Abstract
Objective
Because of its anti-inflammatory, anti-fibrotic, anti-apoptotic and anti-neoplastic properties, the PPAR-γ agonist rosiglitazone is an interesting drug for investigating for use in the prevention and treatment of radiation-induced intestinal damage. We aimed to evaluate the radioprotective effect of rosiglitazone in a murine model of acute intestinal damage, assessing whether radioprotection is selective for normal tissues or also occurs in tumour cells.
Methods
Mice were total-body irradiated (12 Gy), with or without rosiglitazone (5 mg/kg/day). After 24 and 72 hours, mice were sacrificed and the jejunum was collected. HT-29 human colon cancer cells were irradiated with a single dose of 2 (1000 cells), 4 (1500 cells) or 6 (2000 cells) Gy, with or without adding rosiglitazone (20 µM) 1 hour before irradiation. HT-29-xenografted CD1 mice were irradiated (16 Gy) with or without rosiglitazone; tumour volumes were measured for 33 days.
Results
Rosiglitazone markedly reduced histological signs of altered bowel structures, that is, villi shortening, submucosal thickening, necrotic changes in crypts, oedema, apoptosis, and inflammatory infiltrate induced by irradiation. Rosiglitazone significantly decreased p-NF-kB p65 phosphorylation and TGFβ protein expression at 24 and 72 hours post-irradiation and significantly decreased gene expression of Collagen1, Mmp13, Tnfα and Bax at 24 hours and p53 at 72 hours post-irradiation. Rosiglitazone reduced HT-29 clonogenic survival, but only produced a slight reduction of xenograft tumour growth.
Conclusion
Rosiglitazone exerts a protective effect on normal tissues and reduces alterations in bowel structures and inflammation in a radiation-induced bowel toxicity model, without interfering with the radiation effect on HT-29 cancer cells. PPAR-γ agonists should be further investigated for their application in abdominal and pelvic irradiation.
Keywords: Rosiglitazone, PPAR-gamma agonists, radiation-induced toxicity, bowel, radioprotection
Introduction
Ionizing radiation is commonly used to treat a wide variety of cancers. Despite improvements in radiation technology, the delivery of radiotherapy to a tumour unavoidably affects the surrounding normal tissues. Normal tissue toxicity is often a dose-limiting factor, since the therapeutic ratio of radiotherapy is dictated by a balance between normal tissue toxicity and tumour control.1,2 Normal tissue radiation toxicity mainly affects rapidly renewing cell systems, such as bone marrow and gastrointestinal (GI)-tract mucosa. Radiation-induced GI damage is a complication of irradiation (IR) exposure, characterized by early effects, when observed in a few days, or delayed effects, when observed in a few months to years.3,4 Early effects develop as a result of intestinal crypt cell death and disruption of the epithelial barrier.5 Damage to stem cells and decrease in the progenitor cell compartment plays a critical role in intestinal toxicity induced by radiation.6 Early effects are also characterized by a classical inflammatory response involving local leukocyte influx, proinflammatory cytokine production, and the induction of endothelial adhesion receptors.7–9 The delayed effects are characterized by progressive intestinal wall fibrosis and vascular sclerosis.10 Furthermore, in a number of experimental models, it has been shown that excessive inflammatory response after intestinal injury can also lead to multiple organ failure.11 Therefore, early intestinal changes occurring after radiation exposure are particularly promising targets for interventions aimed at preventing or reducing radiation syndromes.12
Currently, effective agents to counteract radiation-induced normal tissue injury are lacking and there is a great need for new agents.
Peroxisome proliferator-activated receptor (PPAR)-γ is a member of the nuclear hormone receptor superfamily, which regulates the expression of several target genes and a multitude of metabolic processes.13 Numerous PPAR-γ synthetic activators have been developed, such as thiazolidinediones, including pioglitazone, rosiglitazone (RGZ), ciglitazone, and trioglitazone, which have shown various metabolic effects,14 including anti-inflammatory,15 anti-fibrotic16 and anti-neoplastic17 effects. Particularly in HT-29 human colorectal cancer cells, RGZ suppresses radiation-induced survival signals and DNA damage response, and enhances the radiation-induced apoptosis signalling cascade.18
Anti-inflammatory properties make PPAR-γ ligands very interesting drugs to investigate for the prevention and treatment of radiation-induced intestinal damage. Moreover, anti-neoplastic activity suggests a possible differential effect between normal and tumour tissues for thiazolidinediones.
Our study aimed to investigate the protective effect of RGZ in a murine model of acute radiation-induced intestinal damage. In addition, we aimed to see if the radioprotective effect of RGZ was selective for normal tissues or if it also occurred in tumour cells.
Materials and methods
Reagents
RGZ was from Alexis Biochemicals (San Diego, CA, USA). Reagents for cells cultures and Western blot were from Sigma-Aldrich (St. Louis, MO, USA). TaqMan Reverse Transcription Reagents kit, primer/probe mix (TaqMan Gene Expression Assays), Bax (ID no. Mm00432050_m1), collagen type I (Col1, ID no. Mm00483888_m1), Mmp13 (ID no. Mm00439491_m1), Tnfα (ID no. Mm00443258_m1), Trp53 (ID no. Mm01731287_m1), Gapdh (ID no. 4352339E) and 1× Universal Master Mix were from Applied Biosystems (Foster City, CA, USA). BCA protein assay kit was from Pierce Biotechnology (Rockford, IL, USA). Rabbit pAbs anti-GAPDH, anti-p-NF-kB p65 (S536) and anti-TGFβ were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Peroxidase-secondary Ab, Immobilon-P PVDF membranes, Immobilon Western Chemiluminescent HRP Substrate, Protease/Phosphatase inhibitor cocktails were from Millipore (Milan, Italy), and DeadEnd(TM) Colorimetric TUNEL System was from Promega Italia Srl (Milan, Italy).
Cell cultures
HT-29 cells were obtained from ATCC (Manassas, VA, USA) and cultured in DMEM with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin at 37℃ in a humidified 5% CO2 atmosphere.
HT-29 (500, 1000, 1500 or 2000 cells/well) were seeded in six-well plates in growth medium. After 24 hours, a single dose of 2 (1000 cells), 4 (1500 cells) or 6 (2000 cells) Gy of IR was delivered, with or without adding RGZ (20 µM) 1 hour before IR. Control cells were plated at 500 cells/well. At 24 hours after IR, growth medium was removed, and fresh drug-free medium was added. Cells were incubated for 12–15 days and then fixed and stained with crystal violet (0.5% in methanol). Only colonies exhibiting at least 50 cells were counted. The surviving fraction was calculated by the formula: (mean colony count/seeded cells) × plating efficiency (PE: mean colony count/cells seeded for unirradiated controls). PE was normalized to the respective control value (RGZ control in combined treatments) for each single experiment. Linear-quadratic survival expression was fitted to the data by nonlinear regression. The radiosensitization enhancement ratio for RGZ at 50% survival (ER50) was as follows: ER50 = dose at 50% survival without RGZ/dose at 50% survival with RGZ.
In vivo studies
Animals and experimental protocol
All applicable international, national, and institutional guidelines for the care and use of animals were followed. Animal experiments were approved by the Animal Ethics Committee of the University of Florence (protocol n° 56/2009).
C57BL/6 J mice and CD1 mice from Charles River Laboratories Italia s.r.l. (Milan, Italy) were housed in plastic cages at controlled temperature (22 ± 2℃), lighting (12 hours) and humidity (60 ± 10%).
Radiation-induced intestinal damage
Mice were exposed to total-body IR (TBI), with a single dose of 12 Gy delivered with Linac, with or without RGZ (5 mg/kg/day, oral gavage) started 24 hours before. Non-irradiated mice were used as control. After 24 and 72 hours, mice were sacrificed and jejunum segments collected.
Human intestinal cancer cells xenograft
HT-29 tumour xenografts were obtained by subcutaneous injection of 6 × 106 cells in the flank of CD1 mice. When the tumour reached a mean diameter of 5 mm, animals were randomly assigned to the following groups: IR, IR + RGZ, RGZ, and saline (control). IR consisted of a dose of 16 Gy selectively delivered to the tumour. The treatment with RGZ (5 mg/kg/day, oral gavage) was started 24 hours before IR and continued until the end of the experiment.
Tumour response was defined as the relative change in tumour volume versus volume before therapy. Tumour volume (mm3) was measured twice a week for 33 days (tumour volume = length (mm) × width2 (mm2)/2).
RNA extraction and real-time PCR
Tissue samples were homogenized using stainless steel beads and a TissueLyser. Total RNA from tissues was extracted with the RNeasy Mini kit according to the manufacturer’s recommendations. Total RNA (400 ng) was reverse transcribed in a final volume of 80 µl containing 500 mMKCl, 0.1 mM EDTA, 100 mM Tris-HCl (pH 8.3), 5.5 mM MgCl2, 500 mM of each dNTP, 2.5 mM random examers, 0.4 U/ml RNase inhibitor, and 1.25 U/ml Multiscribe Reverse Transcriptase. The reverse transcription reaction was performed at 25℃ for 10 minutes, 48℃ for 30 minutes and 95℃ for 3 minutes. Gene expression measurement was performed by quantitative real-time PCR (TaqMan). The amount of target, normalized to an endogenous reference (Gapdh, pre-developed TaqMan assay reagents) and relative to a calibrator (quantitative PCR human reference total RNA), was determined by 2 -ΔΔCt calculation.19 For each sample, 12.5 ng cDNA were added to 10 µl of PCR mix containing each primer/probe mix and 1 × Universal Master Mix. The samples were then subjected to 40 cycles of amplification at 95℃ for 15 seconds and 60℃ for 60 seconds in an ABI Prism 7900 Sequence Detector (Applied Biosystems).
Histological analysis
For each sample, representative sections of 5 µm in thickness were cut from tissue blocks of formalin-fixed, paraffin-embedded tissues and placed on glass slides for morphological evaluation. Sections were stained with hematoxylin and eosin. Measurement of villus height was performed by light microscopy using a calibrated micrometre (100×). The inflammatory cell infiltration was quantified by counting the number of neutrophils in 10 different areas of histological sections from each sample. The average number of neutrophils was divided by the average number in non-irradiated control mice for normalization. The degree of regeneration of jejunal crypts was assessed by counting the number of surviving crypts per circumference using microscopy, as previously described.20
TUNEL assay
The degree of apoptosis was assessed in jejunum sections by TUNEL assay, according to the manufacturer’s instructions. Briefly, the sections were deparaffinized with xylene, dehydrated through a graded alcohol series and treated with permeabilization solution. The labelling reaction was performed using a solution containing terminal deoxynucleotidyl transferase (TdT). The number of TUNEL-positive cells (cells with brown nuclei) per field was counted in three randomly selected fields of each section (at least three sections/animal) at 100 × magnification.
Western blot analysis
Tissues were lysed in 200 μl of ice-cold RIPA buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (v/v) SDS) supplemented with protease/phosphatase inhibitor cocktail. Tissues were centrifuged for 10 minutes at 700 × g at 4℃, and the supernatant was then collected and protein concentration measured. Protein aliquots (30 µg) were processed and separated by 12% SDS-PAGE; the proteins were then transferred onto PVDF membranes, which were incubated for 2 hours at room temperature with primary Abs diluted in Tween Tris-buffered saline (anti-p-NF-kB p65 (S536), anti-TGFβ 1:400, anti-GAPDH 1:1500), followed by peroxidase-conjugated secondary IgG (1:5000). Proteins were revealed using the Immobilion Western Chemiluminescent HRP substrate. The chemiluminescent signal was analysed with Quantity One software on a ChemiDoc XRS instrument (Bio-Rad Laboratories). GAPDH was used as loading control. The densitometric analysis of Western blot bands was performed using the Quantity One software (Bio-Rad Laboratories, Inc., Milan, Italy), normalized to GAPDH, and reported as percentage relative to the respective control, set at 100.
Statistics
The statistical analysis was performed using GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) was used, and a p-value less than 0.05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
Histopathological features
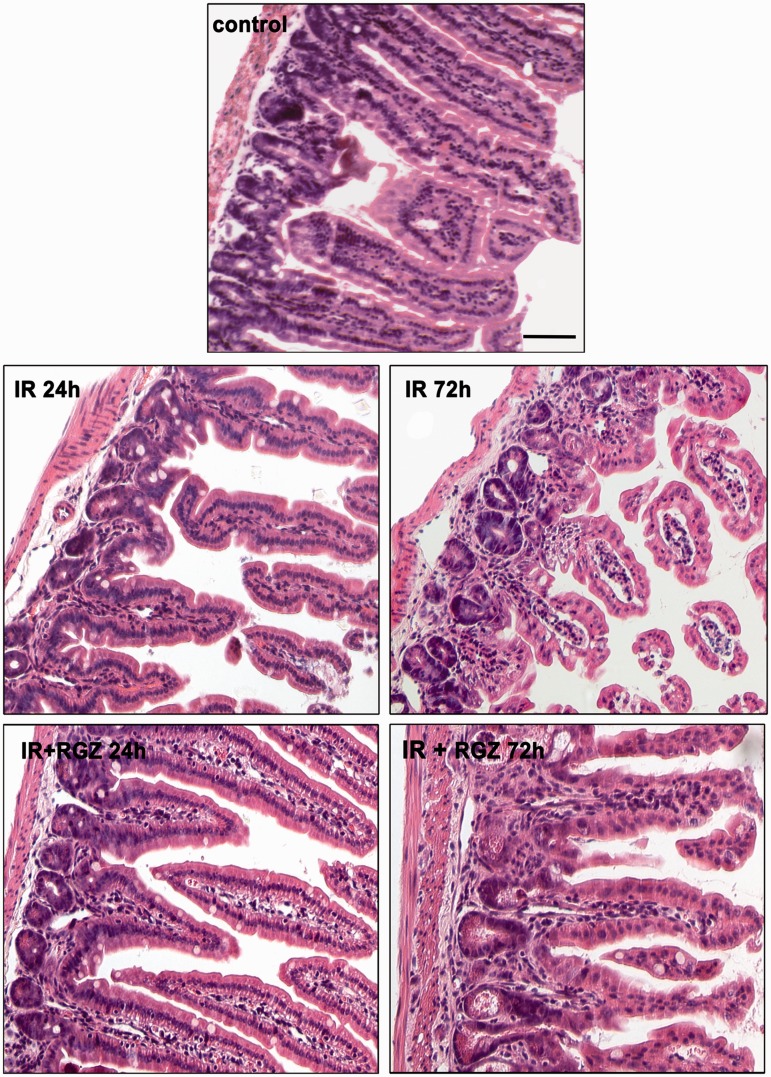
Histopathological analysis of jejunal specimens (Figure 1) showed that the untreated control mice exhibited normal features, with long villi lined with a continuous columnar epithelium and well-developed tubular gland.
Figure 1.
Rosiglitazone reduced histological signs of radiation-induced intestinal damage.
Representative full-thickness images of jejunal wall of mice treated with saline (control), irradiation (IR, 12 Gy) or IR + RGZ (5 mg/kg) after 24 and 72 hours. Bars = 100 µm.
Radiotherapy caused time-dependent injurious effects on the jejunal mucosa, with loss of normal histological structure, as shown by the significant shortening of villi (Table 1), hypoplasia of the tubular glands, and submucosal thickening. A marked inflammatory infiltrate was also observed in irradiated mice (Table 1).
Table 1.
Effect of RGZ on histological changes induced by irradiation in mouse small intestine
| control | IR | IR + RGZ | |
|---|---|---|---|
| Villus height (µm) | 202.90 ± 3.78 | 91.24 ± 2.21 | 137.19 ± 4.28*# |
| Number of crypts/circumference | 94.40 ± 1.05 | 34.55 ± 1.14* | 55.80 ± 1 ± 43*# |
| Inflammatory cell relative number (fold increase vs. control) | 1 ± 0.17 | 8.42 ± 0.39* | 4.26 ± 0.29*# |
Data are shown as mean ± SEM (*p < 0.05 vs control, #p < 0.05 vs. IR).
Moreover, IR reduced the number of regenerating crypts in the jejunum compared with healthy controls (Table 1).
RGZ-treated mice showed a dampened radiation-induced jejunal damage, at both 24 and 72 hours after IR (Figure 1), with increased villi length, improved crypt survival, and a marked reduction of oedema and inflammatory response (Table 1), but preservation of submucosal thickness.
TUNEL findings
TUNEL staining revealed that few apoptotic cells were visible in the tip of villi in control mice (Figure 2). Obvious apoptotic cells were observed in the tip of villi, in the villous stroma and in the crypts of irradiated mice 24 hours after IR (111.25 ± 1.37, p < 0.05 vs. control). Apoptosis was still significant in jejunum of irradiated mice 72 hours after IR (35.26 ± 0.61, p < 0.05 vs. control).
Figure 2.
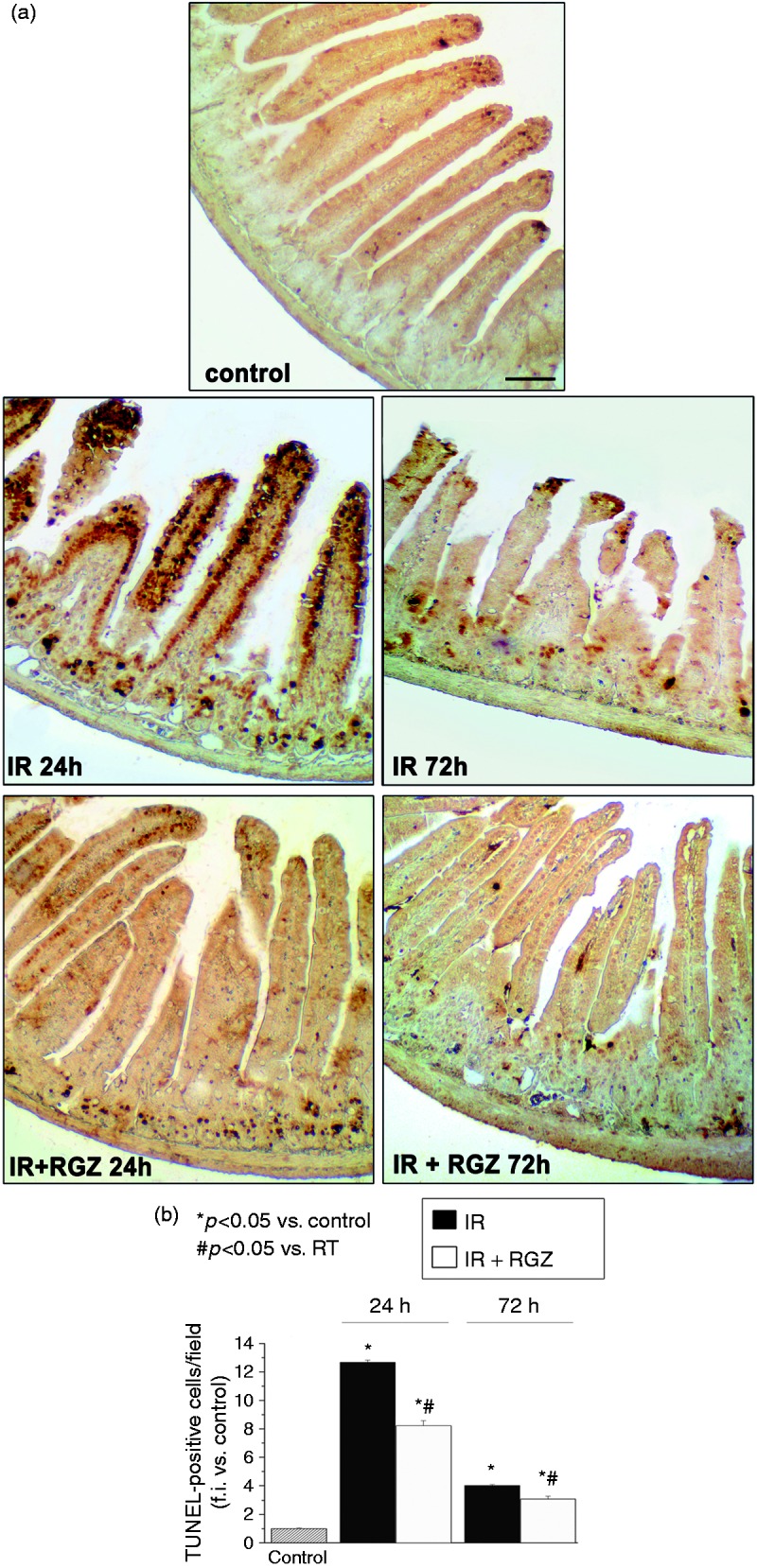
Rosiglitazone decreased radiation-induced apoptosis in bowel tissues.
a. Representative images of TUNEL-stained jejunum sections from mice treated with saline (control), irradiation (IR, 12 Gy) or IR + RGZ (5 mg/kg), after 24 and 72 hours. Bars = 100 µm.
b. Average number of TUNEL-positive cells per field at 100 × magnification. Data, measured as fold increase in expression compared to control (taken as 1), are shown as the mean ± SEM. *p < 0.05 vs. control, #p < 0.05 vs. IR.
Treatment with RGZ significantly reduced the number of TUNEL-positive cells at both time points (RGZ + IR at 24 hours = 72.1 ± 3.21, p < 0.05 vs. IR, p < 0.05 vs. control; RGZ + IR at 72 hours = 26.97 ± 1.63, p < 0.05 vs. IR, p < 0.05 vs. control).
Molecular analysis
To evaluate the anti-inflammatory, anti-fibrotic and anti-apoptotic effects of RGZ, we assessed the changes in several markers by Western blot analysis and real-time RT-PCR in jejunal specimens.
Bowel tissues of irradiated mice exhibited significantly increased protein expression of the fibrosis marker TGFβ and phosphorylation of the p65 subunit of the stress response marker NF-kB, at both 24 and 72 hours, as compared with untreated control (Figure 3). RGZ significantly inhibited the overexpression of radiation-induced p-NF-kB p65 and TGFβ protein expression, at both time points.
Figure 3.
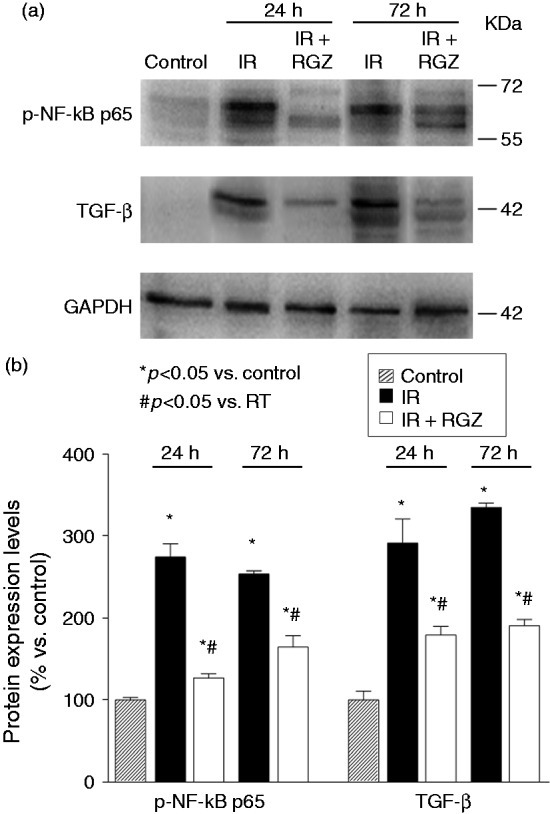
Rosiglitazone decreased overexpression of radiation-induced fibrosis/inflammatory marker proteins in bowel tissues.
a. Representative Western blot analysis of p-NF-kB p65 and TGF-β proteins in jejunum tissue homogenates from mice treated with saline (control), irradiation (IR, 12 Gy) or IR + RGZ (5 mg/kg), after 24 and 72 hours. GAPDH was used as a loading control.
b. Densitometric analysis of Western blot bands, normalized for GAPDH and reported as percentage versus the respective control, set at 100. Data are shown as the mean ± SEM. *p < 0.05 vs. control, #p < 0.05 vs. IR.
In jejunal specimens, IR significantly increased gene expression of Bax, Col1, Mmp13, Tnfα at 24 hours post-TBI, and of Trp53 at 72 hours post-TBI (Figure 4). RGZ treatment significantly reduced these increases in mRNA expression induced by IR (p < 0.05).
Figure 4.
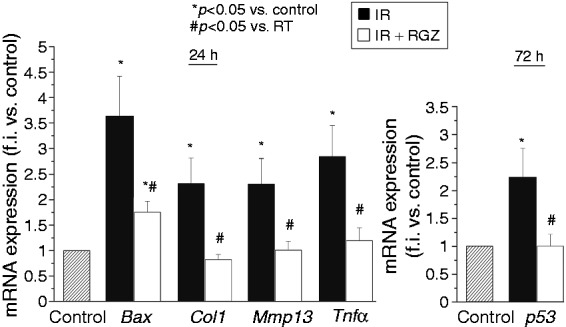
Rosiglitazone decreased the radiation-induced gene expression of fibrosis, inflammatory and apoptosis markers in bowel tissues.
mRNA expression of Bax, Col1, Mmp13 and Tnfα, p53 in jejunum tissues of mice treated with saline (control), irradiation (IR, 12 Gy) or IR + RGZ (5 mg/kg) after 24 and 72 hours. Data, measured as fold increase in expression compared with control (taken as 1), are shown as mean ± SEM. *p < 0.05 vs. control, #p < 0.05 vs. IR.
Effect on irradiated human intestinal cancer cells
To evaluate the effect of RGZ on irradiated cancer cells in vitro, clonogenic assays were performed on HT-29 cells, pre-treated or not with RGZ (20 µM), and irradiated with increasing ionizing radiation doses. According to previous works,18 the addition of RGZ reduced the clonogenic survival of the cancer cells as compared with IR alone (Figure 5): cells treated with RGZ had significantly fewer colonies vs. control cells at each radiation dose (p < 0.05), with an ER50 of 1.93.
Figure 5.
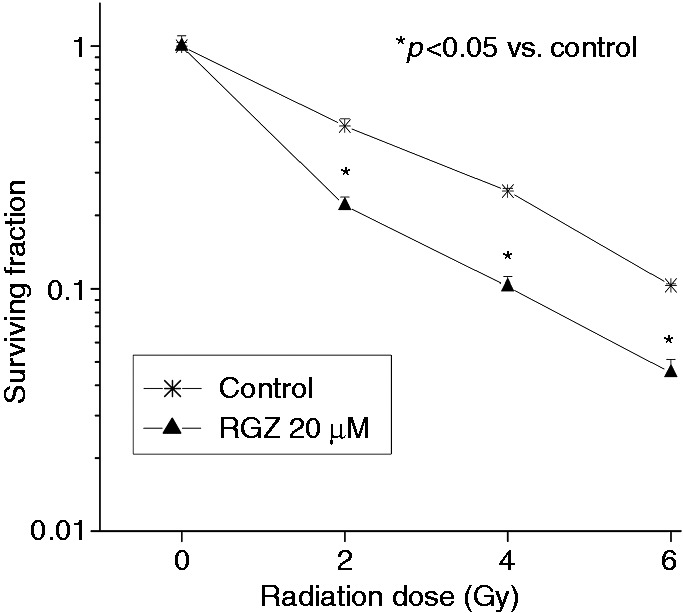
Rosiglitazone significantly reduced clonogenic survival of HT-29 cancer cells following irradiation with increasing radiation doses.
Cells, pre-treated or not with RGZ (20 µM), were irradiated with 0–6 Gy. After 12–15 days, cells were stained and colonies over 50 cells counted. Data are shown as mean ± SEM, corrected by PE. PE was normalized to the respective control value (RGZ control in combined treatments) for each single experiment. *p < 0.05 vs. control.
RGZ effect on cancer cells was also evaluated in a murine HT-29 xenograft model (Figure 6). RGZ alone was not able to significantly reduce tumour growth. A significant control of tumour growth was achieved with IR and the combination of RGZ with IR (p < 0.05 vs. control and vs. RGZ alone), without significant differences between the two groups. Thus, when combined with IR, RGZ did not protect the tumour from the therapeutic effect of radiotherapy.
Figure 6.
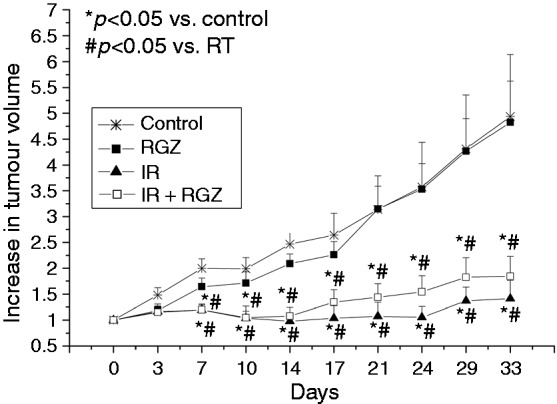
Rosiglitazone did not protect the tumour from irradiation.
When HT-29 tumour xenograft diameter reached 5 mm, mice were treated with vehicle (asterisks), RGZ (5 mg/kg, black squares), 16 Gy irradiation (IR, black triangles) or IR + RGZ (white squares). Tumour volume was measured twice a week for 33 days. Data are mean ± SEM. *p < 0.05 vs. control, #p < 0.05 vs. RGZ.
Discussion
The GI tract has a high radiosensitivity, and bowel constraints represent an important factor in treatment planning. Acute radiation-induced bowel toxicities range from symptoms such as pain, diarrhoea and bloating to obstruction, perforation, fistula and bleeding. Late toxicities include bowel obstruction, diarrhoea, fibrosis, and malabsorption.
Radiation-injury of the GI tract is considered to be one of the critical causes for systemic complications following radiation exposure, and may mediate some effects that lead to multiple organ failure.11,21 Exposure to IR leads to increased oxidative stress, DNA damage, genomic instability and inflammation,2 that induce increased apoptosis and cell loss. When DNA is damaged due to IR exposure, the transcription factor p53 is activated and initiates its signal cascade along the apoptosis pathway,22 in turn activating Bax, a member of the pro-apoptotic Bcl-2 family.23 IR induces proinflammatory cytokines including TNF-α, TGF-β1, IL-1β and IL-6, which have been shown to contribute significantly to the complications associated with radiotherapy.24–27
There is a major need for more effective radioprotective agents, but despite many compounds having been studied,28,29 none has so far been introduced in common clinical practice. An ideal radioprotector must be able to protect healthy tissue from the radiation toxicity without interfering with the therapeutic effect of ionizing radiation on the tumour tissue, and should be well tolerated.
In this study, we evaluated the radioprotective properties of RGZ (5 mg/kg/day started 24 hours before RT) in normal bowel tissue in a murine model of intestinal injury induced by 12-Gy TBI, as previously described.30,31 Histological analysis revealed that IR caused noticeable intestinal damage, with villi shortening, submucosal thickening, crypt necrotic changes, oedema and inflammatory infiltrate, and increased numbers of TUNEL-positive (apoptotic) cells. RGZ produced histological improvement of tissue structure, with normalization of villi and crypts and oedema and reduction of inflammatory infiltrate and apoptotic cell death.
Gene expression analysis revealed that RGZ negatively modulated the radiation-induced expression of several tissue markers of fibrosis (Collagen 1 and Mmp13), inflammation (Tnfα) and apoptosis (Bax, p53) in jejunum. Furthermore, Western blot analysis showed that RGZ reduced protein expression of the fibrosis marker TGF-β and phosphorylation of the p65 subunit of NF-kB – the signalling pathway triggered by the proinflammatory cytokine TNFα.
On the basis of these data, we demonstrated that RGZ reversed the inflammatory and apoptotic changes induced by radiation, and that it had a radioprotective effect on irradiated healthy bowel.
In addition, we wanted to determine if the radioprotective effect of RGZ was selective for normal tissues or if it also occurred in tumour cells. A differential activity between normal and cancer tissues is critical for a putative radioprotective agent. Our in vitro clonogenic survival studies demonstrated a significant decrease in surviving cells irradiated in presence of RGZ, as compared with IR alone. According to previous works,18 RGZ has a radiosensitizing effect on human bowel cancer cells. In vivo studies in a HT-29 xenograft model failed to demonstrate a radiosensitizing effect but showed that RGZ did not protect the tumour from IR.
The anti-inflammatory effect of RGZ on the irradiated bowel and the lack of effect on the therapeutic activity of radiotherapy on irradiated cancer cells suggest that PPAR-γ agonists should be further investigated as a possible therapeutic option for the prevention and treatment of acute radiation-induced bowel injury.
Funding
The present research project was supported by Tuscany Region and Istituto Toscano Tumori (ITT).
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
References
- 1.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol 2009; 85: 539–573. [DOI] [PubMed] [Google Scholar]
- 2.Xiao M, Whitnall MH. Pharmacological countermeasures for the acute radiation syndrome. Curr Mol Pharmacol 2009; 2: 122–133. [DOI] [PubMed] [Google Scholar]
- 3.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol 1990; 58: 925–973. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Zhemg H, Kulkarni A, et al. Regulation of early and delayed radiation responses in rat small intestine by capsaicin-sensitive nerves. Int J Radiat Oncol Biol Phys 2006; 64: 1528–1536. [DOI] [PubMed] [Google Scholar]
- 5.Kunwar A, Bag PP, Chattopadhyay S, et al. Anti-apoptotic, anti-inflammatory, and immunomodulatory activities of 3,3'-diselenodipropionic acid in mice exposed to whole body γ-radiation. Arch Toxicol 2011; 85: 1395–1405. [DOI] [PubMed] [Google Scholar]
- 6.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci 1998; 353: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigneulle RM, Rao S, Fasano A, et al. Structural and functional alterations of the gastrointestinal tract following radiation-induced injury in the rhesus monkey. Dig Dis Sci 2002; 47: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 8.Panes J, Anderson DC, Miyasaka M, et al. Role of leukocyte-endothelial cell adhesion in radiation-induced microvascular dysfunction in rats. Gastroenterology 1995; 108: 1761–1769. [DOI] [PubMed] [Google Scholar]
- 9.Molla M, Panes J. Radiation-induced intestinal inflammation. World J Gastroenterol 2007; 13: 3043–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haydont V, Vozenin-Brotons MC. Maintenance of radiation-induced intestinal fibrosis: Cellular and molecular features. World J Gastroenterol 2007; 13: 2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monti P, Wysocki J, van der Meeren A, et al. The contribution of radiation-induced injury to the gastrointestinal tract in the development of multi-organ dysfunction syndrome or failure. Brit J Radiol 2005; 27: 89–94. [Google Scholar]
- 12.Kim SH, Lee HJ, Kim JS, et al. Protective effect of an herbal preparation (HemoHIM) on radiation-induced intestinal injury in mice. J Med Food 2009; 12: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 13.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol 2004; 5: 419–429. [DOI] [PubMed] [Google Scholar]
- 14.Rosen ED, Spiegelman BM. PPARgamma: A nuclear regulator of metabolism, differentiation, and cell growth. J BiolChem 2001; 276: 37731–37734. [DOI] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, Mazzon E, Dugo L, et al. Reduction in the evolution of murine type II collagen-induced arthritis by treatment with rosiglitazone, a ligand of the peroxisome proliferator-activated receptor gamma. Arthritis Rheum 2003; 48: 3544–3556. [DOI] [PubMed] [Google Scholar]
- 16.Milam JE, Keshamouni VG, Phan SH, et al. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008; 294: L891–L901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanquicett C, Roman J, Hart CM. Thiazolidinediones as anti-cancer agents. Cancer Ther 2008; 6: 25–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu SJ, Hsaio CH, Tseng HH, et al. Rosiglitazone enhances the radiosensitivity of p53-mutant HT-29 human colorectal cancer cells. Biochem Biophys Res Commun 2010; 394: 774–779. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 20.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol 1970; 17: 261–267. [DOI] [PubMed] [Google Scholar]
- 21.François A, Milliat F, Guipaud O, et al. Inflammation and immunity in radiation damage to the gut mucosa. BioMed Res Int 2013; 2013: 123241–123241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jee YH, Jeong WI, Kim TH, et al. P53 and cell-cycle-regulated protein expression in small intestinal cells after fast-neutron irradiation in mice. Mol Cell Biochem 2005; 270: 21–28. [DOI] [PubMed] [Google Scholar]
- 23.Dogu Y, Diaz J. Mathematical model of a network of interaction between p53 and Bcl-2 during genotoxic-induced apoptosis. Biophys Chem 2009; 143: 44–54. [DOI] [PubMed] [Google Scholar]
- 24.Rodemann HP, Blaese MA. Responses of normal cells to ionizing radiation. Semin Radiat Oncol 2007; 17: 81–88. [DOI] [PubMed] [Google Scholar]
- 25.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol 2007; 17: 89–98. [DOI] [PubMed] [Google Scholar]
- 26.Linard C, Marquette C, Mathieu J, et al. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: Effect of an NF-κB inhibitor. Int J Radiat Oncol Biol Phys 2004; 58: 427–434. [DOI] [PubMed] [Google Scholar]
- 27.Chen MF, Keng PC, Lin PY, et al. Differential effects of caffeic acid phenethyl ester for normal lung and lung cancer treated with irradiation: in vitro and in vivo study. BMC Cancer 2005; 5: 158–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasanna PG, Stone HB, Wong RS, et al. Normal tissue protection for improving radiotherapy: Where are the gaps? Transl Cancer Res 2012; 1: 35–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Neta R. Modulation of radiation damage by cytokines. Stem Cells 1997; 15: 287–294. [DOI] [PubMed] [Google Scholar]
- 30.Mangoni M, Sottili M, Gerini C, et al. Protective effect of leuprorelin on radiation-induced intestinal toxicity. Anticancer Res 2015; 35: 3875–3884. [PubMed] [Google Scholar]
- 31.Mangoni M, Vozenin MC, Biti G, et al. Normal tissues toxicities triggered by combined anti-angiogenic and radiation therapies: Hurdles might be ahead. Br J Cancer 2012; 107: 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]