GDNF family neurotrophic factors regulate the development and function of primary sensory neurons. Of these, neurturin has been shown to modulate mechanical and cooling sensitivity behaviorally. Here we show that overexpression of neurturin in basal keratinocytes regulates mechanical responsiveness in A-fiber primary sensory neurons while increasing the overall numbers of cold-sensing units. Results demonstrate a crucial role for cutaneous neurturin in modulating responsiveness to peripheral stimuli at the level of the primary afferent.
Keywords: pain, neurturin, nociceptors, mechanical sensitivity, thermal responsiveness
Abstract
Neurotrophic factors play an important role in the regulation of functional properties of sensory neurons under normal and pathological conditions. The GDNF family member neurturin is one such factor that has been linked to modulating responsiveness to peripheral stimuli. Neurturin binds to the GFRα2 receptor, a receptor found primarily in isolectin B4-expressing polymodal cutaneous nociceptors. Previous work has shown that knockout of GFRα2 alters heat, but not mechanical, responses in dissociated sensory neurons and reduces pain-related behaviors during the second phase of the formalin test. Research has also shown that overexpression of neurturin in basal keratinocytes increases behavioral responsiveness to mechanical stimulation and innocuous cooling of the skin without affecting noxious heat responses. Here we directly examined the impact of neurturin overexpression on cutaneous afferent function. We compared physiological responses of individual sensory neurons to mechanical and thermal stimulation of the skin, using an ex vivo skin-nerve-dorsal root ganglion-spinal cord preparation produced from neurturin-overexpressing (NRTN/OE) mice and wild-type littermate controls. We found that neurturin overexpression increases responsiveness to innocuous mechanical stimuli in A-fiber nociceptors, alters thermal responses in the polymodal subpopulation of C-fiber sensory neurons, and changes the relative numbers of mechanically sensitive but thermally insensitive C-fiber afferents. These results demonstrate the potential roles of different functional groups of sensory neurons in the behavioral changes observed in mice overexpressing cutaneous neurturin and highlight the importance of neurturin in regulating cutaneous afferent response properties.
NEW & NOTEWORTHY GDNF family neurotrophic factors regulate the development and function of primary sensory neurons. Of these, neurturin has been shown to modulate mechanical and cooling sensitivity behaviorally. Here we show that overexpression of neurturin in basal keratinocytes regulates mechanical responsiveness in A-fiber primary sensory neurons while increasing the overall numbers of cold-sensing units. Results demonstrate a crucial role for cutaneous neurturin in modulating responsiveness to peripheral stimuli at the level of the primary afferent.
neurotrophic factors, whose role is well documented in the development and maintenance of primary sensory neurons (Albers et al. 2006; Baudet et al. 2000; Davies 1997; Elitt et al. 2006; Fariñas et al. 2002; Heuckeroth et al. 1999; Kirstein and Fariñas 2002; Zwick et al. 2002), have also been shown to play a significant role in the regulation of nociceptor function after injury and inflammation (Elitt et al. 2006; Jankowski et al. 2009, 2010; Wang et al. 2013). The glial cell line-derived neurotrophic factor (GDNF) family comprises three main ligands that can influence sensory neurons: GDNF, neurturin, and artemin. They exert their action by binding to the Ret receptor tyrosine kinase and the glycophosphatidylinositol-linked family coreceptors GFRα1–3 (see, e.g., Bennett et al. 2000). Interestingly, each of these receptors appears to be expressed differentially across anatomical populations with varying physiological phenotypes. Artemin and its receptor, GFRα3, appear to impact the function of cutaneous peptidergic C-heat neurons (Jankowski et al. 2010), while GDNF and its receptor, GFRα1, appear to impact the function of nonpeptidergic C-polymodal neurons (Molliver et al. 1997).
Comparatively, the role of neurturin in the processing of noxious information from the periphery is not well defined. Previous work has shown that GFRα2 is primarily located in cutaneous neurons with small-diameter cell bodies and unmyelinated axons that bind isolectin B4 (IB4), and functionally transduce mechanical and thermal stimuli (Honma et al. 2010; Kupari and Airaksinen 2014; Stucky et al. 2002), consistent with what is known about C-polymodal afferents. Previously, in vitro electrophysiological recordings of sensory neurons in GFRα2-knockout mice have suggested that neurturin and GFRα2 signaling is required for the transduction of noxious heat but not mechanical stimuli in IB4-binding nociceptors (Stucky et al. 2002). Additionally, GFRα2-knockout mice exhibit attenuated behavioral responses during the second phase of the formalin test (Lindfors et al. 2006). Behaviorally, mice that overexpress neurturin in basal keratinocytes exhibit increased sensitivity to cool temperatures, but not noxious cold, and increased mechanical sensitivity (Wang et al. 2013). While conflicting, these previous results demonstrate that neurturin and GFRα2 activation is required for nociceptive processing across multiple modalities. However, it is not clear how altered neurturin/GFRα2 signaling actually affects the response properties of specific types of cutaneous sensory neurons. To address this question, we used our ex vivo skin-nerve-dorsal root ganglion (DRG)-spinal cord preparation from neurturin-overexpressing (NRTN/OE) mice originally described in Wang et al. (2013) and compared them to their wild-type (WT) littermates. We show that overexpression of neurturin in basal keratinocytes alters afferent responses in a stimulus- and (neuronal) class-specific manner.
MATERIALS AND METHODS
Generation of transgenic mice.
NRTN/OE mice were generated and screened according to procedures described previously (Albers et al. 1994, 1996; Elitt et al. 2006; Wang et al. 2013; Zwick et al. 2002). Briefly, after restriction digest of IMAGE Clone no. 5345262 (GenBank accession no. BC057993; Invitrogen), a 645-bp fragment containing the coding region of mouse NRTN was cloned into the pG4K14 pro-human growth hormone (hGH) vector. Gel- and column-purified fragments containing our NRTN insert along with a 2.3-kb segment of the human K14 keratin promoter sequence and a 1.4-kb portion of the hGH gene-containing intron/exon and poly(A) signal sequences at the 3′ end was injected into fertilized oocytes from C57BL/6 mice. Transgenic development was provided by the Transgenic and Gene Targeting Core Facility at the University of Pittsburgh. Three founder lines were identified by slot blot analysis, and RT-PCR analysis of total RNA isolated from back skin was used to assay the relative level of transgene expression. Only the lines that were found to have the highest expression were used in the analyses presented here. Primers to detect endogenous and transgenic NRTN as well as hGH-specific primers were described in Wang et al. (2013).
Animals.
Experiments were performed on 6- to 8-wk-old male WT and NRTN/OE littermate mice. Mice were housed in group cages except where noted, maintained on a 12:12-h light-dark cycle in a temperature-controlled environment, and given food and water ad libitum. All studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Ex vivo preparation.
The ex vivo skin-nerve-DRG-spinal cord preparation utilized in these experiments is described elsewhere (McIlwrath et al. 2007; Woodbury et al. 2001). Briefly, mice were anesthetized with a 90–10 mg/kg mixture of ketamine and xylazine (im) and were transcardially perfused with oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (aCSF; in mM: 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 d-glucose) containing 253.9 mM sucrose at 12–15°C. The spinal cord and the right hindlimb were then dissected and placed in a bath of cold aCSF, and the hairy skin, saphenous nerve, DRG, and spinal column were dissected in continuity. The preparation was transferred to a separate chamber containing chilled oxygenated aCSF in which the sucrose was replaced with 127.0 mM NaCl. The skin was placed on an elevated grid, such that the epidermis was exposed to air and the dermis was continually perfused. Once the preparation was stabilized, the temperature of the aCSF was warmed to 31°C before recording.
Electrophysiological recording and stimulation.
In all cases, the experimenter was blind to the mouse genotype. Sensory neuron somata were impaled with quartz microelectrodes containing 5% Neurobiotin (Vector Laboratories, Burlingame, CA) in 1 M potassium acetate. Orthograde electrical search stimuli were administered through a suction electrode placed on the saphenous nerve to locate sensory neuron somata innervating the skin. Receptive fields (RFs) were localized with a paintbrush, blunt glass stylus, and/or von Frey filaments. If an electrically driven cell with no mechanical RF was located, a thermal search was performed by applying hot (~51°C) and/or cold (~0°C) 0.9% saline to the skin. Previous work (Jankowski et al. 2009; Lawson et al. 2008) has shown that repeated brief applications of hot saline do not result in nociceptor sensitization.
Response characteristics of DRG neurons were determined by applying digitally controlled mechanical and thermal stimuli. The mechanical stimulator consisted of a tension/length controller (Aurora Scientific, Aurora, ON, Canada) attached to a 1-mm-diameter plastic disk. Computer-controlled 5-s square waves of 1, 5, 10, 25, 50, and 100 mN were applied to the cells’ RF. After mechanical stimulation, a controlled thermal stimulus was applied with a 3-mm2-contact area Peltier element (Yale University Machine Shop, New Haven, CT). The temperature stimulus consisted of first rapid cooling the RF to <5°C for 5 s and then slowly returning to 31°C. Then a 12-s heat ramp from 31°C to 52°C followed by a 5-s holding phase was applied to the RF. The temperature ramped back down to 31°C in 12 s. A 30-s resting period was inserted between stimulus presentations. In some instances, fibers that were unable to be characterized by computer-controlled mechanical and/or thermal stimulation but were phenotyped by von Frey and/or saline stimuli were not included in threshold determination. All elicited responses were recorded digitally for off-line analysis (Spike 2 software, Cambridge Electronic Design, Cambridge, UK). After physiological characterization, select cells were labeled by iontophoretic injection of Neurobiotin. Peripheral conduction velocity was then calculated from spike latency and the distance between stimulation and recording electrodes (measured directly along the saphenous nerve). Thermal thresholds were determined to be the temperature change for fibers that did not exhibit ongoing activity before thermal stimulation. For those fibers that did have some degree of ongoing activity, threshold was determined as the temperature at the second spike of two, where the instantaneous frequency exceeded that present in a 30-s window before thermal stimulation. Peak instantaneous frequencies allow for the measurement of the peak firing capacity of a cell in response to the stimulus. These are calculated as the shortest interval between two spikes detected during the stimulus.
Classification of cutaneous A- and C-fiber sensory neurons.
Sensory neurons with a conduction velocity of <1.2 m/s were classified as C fibers, and all others were classified as A fibers. Conduction velocities between 1.2 and 10 m/s were considered to be in the Aδ range, and those ≥ 10 m/s were classified as conducting in the Aβ range (Koltzenburg et al. 1997; Kress et al. 1992; Lawson et al. 1993; Lawson and Waddell 1991; McIlwrath et al. 2007).
For the present experiments, we have focused our recording and analyses specifically on the A-fiber high-threshold mechanoreceptors (A-HTMRs) and various C-fiber subpopulations. The C fibers specifically were classified as follows: 1) C-polymodal (CPM), meaning those that responded to mechanical and heat stimuli (CMH) and sometimes cool/cold stimuli (CMHC); 2) C-mechano (CM), those that responded only to mechanical stimulation of the skin; 3) C-mechano cool/cold (CMC), those that responded to mechanical and cooling stimuli (but not heating); 4) C-heat (CH), those that were mechanically insensitive but heat sensitive, and 5) C-cooling/cold (CC), those that were mechanically insensitive but responded to cooling of the skin.
Data analysis.
One-way and mixed-design analysis of variance (ANOVA) tests were used to analyze firing rates and peak instantaneous frequencies, as well as mechanical and thermal thresholds. Post hoc analysis was conducted with Tukey’s test. Neurons were sorted by functional type to construct relative distributions. Comparisons between distributions were analyzed by χ2-test. Statistical significance was maintained at α = 0.05. The graphed data are plotted as means ± SE.
RESULTS
Summary of electrophysiological recordings.
In these studies we have analyzed the response properties of 182 characterized cutaneous afferents. Of these 29 were A-HTMRs (WT = 10; NRTN/OE = 19) and 153 were C fibers (WT = 51; NRTN/OE = 102) recorded from 25 mice (WT = 14; NRTN/OE = 11). We first determined the percentage of C fibers that responded to specific modalities in WT and NRTN/OE mice. We found that 90.2% of WT cells were temperature sensitive, with 86.3% responding to heat and 15.7% responding to cold. Comparatively, 79.6% of NRTN/OE cells were temperature sensitive, with 69.9% responding to heat and 30.1% responding to cold (Table 1). The increased percentage of cold-sensitive cells in NRTN/OE mice reached statistical significance (χ2-test, P < 0.05). With respect to mechanical sensitivity, 78.4% of WT cells responded to mechanical stimulation, while 81.6% of NRTN/OE cells responded to mechanical stimulation. A subset of mechanically sensitive fibers was also heat sensitive. For WT cells, 84.3% of mechanically sensitive C fibers were also heat sensitive, while only 68.9% of NRTN/OE mechanically sensitive C fibers also responded to heat. We also found a significant increase in the percentage of cells that were heat, cold, and mechanically responsive in NRTN/OE mice (20.4%) compared with WT control mice (11.8%) (χ2-test, P < 0.05).
Table 1.
Percentages of characterized cells based on modality responsiveness from WT and NRTN/OE mice
| Cell Phenotype | WT % Responders | NRTN/OE % Responders |
|---|---|---|
| Temperature | 90.2 | 79.6 |
| Heat | 86.3 | 69.9 |
| Cold | 15.7 | 30.1* |
| Mechanical | 78.4 | 81.6 |
| Mechanical + heat | 84.3 | 69.0 |
| Mechanical + heat + cold | 11.8 | 20.6* |
P < 0.05 vs. WT.
We then analyzed the various defined populations of C fibers in order to determine the specific subtype responsible for observed alterations in modality prevalence. WT C fibers were comprised of 9.8% CM, 0% CC, 21.6% CH, 3.9% CMC, 52.9% CMH, and 11.8% CMHC from 14 mice. The distribution of NRTN/OE afferents consisted of 19.6% CM, 3.9% CC, 13.7% CH, 5.9% CMC, 36.3% CMH, and 20.6% CMHC from 11 mice (Fig. 1). Comparison of the afferent distributions revealed significant differences in the relative percentages of fibers in each individual class (χ2-test, P < 0.05). This significant difference resulted from an increase in the percentage of CMs and a decrease in the percentage of CMH and CH fibers in NRTN/OE mice.
Fig. 1.
WT and NRTN/OE afferent subpopulation distributions. Analysis revealed significant differences in the distribution of C fibers. This was found to be due to a greater percentage of CMs and a lower percentage of CHs and CMHs in the NRTN/OEs compared with WT controls. *P < 0.05, χ2-test.
Finally, we also analyzed mean C-fiber conduction velocities in WT and NRTN/OE C fibers and found that NRTN/OE C fibers had significantly greater mean conduction velocities than WT C fibers (NRTN/OE = 0.6 ± 0.1 m/s, WT = 0.5 ± 0.1 m/s). This finding complements previous results demonstrating that neurturin overexpression resulted in the hypertrophy of peripheral nerves (Wang et al. 2013).
Neurturin overexpression alters mechanical response properties of A-fiber HTMRs.
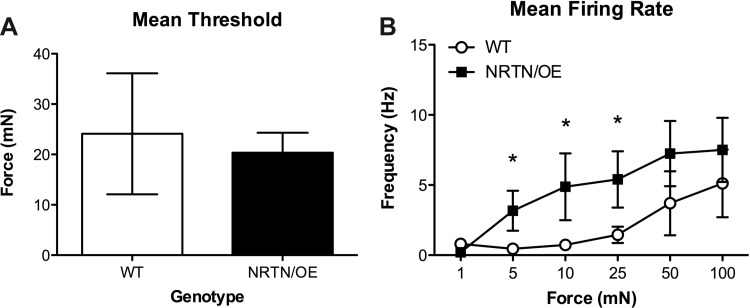
While we were primarily concerned with alterations in response characteristics in unmyelinated afferents, previously published work has shown that overexpression of NRTN in basal keratinocytes increased mechanical responsiveness (Wang et al. 2013). Given this result we also examined firing properties in 10 WT and 19 NRTN/OE myelinated nociceptors. A-fiber nociceptors with Aβ and Aδ conduction velocities were examined in this study; however, data obtained from these separate populations were not found to be statistically different (not shown) and were thus combined for ease of presentation. We did not observe any significant differences in mechanical thresholds (means ± SE) in WT (24.1 ± 12.7 mN) or NTRN/OE (20.4 ± 4.0 mN) mice [t(27) = 0.01, P > 0.05; Fig. 2A]. However, analysis of firing rates over the range of stimulation forces presented did reveal significant differences between the two genotypes. Specifically, NRTN/OE mice exhibited greater mean firing rates at 5, 10, and 25 mN of force delivered to the skin (Fig. 2B). A similar result was obtained when peak instantaneous frequency (IF) was analyzed (all F > 8.35, P < 0.001; data not shown), suggesting that NRTN/OE A-fiber nociceptors are more sensitive to mechanical stimulation, particularly within the innocuous range. Although a few (WT = 3; NRTN/OE = 1) A-HTMRs responded to heat in our recording studies, because of the low numbers acquired we were unable to assess heat responses in these subtypes.
Fig. 2.
Analysis of A-fiber HTMR mean mechanical threshold (A) and mean firing rates (B) from WT and NRTN/OE mice. No differences in mechanical threshold were observed; however, NRTN/OE afferents did exhibit greater response rates than WT afferents at the lower range of mechanical forces delivered to the skin. *P < 0.05 for NRTN/OE (n = 19) vs. WT (n = 10).
Neurturin overexpression alters mechanical response properties in C-mechano afferents.
In addition to the general analysis performed above, we also examined response properties of specific physiological classes of C fibers. We first examined the response properties of characterized CMs. We characterized 5 CM fibers in WT mice and 20 in NRTN/OE mice. Analysis of this limited sample suggests that NRTN/OE CM thresholds were higher than WT CM thresholds (86.0 vs. 25.0 mN; not shown). However, this result should be taken with some caution, as many of the fibers recorded from both conditions have thresholds greater than the calibrated forces we use for phenotypic analysis, leaving a low number of cells with threshold data (NRTN/OE = 5/20 cells; WT = 1/5 cells) and an inability to assess firing rates in response to mechanical stimulation. As mentioned above, we did find a significantly greater percentage of CM fibers in NRTN/OE mice compared with WT control mice (see Fig. 1).
Neurturin overexpression alters thermal response properties in C-heat afferents.
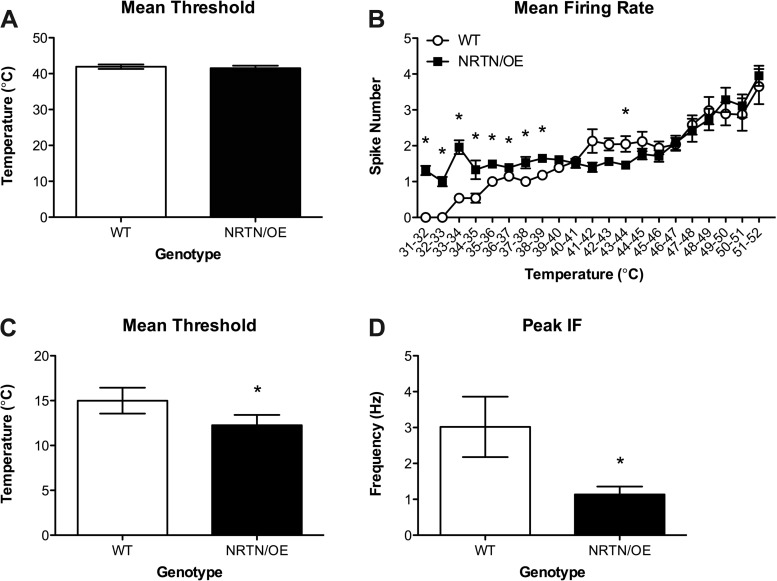
To determine the effect of neurturin overexpression on thermal responses, we next examined response properties of mechanically insensitive but heat-sensitive (CH) neurons. No differences in heat threshold were detected between WT (42.7 ± 1.3°C, n = 8) and NRTN/OE (41.8 ± 1.6°C, n = 11) mice [t(16) = −0.39, P > 0.05; Fig. 3A]. We next examined mean spike numbers over the range of temperatures presented to the skin (35–52°C). Analysis revealed that NRTN/OE CHs fired significantly more spikes from 48°C to 50°C [all t >3.51, P < 0.01; Fig. 3B], suggesting that NRTN/OE CHs are more sensitive to noxious heat than WT CHs within this distinct range of temperatures. Finally, we compared WT (27.6 ± 6.5 Hz) and NRTN/OE (33.8 ± 3.7 Hz) CH maximum peak instantaneous firing frequencies. No significant differences were detected between genotypes [t(16) = −0.9, P > 0.05; data not shown].
Fig. 3.
Analysis of CH mean threshold (A) and mean firing rates across increasing temperatures (B) delivered to the skin for WT and NRTN/OE mice. While there was no difference in heat threshold, we did observe a slight increase in WT CH firing rates at innocuous temperatures and a slight increase in NRTN/OE CH firing rates in response to noxious heat stimulation. *P < 0.05 for NRTN/OE (n = 11) vs. WT (n = 8).
Neurturin overexpression alters thermal response properties in C-polymodal afferents.
Next, we examined how neurturin overexpression impacts responses in polymodal C fibers (CPMs). Specifically, we examined CPMs that were sensitive to mechanical and heat stimulation (CMH; WT n = 27, NRTN/OE n = 37) or those that were sensitive to cold simulation in addition to mechanical and heat stimulation (CMHC; WT n = 6, NRTN/OE n = 21). While these cells have different phenotypic properties, we were only able to characterize six CMHCs from WT animals. Therefore, to increase our statistical power and reliability, we combined CMHs and CMHCs to understand how neurturin overexpression impacts functional characteristics of these polymodal cell types.
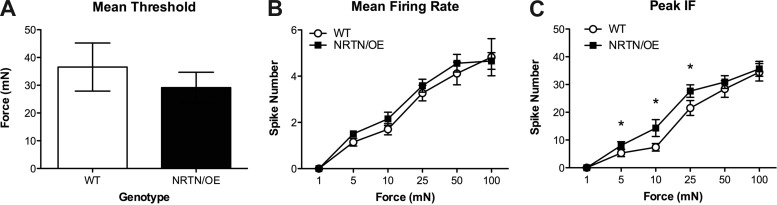
We began our analysis by examining mechanical responses in CPMs from WT and NRTN/OE mice and found no significant difference in mechanical thresholds between WT (30.2 ± 6.9 mN) and NRTN/OE (29.2 ± 5.6 mN) mice [t(85) < 1.00, P > 0.05; Fig. 4A]. While analysis of mean firing rates failed to detect any differences in responsiveness (all F < 1.97, P > 0.05; Fig. 4B), analysis of peak IFs did reveal that NTRN-OE CPMs exhibited significantly higher peak IFs at 5, 10, and 25 mN of stimulation (all t > 2.35, P < 0.05; Fig. 4C).
Fig. 4.
Analysis of CPM mean mechanical threshold (A), mean firing rate across stimulation force (B), and peak IF across stimulation force (C) for WT and NRTN/OE mice. NRTN/OE CPMs did not display any differences in average mechanical threshold or mean firing to mechanical deformation of the skin. However, NRTN/OE CPMs exhibited greater peak IFs from 5 to 25 mN of force than WT CPMs. *P < 0.05 for NRTN/OE (n = 58) vs. WT (n = 33).
Analysis of heat thresholds did not reveal a significant difference between WT (41.76 ± 0.76°C) and NRTN/OE (41.5 ± 0.68°C) mice [t(77) = 0.22, P > 0.05; Fig. 5A]; however, analysis of average spike number per degree of stimulation did reveal that NRTN/OE CPMs exhibited greater firing rates from 31°C to 38°C compared with WT firing rates (all t > 2.58, P < 0.05; Fig. 5B). Interestingly, examination of cooling responses in cold-sensitive CPMs revealed that NRTN/OE CMHCs had lower cold thresholds and maximum peak IFs than WT CMHCs (all t > 2.42, P < 0.05; Fig. 5, C and D). Taken together, these results demonstrate that neurturin overexpression increases CPM sensitivity to innocuous mechanical forces and heating while decreasing sensitivity to cold stimulation within specific afferent groups.
Fig. 5.
Analysis of CPM mean heat threshold (A), average firing rate in response to heating of the skin at various temperatures (B), mean cold threshold (C), and mean peak IF in response to cooling of the skin (D) for WT and NRTN/OE mice. While no differences in heat threshold were observed, we did find a significant increase in the firing rates of NRTN/OE CPMs from 31°C to 38°C. Analysis also revealed that NRTN/OE CPMs possess significantly lower cold thresholds and lower mean peak IFs in response to cooling than WT CPMs. *P < 0.05 for NRTN/OE (n = 58) vs. WT (n = 33).
DISCUSSION
The present experiments examined the effect of neurturin overexpression in basal keratinocytes on the response properties of cutaneous afferents. We found that neurturin overexpression changed the distribution of functional cell types within the DRG, increasing the percentage of identified CMs and decreasing the percentages of identified CHs and CMHs (Fig. 1). Overexpression of neurturin appeared to have the most significant impact on cells that responded to cold stimulation alone and those that responded to mechanical and heat stimulation in addition to cold. Overexpression of neurturin did have an apparent small effect on mechanically insensitive, heat-sensitive cells (Fig. 3), but this was only observed across a small range of noxious temperatures. It is important to note that these differences appear to be due to a reduction of sensitivity in the WT fibers at these temperatures. In addition, these peptidergic CH fibers normally do not express GFRα2. Thus we believe these results do not demonstrate effects on heat sensitivity in this population. However, there was a small but significant increase in the heat response of CPM fibers to a range of innocuous temperatures (Fig. 5). We have previously shown that the majority of these fibers bind IB4 (Lawson et al. 2008), and they have been shown to express GFRα2 (Honma et al. 2010; Kupari and Airaksinen 2014; Stucky et al. 2002), so it is of note that this increase in heat sensitivity is consistent with the decreased in vitro heat sensitivity observed in IB4-binding neurons from GFRα2-knockout mice (Stucky et al. 2002). It should also be noted that while CH fibers (Fig. 3) are firing at higher mean rates than the CPM fibers (Fig. 5) this is not an effect of the overexpression, as we have shown previously that CH fibers normally fire at higher rates (Lawson et al. 2008).
In a previous study, Wang et al. (2013) demonstrated that neurturin overexpression resulted in a decreased sensitivity to noxious cold. Interestingly, they also showed an increased behavioral sensitivity to innocuous cold exposure when mice were presented with a two-temperature discrimination task, whereby NRTN/OE mice preferred the warmer of the two choices. This increased sensitivity was correlated with increased TRPM8 expression in sensory ganglia. When neuronal responses were examined with Ca2+ imaging, cutaneous afferents showed increased Ca2+ influx in response to menthol exposure, further supporting the notion that increased TRPM8 expression played a role in the increased sensitivity observed to cooling sensation.
The present results support these seemingly contradictory observations. First, we have shown that, although there is a significant increase in the percentage of cold-sensitive cells, those classified as polymodal nociceptors (CMHCs; Fig. 1) exhibited decreased cold responsiveness (Fig. 5). This was apparent in both a decrease in average cold threshold and a decrease in peak instantaneous firing frequencies in these neurons, which allows for the assessment of the peak capacity of a cell to fire to a given stimulus. These results could explain the decrease in cold pain sensitivity in the transgenic mice (Wang et al. 2013). Second, we have also found more CC and CMC fibers in the NRTN/OE mice, which could contribute to the increased sensitivity to cool temperature in these mice (Wang et al. 2013). For example, in another as yet unpublished study in our laboratory, we have found that TRPM8-positive/menthol-sensitive cutaneous fibers were always functionally classified as CC or CMC. Therefore, given the known threshold for TRPM8 (Bautista et al. 2007), an increase in the percentage of these fibers in NRTN/OE mice could be contributing to thermal preference observed. However, it should be noted that Campero et al. (2009) and Milenkovic et al. (2014) have suggested that at least a portion of CMHC fibers do express TRPM8. If this is true, the increased expression of TRPM8 in these fibers in the present study did not result in an increase in cold threshold as would be predicted for TRPM8.
The previous study (Wang et al. 2013) also demonstrated behavioral increases in mechanical sensitivity in the NRTN/OE mice. Here we demonstrate increases in mechanical sensitivity in both A-HTMRs as well as CPM fibers. This alteration in mechanical responsiveness was not apparent in mechanical thresholds. Rather, firing rates (mean peak or instantaneous frequencies) were increased in these mechanically sensitive cells, particularly within the range of 5–25 mN of force for A-HTMRs, suggesting that these cells are more sensitive to innocuous intensities of stimulation. As GFRα2 has previously been shown to be expressed in large-, medium-, and small-diameter fibers (Bennett et al. 2000; Wang et al. 2013), it is not surprising that NRTN/OEs have altered mechanical sensitivity in these various fiber types. Interestingly, neurturin overexpression did not seem to impact all mechanically sensitive afferents in the same manner. When response properties from CM fibers were examined we found that the cells had increased response thresholds. The apparent alteration in response properties occurred with a concomitant increase in the number of identified CM fibers, suggesting that neurturin overexpression may have resulted in a switch in neuronal phenotype. Given that fewer CMH afferents were identified in NRTN/OE mice compared with WT mice, it is possible that increased levels of neurturin resulted in a loss of heat sensitivity in a portion of CMHs, driving them into a CM phenotype. It is not yet clear what potentially makes a specific subtype of CMHs susceptible to neurturin or why heat response would be lost. This particular result should be taken with some caution, as this effect was observed in a small number of CM fibers, and additional work will be required to fully understand this effect. Interestingly, a previous study examining the effects of neonatal administration of anti-nerve growth factor (Lewin and Mendell 1994) reported a similar reduction in CMH fibers and the appearance of a novel CM population presumably within the peptidergic (TrkA+) population of nociceptors, albeit with lowered mechanical thresholds.
In their previous study Wang et al. (2013) showed that the increase in behavioral responsiveness to mechanical stimuli was associated with increased expression of the ASIC2a channel. ASIC2a is a sodium channel that is expressed in low-threshold mechanoreceptors (LTMRs) (Price et al. 2000), but in the present experiments we did not find increased responses in LTMRs. However, Wang et al. (2013) hypothesized that increased ASIC2a may have occurred in unmyelinated cutaneous afferents. While we did not examine expression of ASIC2a in individual physiologically characterized afferents, we did observe an increase in the percentage of CMs characterized in the NRTN/OE sensory ganglia, as well as increased mechanical firing rates in myelinated HTMRs and CPMs.
Neurotrophic factors not only influence sensory neuron development but also modulate and maintain sensory neuron phenotype in the adult animal. Prior work has shown that deletion of GDNF reduces the number of DRG neurons (Moore et al. 1996), while overexpression of GDNF has the opposite effect (Zwick et al. 2002). GDNF-sensitive neurons, like the neurturin-sensitive C fibers, tend to be IB4-binding afferents, and overexpression of GDNF resulted in reduced mechanical thresholds compared with WT controls (Albers et al. 2006). Furthermore, in the GDNF/OE mice, virtually all the mechanosensitive C fibers also responded to heat (Albers et al. 2006). Conversely, here we demonstrate an increase in the percentage of C fibers that only respond to mechanical stimulation, suggesting the possibility that GDNF and neurturin could have opposite effects on the phenotype of these IB4-binding neurons. Furthermore, neurturin also regulates sensory neuron proliferation, and deletion of neurturin results in reduced cutaneous innervation by nonpeptidergic fibers (Lindfors et al. 2006). Deletion of neurturin did not alter peptidergic innervation or the detection of noxious heat (Lindfors et al. 2006), suggesting that neurturin may not regulate function of the peptidergic cutaneous nociceptors, such as CH fibers (Lawson et al. 2008). Finally, overexpression of artemin also increases the numbers of sensory neurons, but these neurons express TRPV1 and GFRα3 (Ikeda-Miyagawa et al. 2015), suggesting that these neurons have a peptidergic identity (Jankowski et al. 2009, 2010; Lawson et al. 2008) and that artemin may regulate thermal (Malin and Davis 2008; Malin et al. 2006) and mechanical (Ikeda-Miyagawa et al. 2015) sensitivity of these peptidergic fibers.
Interestingly, the above results from the overexpression of GDNF and artemin resemble what has been previously reported after nerve injury. For example, nerve injury and inflammation impact the balance of neurotrophic factor expression, and prior work has shown that peripheral nerve cut increases GDNF and artemin in the skin as well as GFRα1 and GFRα3 receptors in the DRG (Baudet et al. 2000; Jankowski et al. 2010). Interestingly, nerve transection does not increase neurturin expression in the skin, and subsequent regeneration occurs in the absence of changes in GFRα2 expression (Baudet et al. 2000). This suggests that nerve injury and regeneration-induced changes in the balance of neurotrophic factor receptor expression functionally impact affected primary afferents. Supporting this, the increase in GFRα3 following nerve injury apparently results in an increase in TRPV1 expression during reinnervation of the skin (Jankowski et al. 2010), and these receptors are coexpressed in murine CH fibers (Jankowski et al. 2009, 2010). After injury, some CH afferents exhibit a phenotypic switch and gain mechanical sensitivity, resembling naive CPMs (Jankowski et al. 2009, 2010; Lawson et al. 2008; Woodbury et al. 2004), and this recruitment of CHs is associated with a reduction of CPM thermal thresholds (Jankowski et al. 2010). The reduction in CPM threshold likely occurs from the recruitment of CHs, which are more sensitive to thermal stimulation than the highly mechanically sensitive CPMs. In sum, our results demonstrate that neurotrophic factors, including neurturin, play an important role in nociceptor sensitization and may prove to be valuable targets for therapeutic intervention for patients with various pain states.
GRANTS
This work was funded by National Institute of Neurological Disorders and Stroke Grants R01 NS-23725 (H. R. Koerber) and R01 NS-052848 (H. R. Koerber).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.P.J., T.W., and H.R.K. performed experiments; M.P.J., K.M.B., and T.W. analyzed data; M.P.J., K.M.B., K.M.A., B.M.D., and H.R.K. interpreted results of experiments; M.P.J., K.M.B., and H.R.K. edited and revised manuscript; M.P.J., K.M.B., K.M.A., B.M.D., and H.R.K. approved final version of manuscript; K.M.B. prepared figures; K.M.B. and H.R.K. drafted manuscript.
ACKNOWLEDGMENTS
Present addresses: M. P. Jankowski, Div. of Pain Management, Dept. of Anesthesia, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229; K. M. Baumbauer, Center for Advancement in Managing Pain, University of Connecticut School of Nursing, Storrs, CT 06269.
REFERENCES
- Albers KM, Perrone TN, Goodness TP, Jones ME, Green MA, Davis BM. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J Cell Biol 134: 487–497, 1996. doi: 10.1083/jcb.134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci 26: 2981–2990, 2006. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci 14: 1422–1432, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet C, Mikaels A, Westphal H, Johansen J, Johansen TE, Ernfors P. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development 127: 4335–4344, 2000. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci 20: 427–437, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol 587: 5633–5652, 2009. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM. Studies of neurotrophin biology in the developing trigeminal system. J Anat 191: 483–491, 1997. doi: 10.1046/j.1469-7580.1997.19140483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci 26: 8578–8587, 2006. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fariñas I, Cano-Jaimez M, Bellmunt E, Soriano M. Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia. Brain Res Bull 57: 809–816, 2002. doi: 10.1016/S0361-9230(01)00767-5. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM Jr, Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22: 253–263, 1999. doi: 10.1016/S0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Honma Y, Kawano M, Kohsaka S, Ogawa M. Axonal projections of mechanoreceptive dorsal root ganglion neurons depend on Ret. Development 137: 2319–2328, 2010. doi: 10.1242/dev.046995. [DOI] [PubMed] [Google Scholar]
- Ikeda-Miyagawa Y, Kobayashi K, Yamanaka H, Okubo M, Wang S, Dai Y, Yagi H, Hirose M, Noguchi K. Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons. Mol Pain 11: 4, 2015. doi: 10.1186/s12990-015-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci 29: 1636–1647, 2009. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Koerber HR. Enhanced artemin/GFRα3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci 30: 16272–16283, 2010. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M, Fariñas I. Sensing life: regulation of sensory neuron survival by neurotrophins. Cell Mol Life Sci 59: 1787–1802, 2002. doi: 10.1007/PL00012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol 78: 1841–1850, 1997. [DOI] [PubMed] [Google Scholar]
- Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol 68: 581–595, 1992. [DOI] [PubMed] [Google Scholar]
- Kupari J, Airaksinen MS. Different requirements for GFRα2-signaling in three populations of cutaneous sensory neurons. PLoS One 9: e104764, 2014. doi: 10.1371/journal.pone.0104764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain 9: 298–308, 2008. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull 30: 239–243, 1993. doi: 10.1016/0361-9230(93)90250-F. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435: 41–63, 1991. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol 71: 941–949, 1994. [DOI] [PubMed] [Google Scholar]
- Lindfors PH, Võikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J Neurosci 26: 1953–1960, 2006. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM. Postnatal roles of glial cell line-derived neurotrophic factor family members in nociceptors plasticity. Sheng Li Xue Bao 60: 571–578, 2008. [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 26: 8588–8599, 2006. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci 26: 1801–1812, 2007. doi: 10.1111/j.1460-9568.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- Milenkovic N, Zhao WJ, Walcher J, Albert T, Siemens J, Lewin GR, Poulet JF. A somatosensory circuit for cooling perception in mice. Nat Neurosci 17: 1560–1566, 2014. doi: 10.1038/nn.3828. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861, 1997. doi: 10.1016/S0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79, 1996. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011, 2000. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol 545: 43–50, 2002. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Jing X, DeBerry JJ, Schwartz ES, Molliver DC, Albers KM, Davis BM. Neurturin overexpression in skin enhances expression of TRPM8 in cutaneous sensory neurons and leads to behavioral sensitivity to cool and menthol. J Neurosci 33: 2060–2070, 2013. doi: 10.1523/JNEUROSCI.4012-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol 436: 304–323, 2001. doi: 10.1002/cne.1069. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 24: 6410–6415, 2004. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci 22: 4057–4065, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]