Abstract
Background
Alongside the evolution of interventional endoscopy, the need for a more sophisticated closure tool tailored to the treatment of new challenging indications has been increasing rapidly.
Methods
We here present our collected data on 262 Over-The-Scope-Clip (OTSC®) placements in a total of 233 interventions at our institution. Follow-up was focused on clinically lasting success with regards to different indications.
Results
Immediate success of OTSC® treatment was observed in 87.1% of all sessions (203/233). The success rates per indication were as follows: spontaneous bleeding 84.8% (28/33); iatrogenic bleeding 100% (20/20); acute perforation 90.3% (65/72); prophylaxis for perforation 100% (24/24); anastomotic leakage 61.1% (11/18); fistulae 80.7% (46/57); diameter reduction of the gastrojejunal anastomosis 100% (6/6); and stent fixation 100% (3/3). At 30-day follow-up, the overall success rate was 67.4% (157/233). The success rates per indication were as follows: spontaneous bleeding 69.7% (23/33); iatrogenic bleeding 90% (18/20); acute perforation 86.1% (62/72); prophylaxis for perforation 100% (24/24); anastomotic leakage 33.3% (6/18); fistulae 29.8% (17/57), diameter reduction of the gastrojejunal anastomosis 83.3% (5/6); and stent fixation 66% (2/3).
Conclusions
Our cohort confirms previous data on the clinical usefulness of the OTSC® in daily routine practice.
Keywords: OTSC, perforation, GI bleeding, anastomotic leakage, fistula, stent fixation, gastric bypass
Introduction
Endoscopic procedures regularly exhibit pathologic findings, which demand availability of reliable devices to clip or close wall defects or bleeding sites throughout the gastrointestinal tract from esophagus to rectum. So far, the classical endoscopic tools to treat the majority of gastrointestinal lesions such as fistulae, bleeding vessels or small wall defects have been different types of endoclips and self-expanding metallic stents (SEMS).
The evolution of interventional endoscopy with more aggressive endoluminal procedures such as endoscopic mucosa resection (EMR) and endoscopic submucosal dissection (ESD) was followed by submucosal tunneling procedures such as peroral endoscopic myotomy (POEM). Alongside these novel and progressive options of non-surgical treatments, the incidence of more severe endoscopic complications such as iatrogenic perforations increased over the years. Complex surgical interventions in gastrointestinal oncology together with advanced chemotherapeutics may in addition favor more anastomotic leakages and fistulae. So far, the standard therapeutic options have been endoclips,1 glue injections,2 SEMS,3 and experimental suturing devices with modest success rates.4 Due to this progressive evolution of interventional endoscopy, the need for a more sophisticated closure tool tailored to the treatment of new challenging indications has been increasing rapidly.
In 2007, Kirschniak et al. reported for the first time a retrospective case series on eight therapy-refractory gastrointestinal bleedings and three wall defects being successfully treated with the novel Over-The-Scope-Clip (OTSC®, OVESCO Endoscopy AG, Tübingen).5 Animal studies investigating these OTSC® showed their superiority over conventional endoclips with regard to the closure capacity of iatrogenic perforations.6 Ever since the OTSC® system has been studied in animalistic gastric and colonic models, the possible anatomic sites for OTSC® placement have been increasing along the entire luminal gastrointestinal tract. Over time, the indications for the OTSC® placement have been expanding.7 Recently, broader indications, such as SEMS fixation within the esophagus,8 closure of POEM-access,9 as well as diameter reduction of gastrojejunal anastomosis after gastric bypass,10 have been reported. Yet, the classical indications for OTSC® therapy remain closure or treatment of gastrointestinal perforations,11 leakages,12 fistulae including anorectal lesions,13,14 and uncontrolled bleedings.15 Although different case series have proven the benefit of the OTSC® device in daily endoscopic practice, only one large, multicenter retrospective case series involving 17 centers has been published so far.16
Since 2009, the placement of OTSC® has been established at our institution for the entire spectrum of indications. The standardized approach led to a proper introduction of the device into daily practice over time. Therefore, we present our collected data over six years focusing on indications, anatomic site of OTSC deployment, complications, and immediate and 30-day success rates.
Materials and methods
We collected data of all patients treated with the OTSC® device at our institution including all combined or sequential interventions with overstenting, endo-vacuum treatments, and application of endoclips or injection of different types of glue. Twenty-one of the collected patients were excluded due to prior publication by our group.17–19 Furthermore, patients treated at our institution with the novel FTRD® system (OVESCO Endoscopy AG, Tübingen) were not included in this cohort.
Most of the endoscopies were performed at our endoscopy ward or intensive care units under deep sedation with propofol (NAAP: non-anesthetic application of propofol). Only a limited number of the cases were performed in the operation theater. All procedures were started as diagnostic endoscopies with flexible Olympus® endoscopes. Indication for the need of an OTSC® placement was evaluated directly or after interdisciplinary discussion. The OTSC® placement was performed by one of seven experienced endoscopists.
The used OTSC® system is well described elsewhere.20 The clip size (11, 12, or 14) and the type of clip teeth (traumatic versus atraumatic) were chosen by the procedure-performing endoscopist. The necessity for auxiliary devices such as a double grasping forceps (twingrasper®, OVESCO Endoscopy AG, Tübingen), a three-hook anchoring device (anchor® OVESCO Endoscopy AG, Tübingen), or simple suction into the mounted plastic cap were also assessed by the endoscopist. All procedures were performed using carbon dioxide insufflation instead of ambient air. Intravenous antibiotics (broad spectrum preparations covering Gram-negative and -positive bacteria) were administered if indicated.
Endoscopy reports were standardized with information on the technique of OTSC® deployment, OTSC® size, and occurring complications. Immediate evaluation of success either proven endoscopically or utilizing contrast media was mandatory in fistulae and after perforation closure. These patients were kept on nil per os for 6–24 hours after the initial intervention. The responsible physicians on the ward initiated introduction of per os feeding or necessary imaging depending on signs and symptoms.
Data were analyzed retrospectively considering demographic data. The patients were then allocated to one of the different indication groups for OTSC® placement. Success was registered immediately after clip placement and at 30-day follow-up. Immediate complications and adverse events occurring in the follow-up were collected. Lacking data were collected from patients by phone calls or from their general practitioners. The study was approved by our local ethical committee.
Immediate treatment success was granted if the OTSC® was deployed onto the target tissue as desired by the endoscopist and clinical success was achieved for each particular indication. Additional diagnostic procedures such as cross-section imaging, contrast-enhanced X-rays, or re-endoscopies were optional and at the endoscopist’s discretion.
Data were finally analyzed per patient and per OTSC® session itself.
Results
Over a six-year time period (September 2009–December 2015) at our institution, 202 patients were treated in 233 procedures with a total of 262 OTSC® applications. In 32 cases of the all OTSC® placements, two clips were placed simultaneously during a single session (12.1%). Patient age ranged from 14 to 93 years with a median age of 61 years. The majority of the patients were male (51.5%).
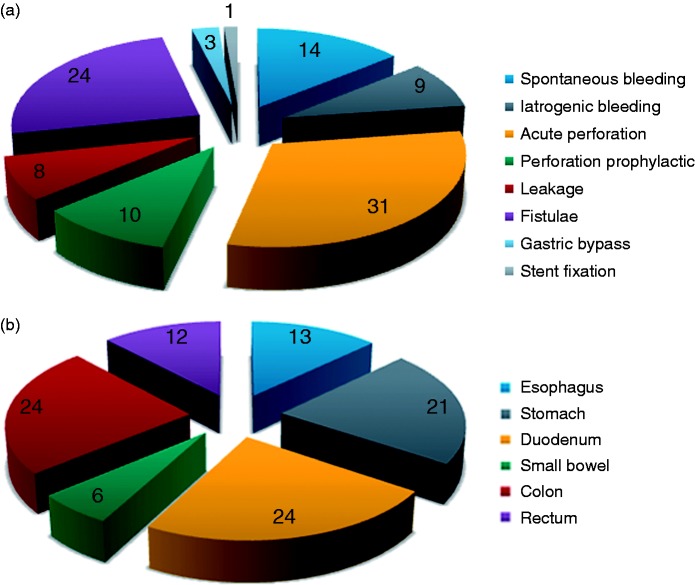
Acute perforation (30.1%, 72/233), fistulae (24.5%; 57/233), spontaneous bleeding (14.2%; 33/233), prophylaxis for perforation (10.3%; 24/233), iatrogenic bleeding (8.6%; 20/233), anastomotic leakage (7.7%; 18/233), diameter reduction of the gastrojejunal anastomosis after bariatric surgery (2.6%; 6/233), and SEMS fixation (1.3%; 3/233) were the eight indication groups for OTSC® placement (Figure 1(a)).
Figure 1.
(a) Indication for OTSC® (%). (b) Anatomic site of OTSC® (%).
The perforation group was subdivided into OTSC® treatment for either acute manifest perforation (75%; 72/96) or prophylactic OTSC® placement (25%; 24/96) after an endoscopic intervention (e.g. polypectomy).
Given the potential difference of tissue quality, a cut-off of 14 days was applied to differentiate between acute anastomotic leakage (diagnosis < 14 d) and chronic fistula (diagnosis > 14 d). Eighteen OTSC® treatments were performed to treat anastomotic leakage (24%; 18/75) whereas 62 were performed for chronic fistulae (76%; 57/75).
The bleeding group was subdivided into patients treated for either spontaneous gastrointestinal bleeding (62.3%; 33/53) or iatrogenic bleeding after an endoscopic intervention (37.7%; 20/53).
The anatomic site of OTSC® deployment was distributed as follows: 12.9% (30/233) in the esophagus, 21.5% (50/233) in the stomach, 23.6% (55/233) in the duodenum, 6% (14/233) in the small bowel, 24% (56/233) in the colon and 12% (28/233) in the rectum (Figure 1(b)).
The majority of the bleeding events (both spontaneous and iatrogenic) occurred in the duodenum. Acute perforations were mainly seen in the colon. Fistulae were evenly distributed among the esophagus, the stomach, the colon and the rectum (data not shown).
A panel of different OTSC® sizes and types were used, although in the majority of the cases either a 12/6t OTSC® (in 47%; 135/286), a 14/6t OTSC® (in 28%; 81/286) or an 11/6t OTSC® (in 15%; 44/286) was chosen. The overall distribution of OTSC® sizes and types is shown in Table 1.
Table 1.
OTSC® subtypes
| OTSC® subtype | Number of OTSC® | Percentage (%) |
|---|---|---|
| 11/3a | 1 | 0 |
| 11/3t | 3 | 1 |
| 11/6a | 6 | 2 |
| 11/6t | 43 | 16 |
| 12/6a | 1 | 0 |
| 12/6t | 129 | 49 |
| 12/6gc | 10 | 4 |
| 14/6a | 2 | 1 |
| 14/6t | 67 | 26 |
| Total | 262 | 100 |
In addition to simple suction through the working channel of the endoscope, auxiliary devices such as a double grasping forceps (twingrasper®, OVESCO Endoscopy AG, Tübingen) (17.6%, 41/233) or a three hook anchoring device (anchor® OVESCO Endoscopy AG, Tübingen) (17.6%, 41/233) were used in order to pull the lesion into the plastic cap of the OTSC® system.
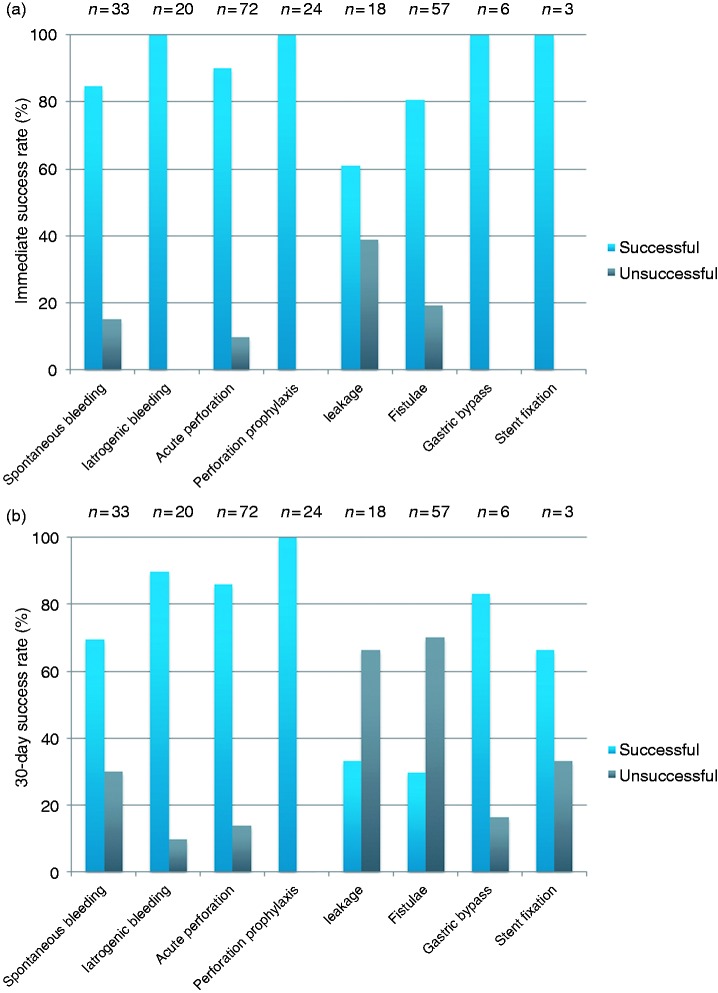
Immediate success of OTSC® treatment judged by the endoscopist was stated in 87.1% of all sessions (203/233). In three cases (1.3%; 3/233), the passage of the mounted OTSC® through the upper esophageal sphincter was not possible, but OTSC®-passage became successful after loading a smaller sized OTSC®. The success rates for the different indication groups were as follows: spontaneous bleeding 85% (28/33); iatrogenic bleeding 100% (20/20); acute perforation 90.3% (65/72); prophylaxis for perforation 100% (24/24); anastomotic leakage 61.1% (11/18); fistulae 80.7% (46/57); diameter reduction of the gastrojejunal anastomosis after bariatric surgery 100% (6/6); and stent fixation 100% (3/3) (Figure 2(a)). The main causes for failure of OTSC® placement (12.9%; 30/233) were non-suitable anatomic structure (30%; 9/30), rigidity of the lesion-surrounding tissue (33.3%; 10/30) and lesion size exceeding the capability of successful approach by an OTSC® (36.7%; 11/30).
Figure 2.
(a) Immediate success of OTSC® deployment as a function of indication. (b) 30-day success of OTSC® deployment as a function of indication.
In case no follow-up endoscopies or radiographic studies were performed at our institution, the patients themselves and/or their family physicians were contacted by telephone and successful outcome was confirmed clinically.
At 30-day follow-up, the success rate was 67.4% (157/233), whereas 32.6% (76/233) of the cases had to be considered as failed treatment.
The success rate 30 days after OTSC® placement in the prophylactic for perforation group was 100%. The success rate in the spontaneous bleeding group remained unchanged at 69.7% (23/33) after 30 days, while the success rate in the iatrogenic bleeding group dropped to 90% (18/20). In the acute perforation group, three cases relapsed in addition to the seven immediate OTSC® failures, thus 30-day success rate was 86.1% (62/72).
These relapses occurred on days 1, 7, and 12 after initial closure. In the small stent fixation group, one out of three clips fell off during the 30 days of follow-up. One out of six patients did not lose any weight after diameter reduction of the gastrojejunal anastomosis at 30-day follow-up and was therefore considered as a failure (success rate 83.3%, 5/6).
Anastomotic leakage and fistulae were the least successful groups, with 33.3% (6/18) and 29.8% (17/57) persistent closure (Figure 2(b)).
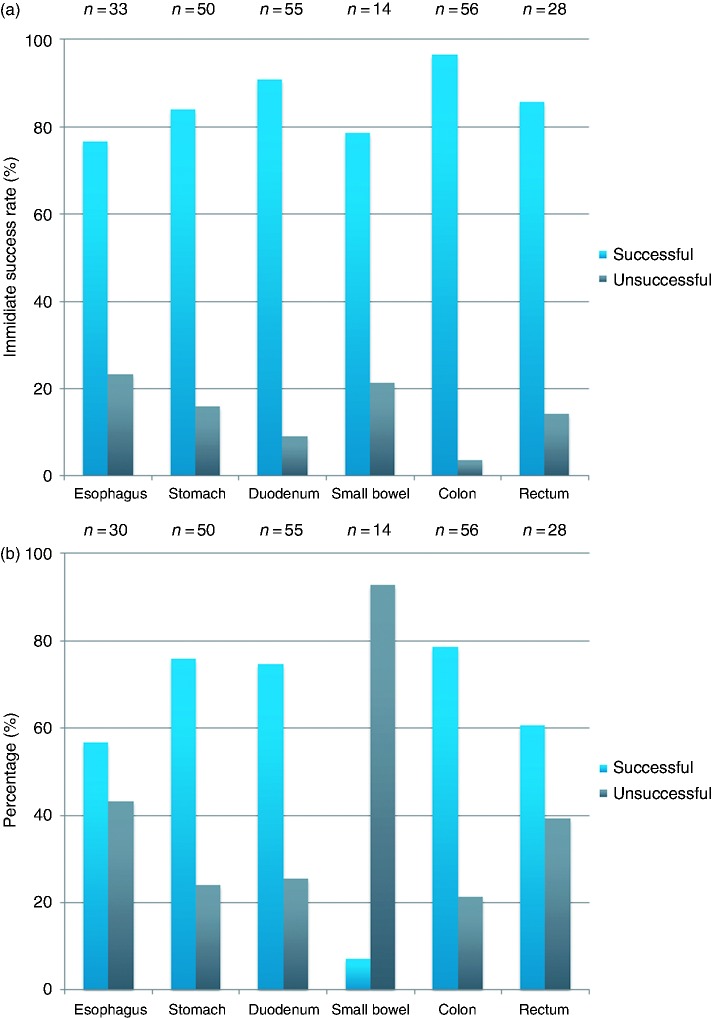
Regarding the OTSC® placement site, the immediate success rates were best in the colon (96.4%; 54/56) and the duodenum 90.9% (50/55), followed by the rectum (85.7%, 24/28) and the stomach (84%; 42/50). The immediate success rates were lowest in the esophagus (76.7%; 23/30) and the small bowel (78.6%; 11/14) (Figure 3(a)).
Figure 3.
(a) Immediate success of OTSC® deployment as a function of anatomic site. (b) 30-day success of OTSC® deployment as a function of anatomic site.
As a function of the anatomic site for OTSC® placement, the 30-day success rates were as follows: esophagus 56.7% (17/30), stomach 76% (38/50), duodenum 74.5% (41/55), small bowel 7.1% (1/14), colon 78.6% (44/56), and rectum 60.7% (17/28) (Figure 3(b)).
Endoscopy-related complications were only noted in 2.6% of cases (6/233). In three cases, the OTSC® was released accidently on the patient’s tongue during withdrawal of the endoscope through the mouthpiece, after the 14-clip was too large for deployment in the upper GI tract (3/6). These clips were then removed by using a side cutter from the emergency ERCP armamentarium. In one particular case, the OTSC® mounted onto the tip of the endoscope led to a wider dehiscence (1/6) of the fresh esophageal anastomosis. In one other case (1/6), the mounted 14-clip caused mucosal laceration of the esophagus without need for further intervention. In one case (1/6), a rectal pseudopolyp including luminal structuring developed after OTSC® treatment causing an outlet obstruction syndrome. Yet, the condition was well manageable with the administration of a laxative.
No anesthesia- or NAAP-associated complications or deaths occurred in our cohort.
Discussion
To our knowledge, we here present the largest cohort of patients treated with a total of 262 OTSC® placements. The initially chosen indications were extended over the course of the observation time and were divided into eight major groups: acute perforation, fistulae, spontaneous bleeding, iatrogenic bleeding, prophylaxis for perforation, anastomotic leakage, diameter reduction of the gastrojejunal anastomosis after bariatric surgery, and metallic stent fixation.
We were able to illustrate that the treatment with an OTSC® is safely feasible in various conditions aiming at high immediate success rates with sustained clinical success at 30-day follow-up.
The group of bleeding conditions was subdivided into two subgroups: bleeding caused by a prior intervention and spontaneously occurring bleeding for instance caused by an ulcer. The fact that slightly lower success rates (immediate and at 30-days) were observed in the latter might be explained due to less favorable features of the surrounding tissue, making the OTSC® adherence more challenging.
Several cases of relevant re-bleeding through the closed claws of the OTSC® were observed, making an additional intervention for hemostasis necessary (data not shown). The fact that the teeth of the OTSC® do not close completely, is indeed a wanted feature to prevent necrosis due to complete interruption of blood perfusion. But it might also cause a need for additional hemostasis interventions in some rare cases. Nevertheless, we were able to prove concordantly with literature,15 that OTSC® treatment is an established rescue manoeuver to stop severe GI bleedings.
We were able to show, that prophylactic OTSC® placement prior to polypectomy works and concludes well. This fact is most likely due to the well-planned placement before the clip is even needed. Certainly, there might also be inclusion of cases where an OTSC® treatment never would have been necessary. However, prophylactic OTSC® placement prior to polypectomy remains a promising indication for avoidance of iatrogenic perforations. The feasibility of this technique has already been demonstrated with the bedside introduction of the full-thickness-resection device (FTRD®).21 Whether the placement of a plain OTSC® prior to resection is reasonable, was not assessed in our cohort at this point. Yet, it is of great importance to aim for clear margins while performing endoscopic polypectomy with prior deployment of an OTSC®. Subsequent resection of residual adenoma tissue is challenging and rarely feasible without prior removal of the clip. Application of cutting current for hot snaring of residual polyps is usually not feasible anymore, due to the electrical conductibility of the OTSC®.
Certainly, OTSC® treatment should always be tried for acute, iatrogenic perforation in the colon, since success rates were astonishingly high in this group. In this particular clinical setting, we emphasized on working with carbon dioxide insufflation and administering broad-spectrum antibiotics from the very beginning.
As assumed beforehand, the success rates were lowest in the leakage and the fistulae group, most likely due to the unfavorable modalities of surrounding tissue in these conditions. The success rates decreased even more at 30-day follow-up probably reflecting the chronicity of these lesions. Secondly, the simple closure of the fistula orificium might not end in total clinical resolution of the condition.
However, treatment success in any individual case of fistulae justifies at least one OTSC® closure attempt due to the lack of reliable alternatives. The combination of different endoscopic modalities, such as stenting, glue injection,13 or even endo-vacuum therapy,12 seems to be reasonable to achieve higher success figures.
Lower success rates were also observed when placing an OTSC® in the esophagus or the small bowel. This fact is most probably due to the tangential mode of operation in a relatively small diameter esophageal lumen. The long way to the small bowel and active peristaltic movements might be hindering further healing in that particular group.
Both, the diameter reduction group of the gastrojejunal anastomosis as well as the SEMS fixation group were orphan indications containing only a limited number of patients making a clear-cut conclusion difficult. Yet, we were able to show, that a treatment approach with an OTSC® in these groups is feasible and promising.
Our cohort did not focus on the OTSC® proctology, which is used to efficiently close anorectal fistulae.22
There are certain circumstances in which the OTSC® removal is necessary. Remaining adenomatous tissue after polypectomy for example will make a clip removal crucial before completing polypectomy or performing full-thickness resection.23 Symptomatic obstruction of the GI tract might be another indication for clip removal, even though we did observe only one such case in our cohort. This female patient developed symptomatic outlet constipation after perforation closure in the lower rectum. Lately, an OTSC® clip cutter system has been developed and introduced by OVESCO Endoscopy AG (Tübingen), which will help to overcome the above discussed difficulties associated with a permanently placed OTSC®.
The upper esophageal sphincter might limit the passage of an OTSC® especially in older women of shorter stature. We therefore tried to abstain from using the largest clip (14-size) in the upper GI tract. In contrast, the anal sphincter never prevented the passage of the largest OTSC® in selected cases of our cohort.
Even though prospective, randomized trials may be the best-recognized instruments to answer clinical questions, particular situations demand simple observation after an intervention with a long-term analysis. Withholding the treatment with a new device in the meantime may be unethical, if no complications have to be feared. As we were able to show in our current cohort, no severe clip-associated complications or deaths occurred during the observation time. Minor complications such as accidental OTSC® release on the patient’s tongue (n = 3) occurred in the first year of use caused by withdrawal of the endoscope after incomplete release of the clip at the targeted site. Thereafter, our endoscopy team started to insist on visualization of the discharged OTSC® prior to withdrawal of the endoscope.
After introduction of the OTSC®, its placement became a valuable new treatment option for many various situations next to the conventional wait-and-see strategy or a surgical revision. To prove its efficacy, immediate and 30-day success rates were evaluated. Superiority over a wait-and-see approach has to be matched with the historical knowledge of the spontaneous course of a certain condition. Therefore, some cases out of our eight indication subgroups may be questioned. Certainly, fistulae represent difficult cases to judge in regard to alternative closing modalities.24 Likewise, contained leakages or perforations into the retroperitoneum or the mesorectum may seal without a closing device or surgical revision.25 On the other hand, free abdominal perforations and ongoing luminal bleedings are always deleterious. In our cohort, the majority (64%; 149/233) of the cases were perforations or bleedings that necessitated a definite action. Prior to the introduction of the OTSC® device, most of these cases would have been subject to interventional radiology or surgery.
Conclusion
Regarding the lack of serious complications, our cohort confirms previous data on the clinical usefulness of the OTSC in daily routine practice. After an instruction course, the OTSC® should be established in every endoscopy venue.
Note
A part of these results was chosen and held as an oral presentation by PV Valli at the UEG Week 2015 in Barcelona.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Yoshikane H, Hidano H, Sakakibara A, et al. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointestinal Endoscopy 1997; 46: 464–466. [DOI] [PubMed] [Google Scholar]
- 2.Samarasena JB, Nakai Y, Park DH, et al. Endoscopic closure of an iatrogenic duodenal perforation: a novel technique using endoclips, endoloop, and fibrin glue. Endoscopy 2012; 44(Suppl 2 UCTN): e424–e425. [DOI] [PubMed] [Google Scholar]
- 3.Dasari BV, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014; 259: 852–860. [DOI] [PubMed] [Google Scholar]
- 4.Raju GS, Shibukawa G, Ahmed I, et al. Endoluminal suturing may overcome the limitations of clip closure of a gaping wide colon perforation (with videos). Gastrointestinal Endoscopy 2007; 65: 906–911. [DOI] [PubMed] [Google Scholar]
- 5.Kirschniak A, Kratt T, Stuker D, et al. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointestinal Endoscopy 2007; 66: 162–167. [DOI] [PubMed] [Google Scholar]
- 6.Von Renteln D, Vassiliou MC, Rothstein RI. Randomized controlled trial comparing endoscopic clips and over-the-scope clips for closure of natural orifice transluminal endoscopic surgery gastrotomies. Endoscopy 2009; 41: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 7.Singhal S, Changela K, Papafragkakis H, et al. Over the scope clip: technique and expanding clinical applications. J Clin Gastroenterol 2013; 47: 749–756. [DOI] [PubMed] [Google Scholar]
- 8.Toshniwal J, Zabielski M, Fry LC, et al. Combination of the “bear claw” (over-the-scope-clip system) and fully covered stent for the treatment of post-operative anastomotic leak. Endoscopy 2012; 44(Suppl 2 UCTN): e288–e289. [DOI] [PubMed] [Google Scholar]
- 9.Saxena P, Chavez YH, Kord Valeshabad A, et al. An alternative method for mucosal flap closure during peroral endoscopic myotomy using an over-the-scope clipping device. Endoscopy 2013; 45: 579–581. [DOI] [PubMed] [Google Scholar]
- 10.Heylen AM, Jacobs A, Lybeer M, et al. The OTSC(R)-clip in revisional endoscopy against weight gain after bariatric gastric bypass surgery. Obesity Surg 2011; 21: 1629–1633. [DOI] [PubMed] [Google Scholar]
- 11.Weiland T, Fehlker M, Gottwald T, et al. Performance of the OTSC system in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc 2013; 27: 2258–2274. [DOI] [PubMed] [Google Scholar]
- 12.Mennigen R, Colombo-Benkmann M, Senninger N, et al. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with the Over-the-Scope Clip (OTSC). J Gastrointestinal Surg 2013; 17: 1058–1065. [DOI] [PubMed] [Google Scholar]
- 13.Mercky P, Gonzalez JM, Aimore Bonin E, et al. Usefulness of over-the-scope clipping system for closing digestive fistulas. Digestive Endoscopy 2015; 27: 18–24. [DOI] [PubMed] [Google Scholar]
- 14.Prosst RL, Joos AK, Ehni W, et al. Prospective pilot study of anorectal fistula closure with the OTSC Proctology. Colorectal Dis 2015; 17: 81–86. [DOI] [PubMed] [Google Scholar]
- 15.Manta R, Galloro G, Mangiavillano B, et al. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endoscopy 2013; 27: 3162–3164. [DOI] [PubMed] [Google Scholar]
- 16.Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointestinal Endoscopy 2014; 80: 610–622. [DOI] [PubMed] [Google Scholar]
- 17.Gubler C, Bauerfeind P. Successful closure of an esophagopericardial fistula with an over-the-scope clip. Endoscopy 2012; 44(Suppl 2 UCTN): e194–e195. [DOI] [PubMed] [Google Scholar]
- 18.Seebach L, Bauerfeind P, Gubler C. “Sparing the surgeon”: clinical experience with over-the-scope clips for gastrointestinal perforation. Endoscopy 2010; 42: 1108–1111. [DOI] [PubMed] [Google Scholar]
- 19.Gubler C, Bauerfeind P. Endoscopic closure of iatrogenic gastrointestinal tract perforations with the over-the-scope clip. Digestion 2012; 85: 302–307. [DOI] [PubMed] [Google Scholar]
- 20.Schurr MO, Arezzo A, Ho CN, et al. The OTSC clip for endoscopic organ closure in NOTES: device and technique. Minim Invasive Ther Allied Technol 2008; 17: 262–266. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy 2015; 47: 719–725. [DOI] [PubMed] [Google Scholar]
- 22.Prosst RL, Ehni W, Joos AK. The OTSC(R) Proctology clip system for anal fistula closure: first prospective clinical data. Minim Invasive Ther Allied Technol 2013; 22: 255–259. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt A, Riecken B, Damm M, et al. Endoscopic removal of over-the-scope clips using a novel cutting device: a retrospective case series. Endoscopy 2014; 46: 762–766. [DOI] [PubMed] [Google Scholar]
- 24.Kumta NA, Boumitri C, Kahaleh M. New devices and techniques for handling adverse events: claw, suture, or cover? Gastrointestinal Endoscopy Clinics North Am 2015; 25: 159–168. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Cecilia D, Arjona-Sanchez A, Gomez-Alvarez M, et al. Conservative management of perforated duodenal diverticulum: a case report and review of the literature. World J Gastroenterol 2008; 14: 1949–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]