Abstract
Background
The adenoma detection rate (ADR) is the main quality indicator of colonoscopy. The ADR recommended in fecal immunochemical testing (FIT)-based colorectal cancer screening programs is unknown.
Methods
Using the COLONPREV (NCT00906997) study dataset, we performed a post-hoc analysis to determine if there was a correlation between the ADR in primary and work-up colonoscopy, and the equivalent figure to the minimal 20% ADR recommended. Colonoscopy was performed in 5722 individuals: 5059 as primary strategy and 663 after a positive FIT result (OC-Sensor™; cut-off level 15 µg/g of feces). We developed a predictive model based on a multivariable lineal regression analysis including confounding variables.
Results
The median ADR was 31% (range, 14%–51%) in the colonoscopy group and 55% (range, 21%–83%) in the FIT group. There was a positive correlation in the ADR between primary and work-up colonoscopy (Pearson’s coefficient 0.716; p < 0.001). ADR in the FIT group was independently related to ADR in the colonoscopy group: regression coefficient for colonoscopy ADR, 0.71 (p = 0.009); sex, 0.09 (p = 0.09); age, 0.3 (p = 0.5); and region 0.00 (p = 0.9). The equivalent figure to the 20% ADR was 45% (95% confidence interval, 35%–56%).
Conclusions
ADR in primary and work-up colonoscopy of a FIT-positive result are positively and significantly correlated.
Keywords: Colorectal adenoma, colorectal neoplasm, fecal immunochemical test, colonoscopy, colorectal cancer screening, adenoma detection rate
Introduction
Colonoscopy plays a key role in colorectal cancer (CRC) screening, either as a primary strategy or work-up examination in other screening modalities (e.g. fecal immunochemical testing (FIT)-based screening programs). Indeed, colonoscopy can detect both premalignant and malignant lesions, and endoscopic polypectomy can effectively reduce CRC incidence and mortality.1,2 However, colonoscopy is limited by low participation, bowel preparation, complications and variable detection rates.3 The adenoma detection rate (ADR) has become the most important quality indicator of screening colonoscopy because it is directly related to key outcome measures, such as interval cancer incidence and mortality.4,5 In addition, the ADR is a marker that indirectly reflects other surrogate quality indicators such as quality of preparation, completeness of colonoscopy, and withdrawal time.
Most CRC screening quality programs recommend that ADR should be, at least, 20% when colonoscopy is the primary screening strategy.6 However, this figure cannot be used in the context of FIT-based CRC screening programs in which the number of adenomas detected in the FIT-based colonoscopy is clearly higher.7 In this setting, although no study has specifically addressed this issue, it has been suggested to raise this figure to 40%.6
The COLONPREV study (NCT00906997) is a multicenter, randomized, controlled trial aimed at comparing the efficacy of one-time colonoscopy and biennial FIT for reducing CRC mortality.8 Colonoscopies were performed by the same endoscopists in both arms in each hospital, following a specific, pre-established quality-assurance program.8,9 The aim of the analysis we present is to determine whether there is a correlation between the ADR in primary and FIT-based screening colonoscopy and, if this correlation does exist, to establish the equivalent figure in FIT-based screening to the well-defined and accepted ADR of 20% in a colonoscopy-based setting.
Material and methods
This is a cross-sectional post-hoc analysis performed within the first round (June 2009–June 2011) of the COLONPREV study.8 As was previously published, this study is being carried out in eight Spanish regions (Aragón, Basque Country, Canarias, Catalonia, Galicia, Madrid, Murcia and Valencia) with the participation of 15 tertiary hospitals. The study protocol was approved by the ethics committee of each hospital, and all participants provided written informed consent. Inclusion and exclusion criteria were described elsewhere.8 In the FIT arm, participants collected one single sample that was analyzed with the automated semiquantitative OC-sensor™ (Eiken Chemical, Tokyo, Japan), without specific diet or medication restrictions. Samples were processed as previously described10 at each regional reference hospital. Individuals with ≥75 ng hemoglobin/ml of buffer solution (≥15 µg/g of feces) were invited for colonoscopy.
In the first round, colonoscopy was performed in 5722 participants (in 5059 individuals as primary strategy and in 663 people as FIT-based examination after a FIT-positive result) by the same endoscopists in both arms in each hospital, and following a specific, pre-established quality-assurance program.8,9 All colonoscopies were performed by experienced endoscopists (individual experience >200 colonoscopies per year). The mean withdrawal time in normal colonoscopies was 8.6 (±3.9) minutes, cecal intubation was achieved in 94.9% of the colonoscopies and colon cleansing was considered adequate in 97.9% of the colonoscopies.9 Colonoscopies were performed using standard white light video equipment. Adenoma was diagnosed by pathological evaluation of retrieved polyps. The ADR was defined as the proportion of individuals with at least one detected adenoma among those tested.
In order to perform this analysis, we calculated the ADR in each age- (50–59 and 60–69 years old), sex- and region-based subgroup both in primary and FIT-based colonoscopy. Before performing a lineal regression analysis, we assessed whether the ADR had a normal distribution with the Kolmogorov-Smirnov test, and whether there were differences in the mean ADR and variance according to the number of colonoscopies (median) in primary and FIT-based colonoscopy arms with the Student t test and the F-test. Thereafter, we calculated the Pearson’s correlation coefficient between both groups. Finally, we developed a predictive model based on a multivariable lineal regression analysis including the confounding variables (i.e. age, sex and region). On the basis of this predictive model, we determined the ADR in FIT-based colonoscopy of a FIT-based screening program equivalent to the most commonly accepted 20% ADR in primary colonoscopy, as well as to the figures recently recommended by the American Society for Gastrointestinal Endoscopy (ASGE) in the same setting (i.e. 25% overall, 30% in men, 20% in women).11 Additionally, we determined if there were differences in the ADR in FIT-based colonoscopy according to the quartile distribution of the ADR in primary colonoscopy and inversely using the Kruskal-Wallis test. Differences were considered statistically significant if the p value was less than 0.05. All analyses were performed using the SPSS statistical software, version 15.0 (SPSS Inc, Chicago, IL, USA).
Results
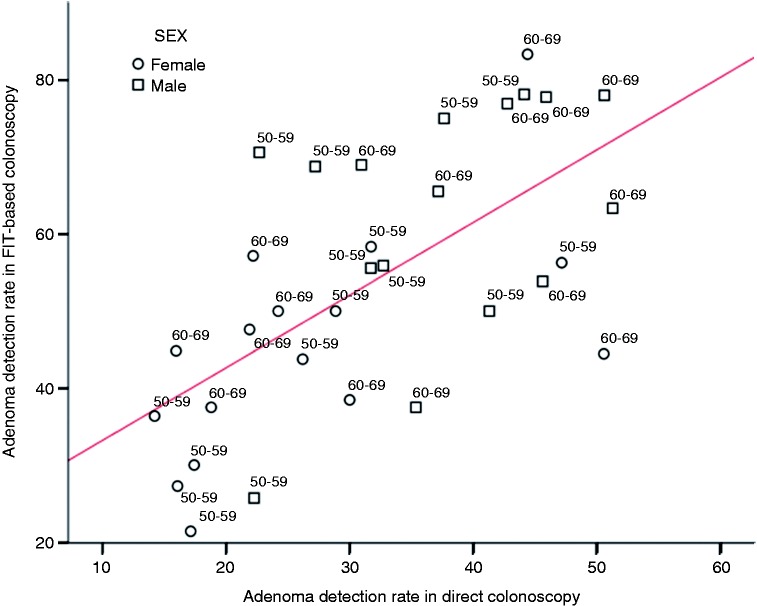
The median number of colonoscopies by age and sex are shown in Table 1. The ADR had a normal distribution in primary and FIT-based colonoscopy groups (p = 0.9), and there were neither statistical significant differences in the variance (p = 0.7) nor in the mean ADR in each group (p = 0.4 and p = 0.7, respectively), according to the number of colonoscopies included in the FIT-based colonoscopy group. There was a positive correlation in the ADR between primary and FIT-based colonoscopy (Pearson’s coefficient, 0.716; 95% confidence interval (95% CI), 0.378–0.819; p < 0.001). In Figure 1, we show the distribution of the ADR in all evaluated subgroups, as well as the corresponding regression line.
Table 1.
Distribution of the adenoma detection rate (ADR) and the number of colonoscopies in each region subgroup according to age and sex and in both work-up and primary colonoscopy groups.
| Work-up colonoscopy |
Primary colonoscopy |
||||
|---|---|---|---|---|---|
| ADR (%) | Number | ADR (%) | Number | ||
| Age (years) | 50–59 | 53 (21–78) | 16 (9–35) | 28 (14–47) | 158.5 (120–288) |
| 60–69 | 56 (38–83) | 22.5 (9–50) | 36 (16–51) | 138 (88–246) | |
| Sex | Male | 67 (26–78) | 17.5 (9–50) | 37 (22–51) | 156 (89–261) |
| Female | 45 (21–83) | 18 (9–29) | 23 (14–51) | 155.5 (88–288) | |
| Overall | 55 (21–83) | 17.5 (9–50) | 55 (21–83) | 155.5 (88–288) | |
Data are expressed as the median and range.
Figure 1.
Distribution of the adenoma detection rate by age group (50–59 and 60–69 years old), sex (women in blue circles and men in green ones) and Spanish region both in primary and fecal immunochemical test (FIT)-based colonoscopy. The regression line is shown.
The coefficient of multiple correlation of the predictive multivariable lineal regression model was 0.68. In this model, the ADR in FIT-based colonoscopy was independently related to the corresponding figure in primary colonoscopy (regression coefficient, 0.71, 95% CI, 0.19–1.22; p = 0.009). The regression coefficients of potential confounders were: sex (male), 0.09 (95% CI, −0.1 to 0.21; p = 0.09); age (60–69 years old), 0.3 (95% CI, −0.07 to 0.13; p = 0.5); and region, 0.00 (95% CI, −0.01 to 0.01; p = 0.9). No collinearity was found among the variables included in the regression model. On the basis of the above-mentioned multivariable regression analysis, estimated ADR in FIT-based colonoscopy equivalent to the 20% ADR in primary colonoscopy was 45% (95% CI, 35%–57%). In addition, estimated ADR in FIT-based colonoscopy equivalent to the figures recommended by the ASGE in primary colonoscopy were 49% (95% CI, 36%–62%) overall, 54% (95% CI, 39%–69%) in men, and 44% (95% CI, 34%–54%) in women.
According to the quartile distribution of the ADR in direct colonoscopy, the ADR in the FIT group ranged from 37.7 ± 11.7% in the lowest quartile to 66.9 ± 14.3% in the highest quartile (p = 0.06). Inversely, the ADR in the primary colonoscopy ranged from 21.3 ± 7.4% in the lowest FIT group quartile to 39.8 ± 9.1% in the highest quartile (p = 0.01).
Discussion
In this cross-sectional post-hoc analysis, we demonstrated that there is a positive and significant correlation between the ADR in primary and FIT-based colonoscopies. According to this correlation, we determined that a 45% ADR in FIT-based CRC screening programs seems equivalent, in terms of quality indicator, to the well-accepted 20% figure in colonoscopy screening. In fact, these findings are concordant with the mean ADR found in other CRC screening programs based on fecal occult blood testing: 44.8% in the Italian screening program (i.e. FIT based) and 46.5% in the National Health System Bowel Cancer Screening Programme in the United Kingdom (UK) (i.e. guaiac based).12,13
Our analysis has two main strengths. First, data were obtained from the two arms of a randomized controlled trial comparing the most widely accepted options for average-risk CRC screening in a population-based scenario,8 thus representing a unique opportunity to match the ADR of both strategies. Second, colonoscopies were performed by the same endoscopists in both arms and followed a strict quality-assurance program,8,9 thus guaranteeing the comparability of results. We are not aware of any other study of similar characteristics from which this comparison could be established.
By contrast, we are aware of some limitations. First, the ADR was calculated by age group, sex and geographic region, but not by each specific endoscopist because of the relatively small number of colonoscopies in the FIT group performed individually. However, this potential weakness was somehow overcome taking into account the large sample size of the COLONPREV study, the statistical analysis employed and, more important, the fact that all colonoscopies were performed by the same group of endoscopists in each center. Second, although there was a strong and independent correlation between the ADR in FIT-based colonoscopy and the corresponding figure in primary colonoscopy, we cannot infer that the selected value for FIT-based screening would also correlate with those outcomes associated with this parameter (i.e. interval cancer and mortality) in the latter setting.4,5 However, while prospective studies are needed to evaluate this aspect and, therefore, to validate the selected value, our data represent a reliable starting point to be used in current CRC screening programs. Finally, it is important to keep in mind that these results were obtained in the first round of a FIT-based screening program using a one-sample strategy with a 15 µg of hemoglobin/g of feces cut-off and, therefore, our correlation should be limited to this scenario. In fact, the positive predictive value of the FIT strategy is modified according to the threshold used and the number of samples analyzed.14–16 In that sense, although the two specific conditions employed in our study are among the most common in FIT-based screening programs, it would be feasible to calculate the specific ADR for other conditions using the corresponding positive predictive value for adenoma as conversion factor. The same approach could be used to correct the fact that our data were derived from the first screening round, in which the prevalence of neoplastic lesions is higher,12 thus universalizing the corresponding figures.
In conclusion, the positive and significant correlation between the ADR in primary and FIT-based colonoscopy provides the rationale for setting this quality indicator at 45% in the first round of FIT-based (i.e. 15 µg of hemoglobin/g of feces cut-off) CRC screening programs.
Acknowledgments
J.C. and A.C. designed the analysis, performed the statistical analysis and wrote the article. M.A, L.B, F.C, R.J, A.L, JD.M, D.S and E.Q. participated in the acquisition of data, interpretation of data, performed critical revision of the manuscript, and obtained funding, technical or material support. All authors approved the final version of the article and decided to send it for publication. Finally, all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article was presented as an oral presentation during the UEG Week that was held in Barcelona, Spain, October 24 to 28, 2015.
Appendix
Investigators of the COLONPREV study
Aragón: Angel Lanas (local coordinator), Francisca González-Rubio, Alberto Moya-Calvo, Mónica Polo-Tomas, Maria Pilar Roncales, Pilar Sebastian-Martínez, María Ángeles Valencia-Doblas, Nieves Valero-Capilla.
Basque Country: Luis Bujanda (local coordinator), María E. Alkiza, Jone Altzibar, Pilar Amiano, Juan Arenas, Edurne Artiñano, Angel Cosme, Isabel Egitegi, Kepa Elorriaga, Jose L. Elósegui, José M. Enriquez-Navascués, Cristina Erce, Inés Gil, María A. Gutiérrez-Stampa, Marta Herreros, Elizabeth Hijona, Mariluz Jaúregui, Eva Laredo, Roberto Martínez, Maria J. Mitxelena, Isabel Montalvo, Carlos Placer, Isabel Portillo, Cristina Sarasqueta.
Canarias: Enrique Quintero (national and local coordinator), Onofre Alarcón, Inmaculada Alonso-Abreu, Marta Carrillo-Palau, Mariola de la Vega-Prieto, María Luisa Díez-Fuentes, Antonio Gimeno-García, Yanira González-Méndez, Manuel Hernández-Guerra, Renata Linertová, David Nicolás-Perez, Juana María Reyes-Melián.
Catalonia: Montserrat Andreu (local coordinator), Cristina Álvarez, Josep M. Augé, Francesc Balaguer, Mercè Barau, Xavier Bessa, Felipe Bory, Andrea Burón, Antoni Castells (national coordinator), Xavier Castells, Mercè Comas, Míriam Cuatrecasas, Maria Estrada, Olga Ferrer, Imma Garrell, Jaume Grau, Rafael Guayta, Cristina Hernández, María López-Cerón, Francesc Macià, Leticia Moreira, Teresa Ocaña, Maria Pellisé, Mercè Piracés, Sandra Polbach, Àngels Pozo, Marc Puigvehí, Cristina Rodríguez, Maria Sala, Agustín Seoane, Anna Serradesanferm, Judith Sivilla, Antoni Trilla.
Galicia: Joaquín Cubiella (local coordinator), Ma Belén Aguado, Susana Aldecoa, Raquel Almazán, Ana Alonso, Inés Castro, Estela Cid, Lucía Cid, Joan Clofent, Ma Luisa de Castro, Pamela Estévez, Ana Belén Fernández, Ma Dolores González, Simoneta González, Ma Carmen González-Mao, Vicent Hernández, Begoña Iglesias, Felipe Iglesias, Pilar Iglesias, Ángeles López-Martínez, Ramiro Macenlle, Alfonso Martínez, David Martínez, Carlos Menéndez, Carmen Méndez, José Antonio Hermo, Isabel Pérez, Carmen Portasany, Mar Rionda, Concepción Rivera, Benito Rodríguez, Rosa Rodríguez, Manuel Rubio, María Isolina Santiago, Miriam Vázquez, José Ángel Vázquez, Pablo Vega, Ma Carmen Vidal, Raquel Zubizarreta.
Madrid: Juan Diego Morillas (local coordinator), Luís Abreu, Francisco Javier Amador, Margarita Barba, José Manuel Blanco, Guillermo Cacho, José Cantero, Juan Carrasco, Beatriz Carrascosa, María Chaparro, José Díaz-Tasende, Nuria Domínguez, José Miguel Esteban, Carlos Fernández, Conrado Fernández, Servando Fernández-Díez, Marta Fernández-Gil, Juan Ferrándiz, Aurelio Garrido, Javier P. Gisbert, Inés Gómez-Molíns, María José González, José Guardiola, Alberto Herreros, Rosario Iglesias, Sonia Izquierdo, Carlos López, Alicia Marín, José Carlos Marín, Daniel Martín, José Luis Martínez, José Andrés Moreno, José Manuel Moreno, Ricardo Moreno, Amelia Nogueiras, María Teresa Pérez, Carmen Plaza, Elena Polentinos, Carmen Poves, Andrés del Pozo, Inmaculada Salces, Fernando Sánchez, Francisco Sánchez-Ceballos, Cecilio Santander, Rocío Sastre, Saoud Tahsin, Vicente Valentín.
Murcia: Fernando Carballo (local coordinator), Miriam Alajarín, Fernando Alberca, Juan Bermejo, Joaquín Carrillo, José Cruzado, Purificación López, Mariano Martínez, María Dolores Navarro, Akiko Ono, Soledad Parra, Francisco Pérez-Riquelme, Pedro Riquelme.
Valencia: Dolores Salas (local coordinator), Mercedes Andrés, Consuelo Calvo, Montserrat Jimenez, Araceli Málaga, Elena Pérez, Antonio Peris, Marta Ponce, Rodrigo Jover, Teresa Sala, Gloria Teruel.
Funding
This work was supported by grants from Asociación Española de Gastroenterología, Fundación Científica de la Asociación Española contra el Cáncer (GCB13131592CAST), Instituto de Salud Carlos III (PI08/90717, PI08/0726, INT-09/208, and PI11/2630), Ministerio de Economía y Competitividad (SAF2014-54453-R), FEDER funds, and Agència de Gestió d’Ajuts Universitaris i de Recerca (2014SGR135). Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas (CIBERehd) is funded by the Instituto de Salud Carlos III. In the Basque Country, the study received additional support with grants from Obra Social de Kutxa, Diputación Foral de Gipuzkoa (DFG 07/5), Departamento de Sanidad del Gobierno Vasco, EITB-Maratoia (BIO 07/CA/19) y Acción Transversal contra el Cáncer del CIBERehd (2008). In Galicia, this work was supported by Dirección Xeral de Innovación e Xestión da Saúde Pública, Conselleria de Sanidade, Xunta de Galicia. OC-Micro instruments and fecal immunochemical tests were kindly provided by Eiken Chemical Co., Ltd., Japan, and its Spanish representatives, Palex Medical and Biogen Diagnóstica; none of them were involved in the design of the study nor in the analysis or interpretation of the results. Maria Rodríguez-Soler is the recipient of a grant from Fundación de la Comunidad Valenciana para la Investigación en el Hospital General Universitario de Alicante.
Conflict of interest
None declared.
References
- 1.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329: 1977–1981. [DOI] [PubMed] [Google Scholar]
- 3.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am J Gastroenterol 2006; 101: 343–350. [DOI] [PubMed] [Google Scholar]
- 4.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 5.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jover R, Herráiz M, Alarcón O, et al. Clinical practice guidelines: Quality of colonoscopy in colorectal cancer screening. Endoscopy 2012; 44: 444–451. [DOI] [PubMed] [Google Scholar]
- 7.Binefa G, García M, Milà N, et al. Colonoscopy quality assessment in a mass population screening programme based on faecal occult blood test. Rev Esp Enferm Dig 2013; 105: 400–408. [DOI] [PubMed] [Google Scholar]
- 8.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366: 697–706. [DOI] [PubMed] [Google Scholar]
- 9.Jover R, Zapater P, Polanía E, et al. Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointest Endosc 2013; 77: 381–389.e1. [DOI] [PubMed]
- 10.Vilkin A, Rozen P, Levi Z, et al. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol 2005; 100: 2519–2525. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81: 31–53. [DOI] [PubMed] [Google Scholar]
- 12.Zorzi M, Senore C, Da Re F, et al. Quality of colonoscopy in an organised colorectal cancer screening programme with immunochemical faecal occult blood test: The EQuIPE study (Evaluating Quality Indicators of the Performance of Endoscopy). Gut 2015; 64: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 13.Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: Experience from the NHS Bowel Cancer Screening Programme. Gut 2012; 61: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 14.Castro I, Cubiella J, Rivera C, et al. Fecal immunochemical test accuracy in familial risk colorectal cancer screening. Int J Cancer 2014; 134: 367–375. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez V, Cubiella J, Gonzalez-Mao MC, et al. Fecal immunochemical test accuracy in average-risk colorectal cancer screening. World J Gastroenterol 2014; 20: 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135: 82–90. [DOI] [PubMed] [Google Scholar]