Abstract
Context:
The health of the skeletal system is important for athletes young and old. From the early benefits of exercise on bones to the importance of osteoporosis prevention and treatment, bone health affects the ability to be active throughout life.
Evidence Acquisition:
PubMed articles dating from 1986 to 2016 were used for the review. Relevant terms such as keywords and section titles of the article were searched and articles identified were reviewed for relevance to this article.
Study Design:
Clinical review.
Level of Evidence:
Levels 1 through 4 evidence included.
Results:
There is strong evidence that exercise benefits bone health at every age and is a critical factor in osteoporosis prevention and treatment. Vitamin D, calcium, and hormones play vital roles in ensuring optimal bone health. When there is an imbalance between exercise and nutrition, as seen in the female athlete triad, bone health is compromised and can lead to bone stress injuries and early osteoporosis. Both of these can lead to morbidity and lost time from training and competition. Thus, early recognition and appropriate treatment of the female athlete triad and other stress fracture risk factors are vital to preventing long-term bone health problems.
Conclusion:
To optimize bone health, adequate nutrition, appropriate weightbearing exercise, strength training, and adequate calcium and vitamin D are necessary throughout life.
Keywords: bone health, stress fractures, exercise, vitamin D, hormones
Overview of Bone
The health of the skeletal system is a critical part of the overall health of the athletic population. Bone health can be affected starting in utero where maternal nutrition and medications can affect the fetal skeleton. Bone mineral density (BMD) peaks in early adulthood and declines after menopause in women, and is largely influenced by genetics. However, a variety of other modifiable factors affect bone health, such as exercise, diet, smoking, alcohol, medications, and calcium intake. Adolescence and young adulthood are the most beneficial times for long-term bone density gains, with nearly 90% of peak bone mass gained by age 18 years.104 However, it is also the most susceptible time, when negative consequences can occur from eating disorders, poor nutrition, hypoestrogenism, and inadequate calcium intake. Physical activity plays a key role, and benefits of bone loading in childhood and adolescence continue into adulthood, where the goal is to maintain bone mass. Maintenance of bone mass can reduce fracture risk by 50% to 80%.48,51,79,80 In older adults, the goals for exercise are fall prevention and ensuring safe exercise with modifications for those with osteoporosis.53
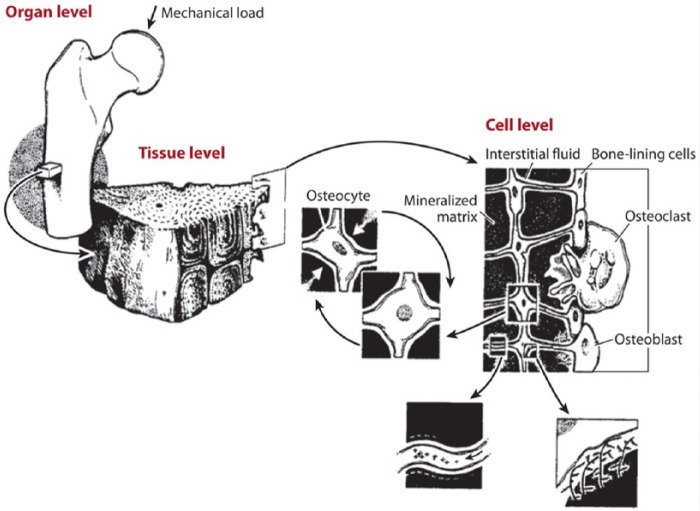
Understanding the benefits of exercise on bone health requires knowledge of bone composition, formation, and adaptation. The adult human skeleton is composed of 80% cortical bone and 20% trabecular, or cancellous, bone. Cortical bone is dense, solid, and surrounds the marrow space, whereas trabecular bone consists of a honeycomb-like network of trabecular plates and rods distributed throughout the bone marrow compartment.29 Mechanotransduction refers to bone’s recognition of and response to loading, with mechanoreceptors primarily on osteocytes, responding to fluid moving in bone’s lacunar-canalicular network (Figure 1). Stresses that cause osteogenesis must be variable, dynamic, and progressive; static loading does not cause osteogenesis.25 A key principle of bone formation and adaptation is that physical deformation of the bone directly stimulates bone formation. Slow bone turnover is the hallmark feature unique to skeletal adaptation. A minimum of 6 to 8 months is necessary to appreciate measurable results, taking into account that one remodeling cycle of bone resorption, formation, and mineralization takes 3 to 4 months to complete.53
Figure 1.
Mechanical loading applied at the whole bone level is transmitted through the bone tissue to the cellular level and causes movement of the interstitial fluid surrounding osteocytes in the mineralized matrix. The osteocytes are distributed throughout bone tissue and connect to each other and to bone-lining cells and osteoblasts on the bone surfaces. Figure adapted from Rubin CT, McLeod KJ, Bain SD. Functional strains and cortical bone adaptation: epigenetic assurance of skeletal integrity. J Biomech. 1990;23(suppl 1):43-54. Reprinted with permission from Elsevier.
Mechanical loading is critical for bone mineral accrual in children and adolescents. Impact or gravitational forces (ie, running, walking, stair climbing) and nonimpact or muscle forces (ie, weight lifting, swimming) are both important for skeletal regulation. Postmenopausal women who participated in either 9 months of nonimpact (ie, weight lifting, rowing) or impact (ie, walking, jogging) exercise training had comparable increases in lumbar spine and total hip BMD, but only the impact group had increases in femoral neck BMD, which is a common site for osteoporotic fractures.54 In addition, when gravitational forces are reduced, such as during bed rest or spaceflight, there is a rapid decline in the quality of bone.56,57
Evaluating Bone Mineral Density
BMD is most commonly measured by dual energy x-ray absorptiometry (DXA), and is the amount of mineral measured per unit area or volume of bone tissue.49 In Caucasian postmenopausal women, osteoporosis is defined as BMD value more than 2.5 SDs below the young adult mean value (t score) with or without fractures.58 Athletes participating in weightbearing sports should have an approximately 10% higher BMD than nonathletes, and athletes in high-impact sports have a higher BMD compared with medium- or low-impact sports.98 Children, adolescents, and premenopausal women require different definitions that take into account age, sport, fracture history, and other risk factors.
While BMD is more frequently measured using DXA in the clinical setting, high-resolution peripheral quantitative computed tomography (HR-pQCT) can better distinguish healthy microarchitecture of bone from suboptimal bone, using 3-dimensional information to provide estimates of bone geometry.54,64 Finite element analysis applied to HR-pQCT images allows estimation of bone strength and mechanical properties, including stiffness and failure load, which are associated with fracture risk, but independent of bone density.23 For example, in 1 study looking at postmenopausal women, those with fractures had 13% to 17% lower stiffness at the tibia and radius than those without fractures, despite similar BMDs measured via DXA scans.93 An additional objective tool to assess bone integrity is the trabecular bone score (TBS), a gray-level textural measurement that indirectly evaluates bone microarchitecture.22 It has the potential to identify differences in DXA scans that show similar BMD measurements. TBS values tend to be lower in postmenopausal women and in men with previous fractures compared with their nonfractured peers.57,83 The advantage of TBS is that it may predict fracture risk in postmenopausal women and is associated with fracture risk in individuals with conditions related to reduced bone mass or bone quality.91
Effects of Exercise on Bone
Weightbearing activity has beneficial effects on bone health throughout life. Being sedentary is a known risk factor for osteoporosis, and physical activity is the only intervention that both increases bone mass and strength and reduces the risk of falls. It is best if exercise involves loading in discrete bouts with recovery in between and is most beneficial during skeletal growth. Exercise should be of high-strain magnitudes and/or rates, such as jumping for the lower body and racquet sports for the upper body.65 To achieve maximum benefits, exercise should:
be dynamic, not static
achieve adequate strain intensity
consist of discrete, intermittent bouts
include variable loading patterns
be supported by optimal nutrition
include adequate intake of calcium and vitamin D.21
Exercise causes larger improvements in bone strength than can be measured by BMD, as new bone formation is often at the bone surface. A 5.4% increase in BMD is equal to a 64% increase in ultimate force and 94% increase in energy to failure.87 Weightbearing activity was found to have a profound impact on BMD in female athletes, with site-specific mechanical loading significantly affecting bone accrual. Both amenorrheic and eumenorrheic athletes were found to have greater total cross-sectional area, trabecular area, and cortical perimeter compared with nonathletes at the distal tibia.1
Osteoporosis
Low bone mass increases the risk for fractures, leading to significant morbidity and mortality. In the United States, 52% of adults older than 50 years have low bone mass at the femoral neck or lumbar spine and 9% meet the diagnostic criteria for osteoporosis at 1 or both sites.61 There are 1.5 million people in the United States annually who sustain an osteoporotic fracture. Mortality increases 2.8 to 4 times during the first 3 months after a hip fracture.73 Thus, it is critical that measures are taken to prevent and treat osteoporosis.
The American College of Sports Medicine recommends that children and adolescents perform impact activities and moderate intensity resistance training at least 10 to 20 minutes twice a day, 3 days a week. The gains in bone density achieved through exercise in childhood are maintained through adulthood. The goal of exercise in adulthood is to maintain BMD, as it is unclear whether density can be increased with exercise in adulthood. However, there is good evidence that exercise can decrease risk of fracture. For adults, the American College of Sports Medicine recommends weightbearing endurance and plyometric exercise 3 to 5 times per week, and resistance exercise of moderate to high loading 2 to 3 times per week for a total of 30 to 60 minutes per day.53 In elderly patients, modifications may be necessary to ensure safety, but exercise should continue to improve balance and prevent falls.
Studies have shown that a variety of different types of exercise can be beneficial, including impact cardiovascular exercises and resistance training; the most beneficial exercise is a combination of different activities. Nonweightbearing high-force exercise, such as progressive resistance strength training for the lower limbs, has the most impact on femoral neck BMD, whereas the most effective intervention for BMD at the spine was a combination exercise program.47 Studies have shown that exercise reduces the risk of hip fracture in older women and decreases the overall incidence of fractures, despite the lack of BMD changes. This may be explained by animal studies that show an increase in bone strength that far exceeds the changes seen in BMD. Therefore, activities in patients with osteoporosis should include muscle-strengthening and balance exercises to reduce fall risk.53
Role of Vitamin D and Calcium
Vitamin D has a significant impact on bone health, immune function, and physical performance. In the deficient state, the athlete may be at an increased risk for stress fractures, respiratory infections, and muscle injuries.45 It is estimated that 1 billion people, including the elderly, young adults, and children, are vitamin D-deficient or insufficient.2,45 Although there is debate about the optimal serum levels of 25-hydroxyvitamin D [25(OH)D], vitamin D deficiency is defined by most experts as a total 25(OH)D level of <20 ng/mL. Vitamin D insufficiency is defined as a level of 20 to 31 ng/mL, and a level of ≥32 ng/mL demonstrates sufficient levels.45,46 The prevalence of vitamin D insufficiency and deficiency in athletes is about 56% overall with a higher incidence in the winter and spring, indoor sports, and mixed sports.37 In a study of NCAA (National Collegiate Athletic Association) athletes, the prevalence was 33.6%, despite taking place in southern California during the summer months.100
The causes of vitamin D deficiency can be multifactorial, including reduced skin synthesis, absorption of dietary vitamin D, and acquired and heritable disorders of vitamin D metabolism.45 The main cause of vitamin D deficiency in the athletic population is the direct result of decreased ultraviolet B (UVB) radiation absorption into the skin, leading to decreased cutaneous synthesis of vitamin D. This has the greatest impact on indoor athletes and those who live and train in latitudes furthest from the equator. The lack of UVB absorption has a similar effect on dark-skinned athletes with increased skin pigmentation.28 Vitamin D maintains calcium and phosphate homeostasis within the body by acting on the intestines, kidneys, parathyroid glands, bone, and skeletal muscle. Sources of dietary vitamin D include fish, dairy, and eggs, although dietary intake makes up a small component of total vitamin D needs. The major source of vitamin D is provided through the interaction of the skin with UVB light, and its production is affected by age, season, geographic location, and skin pigmentation.2,28
Vitamin D is essential for bone growth, density, and remodeling. It plays a central role in skeletal bone mineralization and calcium regulation via 2 distinct pathways: endocrine and autocrine mechanisms.81 Through its endocrine function, in its active form, 1,25(OH)D3 activates intestinal calcium absorption and raises serum calcium concentration. Many studies have identified a direct relationship between serum vitamin D levels and BMD in adults of all races.2 When vitamin D levels fall below 30 ng/mL, parathyroid hormone levels are increased, which triggers an increase in osteoclastic activity in bone.84 Concomitantly, 1,25(OH)D3 directly stimulates osteoblasts to produce receptor activator nuclear factor-κB, which increases osteoclastogenesis and mobilization of calcium from bone.45 Through its autocrine pathway, vitamin D is involved in essential body processes, such as signaling gene response/expression, synthesizing proteins, hormone synthesis, immune/inflammatory response, and cell turnover and synthesis.81
Vitamin D promotes muscle function and reversal of atrophic changes in athletes. The active metabolite, 1,25(OH)D3, promotes gene transcription, increasing cell protein synthesis and growth.28 Muscle biopsies of adults with low vitamin D levels have shown fatty infiltration and fibrosis, but changes appear reversible with vitamin D supplementation.9 In girls aged 12 to 14 years, there is a direct correlation between 25(OH)D3 levels and muscle power, force, velocity, and jump height measured by jump mechanics.101
There still exists controversy over the benefit of vitamin D and calcium supplementation in reducing the risk of fractures.15,18,42 The role of calcium in strengthening bones is unclear. On one hand, Americans have among the highest calcium intake in the world, but also one of the highest rates of osteoporosis.44 The recommendations for daily calcium intake are 1300 mg for adolescents, 1000 mg for women aged 19 to 50 years, and 1200 mg for women older than 50 years.31 Dietary dairy is the best source of calcium as not only is it the most bioavailable but is also an energy-dense food source.62 Intake of calcium through diet is preferable considering the increased risks of adverse effects, particularly kidney stones and cardiovascular events, even in dosages as low as 500 mg daily. Patients should be informed of potential harm with supplementation. Consuming calcium in diet, such as dairy products, however, does not seem to pose risks for kidney stones or cardiovascular events as seen with artificial forms of calcium supplementation.19,32
On the other hand, both calcium and vitamin D supplementation can decrease the incidence of stress fractures, as seen among female military recruits.55 In older populations (age ≥65 years), high-dose vitamin D supplementation (>800 IU daily) prevented hip and nonvertebral fractures.15 A recent study of professional football players also showed a higher incidence of fractures in vitamin D-deficient players and, perhaps, decreased performance.66
Impact of Hormones on Bone Health
Estrogen plays an important role in skeletal homeostasis, with well-recognized benefits on BMD. At the cellular level, estrogen affects both osteoclasts and osteoblasts. It inhibits bone turnover and maintains balance between bone resorption and formation.86 Estrogen acts on osteoblasts and osteoclasts in a receptor-mediated fashion and has indirect effects on other hormones, including calcitonin, parathyroid hormone, cytokines, and growth factors.5,60
Adolescence is a particularly crucial period for skeletal development. Because of the significant impact puberty has on bone growth, there is up to a 50% increase in total body bone mass between the ages of 12 and 18 years.89 Increases in growth hormone, insulin-like growth factor 1, and estrogen that occur between Tanner stages 2 and 4 coincide with maximal rates of bone mineral gains. Testosterone and estradiol are known to have positive effects on bone health; however, absence of these hormones is linked to osteoporosis.61,104 These hormones protect against bone loss by having antiapoptotic effects on osteocytes and osteoblasts, and apoptotic effects on osteoclasts.68 In addition, these hormones slow the rate of bone remodeling, and the number of remodeling cycles decreases.68,104
In the athletic population, use of hormones in treatment of amenorrhea, oligomenorrhea, and low bone density has been controversial or inconclusive.30,33,35,78 The impact of oral hormones on insulin-like growth factor 1 in the liver may negate the benefit of estrogen on bone.33,35,78 Insulin-like growth factor 1 is a bone trophic hormone and oral estrogen decreases its systemic concentration but transdermal estrogen does not, thus leading researchers to investigate the effects of transdermal estrogen on BMD.50,70,103 When low BMD is from low energy availability and a hypoestrogenic state as seen in the female athlete triad (Triad), the emphasis for treatment should be to improve BMD and resume normal menses through nutritional and behavioral changes. However, if bone density remains low despite at least 1 year of treatment, transdermal estrogen should be considered.33
Other hormonal medications have an impact on bone health, such as depot medroxyprogesterone acetate (Depo-Provera) injections for birth control and hormone replacement therapy (HRT) in menopausal treatment. The mechanism for loss in BMD among Depo-Provera users is estrogen deficiency because of suppression of the hypothalamic-pituitary-ovarian axis without exogenous estrogen replacement.26 A higher body weight correlates with higher BMD, regardless of treatment with hormonal contraception, suggesting that body weight and body fat may override potential detrimental effects of Depo-Provera.20 HRT improves BMD, decreases fracture risk, and improves symptoms in postmenopausal women and was a more commonly used therapy for osteoporosis prior to the publication of the Women’s Health Initiative and the Million Women Study, which raised some concerns about potential risks of breast cancer, cardiac disease, and stroke. Because of these concerns and the availability of other osteoporosis medications, estrogen has a role but is no longer a first-line agent for postmenopausal osteoporosis.14,16,24,88
Female Athlete Triad
The Triad was originally described in 1992 as 3 interrelated components: disordered eating, amenorrhea, and osteoporosis.82,105 This definition has expanded to now describe a spectrum of pathology that includes less severe forms of these 3 factors.77 The goal of describing the Triad as a spectrum is that athletes with subclinical versions of these factors can be identified and treated early before negative consequences—such as bone stress injuries, traumatic fractures, early osteoporosis, cardiovascular issues, and infertility issues—can occur. The crux of the Triad is low energy availability with or without disordered eating. Low energy availability often causes menstrual dysfunction and hypoestrogenism, leading to negative effects on bone health and endothelial cell dysfunction.34,85 Bone stress injuries, which include stress reactions and fractures, are more often seen in athletes with menstrual irregularities and/or low BMD.6,7,40,52,96 Athletes with amenorrhea are 2 to 4 times more likely to suffer a stress fracture than eumenorrheic subjects.11 In a study of college athletes, the lower the BMD the longer the recovery from a stress injury.76 The risk of incurring a stress injury increases as the number of Triad-related risk factors increases.6 Although the Triad is specifically describing the syndrome seen in women, a parallel syndrome is seen in men with low energy availability, hypogonadism, and low BMD, with further research needed in this area.95
The fact that the Triad is a spectrum of disease makes determining prevalence challenging. Up to 15.9% of athletes have all 3 most severe components of the Triad. The prevalence increases when evaluating those with less severe components or those with 1 or 2 components. Estimated prevalence of menstrual disturbance in female athletes is up to 60%, eating disorder/disordered eating is up to 89.2%, and low BMD is up to 39.8%.39
Early detection is critical to prevent the negative consequences of the Triad. A series of questions has been suggested as part of screening questionnaires used for the preparticipation evaluation (Table 1). It is important to recognize that normal menses does not mean the Triad may not be present, so all factors of the Triad need to be investigated. Among high school athletes, those taking hormonal contraceptive pills were more likely to have disordered eating despite the same injury and menstrual patterns as those not taking hormonal contraceptive pills.97 If there are any concerns about the responses to the questions, further in-depth evaluation using a team of health professionals is recommended. This team should include the team physician, sports dietitian, and mental health professionals, as indicated. The physical examination should include height, weight, body mass index (BMI), and signs of eating disorders (lanugo, parotid gland enlargement, calluses on knuckles, and tooth enamel erosions).33 There are a variety of ways to assess energy availability and an experienced sports dietitian or exercise physiologist can assist. Energy availability is defined as energy intake (kcal) minus exercise energy expenditure (kcal) divided by kilograms of fat-free mass or lean body mass.63 These components can be difficult to measure outside the research setting but estimates and less precise measurements can be done and an energy availability calculator is available on the Female Athlete Triad Coalition website (http://www.femaleathletetriad.org/calculators). Functional hypothalamic amenorrhea is the disruption of the hormonal axis function secondary to low energy availability and is a diagnosis of exclusion. Laboratory analysis should include tests to evaluate for pregnancy, thyroid dysfunction, hyperprolactinemia, primary ovarian failure, and other endocrine disorders.33 Evaluation with a DXA scan should be considered in the following instances:
Table 1.
Screening questions for the female athlete triada
| ● Have you ever had a menstrual period? ● How old were you when you had your first menstrual period? ● When was your most recent menstrual period? ● How many periods have you had in the past 12 months? ● Are you presently taking any female hormones (estrogen, progesterone, birth control pills)? ● Do you worry about your weight? ● Are you trying to or has anyone recommended that you gain or lose weight? ● Are you on a special diet or do you avoid certain types of food or food groups? ● Have you ever had an eating disorder? ● Have you ever had a stress fracture? ● Have you ever been told you have low bone density (osteopenia or osteoporosis)? |
Adapted with permission from BMJ Publishing Group Ltd, Br J Sports Med.33
diagnosis of an eating disorder
BMI ≤17.5 kg/m2, <85% estimated weight, or recent weight loss of ≥10% in 1 month
menarche ≥16 years
current or history of <6 menses over 12 months
2 prior bone stress injuries, 1 high-risk stress injury, or a low-energy fracture
prior Z-score ≤ −2.0.
If multiple risk factors are present but not as significant or the athlete is taking medications known to negatively impact the bone, the physician should also consider ordering a DXA scan (Table 2).33
Table 2.
Medications with adverse effects on bone75
| Aluminum-containing antacids |
| Antiseizure medicines (ie, phenytoin) |
| Aromatase inhibitors (ie, anastrozole) |
| Cancer chemotherapy agents |
| Glucocorticoids (ie, prednisone) |
| Gonadotropin-releasing hormone (ie, leuprolide acetate) |
| Heparin |
| Lithium |
| Medroxyprogesterone acetate injectable solution |
| Proton pump inhibitors |
| Selective serotonin uptake inhibitors |
| Tamoxifen |
| Thiazolidinediones |
| Thyroid medication in excess |
The best treatment for the Triad is prevention. Work must be done to continue to change the culture in some sports as it relates to weight and body image. Creating a healthy approach to exercise and nutrition at home and in schools will hopefully achieve this. Screening to identify those athletes with the Triad or at risk for the Triad will allow early intervention to minimize risk to the athlete. Treatment of the Triad is focused on correcting the low energy availability and requires a multidisciplinary approach in which education of the athlete is the key component. Nutrition counseling, exercise counseling, and psychological therapy may all be necessary aspects of treatment. Restoration of weight and resumption of normal menses are the main goals of treatment and are important in preventing further bone loss.33,71 The majority of athletes can be treated successfully with a nonpharmacologic approach, but in those with osteoporosis and/or history of multiple fractures who do not respond to nonpharmacologic treatment for 1 year or who continue to fracture, pharmacologic treatment should be considered. To address the hypoestrogenic state, estrogen replacement can be considered and has been shown to have beneficial effects on bone mass when given via the transdermal route. Combined oral contraceptive pills do not improve bone density in young women.30,102 For athletes in whom the Triad has been diagnosed, clearance and return to play can be challenging, and clinicians want to ensure participation is safe for the athlete. Guidelines using a risk stratification tool have been described in the 2014 Female Athlete Triad Consensus Statement on Treatment and Return to Play of the Female Athlete Triad.33 Magnitude of risk for BMI, energy availability, BMD, menstrual function, and bone stress injuries is evaluated. The total points are then used to help guide clinicians on whether an athlete should be cleared with or without provisions or modifications to training and competition, or even disqualified from participation. A written contract should be used that details the specific criteria that need to be met for the athlete to be cleared.33
Stress Injuries
Bone stress injuries occur over a spectrum, which encompasses stress reactions and stress fractures. They occur because of a disturbance in the equilibrium between osteoblastic bone formation and osteoclastic bone resorption. If bone is unable to withstand repetitive mechanical stresses and edema (reaction), fracture can occur. Not all stress fractures are the same; cancellous bone stress fractures occur more often in patients with osteopenia, compared with cortical stress fractures.67,76 More severe stress fractures by magnetic resonance grading are correlated with lower BMD. High-risk stress fractures such as those seen in the sacrum, pelvis, and femoral neck are associated with more risk factors such as the Triad.76
Bone stress injuries are a common cause of missed training and competition and have a higher prevalence in track and field athletes and military recruits. The prevalence of stress fractures is 0.7% to 21% in athletes.12 Stress injuries of the foot and lower leg are most common,3,68 but they can occur in any bone that is subjected to repetitive stress. Risk factors for stress injuries are often described as intrinsic and extrinsic (Table 3). Specifically, factors of the Triad, such as disordered eating,13 menstrual irregularities,8,13 and low bone density13,74 are associated with stress injuries (Figure 2). Runners with oligomenorrhea were 6 times more likely to sustain a stress injury.13
Table 3.
Stress injury risk factors
| Intrinsic | Extrinsic |
|---|---|
| Female sex | Type of sport (eg, distance running) |
| Biomechanical abnormalities | Training changes (eg, increased mileage, intensity, type of exercise) |
| Low bone density | Terrain/running surface |
| Female athlete triad | Inadequate recovery time |
| Nutritional deficiencies | Inappropriate or sudden change in shoe wear |
Figure 2.
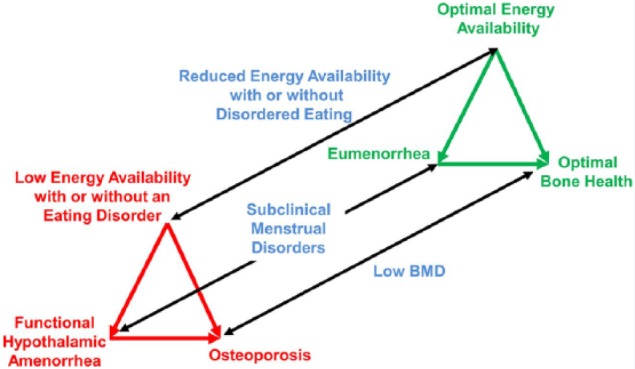
Schematic of the spectrum of the female athlete triad, composed of 3 interrelated components of energy availability, menstrual function, and bone health. BMD, bone mineral density. Used with permission from Lippincott Williams & Wilkins/Wolters Kluwer Health.77 As published in Br J Sports Med.33
Stress injuries can be diagnosed clinically but imaging is often used to assist with the diagnosis of certain stress injuries. Plain radiographs may be normal, particularly in certain stress injuries such as those of the femoral neck (Figure 3), and magnetic resonance imaging (MRI) or bone scan may be indicated to diagnose the stress injury. MRI grading can be helpful for prognosis as well4,72,76; in college athletes, the higher the MRI grade, the longer the recovery.76
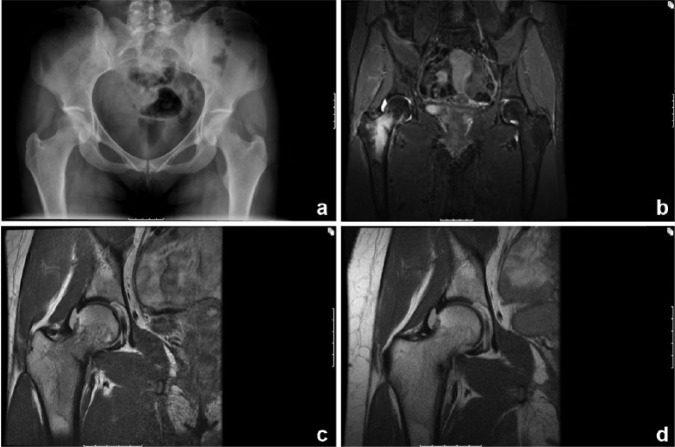
Figure 3.
Imaging studies of a right compression-sided femoral neck stress fracture in a 26-year-old female runner with the female athlete triad. Anteroposterior pelvis radiograph (a) does not show any abnormality. Coronal inversion recovery (b) and proton density–weighted (c) magnetic resonance images show bone marrow edema with a fracture line that involves approximately 50% of the diameter of the neck. A repeat coronal proton density–weighted magnetic resonance imaging done 6 weeks later (d) shows interval healing with decreased fracture line.
The focus of stress injury treatment is on minimizing weightbearing activity to allow healing as well as identification and treatment of underlying risk factors. Sometimes, to assist with minimizing weightbearing, devices such as crutches, walking boots, or long pneumatic air splints are used. Treatment must be individualized and address the current activity level and plans for safely returning to sport. Factors that need to be taken into account for return to sport include location and severity of stress injury, duration of symptoms, underlying risk factors, including low energy availability, response to initial treatment, type and level of sport, and upcoming training/competition goals.41 Trabecular bone and higher grade (by MRI) stress fractures are associated with a longer return to play.76 Surgery is rarely indicated but may be necessary in high-risk fracture sites such as the tension side of the femoral neck or anterior tibia.10,17,36,38,90 Supplementation of calcium and vitamin D in a diet that is deficient in these reduces the incidence of stress fractures by as much as 20%.55
For stress fractures with delayed healing and/or in the setting of low BMD, case studies describe adjunctive medications (nasal calcitonin,43 bisphosphonates,69,92,94 and recombinant parathyroid hormone Forteo)92 but the evidence of their effectiveness and safety in human clinical trials is lacking. In addition, bisphosphonates have teratogenic effects that last for many years in the bone so they should be avoided in women in their childbearing years. There has been some promising evidence for recombinant parathyroid hormone improving BMD, bone mineral content, and stress fracture healing in animal studies but further research is needed in humans.92 External bone stimulators have also been used, although human evidence is lacking for their effectiveness in stress fractures and data are primarily found in studies of traumatic fractures.27,59,99
Conclusions
Exercise plays a vital role in achieving optimal bone health in athletes. Exercise has a variable impact at different stages of life, and adolescence is a critical time during bone mass development. To prevent osteoporosis and bone stress injuries, adequate calcium and vitamin D intake and a healthy balance between exercise and nutrition are necessary. The components of the Triad are associated with lower BMD and stress injuries. Therefore, when a stress injury is diagnosed, part of the treatment plan should include evaluation for and treatment of risk factors to identify those who could carry negative consequences on bone health.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Ackerman KE, Nazem T, Chapko D, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96:3123-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angeline ME, Gee AO, Shindle M, Warren RF, Rodeo SA. The effects of vitamin D deficiency in athletes. Am J Sports Med. 2013;41:461-464. [DOI] [PubMed] [Google Scholar]
- 3. Arendt E, Agel J, Heikes C, Griffiths H. Stress injuries to bone in college athletes: a retrospective review of experience at a single institution. Am J Sports Med. 2003;31:959-968. [DOI] [PubMed] [Google Scholar]
- 4. Arendt EA, Griffiths HJ. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. 1997;16:291-306. [DOI] [PubMed] [Google Scholar]
- 5. Balasch J. Sex steroids and bone: current perspectives. Hum Reprod Update. 2003;9:207-222. [DOI] [PubMed] [Google Scholar]
- 6. Barrack MT, Gibbs JC, De Souza MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med. 2014;42:949-958. [DOI] [PubMed] [Google Scholar]
- 7. Barrack MT, Rauh MJ, Nichols JF. Cross-sectional evidence of suppressed bone mineral accrual among female adolescent runners. J Bone Miner Res. 2010;25:1850-1857. [DOI] [PubMed] [Google Scholar]
- 8. Barrow GW, Saha S. Menstrual irregularity and stress fractures in collegiate female distance runners. Am J Sports Med. 1988;16:209-216. [DOI] [PubMed] [Google Scholar]
- 9. Bartoszewska M, Kamboj M, Patel DR. Vitamin D, muscle function, and exercise performance. Pediatr Clin North Am. 2010;57:849-861. [DOI] [PubMed] [Google Scholar]
- 10. Behrens SB, Deren ME, Matson A, Fadale PD, Monchik KO. Stress fractures of the pelvis and legs in athletes: a review. Sports Health. 2013;5:165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28:91-122. [DOI] [PubMed] [Google Scholar]
- 12. Bennell KL, Brukner PD. Epidemiology and site specificity of stress fractures. Clin Sports Med. 1997;16:179-196. [DOI] [PubMed] [Google Scholar]
- 13. Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in female track-and-field athletes: a retrospective analysis. Clin J Sport Med. 1995;5:229-235. [DOI] [PubMed] [Google Scholar]
- 14. Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419-427. [DOI] [PubMed] [Google Scholar]
- 15. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40-49. [DOI] [PubMed] [Google Scholar]
- 16. Black DM, Rosen CJ. Clinical practice. postmenopausal osteoporosis. N Engl J Med. 2016;374:254-262. [DOI] [PubMed] [Google Scholar]
- 17. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8:344-353. [DOI] [PubMed] [Google Scholar]
- 18. Bohon TM, Goolsby MA. The role of vitamin D supplements in women’s health. Clin Med Insights Womens Health. 2013;6:67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonny AE, Secic M, Cromer BA. Relationship between weight and bone mineral density in adolescents on hormonal contraception. J Pediatr Adolesc Gynecol. 2011;24:35-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35:779-830. [DOI] [PubMed] [Google Scholar]
- 22. Bousson V, Bergot C, Sutter B, Levitz P, Cortet B, Scientific Committee of the Groupe de Recherche et d’Information sur les Osteoporoses. Trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int. 2012;23:1489-1501. [DOI] [PubMed] [Google Scholar]
- 23. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508-6515. [DOI] [PubMed] [Google Scholar]
- 24. Bowring CE, Francis RM. National Osteoporosis Society’s position statement on hormone replacement therapy in the prevention and treatment of osteoporosis. Menopause Int. 2011;17:63-65. [DOI] [PubMed] [Google Scholar]
- 25. Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781-786. [DOI] [PubMed] [Google Scholar]
- 26. Busen NH, Britt RB, Rianon N. Bone mineral density in a cohort of adolescent women using depot medroxyprogesterone acetate for one to two years. J Adolesc Health. 2003;32:257-259. [DOI] [PubMed] [Google Scholar]
- 27. Busse JW, Kaur J, Mollon B, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ. 2009;338:b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41:1102-1110. [DOI] [PubMed] [Google Scholar]
- 29. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cobb KL, Bachrach LK, Sowers M, et al. The effect of oral contraceptives on bone mass and stress fractures in female runners. Med Sci Sports Exerc. 2007;39:1464-1473. [DOI] [PubMed] [Google Scholar]
- 31. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2010. [Google Scholar]
- 32. Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497-504. [DOI] [PubMed] [Google Scholar]
- 33. De Souza MJ, Nattiv A, Joy E, et al. 2014. female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48:289. [DOI] [PubMed] [Google Scholar]
- 34. De Souza MJ, West SL, Jamal SA, Hawker GA, Gundberg CM, Williams NI. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone. 2008;43:140-148. [DOI] [PubMed] [Google Scholar]
- 35. De Souza MJ, Williams NI. Beyond hypoestrogenism in amenorrheic athletes: energy deficiency as a contributing factor for bone loss. Curr Sports Med Rep. 2005;4:38-44. [DOI] [PubMed] [Google Scholar]
- 36. Diehl JJ, Best TM, Kaeding CC. Classification and return-to-play considerations for stress fractures. Clin Sports Med. 2006;25:17-28, vii. [DOI] [PubMed] [Google Scholar]
- 37. Farrokhyar F, Tabasinejad R, Dao D, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45:365-378. [DOI] [PubMed] [Google Scholar]
- 38. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309-325. [DOI] [PubMed] [Google Scholar]
- 39. Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc. 2013;45:985-996. [DOI] [PubMed] [Google Scholar]
- 40. Goolsby MA, Barrack MT, Nattiv A. A displaced femoral neck stress fracture in an amenorrheic adolescent female runner. Sports Health. 2012;4:352-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goolsby M, Hough L, Safran M. Stress fractures of the hip and pelvis. In: Kelly B, Bedi A, Larson C, O’Sullivan E. eds. Sports Hip Injuries: Diagnosis and Management. Thorofare, NJ: Slack; 2015:171-186. [Google Scholar]
- 42. Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (randomised evaluation of calcium or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621-1628. [DOI] [PubMed] [Google Scholar]
- 43. Hegde V, Jo JE, Andreopoulou P, Lane JM. Effect of osteoporosis medications on fracture healing. Osteoporos Int. 2016;27:861-71. [DOI] [PubMed] [Google Scholar]
- 44. Hegsted DM. Calcium and osteoporosis. J Nutr. 1986;116:2316-2319. [DOI] [PubMed] [Google Scholar]
- 45. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [DOI] [PubMed] [Google Scholar]
- 46. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S-1688S. [DOI] [PubMed] [Google Scholar]
- 47. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333. [DOI] [PubMed] [Google Scholar]
- 48. Johnston CC, Jr, Slemenda CW. Peak bone mass, bone loss and risk of fracture. Osteoporos Int. 1994;4(suppl 1):43-45. [DOI] [PubMed] [Google Scholar]
- 49. Kahn K, McKay H, Kannus P, Bailey D, Wark J, Bennell K. Physical Activity and Bone Health. Champaign, IL: Human Kinetics; 2001. [Google Scholar]
- 50. Kam GY, Leung KC, Baxter RC, Ho KK. Estrogens exert route- and dose-dependent effects on insulin-like growth factor (IGF)-binding protein-3 and the acid-labile subunit of the IGF ternary complex. J Clin Endocrinol Metab. 2000;85:1918-1922. [DOI] [PubMed] [Google Scholar]
- 51. Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989-995. [DOI] [PubMed] [Google Scholar]
- 52. Kelsey JL, Bachrach LK, Procter-Gray E, et al. Risk factors for stress fracture among young female cross-country runners. Med Sci Sports Exerc. 2007;39:1457-1463. [DOI] [PubMed] [Google Scholar]
- 53. Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR; American College of Sports Medicine. American College of Sports Medicine position stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985-1996. [DOI] [PubMed] [Google Scholar]
- 54. Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24:35-39. [DOI] [PubMed] [Google Scholar]
- 55. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741-749. [DOI] [PubMed] [Google Scholar]
- 56. LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact. 2007;7:33-47. [PubMed] [Google Scholar]
- 57. Leib E, Winzenrieth R, Aubry-Rozier B, Hans D. Vertebral microarchitecture and fragility fracture in men: a TBS study. Bone. 2014;62:51-55. [DOI] [PubMed] [Google Scholar]
- 58. Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone. 2008;43:1115-1121. [DOI] [PubMed] [Google Scholar]
- 59. Li J, Waugh LJ, Hui SL, Burr DB, Warden SJ. Low-intensity pulsed ultrasound and nonsteroidal anti-inflammatory drugs have opposing effects during stress fracture repair. J Orthop Res. 2007;25:1559-1567. [DOI] [PubMed] [Google Scholar]
- 60. Liu SL, Lebrun CM. Effect of oral contraceptives and hormone replacement therapy on bone mineral density in premenopausal and perimenopausal women: a systematic review. Br J Sports Med. 2006;40:11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Looker AC, Borrud LG, Dawson-Hughes B, Shepherd JA, Wright NC. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005-2008. NCHS Data Brief. 2012;(93):1-8. [PubMed] [Google Scholar]
- 62. Lorincz C, Manske SL, Zernicke R. Bone health: part 1, nutrition. Sports Health. 2009;1:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Loucks AB. Low energy availability in the marathon and other endurance sports. Sports Med. 2007;37:348-352. [DOI] [PubMed] [Google Scholar]
- 64. MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29:1096-1105. [DOI] [PubMed] [Google Scholar]
- 65. Manske SL, Lorincz CR, Zernicke RF. Bone health: part 2, physical activity. Sports Health. 2009;1:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maroon JC, Mathyssek CM, Bost JW, et al. Vitamin D profile in National Football League players. Am J Sports Med. 2015;43:1241-1245. [DOI] [PubMed] [Google Scholar]
- 67. Marx RG, Saint-Phard D, Callahan LR, Chu J, Hannafin JA. Stress fracture sites related to underlying bone health in athletic females. Clin J Sport Med. 2001;11:73-76. [DOI] [PubMed] [Google Scholar]
- 68. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15:46-58. [DOI] [PubMed] [Google Scholar]
- 69. Milgrom C, Finestone A, Novack V, et al. The effect of prophylactic treatment with risedronate on stress fracture incidence among infantry recruits. Bone. 2004;35:418-424. [DOI] [PubMed] [Google Scholar]
- 70. Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Misra M, Prabhakaran R, Miller KK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93:1231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moran DS, Evans RK, Hadad E. Imaging of lower extremity stress fracture injuries. Sports Med. 2008;38:345-356. [DOI] [PubMed] [Google Scholar]
- 73. Moyer VA, U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158:691-696. [DOI] [PubMed] [Google Scholar]
- 74. Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113:754-759. [DOI] [PubMed] [Google Scholar]
- 75. National Osteoporosis Foundation. Medicines that may cause bone loss. http://nof.org/articles/6. Accessed February 2, 2016.
- 76. Nattiv A, Kennedy G, Barrack MT, et al. Correlation of MRI grading of bone stress injuries with clinical risk factors and return to play: a 5-year prospective study in collegiate track and field athletes. Am J Sports Med. 2013;41:1930-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882. [DOI] [PubMed] [Google Scholar]
- 78. Nazem TG, Ackerman KE. The female athlete triad. Sports Health. 2012;4:302-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nichols DL, Sanborn CF, Essery EV. Bone density and young athletic women. An update. Sports Med. 2007;37:1001-1014. [DOI] [PubMed] [Google Scholar]
- 80. Nordstrom A, Karlsson C, Nyquist F, Olsson T, Nordstrom P, Karlsson M. Bone loss and fracture risk after reduced physical activity. J Bone Miner Res. 2005;20:202-207. [DOI] [PubMed] [Google Scholar]
- 81. Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013;5:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 1997;29(5):i-ix. [DOI] [PubMed] [Google Scholar]
- 83. Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom. 2009;12:170-176. [DOI] [PubMed] [Google Scholar]
- 84. Powers S, Nelson WB, Larson-Meyer E. Antioxidant and vitamin D supplements for athletes: sense or nonsense? J Sports Sci. 2011;29(suppl 1):S47-S55. [DOI] [PubMed] [Google Scholar]
- 85. Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. J Clin Endocrinol Metab. 2005;90:1354-1359. [DOI] [PubMed] [Google Scholar]
- 86. Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279-302. [DOI] [PubMed] [Google Scholar]
- 87. Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545-1554. [DOI] [PubMed] [Google Scholar]
- 88. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [DOI] [PubMed] [Google Scholar]
- 89. Sabatier JP, Guaydier-Souquieres G, Benmalek A, Marcelli C. Evolution of lumbar bone mineral content during adolescence and adulthood: a longitudinal study in 395 healthy females 10-24 years of age and 206 premenopausal women. Osteoporos Int. 1999;9:476-482. [DOI] [PubMed] [Google Scholar]
- 90. Shin AY, Gillingham BL. Fatigue fractures of the femoral neck in athletes. J Am Acad Orthop Surg. 1997;5:293-302. [DOI] [PubMed] [Google Scholar]
- 91. Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518-530. [DOI] [PubMed] [Google Scholar]
- 92. Sloan AV, Martin JR, Li S, Li J. Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone. 2010;47:235-240. [DOI] [PubMed] [Google Scholar]
- 93. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J Clin Endocrinol Metab. 2011;96:2041-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stewart GW, Brunet ME, Manning MR, Davis FA. Treatment of stress fractures in athletes with intravenous pamidronate. Clin J Sport Med. 2005;15:92-94. [DOI] [PubMed] [Google Scholar]
- 95. Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the female athlete triad in male athletes. Sports Med. 2016;46:171-82. [DOI] [PubMed] [Google Scholar]
- 96. Tenforde AS, Sayres LC, McCurdy ML, Sainani KL, Fredericson M. Identifying sex-specific risk factors for stress fractures in adolescent runners. Med Sci Sports Exerc. 2013;45:1843-1851. [DOI] [PubMed] [Google Scholar]
- 97. Thein-Nissenbaum JM, Carr KE, Hetzel S, Dennison E. Disordered eating, menstrual irregularity, and musculoskeletal injury in high school athletes: a comparison of oral contraceptive pill users and nonusers. Sports Health. 2014;6:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Torstveit MK, Sundgot-Borgen J. Low bone mineral density is two to three times more prevalent in non-athletic premenopausal women than in elite athletes: a comprehensive controlled study. Br J Sports Med. 2005;39:282-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Uchiyama Y, Nakamura Y, Mochida J, Tamaki T. Effect of low-intensity pulsed ultrasound treatment for delayed and non-union stress fractures of the anterior mid-tibia in five athletes. Tokai J Exp Clin Med. 2007;32:121-125. [PubMed] [Google Scholar]
- 100. Villacis D, Yi A, Jahn R, et al. Prevalence of abnormal vitamin D levels among division I NCAA athletes. Sports Health. 2014;6:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94:559-563. [DOI] [PubMed] [Google Scholar]
- 102. Warren MP, Miller KK, Olson WH, Grinspoon SK, Friedman AJ. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in women with hypothalamic amenorrhea and osteopenia: an open-label extension of a double-blind, placebo-controlled study. Contraception. 2005;72:206-211. [DOI] [PubMed] [Google Scholar]
- 103. Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72:374-381. [DOI] [PubMed] [Google Scholar]
- 104. Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004;134:696S-700S. [DOI] [PubMed] [Google Scholar]
- 105. Yeager KK, Agostini R, Nattiv A, Drinkwater B. The female athlete triad: disordered eating, amenorrhea, osteoporosis. Med Sci Sports Exerc. 1993;25:775-777. [DOI] [PubMed] [Google Scholar]