 2007. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeese MR, Wehr M, Zador AM. Binary spiking in auditory cortex. J Neurosci 23: 7940–7949, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Arabzadeh E. Whisker sensory system - from receptor to decision. Prog Neurobiol 103: 28–40, 2013. doi: 10.1016/j.pneurobio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Arabzadeh E. Whisker-mediated texture discrimination. PLoS Biol 6: e220, 2008. doi: 10.1371/journal.pbio.0060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Clancy KB, Jafri H, Akimenko I, Feldman DE. Cellular mechanisms for response heterogeneity among L2/3 pyramidal cells in whisker somatosensory cortex. J Neurophysiol 112: 233–248, 2014. doi: 10.1152/jn.00848.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Brecht M, Helmchen F, Petersen CC, Poulet JF, Staiger JF, Luhmann HJ, Schwarz C. Barrel cortex function. Prog Neurobiol 103: 3–27, 2013. doi: 10.1016/j.pneurobio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 50: 617–629, 2006. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Garion L, Dubin U, Rubin Y, Khateb M, Schiller Y, Azouz R, Schiller J. Texture coarseness responsive neurons and their mapping in layer 2-3 of the rat barrel cortex in vivo. eLife 3: e03405, 2014. doi: 10.7554/eLife.03405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron 65: 422–435, 2010. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 15: 607–612, 2012. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Bergner CG, Stüttgen MC, Waiblinger C, Schwarz C. Discrimination of vibrotactile stimuli in the rat whisker system: behavior and neurometrics. Neuron 65: 530–540, 2010. doi: 10.1016/j.neuron.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Barth AL. Stimulus intensity determines experience-dependent modifications in neocortical neuron firing rates. Eur J Neurosci 41: 410–419, 2015. doi: 10.1111/ejn.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick CA, Millard DC, Ortiz AD, Bellamkonda RV, Stanley GB. Response reliability observed with voltage-sensitive dye imaging of cortical layer 2/3: the probability of activation hypothesis. J Neurophysiol 115: 2456–2469, 2016. doi: 10.1152/jn.00547.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 324: 361–364, 1986. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature 493: 97–100, 2013. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature 503: 51–58, 2013. doi: 10.1038/nature12654. [DOI] [PubMed] [Google Scholar]
- Henze DA, Buzsáki G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience 105: 121–130, 2001. doi: 10.1016/S0306-4522(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Hires SA, Gutnisky DA, Yu J, O’Connor DH, Svoboda K. Low-noise encoding of active touch by layer 4 in the somatosensory cortex. eLife 4: e06619, 2015. doi: 10.7554/eLife.06619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Some quantitative aspects of vibrissa-driven neuronal responses in rat neocortex. J Neurophysiol 46: 705–715, 1981. [DOI] [PubMed] [Google Scholar]
- Ito M. Processing of vibrissa sensory information within the rat neocortex. J Neurophysiol 54: 479–490, 1985. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Feldman DE. Texture coding in the whisker system. Curr Opin Neurobiol 20: 313–318, 2010. doi: 10.1016/j.conb.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Wolfe J, Feldman DE. Sparse temporal coding of elementary tactile features during active whisker sensation. Nat Neurosci 12: 792–800, 2009. doi: 10.1038/nn.2328. [DOI] [PubMed] [Google Scholar]
- Kerr JND, de Kock CP, Greenberg DS, Bruno RM, Sakmann B, Helmchen F. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J Neurosci 27: 13316–13328, 2007. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SE, Yang H, Minamisawa G, O’Connor DH. Sensory and decision-related activity propagate in a cortical feedback loop during touch perception. Nat Neurosci 19: 1243–1249, 2016. doi: 10.1038/nn.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Diamond ME, Arabzadeh E. Sensory prioritization in rats: behavioral performance and neuronal correlates. J Neurosci 36: 3243–3253, 2016. doi: 10.1523/JNEUROSCI.3636-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Simons DJ. Angular tuning and velocity sensitivity in different neuron classes within layer 4 of rat barrel cortex. J Neurophysiol 91: 223–229, 2004. doi: 10.1152/jn.00541.2003. [DOI] [PubMed] [Google Scholar]
- Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61: 301–316, 2009. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol 13: 493–497, 2003. doi: 10.1016/S0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein SH, Carvell GE, Simons DJ. Responses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosens Mot Res 7: 47–65, 1990. doi: 10.3109/08990229009144697. [DOI] [PubMed] [Google Scholar]
- Lottem E, Azouz R. Mechanisms of tactile information transmission through whisker vibrations. J Neurosci 29: 11686–11697, 2009. doi: 10.1523/JNEUROSCI.0705-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc Biol Sci 274: 1035–1041, 2007. doi: 10.1098/rspb.2006.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1: 31–37, 2004. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- O’Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. Vibrissa-based object localization in head-fixed mice. J Neurosci 30: 1947–1967, 2010a. doi: 10.1523/JNEUROSCI.3762-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67: 1048–1061, 2010b. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Ollerenshaw DR, Bari BA, Millard DC, Orr LE, Wang Q, Stanley GB. Detection of tactile inputs in the rat vibrissa pathway. J Neurophysiol 108: 479–490, 2012. doi: 10.1152/jn.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerenshaw DR, Zheng HJ, Millard DC, Wang Q, Stanley GB. The adaptive trade-off between detection and discrimination in cortical representations and behavior. Neuron 81: 1152–1164, 2014. doi: 10.1016/j.neuron.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol 14: 481–487, 2004. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Peron SP, Freeman J, Iyer V, Guo C, Svoboda K. A cellular resolution map of barrel cortex activity during tactile behavior. Neuron 86: 783–799, 2015. doi: 10.1016/j.neuron.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Crochet S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron 78: 28–48, 2013. doi: 10.1016/j.neuron.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Hartings JA, Brumberg JC, Simons DJ. Cortical damping: analysis of thalamocortical response transformations in rodent barrel cortex. Cereb Cortex 13: 33–44, 2003. doi: 10.1093/cercor/13.1.33. [DOI] [PubMed] [Google Scholar]
- Pinto DJD, Brumberg JCJ, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol 83: 1158–1166, 2000. [DOI] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci 12: 1577–1585, 2009. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454: 881–885, 2008. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Kreiman G, Koch C, Fried I. Sparse but not ‘grandmother-cell’ coding in the medial temporal lobe. Trends Cogn Sci 12: 87–91, 2008. doi: 10.1016/j.tics.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Pnevmatikakis EA, Merel J, Paninski L, Miller KD, Bruno RM. Spatiotemporal receptive fields of barrel cortex revealed by reverse correlation of synaptic input. Nat Neurosci 17: 866–875, 2014. doi: 10.1038/nn.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57: 599–613, 2008. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ. Sparseness of the neuronal representation of stimuli in the primate temporal visual cortex. J Neurophysiol 73: 713–726, 1995. [DOI] [PubMed] [Google Scholar]
- Sachdev RNS, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol 92: 3511–3521, 2004. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y, Petersen CCH. Membrane potential correlates of sensory perception in mouse barrel cortex. Nat Neurosci 16: 1671–1677, 2013. doi: 10.1038/nn.3532. [DOI] [PubMed] [Google Scholar]
- Sato TR, Gray NW, Mainen ZF, Svoboda K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol 5: e189, 2007. doi: 10.1371/journal.pbio.0050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, O’Connor DH, Segev R. How silent is the brain: is there a “dark matter” problem in neuroscience? J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 777–784, 2006. doi: 10.1007/s00359-006-0117-6. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol 41: 798–820, 1978. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol 54: 615–635, 1985. [DOI] [PubMed] [Google Scholar]
- Spanne A, Jörntell H. Questioning the role of sparse coding in the brain. Trends Neurosci 38: 417–427, 2015. doi: 10.1016/j.tins.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Taub AH, Katz Y, Lampl I. Cortical balance of excitation and inhibition is regulated by the rate of synaptic activity. J Neurosci 33: 14359–14368, 2013. doi: 10.1523/JNEUROSCI.1748-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7: 27–41, 1998. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Waiblinger C, Brugger D, Whitmire CJ, Stanley GB, Schwarz C. Support for the slip hypothesis from whisker-related tactile perception of rats in a noisy environment. Front Integr Neurosci 9: 53, 2015. doi: 10.3389/fnint.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Webber RM, Stanley GB. Thalamic synchrony and the adaptive gating of information flow to cortex. Nat Neurosci 13: 1534–1541, 2010. doi: 10.1038/nn.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci 24: 3985–3998, 2004. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Stimulus-dependent changes in spike threshold enhance feature selectivity in rat barrel cortex neurons. J Neurosci 25: 2983–2991, 2005a. doi: 10.1523/JNEUROSCI.4906-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci 8: 1364–1370, 2005b. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol 6: e215, 2008. doi: 10.1371/journal.pbio.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J, Houweling AR, Brecht M. Sparse and powerful cortical spikes. Curr Opin Neurobiol 20: 306–312, 2010. doi: 10.1016/j.conb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci 29: 70–85, 2009. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Shu Y, McCormick DA. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J Neurosci 28: 7260–7272, 2008. doi: 10.1523/JNEUROSCI.1613-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol 81: 1171–1183, 1999. [DOI] [PubMed] [Google Scholar]