Abstract
Background
Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) and quantitative-elastography endoscopic ultrasound (QE-EUS) are considered useful tools for the evaluation of solid pancreatic tumors (SPT). The aim of our study was to evaluate the diagnostic accuracy of CEH-EUS, QE-EUS, and the combination of both for the differential diagnosis of SPT.
Methods
Sixty-two consecutive patients (mean age 64.3 years, range 32–89 years, 44 male) who underwent EUS for the evaluation of SPT were prospectively included. EUS was performed with a linear Pentax-EUS and a Hitachi-Preirus processor. The mass (area A) and a reference area B were selected during QE-EUS, and results expressed as B/A (strain ratio). A strain histogram of the mass was also evaluated. Microvascularization of the tumor was evaluated over 2 min during CEH-EUS after intravenous injection of 4.8 mL SonoVue. Final diagnosis was based on histopathology of surgical specimens or EUS-guided tissue acquisition and clinical follow-up in non-operated cases. Diagnostic accuracy of CEH-EUS, QE-EUS, and their combination was calculated.
Results
Median size of the masses was 32 mm (range 12-111). Final diagnosis was pancreatic adenocarcinoma (n = 45), neuroendocrine tumor (n = 3), inflammatory mass (n = 10), pancreatic metastasis (n = 2), autoimmune pancreatitis (n = 1), and a mucinous cystadenocarcinoma (n = 1). Overall accuracies for determination of malignancy using QE-EUS, CEH-EUS, their combination, and EUS-guided tissue acquisition were 98.4% (95% confidence interval (CI): 91.4–99.7), 85.5% (95% CI: 74.7–92.2), 91.9% (95% CI: 82.5–96.5), and 91.5% (95% CI: 83.6–99.5), respectively.
Conclusion
The combination of QE-EUS and CEH-EUS is a useful tool for the differential diagnosis of SPT, giving complementary information. However, this combination does not significantly increase the diagnostic accuracy of either of the techniques performed alone.
Keywords: Solid pancreatic lesion, stiffness, vascularization, tissue acquisition, endoscopic ultrasound
Introduction
Endoscopic ultrasound (EUS) provides high-resolution images from the pancreatic parenchyma and ductal system, and is considered a highly reliable diagnostic tool in the evaluation of pancreatic disease.1–3 Nevertheless, differentiation between different types of pancreatic lesions remains a challenge. EUS-guided biopsy (EUS-FNB) is one method to optimize the diagnostic yield of pancreatic EUS.4,5 However, EUS-FNB may be technically demanding, and false negative results can be a problem.6 EUS-FNB is also associated with a low but not negligible risk of complications.7
During recent years, non-invasive complementary methods with the ability to increase the diagnostic yield of EUS have emerged. Among these, elastography and contrast enhancement are the most widely used. Elastography investigates tissue stiffness by detection of small structure deformations within the B-mode image caused by compression.8,9 Strain differs among different tissues and the degree of deformation is used as an indicator of the hardness or stiffness of the tissue. Different elasticity values are translated to a color scale from dark blue to cyan, green, yellow, and red, where dark blue is the hardest and red is the softest tissue. Elastography colors are superimposed over the conventional gray-scale EUS image. Elastography findings have been related to histopathological features,10 and therefore denominated as a virtual biopsy.11 This novel technique has been initially used for the analysis of superficial organ lesions, e.g. breast and prostate.12,13 However, nowadays, several studies on the diagnostic accuracy of EUS in the differentiation between malignant and benign pancreatic solid lesions have been published and recently analyzed in a pooled meta-analysis.14 Qualitative elastography has some inherent shortcomings: the interpretation of rapidly changing colors is subjective, not quantifiable, and sometimes difficult. This problem has been addressed in the development of strain histogram and strain ratio analysis,8 two alternative methods for quantification of tissue stiffness based on the qualitative EUS data. The strain histogram is a mean value of elasticity strains in a selected region of interest,15 and the strain ratio is calculated as the ratio of the mean strain in a region of interest over the mean strain in a reference area that usually is selected from the gut wall.8
Contrast-enhanced ultrasound is today a part of clinical routine and plays a pivotal role in the differential diagnosis of solid lesions by providing information on tissue microvasculature and perfusion. Ultrasound contrast agents consist of small microbubbles that backscatter the ultrasound signal and oscillate in response to sound pressure. In EUS, the use of contrast media was initially limited to contrast-enhanced power Doppler EUS.16 Due to recent development of new linear echoendoscopes and second-generation contrast enhancement agents, it is now possible to perform contrast-enhanced harmonic EUS (CEH-EUS), based on a low mechanical index with substantially higher resolution.16 Studies on CEH-EUS in the diagnosis of pancreatic cancer have been analyzed in a recent meta-analysis indicating high sensitivity and specificity for pancreatic adenocarcinoma.17 However, a direct comparison between elastography and CEH-EUS has only been made in one small study to date and there is little information on how the results of both techniques can be combined in the differential diagnosis of solid pancreatic masses.18
The aim of the present study was to evaluate the diagnostic accuracy of CEH-EUS, the two different methods for quantitative elastography endoscopic ultrasound (QE-EUS), and the combination of CEH-EUS and QE-EUS in the differentiation between benign and malignant lesions, and in the differentiation between pancreatic cancer and chronic pancreatitis.
Material and methods
Design
A prospective single-center study is reported on the sensitivity, specificity, and predictive values of QE-EUS and CEH-EUS in the differential diagnosis of solid pancreatic masses. The study was approved by the local ethical committee and conducted in accordance with the Declaration of Helsinki and its amendments, and Good Clinical Practice guidelines. All patients provided written informed consent to the study.
Subjects
All patients referred to the EUS Unit of the Department of Gastroenterology at the University Hospital of Santiago de Compostela, presenting a solid-appearing pancreatic mass at EUS, over a period of six months, were prospectively included into the study. Exclusion criteria were the presence of a predominantly cystic lesion and contraindications for the administration of the ultrasound contrast agent SonoVue (Bracco, Milan, Italy), i.e. uncontrolled hypertension, pulmonary hypertension, known right-to-left shunt, heart failure, and known hypersensitivity to SonoVue.
Endoscopic ultrasound
EUS was performed using a linear probe (EG-3870-UTK; Pentax Europe GmbH, Hamburg, Germany) and the platform Preirus (Hitachi Medical Systems Europe, Zug, Switzerland) equipped with real-time tissue elastography and low-index contrast-enhanced harmonic modules. All EUS examinations were performed under conscious sedation by a single expert endosonographer (JIG) with >9000 procedures performed. The result of the EUS-guided elastography and the CEH-EUS was interpreted and recorded during the examination, before any cyto- or histopathological evaluation had been performed.
Endoscopic ultrasound-guided elastography
The following settings for the EUS-guided elastography software were used (1/-/-/2/3/4 T-Elasto-H): reject function 1, e-smoothing 2, persistence 3, and e-dynamic range 4. The EUS probe was applied to the gut wall just exerting the pressure needed for an optimal and stable B-mode image at 7.5 MHz. The region of interest (ROI) for the elastographic evaluation was manually selected so that the lesion is centered and occupies <50% of total ROI area. Large vessels and areas at depth with no B-mode signal area were excluded. Maximal sensitivity for elastographic registration was consistently used in the study. For calculation of strain ratio, two different areas were selected, areas A and B. The largest possible area of the investigated lesion was selected as area A. Area B was selected in soft (red) peripancreatic tissue outside the tumor (since relative size of this reference area is considered not to be important, minimum size was defined automatically by the system). Strain ratio was automatically calculated as the quotient B/A. To make strain histogram measurement, the largest measurement box touching inside boundary of lesions was selected.
Contrast-enhanced harmonic endoscopic ultrasound
The extended pure harmonic detection mode, which combines receiving frequencies of filtered fundamental and second harmonic components, was used for CEH–EUS. A low mechanical index procedure (dynamic wide-band contrast harmonic imaging mode) was used, with a mechanical index of 0.08–0.25 and corresponding powers. In all cases, SonoVue, a second-generation ultrasound contrast agent containing phospholipid-stabilized microbubbles of sulfur hexafluoride, was used. Two phases of contrast enhancement were analyzed: an early/arterial phase (starting from 10 to 30 s) and a venous/late phase (from 30 to 120 s) in accordance with the European Federation Societies of Ultrasound in Medicine and Biology guidelines and recommendations.19 A two-panel image was used with the conventional gray-scale B-mode EUS image on the right side and the contrast harmonic image on the left side. Starting time was defined as the moment when intravenous contrast was injected (SonoVue 4.8 mL), followed by the infusion of 10 cc of saline. SonoVue uptake and washout were evaluated for a minimum of 120 s. The enhancement pattern of lesions was compared with that of the adjacent normal parenchyma. After injection of contrast agent, the beginning of the arterial phase was defined as when a hyperechogenic appearance of the aorta and other major perilesional arteries was observed. Hyperechogenicity of the splenomesenteric-portal vessels was considered as indicating the start of the venous phase. The degree of enhancement was evaluated during the arterial phase starting from the first arrival of contrast (usually in 10–20 s) until approximately 30–45 s. Lesions were classified based on their overall degree of enhancement in comparison with the surrounding structures, as one of three types: hypo-enhanced, iso-enhanced, or hyper-enhanced. Lesions were classified as malignant if a hypo- or hyper-enhanced pattern was observed and as non-malignant in the case of iso-enhancement.
Assessment of final diagnosis
Cases were classified as benign or malignant using histology of surgical specimens as reference method in operated cases. Final diagnosis was classified as malignant in non-operated cases if histology and/or cytology from EUS guided tissue acquisition demonstrated malignancy. Non-operated cases were classified as benign if EUS guided tissue sampling did not reveal signs of malignancy and/or if computed tomography and clinical follow-up for a minimum of 24 months were compatible with a benign diagnosis.20
Data analysis
Data are shown as mean and standard deviation (SD) or percentage as appropriate. Optimal cutoff values for detection of malignancy by strain ratio and hue histogram were identified by receiver operating characteristic (ROC) curve analysis. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for QE-EUS (based on strain ratio or strain histogram), CEH-EUS, and the combination of the both methods for the detection of malignancy were calculated with 95% confidence intervals (CIs). In the analysis of the combined use of QE-EUS and CEH-EUS, a pathological result was defined as both QE-EUS and CEH-EUS pathological and a normal result was defined as at least one of the two methods displaying a normal result. Accuracy of EUS-guided tissue acquisition was also performed, according to the established gold-standard. Diagnostic performance was compared between the methods using Fishers exact test. A p-value < 0.05 was considered as statistically significant. Statistical analyses were performed using the software SPSS 19.0 (Chicago, Illinois).
Results
Out of 832 EUS examinations performed over the study period, a solid-appearing pancreatic mass was detected in 62 patients (mean age 65.2 years (SD 15.1), 44 male). Baseline characteristics of benign versus malignant lesions are reported in Table 1. All these cases were included in the study. Median size of pancreatic masses was 32 mm (range 13–111 mm). Tumors were located in the head (n = 45 (73%)), body (n = 15 (24%)), and tail (n = 2 (3%)) of the pancreas. The final diagnosis was based on surgery in 14 (23%) cases and on EUS-FNA and clinical follow-up in 48 (77%) cases. FNA was performed in all but three cases (one operated and two non-operated cases) using 19-gauge histology (n = 4), 22-gauge (n = 20), 22-gauge histology (n = 10), and 25-gauge (n = 25) needles.
Table 1.
General characteristics of included patients
| Benign (n = 13) | Malignant (n = 49) | ||
|---|---|---|---|
| Sex, male, n (%) | 11 (84.6%) | 33 (67.3%) | |
| Age, mean (SD) | 50.8 (11.7) | 69.1 (13.5) | |
| Tumor location | Head/body/tail | 8/3/2 | 37/12/0 |
| Tumor size (mm), median (range) | 29 (13–51) | 32 (21–111) | |
| Final diagnosis (n) | Autoimmune pancreatitis | 1 | |
| Chronic pancreatitis | 10 | ||
| Benign NET | 2 | ||
| Malignant NET | 1 | ||
| Pancreatic adenocarcinoma | 45 | ||
| Mucinous cystadenocarcinoma | 1 | ||
| Metastasis | 2 |
CEH-EUS pattern, elastography strain ratio, and mean strain histogram value in relation to final diagnosis are presented in Table 2. A strain ratio >10 and a mean strain histogram value <50 were identified as optimal cutoff values for classification of lesions as malignant based on ROC curve analysis (Figure 1). A perfect correlation between strain ratio and strain histogram was observed when these cutoff values were applied (Figure 2). Therefore, cases with strain ratio >10 and mean strain histogram <50 are denominated as QE-EUS positive for malignancy hereafter.
Table 2.
Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) pattern and elastography strain ratio and strain histogram in relation to final diagnosis
| Neuroendocrine tumors |
|||||||
|---|---|---|---|---|---|---|---|
| Inflammatory mass | Benign | Malignant | Pancreatic cancer | Metastasis | |||
| Total (n) | 11 | 2 | 1 | 46 | 2 | ||
| Strain ratio elastography | Median (range)a | 6.00 (2.15–43.22) | 3.41 and 5.18 | 32.33 | 32.83 (14.27–106.0) | 38.00 and 77.25 | |
| Non-malignant | <10 (n) | 10 | 2 | 0 | 0 | 0 | |
| Malignant | >10 (n) | 1 | 0 | 1 | 46 | 2 | |
| Strain histogram | Median (range)a | 77.7 (22.7–118.1) | 96.2 and 111.3 | 13.2 | 30.0 (7.5–42.7) | 22.8 and 30.3 | |
| Non-malignant | >50 | 10 | 2 | 0 | 0 | 0 | |
| Malignant | <50 | 1 | 0 | 1 | 46 | 2 | |
| CEH-EUS | Non-malignant | Isovascular | 9 | 0 | 0 | 4 | 1 |
| Malignant | Hypovascular | 2 | 1 | 0 | 42 | 1 | |
| Malignant | Hypervascular | 0 | 1 | 1 | 0 | 0 | |
Individual values are reported for neuroendocrine tumors and pancreatic metastasis due to low number of cases.
Figure 1.
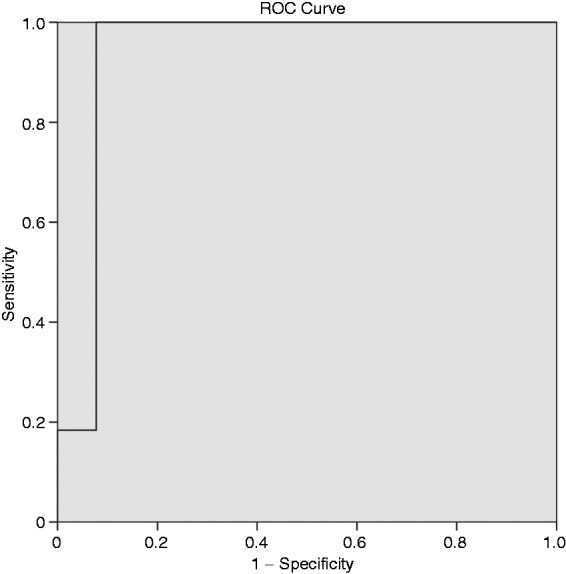
Receiver operating characteristic (ROC) curves drawn to obtain the optimal cutoff point to determine malignancy with strain ratio and strain histogram.
Figure 2.
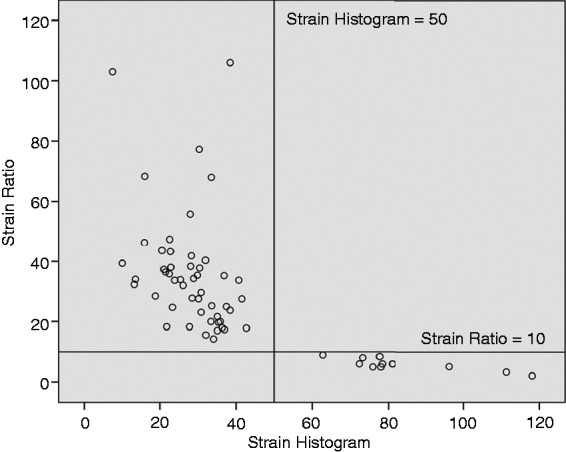
Correlation between strain ratio and strain histogram. Black lines in the figure represent cut-of values for differentiation between benign and malignant lesions (strain ratio >10 and strain histogram <50) used in the current study.
CEH-EUS pattern and quantitative elastography measurement in relation to diagnosis
Strain ratio was above 10 and strain histogram was below 50 in all of the 46 cases classified as pancreatic adenocarcinomas (including one case of a mucinous cystadenocarcinoma) (Figure 3). The CEH-EUS pattern was hypovascular in 42 cases (including the mucinous cystadenocarcinoma) and isovascular in four (Figure 4).
Figure 3.
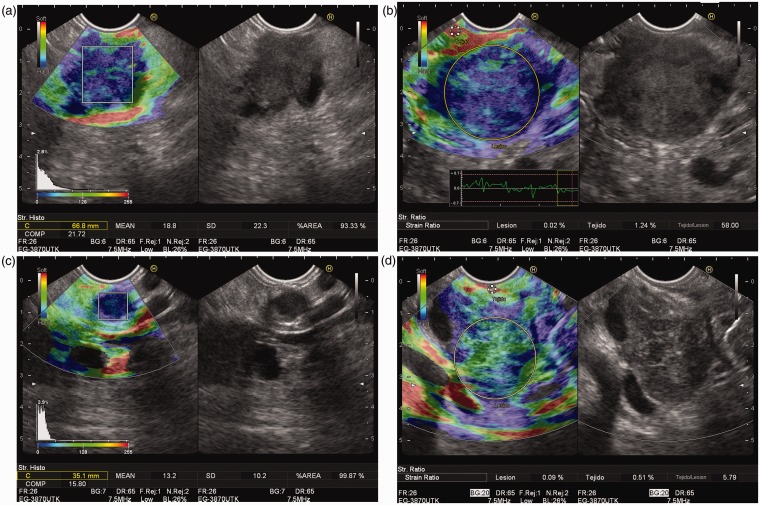
Endoscopic ultrasound guided elastographic images of different pancreatic solid tumors. (a) Pancreatic adenocarcinoma, with a predominant blue pattern in qualitative image, with a mean in the strain histogram of 18.8. (b) Pancreatic adenocarcinoma, with a predominant blue pattern in qualitative image, with a strain ratio of 58.00. (c) Solid pancreatic tumor, corresponding to a neuroendocrine lesion, with a blue pattern in qualitative image, and a strain histogram of 13.2. (d) Inflammatory mass located at pancreatic head, showing a heterogeneous green predominant pattern in qualitative image, and a strain ratio of 5.79.
Figure 4.
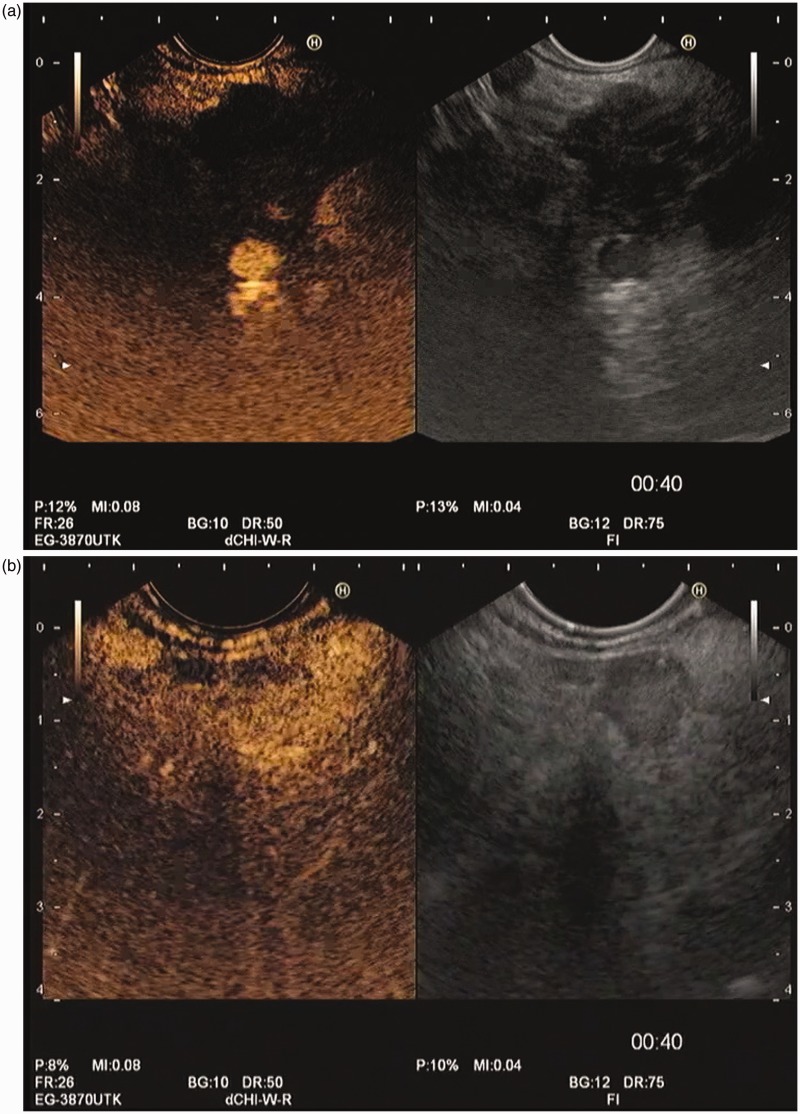
Contrast-enhanced harmonic endoscopic ultrasound evaluation of pancreatic solid masses, both at the end of arterial phase, beginning of venous phase (00:40 minutes). (a) Pancreatic adenocarcinoma, showing the typical hypovascular pattern. (b) Neuroendocrine pancreatic tumor, showing a hypervascular pattern.
There were 11 inflammatory masses; all but one presented strain ratio <10 and mean strain histogram >50. The inflammatory mass with pathological strain ratio and mean strain histogram value was a case of autoimmune pancreatitis with a strain ratio of 43.2, strain histogram of 22.7, and an iso-enhanced appearance on CEH-EUS. Median strain ratio and mean strain histogram for inflammatory masses were 6.00 (range 2.15–8.95) and 77.9 (range 62.9–118.1) if the case of autoimmune pancreatitis was excluded.
There were three neuroendocrine tumors (NETs), one classified as malignant and two as benign. The malignant NET was a lesion of 31 mm in the head of the pancreas, with a strain ratio of 32.3, a strain histogram of 13.2, and a hypervascular appearance (Table 2). This lesion was surgically removed and pathology demonstrated a high-grade (G3) NET, according to the World Health Organization (WHO) classification. The first NET classified as benign was a 13 mm tumor in the body of the pancreas with a hyper-enhanced pattern on CEH-EUS, a strain ratio of 3.41, and a mean strain histogram value of 111.3. This tumor was surgically resected and pathology demonstrated a highly differentiated benign insulinoma with low mitotic activity (<2/high power field), consistent with a low-grade NET (G1) according to the WHO classification. The second NET classified as benign was a 13 mm lesion at pancreatic body with a hypo-enhanced pattern on CEH-EUS, a strain ratio of 5.18, and a men strain histogram value of 96.2. This lesion was not punctured. This patient had a known multiple endocrine neoplasia syndrome type 1 and a previous pancreatic resection for a NET (highly differentiated with low grade malignancy according to the WHO classification). The patient was followed for 20 months without signs of increase in size or malignant transformation of the present lesion.
There were two pancreatic metastases, one from a lung cancer and one from a colonic cancer, both presenting clear malignant features on CEH-EUS and elastography (Table 1).
Diagnostic performance of CEH-EUS and elastography
Sensitivity, specificity, positive and negative predictive value, and accuracy for CEH-EUS, elastography strain ratio, elastography mean strain histogram value, and the combination of CEH-EUS and elastography strain ratio in the differentiation between benign and malignant lesions, and in the differentiation between pancreatic adenocarcinoma and chronic pancreatitis, are given in Table 3. The sensitivity for malignancy of elastography EUS was significantly higher than that of CEH-EUS (100% (95% CI: 92.7–100) versus 89.8% (95% CI: 78.2–95.6), p = 0.02). The specificity for malignancy of QE-EUS was higher than that of CEH-EUS but this difference was not statistically significant (92.3% (95% CI: 66.7–98.6) versus 69.2% (95% CI: 42.4–87.3), p = 0.14) (Table 3). Restricting the analysis to only cases with a final diagnosis of either pancreatic adenocarcinoma or chronic pancreatitis gave similar results (Table 3) with a higher sensitivity but not specificity of QE-EUS compared to CEH-EUS (p = 0.04 and p = 0.54, respectively).
Table 3.
Diagnostic performance of contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS), elastography strain ratio, and strain histogram
| Benign versus malignant |
Pancreatic cancer versus chronic
pancreatitis |
|||||
|---|---|---|---|---|---|---|
| Elastographya | CEH-EUS | Combinationb | Elastographya | CEH-EUS | Combinationb | |
| Sensitivity (95% CI) | 100 (92.7–100) | 89.8 (78.2–95.6) | 89.8 (78.2–95.6) | 100 (92.3–100) | 91.3 (79.7–96.6) | 91.3 (79.7–96.6) |
| Specificity (95% CI) | 92.3 (66.7–98.6) | 69.2 (42.4–87.3) | 100 (77.2–100) | 90.9 (62.3–98.4) | 81.8 (52.3–94.9) | 100 (74.1–100) |
| Positive predictive value (95% CI) | 98.0 (89.5–99.7) | 91.7 (80.5–96.7) | 100 (92.0–100) | 97.9 (88.9–99.6) | 95.5 (84.9–98.7) | 100 (91.6–100) |
| Negative predictive value (95% CI) | 100 (75.8–100) | 64.3 (38.8–83.7) | 72.2 (49.1–87.5) | 100 (72.3–100) | 69.2 (42.4–87.3) | 73.3 (48.1–89.1) |
| Accuracy (95% CI) | 98.4 (91.4–99.7) | 85.5 (74.7–92.2) | 91.9 (82.5–96.5) | 98.3 (90.7–99.7) | 89.5 (78.9–95.1) | 93.0 (83.3–97.2) |
CI: confidence interval.
Diagnostic performance of strain ratio and mean strain histogram was identical and these modalities are therefore presented together.
Cases were classified as positive if both CEH-EUS and elastography were positive.
Given the very high diagnostic accuracy in the differentiation between malignant and benign lesions by elastography, a possible additional value of CEH-EUS could be to further characterize the type of malignancy in a lesion with a malignant appearance on elastography. A total of 50 cases were positive according to elastography strain ratio/mean strain histogram. Out of these, 43 were hypo-enhanced (42 pancreatic adenocarcinomas and 1 metastasis), six were iso-enhanced (4 pancreatic adenocarcinomas, 1 inflammatory mass, and 1 metastasis), and one was hyper-enhanced (1 malignant NET) on CEH-EUS. Hence, our results indicate that a lesion with a positive elastography in combination with a hyper-enhanced pattern on CEHEUS should raise the suspicion of a malignant NET. However, this statement is based on a single observation and warrants further studies. Overall, one of the most important findings of the current study is that combining both techniques results in an acceptable sensitivity (89.8%), but a very high specificity and positive predictive value (100%).
Diagnostic performance of EUS-guided tissue acquisition
EUS-guided tissue acquisition was performed in 59 cases (95.2%). Sensitivity, specificity, positive and negative predictive value, and accuracy for detecting malignancy was 89.80% (80.3–99.3), 100% (95.0–100), 100% (98.9–100), 66.67% (39.5–93.9), and 91.53% (83.6–99.5), respectively. There were five cases with a false negative result at EUS-guided tissue acquisition. Four of these cases finally corresponded to a pancreatic adenocarcinoma; all four cases presented a QE-EUS and CEH-EUS compatible with malignancy, whereas the final cases corresponded to a metastasis from a lung cancer, with a QE-EUS compatible with malignancy and a CEH-EUS showing an isovascular lesion.
Discussion
In the present study, we demonstrate that QE-EUS and CEH-EUS are accurate and useful tools in the differential diagnosis of solid pancreatic lesions. A positive elastography finding (defined as strain ratio >10 or strain histogram level <50) classified lesions as malignant with 100% sensitivity and 92.3% specificity. Demonstration of a hypo- or hypervascular pattern on CEH-EUS detected malignancy with 89.8% sensitivity and 69.2% specificity. The sensitivity decreased to 89.8% and the specificity increased to 100% if a pathological finding on both modalities was required to define a lesion as malignant. In addition, both techniques provided information that may aid in the differential diagnosis between different types of malignant lesions.
Distinguishing pancreatic adenocarcinoma from other pancreatic masses is challenging with current imaging techniques.1,21,22 The ability of EUS to differentiate between benign and malignant solid lesions of the pancreas is limited, despite recent improvements in image quality and resolution. EUS-guided tissue sampling has improved the diagnostic accuracy of EUS.4,5,23 However, EUS-guided tissue sampling is associated with certain specific limitations itself, notably the risk of false negative results,6 and a small, but not negligible morbidity.7 Furthermore, a puncture can be technically difficult or even impossible in some cases, due to difficulties in positioning the instrument, interposed malignant tissue or vascular structures.24 Considering the risks and shortcomings of EUS-guided tissue sampling, a need for more accurate non-invasive EUS diagnosis is clearly present. CEH-EUS and QE-EUS are two of the most promising alternatives in this context.
The development of contrast-enhanced EUS started with contrast-enhanced power Doppler EUS. Initial reports were promising,25–28 but the method had some inherent problems notably with artifacts in the presence of turbulent flow.16 Today, the technique of contrast-enhanced ultrasonography has been further developed and CEH-EUS based on a low mechanical index is now available. This technology can detect signals from microbubbles in vessels with very slow flow and without Doppler-related artifacts. Dietrich et al. published the first report on the use of contrast-enhanced, low-mechanical index, real-time EUS.29 The diagnostic performance of modern CEH-EUS has been evaluated in four recent studies of 35, 90, 277, and 100 patients, respectively.30–33 The reported sensitivity and specificity from adenocarcinoma ranged from 89–95% and 64–96%, respectively, in these studies.30–33 The typical picture of a pancreatic adenocarcinoma is described as a heterogeneous hypovascular lesion and a lower density of vessels relative to the surrounding pancreatic tissue. Contrast enhancement patterns that we have obtained in the current study are in accordance with the previous reports. Pancreatic adenocarcinomas appeared as hypovascular lesions, inflammatory masses were mainly isovascular, and neuroendocrine tumors demonstrated a hypervascular pattern. We found sensitivity for detection of malignancy that was highly similar to previous studies (90% versus 89–96%). The specificity found in the current study (69%) was lower compared to studies by Kitano et al. (89%),32 Napoleon et al. (88%),30 and Gincul et al.,33 but similar to the study by Fusaroli et al. (64%).31 In the current study, both hyper- and hypo-enhancing lesions were classified as malignant since priority was given to a high sensitivity to detect malignancy in the design of the study. It is well known that many of the malignant lesions that can be found in the pancreas that are not adenocarcinomas have a hypervascular pattern (i.e., NETs and metastases). Hence, a further use of CEH-EUS is to guide in the evaluation of etiology of a lesion that already has been judged as malignant on other grounds. However, evaluation of the use of CEH-EUS in that differential diagnosis was beyond the scope of this study.
A new technology associated to CEH-EUS has been developed, allowing quantitative CEH-EUS evaluation. Saftoiu et al. have recently published their experience, reporting a time quantitative assessment of CEH-EUS of 87.5% sensitivity, 92.72% specificity, 96.07% positive predictive value, and 78.46% negative predictive value. By adding an artificial neural network classification model, sensitivity was 94.64%, specificity 94.44%, positive predictive value 97.24%, and negative predictive value 89.47%.34
EUS-guided elastography is a new technique for assessment of tissue stiffness.9 Many pathological conditions, including cancer, can alter the mechanical properties of tissue by fibrosis and extracellular matrix remodeling. Since the first study by Giovannini et al. in 2006,11 several studies on the usefulness of EUS-guided elastography in the differential diagnosis between benign and malignant lesions of the pancreas have been published and the literature has recently been reviewed,8,35 and analyzed in meta-analyses.3,14,36 Early studies used a qualitative elastography evaluation by classification of lesions according to predominant color pattern. In an attempt to address the problem with the subjective nature of the qualitative evaluation, later studies that have applied a semi-quantitative elastography evaluation based on either strain-histograms or strain ratios.8 High sensitivity, specificity, and overall accuracy have been reported for both qualitative and quantitative elastography,8 with some exceptions in the literature.37,38 In the current study we found a sensitivity of 100% for detection of malignancy by EUS-guided elastography. A sensitivity of 100% has been reported in several previous studies,10,11,39–41 and is only moderately higher than what has been found in meta-analyses (95–99%).3,14,36 The specificity of 92% found in the current study is significantly higher than what has been reported in meta-analyses (67–73%),3,14,36 but highly similar to a previous publication from our own institution (93%).42
To the best of our knowledge, there are only two studies published that directly compare contrast-enhanced power Doppler EUS and EUS-guided elastography. Saftoiu et al. made the comparison in 54 patients with solid pancreatic lesions and found no significant difference in sensitivity (91% versus 85%), specificity (71% versus 76%), or accuracy (83% versus 82%).43 Combination of the two techniques improved specificity to 95% and positive predictive value to 96%.43 Figueiredo et al. performed a similar study, comparing contrast-enhanced power Doppler EUS and EUS-guided elastography in 47 cases with solid pancreatic lesions.18 Similar to what was observed in the study by Saftoiu et al., no technique was demonstrated to be superior to the other. Sensitivity, specificity, and accuracy were 93% versus 90%, 67% versus 75%, and 79% versus 82% for contrast-enhanced power Doppler EUS versus EUS elastography.18 Several differences between the current study and the two mentioned above have to be taken into account. First, both previous studies use the power Doppler technique for contrast-enhanced EUS. Our study is the first to compare the improved CEH-EUS technique, using low mechanical index. Second, classification of elastography and contrast-enhancement findings was computerized in the study by Saftoiu et al., as opposed to the manual direct classification in our study and the study by Figueiredo et al. Furthermore, Saftoiu et al. applied a binary classification of contrast enhancement (hypo- or hypervascular) without any category for iso-enhancement. This is relevant since it is well known that the majority of neuroendocrine tumors are hypervascular and will hence be classified as pathological (hypervascular) using our definition but normal (not hypovascular) using the definition applied by Saftoiu et al.43 Figueiredo et al. avoided this problem in the study by excluding neuroendocrine tumors.18 Despite being the largest out of the three above-mentioned studies, the limited number of patients in our study precludes firm conclusions on minor differences in diagnostic performance between QE-EUS and CEH-EUS. Future studies and meta-analyses will hopefully shed further light on this issue.
The current study has several strong points. Patient selection bias was minimized by consecutive inclusion of all patients with pancreatic masses during the study period. In addition, there were no exclusions due to technical problems to perform QE-EUS or CEH-EUS. This has resulted in a representative distribution of malignant lesions including not only adenocarcinomas and an important proportion of benign lesion, increasing the generalizability of our findings. We applied a well-defined and established method for classification of final diagnosis that has been used in previous studies.20
The present study has some limitations. Despite being one of the lager series published comparing QE-EUS and CEH-EUS, the statistical power was still limited. This has resulted in relatively wide confidence intervals of diagnostic performance estimates. Furthermore, there was a relatively important numerical difference in specificity comparing QE-EUS and CEH-EUS (92.3% (95% CI 66.7–98.6) versus 69.2% (42.4–87.3)) but this difference did not reach statistical significance (p = 0.14). This may be due to poor statistical power. The QE-EUS has been brought forward as a technique that is more objective than qualitative elastography, avoiding the subjective and sometimes difficult interpretation of predominant color-pattern. However, QE-EUS is nevertheless dependent on proper selection of a representative area from the lesion (as well as a reference area in the calculation of strain ratios). The internal validity and inter-observer agreement of QE-EUS as well as the hypothesis that QE-EUS is less operator-dependent than qualitative EUS need to be investigated in future studies. The generalizability of our results is also limited to some degree due to the fact that this was a single-center study with only one highly experienced endosonographer involved. Additional multicenter studies with endosonographers with different degree of experience are warranted. Last, the distribution of final diagnoses affects estimates of diagnostic performance. In the current series, there was a high proportion of pancreatic adenocarcinomas, reflecting the normal flow of patients referred for EUS at our institution. This needs to be taken into account when our results are compared to other; this may reflect the clinical routine in the workup diagnosis of solid pancreatic lesions. Further studies including different type of lesions are necessary to highlight the importance of this technique.
In conclusion, this study has indicated that QE-EUS can differentiate between benign and malignant pancreatic lesions with a high sensitivity that exceeds that of CEH-EUS. The use of CEH-EUS may be to differentiate between different types of malignant lesions. The combination of both techniques may even optimize the characterization of solid pancreatic masses, by guiding to the final diagnosis, mainly to differentiate between autoimmune pancreatitis and pancreatic cancer or between different types of NET lesions. Further research is needed to confirm our results.
Funding
This work was partially funded by the Galician Development and Technological Innovation Research Plan 2006–2010 (project number 09CSA041918PR).
Conflict of interest
JE Domínguez-Muñoz has acted as scientific advisor for Pentax Medical Company. Other authors declare no conflicts of interest with no disclosures to be made.
References
- 1.Iglesias-Garcia J, Lindkvist B, Larino-Noia J, et al. The role of EUS in relation to other imaging modalities in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and ductal pancreatic adenocarcinoma. Rev Esp Enferm Dig 2012; 104: 315–321. [DOI] [PubMed] [Google Scholar]
- 2.Seicean A. Endoscopic ultrasound in chronic pancreatitis: where are we now? World J Gastroenterol 2010; 16: 4253–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Xu W, Shi J, et al. Endoscopic ultrasound elastography for differentiating between pancreatic adenocarcinoma and inflammatory masses: a meta-analysis. World J Gastroenterol 2013; 19: 6284–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas 2013; 42: 20–26. [DOI] [PubMed] [Google Scholar]
- 5.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2011; 43: 897–912. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 1995; 27: 171–177. [DOI] [PubMed] [Google Scholar]
- 7.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006; 63: 622–629. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias-Garcia J, Lindkvist B, Larino-Noia J, et al. Endoscopic ultrasound elastography. Endosc Ultrasound 2012; 1: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Parker KJ, Lerner RM, et al. Imaging of the elastic properties of tissue: a review. Ultrasound Med Biol 1996; 22: 959–977. [DOI] [PubMed] [Google Scholar]
- 10.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc 2009; 70: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini M, Hookey LC, Bories E, et al. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy 2006; 38: 344–348. [DOI] [PubMed] [Google Scholar]
- 12.Cochlin DL, Ganatra RH, Griffiths DF. Elastography in the detection of prostatic cancer. Clin Radiol 2002; 57: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 13.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of us elastography for diagnosis. Radiology 2006; 239: 341–350. [DOI] [PubMed] [Google Scholar]
- 14.Mei M, Ni J, Liu D, et al. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc 2013; 77: 578–589. [DOI] [PubMed] [Google Scholar]
- 15.Saftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc 2008; 68: 1086–1094. [DOI] [PubMed] [Google Scholar]
- 16.Saftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy 2012; 44: 612–617. [DOI] [PubMed] [Google Scholar]
- 17.Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc 2012; 76: 301–309. [DOI] [PubMed] [Google Scholar]
- 18.Figueiredo FA, da Silva PM, Monges G, et al. Yield of contrast-enhanced power Doppler endoscopic ultrasonography and strain ratio obtained by EUS-elastography in the diagnosis of focal pancreatic solid lesions. Endosc Ultrasound 2012; 1: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) – update 2008. Ultraschall Med 2008; 29: 28–44. [DOI] [PubMed] [Google Scholar]
- 20.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc 2011; 73: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 21.Taylor B. Carcinoma of the head of the pancreas versus chronic pancreatitis: diagnostic dilemma with significant consequences. World J Surg 2003; 27: 1249–1257. [DOI] [PubMed] [Google Scholar]
- 22.Frulloni L, Falconi M, Gabbrielli A, et al. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis 2010; 42(Suppl. 6): S381–S406. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011; 106: 1705–1710. [DOI] [PubMed] [Google Scholar]
- 24.DeWitt J, McGreevy K, Sherman S, et al. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc 2008; 67: 610–619. [DOI] [PubMed] [Google Scholar]
- 25.Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc 2001; 53: 784–789. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol 2008; 6: 590–597. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto H, Kitano M, Suetomi Y, et al. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol 2008; 34: 525–532. [DOI] [PubMed] [Google Scholar]
- 28.Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol 2006; 12: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol 2005; 43: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 30.Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy 2010; 42: 564–570. [DOI] [PubMed] [Google Scholar]
- 31.Fusaroli P, Spada A, Mancino MG, et al. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol 2010; 8: 629–634. [DOI] [PubMed] [Google Scholar]
- 32.Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol 2012; 107: 303–310. [DOI] [PubMed] [Google Scholar]
- 33.Gincul R, Palazzo M, Pujol B, et al. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: a prospective multicenter trial. Endoscopy 2014; 46: 373–379. [DOI] [PubMed] [Google Scholar]
- 34.Saftoiu A, Vilmann P, Dietrich CF, et al. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc 2015; 82: 59–69. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich CF, Saftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS): a comprehensive review. Eur J Radiol 2014; 83: 405–414. [DOI] [PubMed] [Google Scholar]
- 36.Pei Q, Zou X, Zhang X, et al. Diagnostic value of EUS elastography in differentiation of benign and malignant solid pancreatic masses: a meta-analysis. Pancreatology 2012; 12: 402–408. [DOI] [PubMed] [Google Scholar]
- 37.Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy 2008; 40: 910–917. [DOI] [PubMed] [Google Scholar]
- 38.Janssen J, Schlorer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc 2007; 65: 971–978. [DOI] [PubMed] [Google Scholar]
- 39.Schrader H, Wiese M, Ellrichmann M, et al. Diagnostic value of quantitative EUS elastography for malignant pancreatic tumors: relationship with pancreatic fibrosis. Ultraschall Med 2012; 33: E196–E201. [DOI] [PubMed] [Google Scholar]
- 40.Dawwas MF, Taha H, Leeds JS, et al. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, single-center study. Gastrointest Endosc 2012; 76: 953–961. [DOI] [PubMed] [Google Scholar]
- 41.Iglesias-García J, Castiñeira-Alvariño M, Lariño-Noia J, et al. Usefulness of quantitative endoscopic ultrasound (EUS) elastography for diagnosing chronic pancreatitis. Pancreatology 2010; 10: 298–298. [Google Scholar]
- 42.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology 2010; 139: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 43.Saftoiu A, Iordache SA, Gheonea DI, et al. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc 2010; 72: 739–747. [DOI] [PubMed] [Google Scholar]