Abstract
Objective
To determine the survival outcome and prognostic factors of hepatocellular carcinoma (HCC) survival in patients who underwent radiofrequency ablation (RFA).
Methods
The Surveillance, Epidemiology and End Results (SEER) database was queried: There were 2588 HCC patients from 2004 to 2012 who underwent RFA. The Kaplan-Meier curves and the multivariate Cox regression analysis were used to assess the prognostic factors.
Results
With a median follow-up of 20 months, the 1-, 3- and 5-year overall survival (OS) rates were: 83%, 51% and 33%. Patients with a tumor size ≤5 cm in diameter had a better 5-year OS, as compared to patients with a tumor size >5 cm. The 5-year OS was significantly higher among patients with a normal level of alpha-fetoprotein (AFP), compared with those having elevated AFP. In an adjusted multivariate Cox regression analysis, those with ≥60 years of age (HR: 1.19; 95% CI 1.05–1.36), non-Asian race (HR: 1.53; 95% CI 1.30–1.81), tumor size >5 cm (HR: 1.43; 95% CI 1.24–1.65), elevated AFP (HR: 1.42; 95% CI 1.22–1.64), American Joint Committee on Cancer (AJCC) stages II-III (HR: 1.30; 95% CI 1.14–1.48) and the year of diagnosis from 2004–2007 (HR: 1.22; 95% CI 1.07–1.40) were significantly associated with a poor prognosis.
Conclusions
Age, race, tumor size, AFP level, AJCC stage and year of diagnosis were prognostic factors for OS in HCC patients who underwent RFA.
Keywords: Alpha fetoprotein, database analysis, hepatocellular carcinoma, liver cancer, prognostic factors, radiofrequency ablation, survival, SEER database
Introduction
Liver cancer in men is the second leading cause of cancer death worldwide, with an estimated 748,300 newly diagnosed cases in 2008.1 In the USA, the incidence of hepatocellular carcinoma (HCC) tripled since 1980, to become 6 per 100,000 in 2010.2 The increased incidence of HCC is related to many risk factors, but the majority of cases are due to metabolic disorders, followed by: Alcoholic-related disorders, hepatitis C virus, hepatitis B virus and rare genetic disorders.3,4
For early-stage HCC patients, surgical resection, liver transplantation and radiofrequency ablation (RFA) are the best options as potentially curative therapies.5 Using the Milan criteria, the 5-year overall survival after liver transplantation for HCC is about 70%; however, in an organ shortage and with patients having unfitness for surgery, RFA is increasingly utilized in the USA.6 Moreover, recent randomized controlled trials (RCT) concluded that RFA was as effective as hepatic resection in the treatment of early HCCs.7–10 For these reasons, some centers have started to use RFA as the first-line modality for those patients.11,12
Several studies that have analyzed the prognostic factors for survival after RFA have included tumor size,13,14 fibrosis stage, alpha-fetoprotein (AFP)15,16 level and race17; however, a large population-based study is still needed to assess the impact of these prognostic factors.
The aim of this US population-based study was to determine the survival outcome and survival risk factors in 2588 patients with primary HCC who underwent RFA between the years 2004–2012. Evaluation of the prognostic factors is important for selecting RFA patients and improving the treatment outcome.
Methods
Data source
The Surveillance, Epidemiology and End Results (SEER) database currently collects and publishes cancer incidence and survival data from 18 population-based cancer registries, covering approximately 28% of the US population. The SEER’s 18 registries are in: Metropolitan Atlanta, CT, Detroit, HI, IA, NM, San Francisco-Oakland, Seattle-Puget Sound, UT, Los Angeles, San Jose and Monterrey, rural GA, the Alaska Native Tumor Registry, greater CA, KY, LA, NJ and greater GA. SEER regions are more urban and have a higher proportion of foreign-born persons, compared to the general US population.
Study subjects
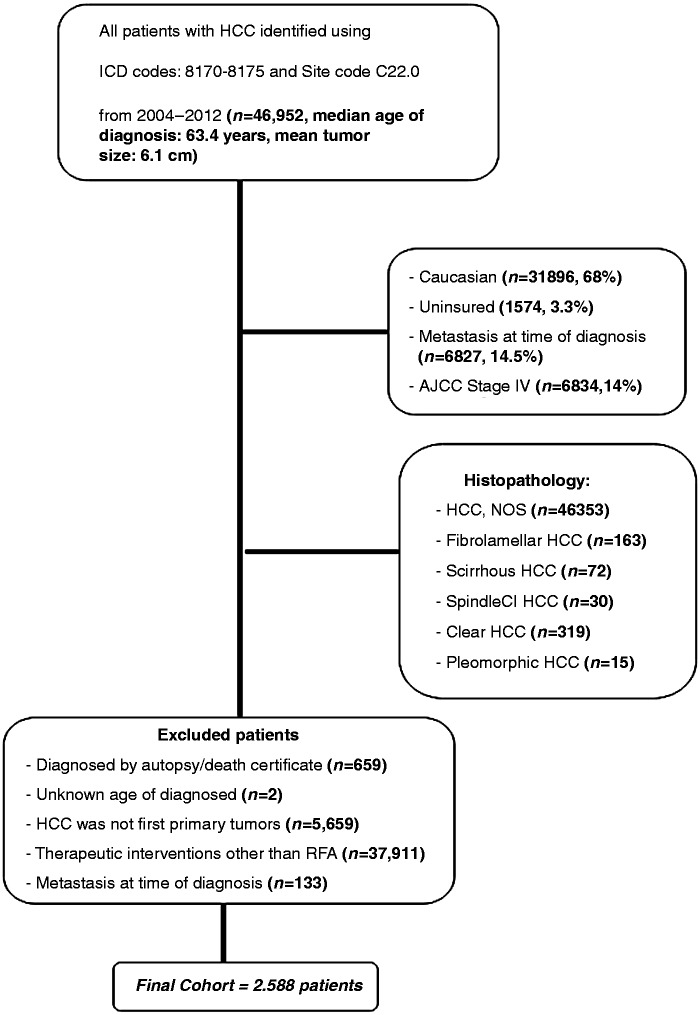
From 18 SEER registries, we extracted the data of 46,952 patients with histological diagnosis consistent with HCC [ICD-O-3 morphology codes 8170–8175] between the years 2004–2012 (including two diagnostic time periods: 2004–2007 and 2008–2012).18 Of 46,952 patients, sequential exclusions (Figure 1) were then made for:
Diagnosed by autopsy/death certificate (n = 659);
Unknown age of diagnosis (n = 2);
HCC was not first primary tumor (n = 5659). Thus, sequence number codes other than 0 or 1 were excluded;
Underwent therapeutic interventions other than RFA (n = 37,911), as per the SEER Program Coding and Staging Manuals … the patients with code 16 (RFA) were included in SEER site-specific surgery; and
As metastasis has an impact on survival, cases with a distant metastasis at the time of diagnosis were excluded (n = 133).
Figure 1.
Flow diagram of patient selection out of a total of 46,925 patients identified with HCC, to arrive at those patients who were to be included in our survival analysis, within the SEER database during 2004–2012.
HCC: hepatocellular cancer; ICD: International Classification of Diseases for Oncology; SEER: Surveillance, Epidemiology and End Results database.
This gave a final cohort of 2588 non-metastatic HCC patients who underwent RFA (Figure 1). The primary endpoints of the study were overall survival and the prognostic factors for survival in patients who underwent RFA for HCC. Overall survival was defined as the interval between the date of diagnosis to the date of death (followed for vital status) or the date of last follow-up (median follow-up time: 20 months). Data were censored at 31 December 2012, from the date of HCC diagnosis.
Clinicopathological factors included: Patient age at HCC diagnosis, gender, race, year of diagnosis, AFP level, tumor multiplicity and tumor size; these could potentially affect patient survival and were included in the analysis. Age was categorized by the median (<60 years and ≥60). The patient’s race was classified by the SEER coding scheme and defined as: White, black, American indian/AK native, Asian or Pacific Islander, or other unspecified and unknown.
For patients with liver pathology, the SEER database captures the degree of liver fibrosis in a binary variable (SEER Fibrosis Score: F0 or F1). The 6-point fibrosis score developed by Ishak et al.19 had a good prognostic significance, according to the American Joint Committee on Cancer (AJCC). Scores of 0–4 represent an absent-to-moderate degree of fibrosis (SEER Fibrosis Score: F0); whereas scores of 5–6 represent severe fibrosis or cirrhosis (SEER Fibrosis Score: F1).
AFP in the SEER database is categorized as positive, negative and other (test not done, borderline, not applicable and unknown). We categorized a tumor size as ≤5 cm or >5 cm, as tumor size >5 cm is a predictor of death in HCC.20 The SEER data only allows for categorization of the number of lesions as 1 or >1. Tumor stages were identified by the tumor, node and metastasis (TNM) classification system according to the AJCC cancer staging manual. (derived from the AJCC Stage Group’s 6th edition of variables in data).21
Statistical analysis
Statistical analyses were performed using the statistical software package SPSS for Windows, version 15 (SPSS Inc., Chicago, IL, USA). Clinical and demographic characteristics were expressed as proportions (%) and frequencies (n) for categorical variables, or as the median and range for continuous variables. We calculated the 1-year, 3-year and 5-year overall survival and their 95% CIs. Survival curves were generated using the Kaplan-Meier method and compared by log-rank test. Patient survival was censored at death or at the end of follow-up. Univariate analysis was performed to determine the significant prognostic factors for overall survival, using the Cox proportional hazards model. Variables with p < 0.05 were included in the multivariable Cox regression models. In the univariate and multivariate analyses, we excluded cases with unknown or missing data for tumor size, AFP, race, tumor multiplicity and AJCC staging from the data analysis. Results were considered statistically significant when a 2-tailed test p-value of <0.05 was achieved.
Results
Patient and tumor characteristics
From the 18 SEER registries, we extracted data of 46,952 patients with a HCC, between the years 2004–2012. Of these, the median age of diagnosis was 63.4 years, 76.8% were male and 14.5% had metastasis at the time of HCC diagnosis. Fibro-lamellar HCC represented only 0.3% of all HCC patients (163/46,952).
According to the inclusion and exclusion criteria for the study (Figure 1), we identified 2588 patients whom were diagnosed with non-metastatic HCC between the years 2004 and 2012. The characteristics of the patients included in this analysis are summarized in Table 1. The median age at HCC diagnosis was 61 years (mean ± SD: 62.2 ± 9.8). The study population showed a predominance of the male gender (75.6%). The racial distribution was: 64.8% Caucasian, 21.7% Asian, 11.6% African-American, 1.6% American Indian/AK native and 0.3% unknown. Across the 9-year period of the study, the number of RFA-treated HCC patients in this cohort study increased from 942 to 1247, from 2004–2007 to 2008–2011, respectively. The median follow-up time was 20 months (Table 1).
Table 1.
Patient and tumor characteristics (n = 2588)
| Variable | n (%) |
|---|---|
| Age at diagnosis of HCC | |
| <60 | 1152 (44.5) |
| ≥60 | 1436 (55.5) |
| Median age (range) | 61 (21–91) |
| Median follow-up time | 20 months |
| Gender | |
| Male | 1957 (75.6) |
| Female | 631 (24.4) |
| Male: female ratio | 3:1 |
| Race | |
| Caucasian | 1676 (64.8) |
| African-American | 299 (11.6) |
| American Indian/Alaska Native | 42 (1.6) |
| Asian or Pacific Islander | 562 (21.7) |
| Unknown | 9 (0.3) |
| Year of diagnosis of HCC | |
| 2004–2007 | 942 (36.4) |
| 2008–2012 | 1646 (63.6) |
| Tumor size | |
| ≤5 cm | 2171 (83.9) |
| >5 cm | 253 (9.8) |
| Unknown | 164 (6.3) |
| Median tumor size | 2.9 cm |
| Tumor multiplicity | |
| Single lesion | 1566 (60.5) |
| Multiple lesions | 678 (26.2) |
| Not otherwise specified (NOS) | 344 (13.3) |
| AFP | |
| Positive | 1515 (58.5) |
| Negative | 603 (23.3) |
| Othera | 470 (18.2) |
| Fibrosis scoreb | |
| Non/moderate (F0-F4) | 147 (5.7) |
| Severe/cirrhosis (F5-F6) | 731 (28.2) |
| Unknown | 1710 (66.1) |
| AJCC staging | |
| Stage I | 1645 (63.6) |
| Stage II | 640 (24.7) |
| Stage III | 174 (6.7) |
| Unknown | 129 (5.0) |
Not done-not ordered-bordeline-unknown.
Fibrosis score as described by Ishak et al., 1995.
AFP: alpha-fetoprotein;AJCC: American Joint Committee on Cancer; NOS: not otherwise specified
There were 10% (253/2424) of the patients who had a tumor size >5 cm (median tumor diameter: 2.9 cm), 30% (678/2244) of the patients had >1 lesion, 71.5% (1515/2118) of the patients had an elevated AFP and 83.3% (731/878) of the patients had advanced liver fibrosis (F5-F6). We excluded patients with unknown information about the tumor characteristics from data analysis. The median of tumor diameter decreased each year, from 35 mm in 2004 to 29 mm in 2012. More than one-half of the patients (63.6%) had an early HCC stage (American Joint Committee on Cancer (AJCC) Stage I); while 24.7% had Stage II, 6.7% had Stage III and 5.0% had an unknown HCC stage (Table 1).
Survival and prognostic factors for OS
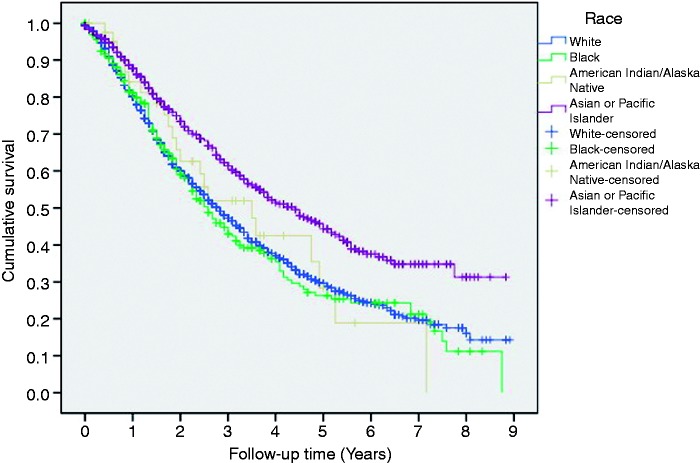
With a median follow-up of 20 months (Interquartile (IQ) range: 9–38), the overall post-RFA 1 - year, 3-year and 5-year survival rates were 83%, 51% and 33%, respectively. Male patients had a non-significant poor 5-year OS, compared to female patients following RFA (32% versus 37% and p = 0.2, respectively). Patients ≥60 years of age had significantly poor survival, compared to patients aged <60 years (at 5 years, 30% versus 37% and p = 0.02, respectively). The Asian or Pacific Islander cases had a better 5-year survival (46%) than African-American (26%), Caucasian (30%) or American Indian/AK native (30%) cases (Figure 2). The 1-year, 3-year and 5-year overall survival rates had improved in recent years; as they were 78%, 47% and 31% respectively in 2004–2007, as compared to 87%, 54% and 36% respectively, in 2008–2012 (p = 0.001).
Figure 2.
Overall survival in post-RFA patients, categorized by race.
RFA: radiofrequency ablation
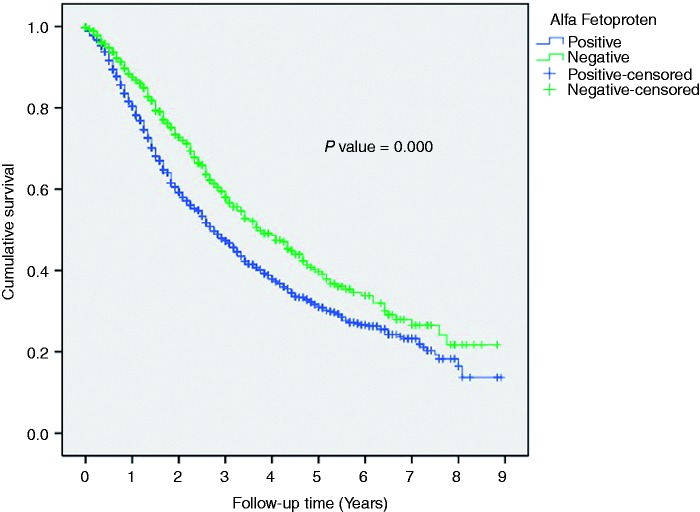
Patients with a tumor size ≤5 cm had a better 5-year OS, compared to patients with a tumor size >5 cm (35 versus 21%; p = 0.000). Furthermore, patients with a single lesion had significantly higher 5-year OS than patients with multiple lesions (38 versus 27%; p = 0.000). The 5-year OS was significantly higher among patients with a normal level of AFP, compared with an elevated AFP (40 versus 32%; p = 0.000, respectively) (Figure 3).
Figure 3.
Overall survival in Post-RFA patients categorized by AFP.
AFP: alpha feto-protein; RFA: radiofrequency ablation
Among the 878 patients for whom the data about liver fibrosis was available, the patients with a low fibrosis score (F0-F4) experienced non-significantly better OS, compared with patients with a high fibrosis score (42% versus 34%; p = 0.078, respectively). In the patients with Stage I HCC (AJCC staging), the 1-, 3- and 5-year survival rates were 86%, 57% and 37%, respectively; which were higher than those of the patients with Stage II-III tumors (66–82%, 24–45% and 11–31%, respectively) (Table 2).
Table 2.
Median and overall 5-year survival rates after RFA, according to the patient and tumor characteristics
| Variable | Overall 5-year survival rate | Median OS months | p-valuea |
|---|---|---|---|
| All patients | 33% | 36.6 mo | |
| Age | |||
| <60 | 37% | 39.7 mo | 0.02 |
| ≥60 | 30% | 34.2 mo | |
| Gender | |||
| Male | 32% | 35.7 mo | 0.2 |
| Female | 37% | 40.4 mo | |
| Race | |||
| Caucasian | 30% | 33.9 mo | 0.001 |
| Black-American | 26% | 31.0 mo | |
| American indian/AK native | 30% | 42.4 mo | |
| Asian or Pacific Islander | 46% | 53.0 mo | |
| Year of diagnosis of HCC | |||
| 2004–2007 | 31% | 33.0 mo | 0.001 |
| 2008–2012 | 36% | 39.3 mo | |
| Tumor size (n = 2424)b | |||
| ≤5 cm | 35% | 38.7 mo | 0.000 |
| >5 cm | 21% | 22.7 mo | |
| Tumor multiplicity (n = 2244)b | |||
| Single lesion | 38% | 43.7 mo | 0.000 |
| Multiple lesions | 27% | 28.6 mo | |
| AFP (n = 2118)b | |||
| Positive | 32% | 33.5 mo | 0.000 |
| Negative | 40% | 45.3 mo | |
| Fibrosis score (n = 878)b | |||
| Non/moderate (F0-F4) | 42% | 49.7 mo | 0.078 |
| Severe/cirrhosis (F5-F6) | 34% | 38.6 mo | |
| AJCC Stage (n = 2459)b | |||
| Stage I | 37% | 42.3 mo | 0.000 |
| Stage II | 31% | 31.3 mo | |
| Stage III | 11% | 18.8 mo |
Determined using the log-rank test.
Cases with unknown data were excluded.
AFP: alpha feto-protein; AJCC: American Joint Committee on Cancer; AK: Alaska; HCC: hepatocellular carcinoma; mo: months; OS: overall survival; RFA: radiofrequency ablation
The 1-, 3- and 5- year OS rates were 88%, 59% and 39%, respectively, in the solitary HCC lesions with a diameter ≤5 cm (Milan criteria); compared to 77%, 43% and 30%, respectively, in tumors with a diameter >5 cm.
In the univariate Cox regression model, an age ≥60 years, non-Asian race, a tumor size >5 cm, advanced tumor stages (II and III), an elevated AFP and the year of diagnosis (2004–2007) were regarded as significant risk factors for a poorer prognosis (Table 3). In the adjusted multivariate Cox regression model, all significant risk factors except for gender in the univariate analysis were found to be independent prognostic factors; including patients with age ≥60 years (p < 0.01), non-Asian race (p < 0.001), an elevated AFP level (p < 0.001), a tumor size >5 cm (p < 0.001), advanced tumor stages including stages II-III (p < 0.001), and an earlier year of diagnosis (p < 0.01) (Table 3).
Table 3.
Univariable and multivariable analyses of independent prognostic factors of overall survival (n = 1981)a
| Parameters | Multivariate |
Univariate |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Tumor size > 5 cm (ref, ≤ 5 cm) | 1.43 (1.24–1.65) | 0.000 | 1.52 (1.34–1.73) | 0.000 |
| Elevated AFP | 1.42 (1.22–1.64) | 0.000 | 1.37 (1.19–1.58) | 0.000 |
| Stage II-III versus Stage I | 1.30 (1.14–1.48) | 0.000 | 1.45 (1.29–1.63) | 0.000 |
| Non-Asian race versus Asian race | 1.53 (1.30–1.81) | 0.000 | 1.56 (1.35–1.80) | 0.000 |
| Year of diagnosis (2003–2007) | 1.22 (1.07–1.40) | 0.004 | 1.23 (1.09–1.38) | 0.001 |
| Age at diagnosis (≥ 60) | 1.19 (1.05–1.36) | 0.008 | 1.19 (1.07–1.33) | 0.002 |
| Gender (male) | – | – | 1.08 (0.95–1.23) | 0.24 |
| Multiple lesions | –b | – | 1.45 (1.28–1.64) | 0.000 |
| Advanced fibrosis (F5-F6) | –b | – | 1.28 (0.97–1.69) | 0.08 |
Cases with unknown data were excluded from the multivariate analysis.
Excluded from a multivariate, as they are a part of tumor stage.
AFP: alpha fetoprotein; HR: hazard ratio
Discussion
In this US population-based study, we determined the survival outcome and survival risk factors in 2588 patients with primary HCC, who underwent RFA between the years 2004–2012. More than two-thirds (75.6%) of the patients were male and 55.5% of them were aged >60 years. These findings reflect common trends in the USA, where HCC is 2–4 times more common in men than in women,22 and the average age of HCC diagnosis was 65 years.23 The 5-year survival rate at age ≥60 was 30% in our analysis, which was better than 17%, which was previously reported in a SEER-based analysis in the elderly >66 years.24 Furthermore, comparable to the findings of previous reports,25–27 old age was a risk factor for survival in this study (HR: 1.19; 95% CI 1.05–1.36; p = 0.008). In a large, single-center retrospective study involving 1170 HCC patients who underwent RFA, an increasing age was significantly associated with OS (HR: 1.03; 95% CI 1.02–1.04; p < 0.0001).28
In the USA, the age-adjusted HCC incidence rates in those with Asian/Pacific Islander ethnicity was about three times higher than in Caucasians.29 In our study, the Asians and Pacific islanders had the highest survival rates among all other races that underwent RFA, as the non-Asians had a higher (53%) risk of death, compared with Asians, after adjusting for age, AJCC stage and AFP. This was consistent with a prior SEER-based study that found Asians and Pacific islanders had the highest survival rates among HCC patients, whom either had a resection, ablation or no intervention.30 The best survival among Asians or Pacific Islanders, compared to other racial groups, could be explained by differences in their risk factors or HCC screening.31 As 50% of Asians in the USA who were diagnosed with HCC were Hepatitis B surface antigen (HBsAg) positive, screening and an early detection may play a role in a good HCC survival.32 Moreover, Hepatitis B virus (HBV) is an oncogenic virus that induces HCC on a normal liver background, which contributes to better outcomes after curative modalities.26,31
In the current study, gender was not a predictor of poor survival, which was consistent with other studies25,26; however, Tangkijvanich et al.33 report that women with HCC tend to have a longer survival than men, as women have less aggressive tumor behavior in terms of tumor size, multiplicity and vascular invasion. In an experimental model, estrogen inhibits the induction of IL-6 produced by Kupffer cells, which can abolish hepatocarcinogensis in mice.34
Several reports reveal that 5-year OS in HCC ≤ 5 cm in diameter ranges, after RFA, from 38% to 55%.35–37 In a single-center study including 235 Western patients with HCCs ≤5 cm and up to three lesions, the Child-Pugh A/B revealed that the median OS was 48 months and the 5-year OS was 40%, after RFA.36 In a recent multicenter randomized controlled trial (RCT) that compared RFA with percutaneous cryoablation for the treatment of HCC with Child-Pugh Class A or B cirrhosis, and one or two HCC lesions ≤4 cm, the 5-year OS and 5-year tumor-free survival rates were 38% and 34% in the RFA group, respectively.35
In this cohort study, single lesions were observed in 1566 HCC patients (60.5%), while multiple lesions presented in 26.2% with a significantly longer median; and the 5-year survival among patients who had single lesions (43.7 months and 38% versus 28.6 months and 27%, respectively), which was consistent with many reports from China,38 France,36 Taiwan26 and Turkey.27 In addition to that, multiple lesions were associated with a 1.45-fold increased risk of mortality in a univariate analysis, as the number of nodules is a risk factor for the aggressive behavior of a tumor, including: microvascular invasion, poor differentiation39,40 and the risk of recurrence.36 In a retrospective study including 866 patients with Child-Pugh Class A-B cirrhosis and HCC within the Milan criteria who underwent cryoablation, the hazard ratios (HRs) of mortality were 1.62 and 2.29, in patients with two and three HCC lesions, respectively.41
A multicenter international collaborative study in patients with HCC who underwent resection reports that a tumor size >5 cm correlates with a higher incidence of microvascular invasion, compared with smaller tumors.39 In a retrospective study of 618 patients who underwent partial hepatectomy for solitary HCC, a 3-cm cutoff seemed to best determine biological behavior and clinical prognosis.42 In contrast, N'Kontchou et al.36 report that tumor size is not associated with overall and tumor-free survival after RFA. The present study verified the relationship between small HCC tumor size and OS; as in solitary HCC lesions, the 1-, 3- and 5-year OS rates were 88%, 59% and 39%, respectively, in tumors sized ≤5 cm; as compared to 77%, 43% and 30%, respectively, in tumors sized >5 cm.
In the present report, the patients with HCC of AJCC Stage I had better 5-year OS than the Stage II-III patients (37% versus 11–31%, respectively), and the patients with Stage II-III had a 30% increased risk of death. Other reports demonstrate similar findings.38,43 A SEER data-base study reveals improved survival in the early stages of unresectable HCC patients who had more favorable socio-demographic factors.44 Furthermore, this study’s multivariate analysis indicated that the AJCC stage was an independent prognostic factor associated with survival, which was consistent with the results of a multicenter study conducted in Mongolia.45
In our data analysis among the 2118 patients for whom the results of AFP level were available, 1515 (83.3%) patients had an elevated AFP. A multicenter survey found that even in advanced stages of HCC, serum AFP remains normal in 40% of patients.46 We are not surprised that patients with normal-level AFP had higher OS than patients with an elevated AFP (40% versus 32%, p = 0.000), and risk of mortality increased 1.4-fold in patients with an elevated AFP (95% CI 1.22–1.64). According to these findings, incorporation of AFP in an AJCC staging system may be beneficial in giving the prognosis after RFA. A high AFP level is indicative of tumor burden-activity47 and a prognostic factor for HCC with poor survival and recurrence.16,48
The results of this study indicated that the 1-, 3- and 5-year OS rates had improved in the recent years, as they were 78%, 47% and 31% respectively in 2004–2007, as compared to 87%, 54% and 36%, respectively, in 2008–2012 (p = 0.001). This may be explained by the development of guidelines that enhance surveillance and enhanced screening for high-risk groups of HCC, which leads to early detection and intervention with subsequent improvement of survival, also the development of experience and a good selection of candidates for RFA.24,49,50
Conclusions
Although this SEER analysis had limitations, such as the lack of information regarding the etiology of liver disease, liver background status and retrospective study design, it provided guidance on how to select patients who are candidates for RFA.
In conclusion, our results showed that a tumor size ≤5 cm, an early AJCC stage, normal AFP levels, a more recent year of diagnosis, and an Asian or Pacific Islander race were independent predictors for both long median and 5-year survival in HCC patients who underwent RFA. Further studies are recommended to address the integration of AFP in AJCC staging, as it has a prognostic role.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Jemal A, Bray F, Ferlay J. Global cancer statistics: 2011. CA Cancer J Clin 1999; 49: 1, 33–64. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014; 60: 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol 2013; 108: 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016; 122: 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forner A, Reig ME, De Lope CR, et al. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis 2010; 30: 61–74. [DOI] [PubMed] [Google Scholar]
- 6.Massarweh NN, Park JO, Farjah F, et al. Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg 2010; 210: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M-S, Li J-Q, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lü M, Kuang M, Liang L, et al. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: A randomized clinical trial]. Zhonghua Yi Xue Za Zhi 2006; 86: 801–805. [PubMed] [Google Scholar]
- 9.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010; 252: 903–912. [DOI] [PubMed] [Google Scholar]
- 10.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57: 794–802. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M. Local ablation therapy for hepatocellular carcinoma: Current status and future perspectives. J Gastroenterol 2004; 39: 205–214. [DOI] [PubMed] [Google Scholar]
- 12.Livraghi T, Meloni F, Morabito A, et al. Multimodal image-guided tailored therapy of early and intermediate hepatocellular carcinoma: Long-term survival in the experience of a single radiologic referral center. Liver Transpl 2004; 10: S98–106. [DOI] [PubMed] [Google Scholar]
- 13.Pompili M, Saviano A, De Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013; 59: 89–97. [DOI] [PubMed] [Google Scholar]
- 14.Yin XY, Xie XY, Lu M De, et al. Percutaneous thermal ablation medium and large hepatocellular carcimoma. Cancer 2009; 115: 1914–1923. [DOI] [PubMed] [Google Scholar]
- 15.Seo JY, Kim W, Kwon JH, et al. Noninvasive fibrosis indices predict intrahepatic distant recurrence of hepatitis B-related hepatocellular carcinoma following radiofrequency ablation. Liver Int 2013; 33: 884–893. [DOI] [PubMed] [Google Scholar]
- 16.Dohi C, Nouso K, Miyahara K, et al. Potential of alpha-fetoprotein as a prognostic marker after curative radiofrequency ablation of hepatocellular carcinoma. Hepatol Res 2015. doi: 10.1111/hepr.12636. Available at: http://onlinelibrary.wiley.com/doi/10.1111/hepr.12636/abstract;jsessionid=DB66B151AA325B3F2B3A975F844C1B14.f01t02. [DOI] [PubMed] [Google Scholar]
- 17.Tong MJ, Chavalitdhamrong D, Lu DSK, et al. Survival in Asian Americans after treatments for hepatocellular carcinoma: A seven-year experience at UCLA. J Clin Gastroenterol 2010; 44: e63–e70. [DOI] [PubMed] [Google Scholar]
- 18.Fritz AG. International Classification of Diseases for Oncology (ICD-O). World Heal Organ 2000; 240. Available at: http://apps.who.int/iris/bitstream/10665/96612/1/9789241548496_eng.pdf.
- 19.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696–699. [DOI] [PubMed] [Google Scholar]
- 20.Vauthey J-N, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002; 20: 1527–1536. [DOI] [PubMed] [Google Scholar]
- 21.Egner JR. AJCC cancer staging manual. JAMA 2010; 304: 1726–1727.
- 22.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 23.El-Serag HB. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology 2004; 127: S27–34. [DOI] [PubMed] [Google Scholar]
- 24.Massarweh NN, Park JO, Yeung RSW, et al. Comparative assessment of the safety and effectiveness of radiofrequency ablation among elderly medicare beneficiaries with hepatocellular carcinoma. Ann Surg Oncol 2012; 19: 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facciorusso A, Del Prete V, Antonino M, et al. Conditional survival analysis of hepatocellular carcinoma patients treated with radiofrequency ablation. Hepatol Res 2015; 45: E62–72. [DOI] [PubMed] [Google Scholar]
- 26.Kao W-Y, Chiou Y-Y, Hung H-H, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: The clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol 2011; 23: 528–536. [DOI] [PubMed] [Google Scholar]
- 27.Gokcan H, Savaş N, Oztuna D, et al. Predictors of survival in hepatocellular carcinoma patients. Ann Transplant 2015; 20: 596–603. [DOI] [PubMed] [Google Scholar]
- 28.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-Year outcome and prognostic factors. Am J Gastroenterol 2011; 107: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27: 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong RJ, Corley DA. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig Dis Sci 2009; 54: 2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClune AC, Tong MJ. Chronic hepatitis B and hepatocellular carcinoma. Clin Liver Dis 2010; 14: 461–476. [DOI] [PubMed] [Google Scholar]
- 32.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Hepatitis C-related hepatocellular carcinoma in the United States: Influence of ethnic status. Am J Gastroenterol 2003; 98: 2060–2063. [DOI] [PubMed] [Google Scholar]
- 33.Tangkijvanich P, Mahachai V, Suwangool P, et al. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World J Gastroenterol 2004; 10: 1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317: 121–124. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015; 61: 1579–1590. [DOI] [PubMed] [Google Scholar]
- 36.N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009; 50: 1475–1483. [DOI] [PubMed] [Google Scholar]
- 37.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complication rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008; 47: 82–89. [DOI] [PubMed] [Google Scholar]
- 38.Yan K, Chen MH, Yang W, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term outcome and prognostic factors. Eur J Radiol 2008; 67: 336–347. [DOI] [PubMed] [Google Scholar]
- 39.Pawlik TM, Delman KA, Vauthey J, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005; 11: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 40.McHugh PP, Gilbert J, Vera S, et al. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. Hpb 2010; 12: 56–61. Available at: http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1477-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong G, Bai W, Dong Z, et al. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One 2015; 10: e0123065–e0123065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X-Y, Xi T, Lau W-Y, et al. Pathobiological features of small hepatocellular carcinoma: Correlation between tumor size and biological behavior. J Cancer Res Clin Oncol 2011; 137: 567–575. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Chen M, Yan K, et al. Effect and prognostic analysis of radiofrequency ablation in treating advanced hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 2012; 92: 735–738. [PubMed] [Google Scholar]
- 44.Kokabi N, Xing M, Duszak R, Jr, et al. Sociodemographic impact on survival in unresectable hepatocellular carcinoma: A survival epidemiology and end results study. Future Oncol 2016; 12: 183–198. [DOI] [PubMed] [Google Scholar]
- 45.Baatarkhuu O, Kim DY, Nymadawa P, et al. Clinical features and prognosis of hepatocellular carcinoma in Mongolia: A multicentre study. Hepatol Int 2012; 6: 763–769. [DOI] [PubMed] [Google Scholar]
- 46.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: Both or neither? Am J Gastroenterol 2006; 101: 524–532. [DOI] [PubMed] [Google Scholar]
- 47.Chan SL, Mo FKF, Johnson PJ, et al. New utility of an old marker: Serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol 2009; 27: 446–452. [DOI] [PubMed] [Google Scholar]
- 48.Li P, Wang S-S, Liu H, et al. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol 2011; 17: 4563–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236. [DOI] [PubMed] [Google Scholar]
- 50.Lau WY, Lai ECH. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: A systematic review. Ann Surg 2009; 249: 20–25. [DOI] [PubMed] [Google Scholar]