Abstract
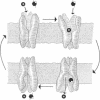
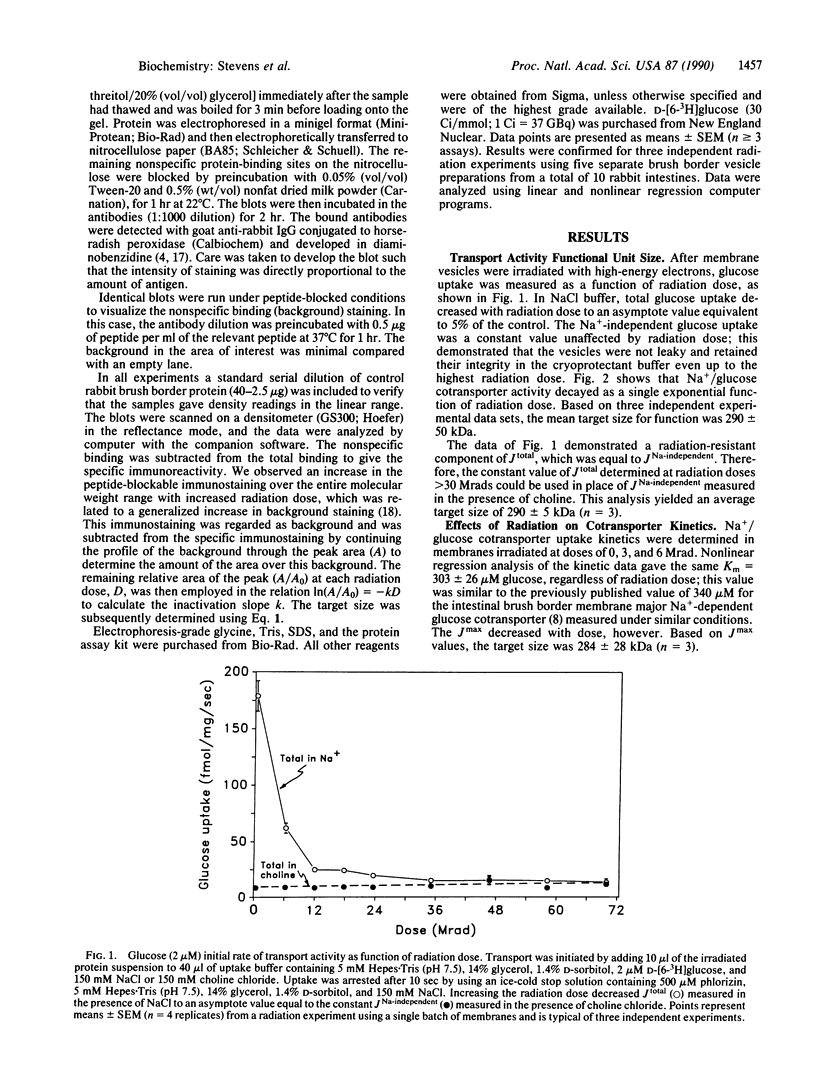
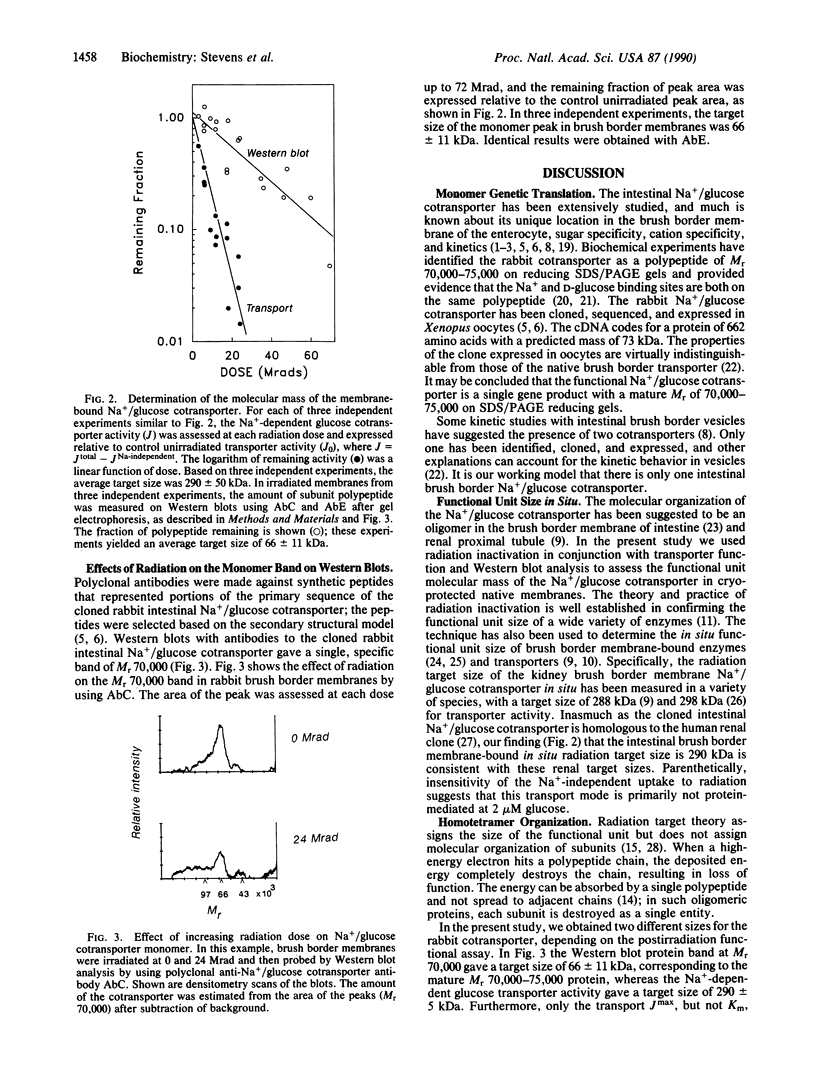
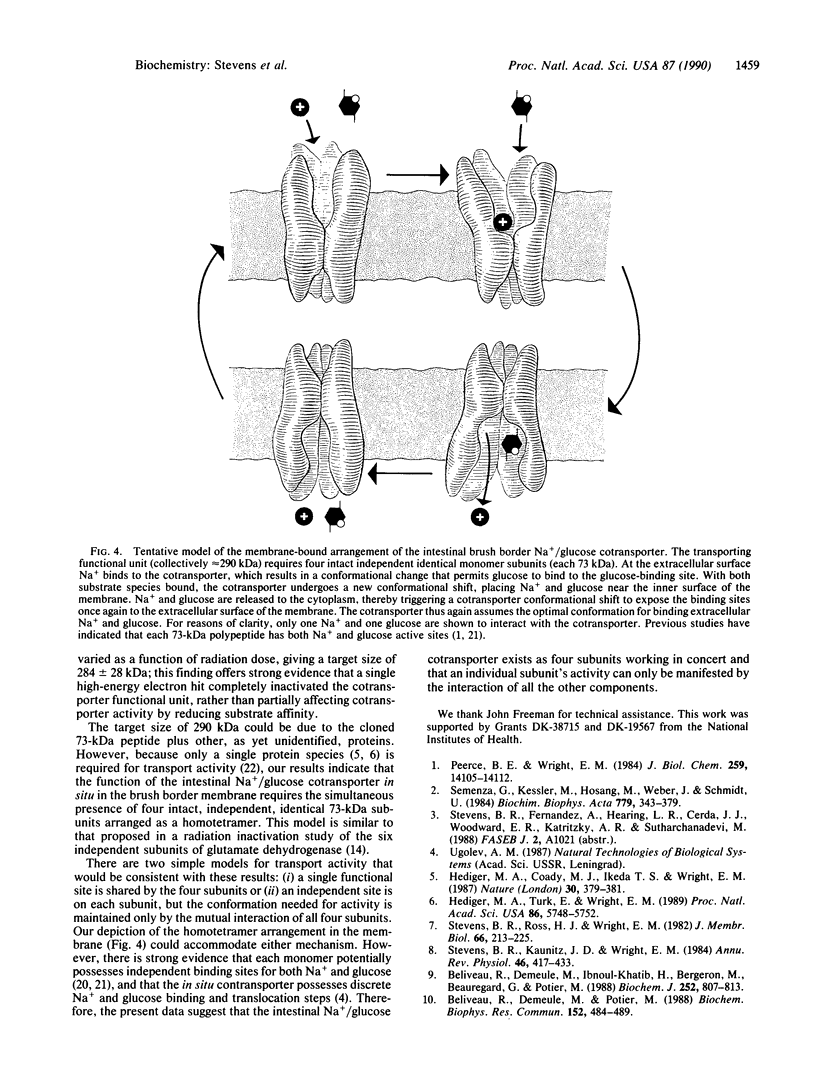
The functional unit molecular size of the intestinal brush border membrane-bound Na+/glucose cotransporter was determined by radiation inactivation. Purified brush border membrane vesicles preserved in cryoprotectant buffer were irradiated (-135 degrees C) with high-energy electrons from a 13-MeV (1 eV = 1.602 x 10(-19) J) linear accelerator at doses from 0 to 70 Mrad (1 rad = 0.01 Gy). After each dose, the cotransporter was investigated with respect to (i) Na(+)-dependent transport activity and (ii) immunologic blot analysis with antibodies against the cloned rabbit intestinal cotransporter. Increasing radiation decreased the maximal Na(+)-dependent cotransporter activity Jmax without affecting apparent Km. The size of the transporting functional unit was 290 +/- 5 kDa. Immunologic blot analysis of brush border membranes gave a single band of Mr 70,000, which decreased in intensity with increased radiation dose and gave a target size of 66 +/- 11 kDa. We conclude that activity of the intestinal Na+/glucose cotransporter in situ in the brush border membrane requires the simultaneous presence of four intact, independent, identical subunits arranged as a homotetramer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Béliveau R., Demeule M., Ibnoul-Khatib H., Bergeron M., Beauregard G., Potier M. Radiation-inactivation studies on brush-border-membrane vesicles. General considerations, and application to the glucose and phosphate carriers. Biochem J. 1988 Jun 15;252(3):807–813. doi: 10.1042/bj2520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béliveau R., Demeule M., Potier M. Molecular size of the Na+-H+ antiport in renal brush border membranes, as estimated by radiation inactivation. Biochem Biophys Res Commun. 1988 Apr 15;152(1):484–489. doi: 10.1016/s0006-291x(88)80739-3. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Ingram J., Kenny A. J. Radiation inactivation analysis of kidney microvillar peptidases. FEBS Lett. 1986 Sep 15;205(2):323–327. doi: 10.1016/0014-5793(86)80921-8. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Nielsen T. B., Kempner E. S. Molecular weight determinations from radiation inactivation. Methods Enzymol. 1985;117:65–94. doi: 10.1016/s0076-6879(85)17008-4. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Turk E., Wright E. M. Homology of the human intestinal Na+/glucose and Escherichia coli Na+/proline cotransporters. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5748–5752. doi: 10.1073/pnas.86.15.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T. S., Hwang E. S., Coady M. J., Hirayama B. A., Hediger M. A., Wright E. M. Characterization of a Na+/glucose cotransporter cloned from rabbit small intestine. J Membr Biol. 1989 Aug;110(1):87–95. doi: 10.1007/BF01870995. [DOI] [PubMed] [Google Scholar]
- Kano-Kameyama A., Hoshi T. Purification and reconstitution of Na+/D-glucose cotransport carriers from guinea pig small intestine. Jpn J Physiol. 1983;33(6):955–970. doi: 10.2170/jjphysiol.33.955. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Fleischer S. Radiation inactivation of membrane components and molecular mass determination by target analysis. Methods Enzymol. 1989;172:410–439. doi: 10.1016/s0076-6879(89)72027-9. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Haigler H. T. The influence of low temperature on the radiation sensitivity of enzymes. J Biol Chem. 1982 Nov 25;257(22):13297–13299. [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H. Radiation inactivation of glutamate dehydrogenase hexamer: lack of energy transfer between subunits. Science. 1983 Nov 11;222(4624):586–589. doi: 10.1126/science.6635656. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Miller J. H. Radiation-damaged tyrosinase molecules are inactive. Biophys J. 1989 Jan;55(1):159–162. doi: 10.1016/S0006-3495(89)82787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempner E. S. Molecular size determination of enzymes by radiation inactivation. Adv Enzymol Relat Areas Mol Biol. 1988;61:107–147. doi: 10.1002/9780470123072.ch3. [DOI] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Distance between substrate sites on the Na-glucose cotransporter by fluorescence energy transfer. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8092–8096. doi: 10.1073/pnas.83.21.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Evidence for tyrosyl residues at the Na+ site on the intestinal Na+/glucose cotransporter. J Biol Chem. 1985 May 25;260(10):6026–6031. [PubMed] [Google Scholar]
- Peerce B. E., Wright E. M. Sodium-induced conformational changes in the glucose transporter of intestinal brush borders. J Biol Chem. 1984 Nov 25;259(22):14105–14112. [PubMed] [Google Scholar]
- Rabon E. C., Gunther R. D., Bassilian S., Kempner E. S. Radiation inactivation analysis of oligomeric structure of the H,K-ATPase. J Biol Chem. 1988 Nov 5;263(31):16189–16194. [PubMed] [Google Scholar]
- Semenza G., Kessler M., Hosang M., Weber J., Schmidt U. Biochemistry of the Na+, D-glucose cotransporter of the small-intestinal brush-border membrane. The state of the art in 1984. Biochim Biophys Acta. 1984 Sep 3;779(3):343–379. doi: 10.1016/0304-4157(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Kaunitz J. D., Wright E. M. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Kempner E. S., Wright E. M. Radiation inactivation probe of membrane-bound enzymes: gamma-glutamyltranspeptidase, aminopeptidase N, and sucrase. Anal Biochem. 1986 Nov 1;158(2):278–282. doi: 10.1016/0003-2697(86)90550-6. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Ross H. J., Wright E. M. Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J Membr Biol. 1982;66(3):213–225. doi: 10.1007/BF01868496. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Wright S. H., Hirayama B. S., Gunther R. D., Ross H. J., Harms V., Nord E., Kippen I., Wright E. M. Organic and inorganic solute transport in renal and intestinal membrane vesicles preserved in liquid nitrogen. Membr Biochem. 1982;4(4):271–282. doi: 10.3109/09687688209065436. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Malathi P., Preiser H., Jung C. Y. Radiation inactivation studies on the rabbit kidney sodium-dependent glucose transporter. J Biol Chem. 1985 Sep 5;260(19):10551–10556. [PubMed] [Google Scholar]