Abstract
Summary:Huntington’s disease (HD) is a dominantly transmitted neurodegenerative disorder with wide variation in onset age but with an average age at onset of 40 years. Children of HD gene carriers have a 50% chance of inheriting the disease. The characteristic symptoms of HD are involuntary choreiform movements, cognitive impairment, mood disorders, and behavioral changes which are chronic and progressive over the course of the illness. HD is a “trinucleotide repeat” disorder, which is caused by an increase in the number of CAG repeats in the HD gene. Repeats of 40 or larger are associated with disease expression, whereas repeats of 26 and smaller are normal. Intermediate numbers of repeats, between 27 and 35, are not associated with disease expression but may expand in paternal transmission, resulting in the disease in descendents. Repeats of 36–39 are associated with reduced penetrance whereby some develop HD and others do not. The identification of the genetic defect in HD permits direct genetic testing for the presence of the gene alteration responsible for the disease. Tests may be performed in three circumstances: (1) confirmation of diagnosis, (2) predictive testing of persons at genetic risk for inheriting HD, and (3) prenatal testing. Testing is widely available and much experience has been gained with protocols that assist the individual in making an informed choice about test options, and minimize the occurrence of adverse emotional outcomes.
Keywords: Huntington’s disease, genetics, trinucleotide repeat, genetic testing, genetic modifiers
INTRODUCTION
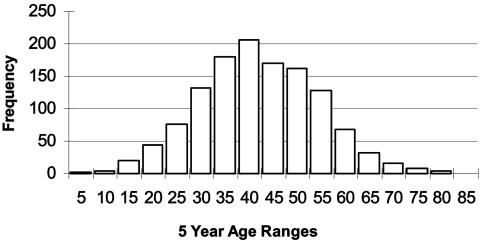
This chapter addresses two aspects of Huntington’s disease (HD). The first section reviews salient genetics features of HD, and the second section addresses the utilization of genetic testing for the illness. HD is a dominantly transmitted neurodegenerative disorder involving the basal ganglia and cerebral cortex that typically strikes in midlife but can occur as young as age 2 or 3 and as old as age 80 or more (FIG. 1). Survival from onset to death averages 17–20 years with some evidence that later onset is associated with slower progression. Children of HD gene carriers have a 50% chance of inheriting the gene, and because penetrance is full, those who inherit the gene eventually develop the disease, given that they do not die of other causes before onset. Because most persons at risk for HD will have known an affected parent, they often have personal experiences which shape their views and influence major life choices. The characteristic symptoms of HD are involuntary choreiform movements, cognitive impairment, mood disorders, and behavioral changes that are chronic and progressive over the course of the illness. Although substantial strides are being made in the development of treatments for HD, at this time treatments to slow the progression or delay the onset of the disease remain inadequate.
FIG. 1.
Huntington’s disease onset ages. The age at onset distribution in Huntington’s disease is very broad and may vary ffrom as young as 3 or 4 years of age to as old as 85. Onset presented here represents initial signs of motor impairment.
The HD gene
The nature of the genetic defect in the HD gene explains many of the genetic features of the disorder, including the variability in age at onset, the tendency for juvenile disease to be inherited from fathers, and the sporadic appearance of new mutations to HD.1 The gene is located on chromosome 4p16.32 and the genetic alteration which causes the disease is an increase of the number of repetitions of three nucleic acids (C, A, and G) in the coding region of the first exon of the HD gene.3 This CAG “triplet” is normally repeated about 20 times, but an approximate doubling in the number of repeats to 40 or more results in the expression of the disease.3,4 Figure 2 shows the distribution of normal repeats, from 10 to 26, and of HD repeats, from 40 to about 80. Repeats between 27 and 35 can be meiotically unstable in paternal transmission. Descendents of men with repeats in this range have been known to inherit disease-associated repeats of 40 or more. In a sample of 1260 persons ascertained from HD families studied in the New England Huntington’s Disease Research Center Without Walls (Boston, MA), repeats between 27 and 35, represented about 3.2% of all repeats. Because few of these repeats have been observed, and their frequency in a non-HD sample has not been adequately established, the frequency with which these expansions occur is difficult to estimate. An estimates of 6% has been offered.5,6 Repeats between 36 and 39 are also rare (2.7% in this series), and are associated with reduced penetrance, whereby some with repeats in this range develop HD and others do not. Again the estimates of penetrance are poor because of the small numbers of observations and because most of those observed are among persons who develop HD whereas those who do not manifest symptoms may escape detection.
FIG. 2.
Normal and expanded HD repeat sizes. The distribution of repeats for Huntington’s disease may be divided into four categories. Repeats of 26 or fewer are normal. Repeats between 27 and 35 are rare and are not associated with the expression of the disease, but occasionally fathers with repeats in this range will transmit a repeat to descendants that is expanded to the range for expression of the illness. Repeats between 36 and 39 are associated with reduced penetrance whereby some individuals will develop HD and others will not. Repeats of 40 or larger are associated with the expression of HD. Persons carrying repeats in this range will develop HD, assuming they do not die of other causes before onset.
Modification of disease expression by repeat size
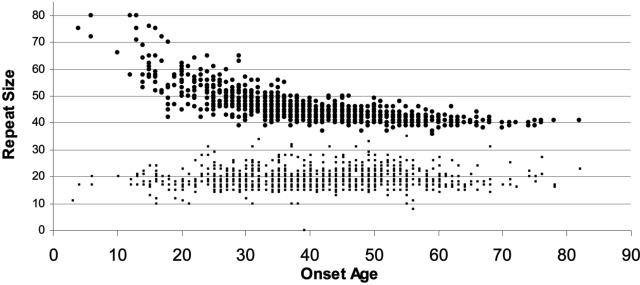
HD is a disorder with highly variable clinical expression, as exemplified by the wide variation in onset age. The strong inverse relationship between age at onset and number of CAG repeats is unequivocal.1 For the 1165 HD cases depicted in Figure 3, the correlation between repeat size and onset age is r = −0.81 and accounts for about 66% of the variance in onset age. Much of the strength of this correlation derives from the small sample of persons (about 5% of the sample) who have very large repeat sizes and very young onset ages. Thus, although the correlation between repeat size and onset age is strong, it is widely acknowledged that the repeat size is a poor predictor of onset age. The predictive shortcomings can be appreciated by examining the range of onset age for persons with a particular number of repeats. For example, persons represented in Figure 3 with 44 CAG repeats exhibit onset ages as young as 31 and as old as 66 years of age. The 34-year span in onset demonstrates not only the poor predictive power for onset of the repeat size, but also the substantial variation in onset age that is not explained by the HD repeat. It is important therefore to recognize that predictive HD gene testing will not reveal meaningful information about when an individual is likely to develop symptoms of the illness and this should be made clear in pretest counseling.
FIG. 3.
HD repeat size and onset age. The relationship between the repeat size and the age at onset is presented. Persons with repeats of 60 or larger commonly have very young onset, before the age of 20, and among these large repeats there is a clear relationship between repeat size and onset age. For persons with 55 repeats or fewer, the relationship between repeat size and onset age is much weaker and the repeat size is not predictive of onset age.
Sex of the affected parent
Merritt et al.7 first observed that a disproportionate number of cases with onset before the age of 21 had inherited the HD gene from affected fathers. The observation of earlier ages at onset in successive generations, termed “anticipation”, is seen in several of the trinucleotide repeat disorders. Meiotic instability of the HD repeat in paternal transmission explains the observation of anticipation in HD. Although meiotic instability may occur in both maternal and paternal transmission, in paternal transmission there is a propensity toward larger repeat expansion. For maternal transmission, nearly equal numbers of expansions and contractions are seen, and the shifts are small, ranging from 1 to 3 repeats. The observation that paternally transmitted repeats are prone to large increases in size4,8,9 explains why most juvenile onset HD is inherited through the male germline.
Genetic modifiers of HD expression
Significant familial aggregation for the age at onset in HD has been reported.10,11 Using onset ages adjusted for the size of the HD repeat mutation, pairs of affected siblings were found to have remarkably similar onset ages independent of the size of the HD repeat. Estimates of heritability of onset age after adjustment for the repeat size range from 56% to 65%.10,11 Thus 56% to 65% of the variation in onset age, which is not attributable to the repeat size, can be attributed to modifier genes. In addition to the familial aggregation in onset age, Djoussé et al.10 also found that the size of the normal repeat influenced onset age. Although the effect is small and explains about 1% of the variation in onset age, it is noteworthy that in contrast to the HD repeat itself, larger normal repeats are associated with later onset.
The evidence for strong heritability for the repeat-adjusted onset age led to a genome scan to identify loci that may influence the expression of HD.12 Suggestive evidence for linkage was found at 4p16 (LOD = 1.93), 6p21–23 (LOD = 2.29), and 6q24–26 (LOD = 2.28), which may be useful for the investigation of genes that modify age at onset of HD. Li et al.12 noted evidence for a genetic modifier at the HD locus itself. The further investigation of genes that modify disease expression may lead to novel therapeutic interventions to delay the onset of disease.
The cloning of the HD gene in 19933 led to important advances in the understanding of the mechanisms for gene expression. One important aspect of HD, which is not addressed in this chapter, is the use of transgenic mice for study of therapeutic intervention and for understanding of the pathogenesis of the disease. Fortunately these have recently been described in other reviews.13–15
DIRECT GENETIC TESTING
Huntington’s disease has become a model for genetic testing in other adult-onset inherited disorders because the illness is relatively common, and there is widespread experience with it. Genetic testing programs began in 1986 with linkage testing and evolved to direct gene testing shortly after the gene was cloned in 1993. There are three main types of HD genetic testing:1 diagnostic testing to confirm or rule out disease,2 presymptomatic testing to determine the carrier status of an individual at genetic risk for inheriting the disease, and3 prenatal testing to determine the carrier status of a fetus. These three test circumstances necessitate the imparting of different information to the individual seeking the test.
Persons at risk for HD often seek presymptomatic testing to assist in making decisions about marriage, procreation, or career. Nevertheless, the emotional impact of the result can be difficult to anticipate and can evoke substantial adverse emotional reactions.16,17 Appropriate pretest counseling is important to assist the at-risk individual in considering the risks and benefits of genetic testing for diseases such as HD for which available treatment does not justify testing.
The HD genetic test is widely available, and can be ordered as a clinical diagnostic procedure by sending a blood specimen to one of the many DNA diagnostic laboratories in North America. Since 1994, when the direct gene test was first offered, approximately 300 presymptomatic tests per year have been performed in the United States.18 Although it is estimated that ∼120,000 persons are at risk for HD in the US, about one-third are minors and one-third are older than their expected onset age, leaving ∼40,000–60,000 in the age range where predictive testing is sought. Of the adults at risk for HD, approximately 5% to 7% have been tested. At the rate of 0.5% to 0.7% per year, currently performed each year through the more than 50 HD testing programs in the US, it is expected that 10% to 15% of persons at risk for HD will be tested, making it the most widely used genetic test for adult-onset disease.
A common factor that is shared by many individuals who seek predictive testing and follow through to completion of the test is that there is someone else for whom the test has significant implications. These may be divided into three common scenarios. The first situation is the young adult who is contemplating marriage, is in a serious romantic relationship and is seeking to learn what to tell the prospective mate about his or her genetic risk for HD. For these young people the emotional impact of a gene-positive result can be very profound because it may seem to close the door to many of the common desires for the future, including marriage and family. Testing among young adults can lead to bitter disappointment and a long period of recovery and adjustment.
A second situation involves the person who is already married but is contemplating having children. For these individuals, a gene-positive result can raise questions about other options for having children, including adoption, artificial insemination, or prenatal testing. Prenatal testing is discussed later in this chapter, but it is worth noting that in some instances adoption can be difficult when one parent is recognized to carry a gene predisposing to a seriously disabling disease such as HD.
The third circumstance is the individual who is already married and has children and is seeking to learn what to tell the children about their risk for HD. Commonly, these individuals have the most resources to draw upon. They may have a stable marriage with a supportive spouse and they may have more maturity and experience in dealing with disappointment. Conversely, they may be closer to their age at onset and may have less time to adjust to the information of a gene-positive result before symptoms appear.
In some instances, individuals seek testing with the motivation being verbalized as “I just need to know, it’s been on my mind a lot and I would rather know one way or the other.” When the individual does not identify a concrete motivation for testing, one must consider the possibility that he or she may have had one or more experiences prompting them to suspect they may be symptomatic. It can be difficult for the individual seeking testing to acknowledge their suspicions because such symptoms may bring them face to face with their worst fears of the disease. Persons who believe they are symptomatic may be at increased risk for suicide with a gene-positive result.19,20 Although the risk for suicide was extensively discussed at the time at which linkage testing was implemented, in practice it has proved to be a rare event. International studies suggest that suicide among persons who learn they are gene-positive may occur around the time of onset.16 A neurological exam to address suspicions for possible symptoms may be helpful for the individual who has these concerns.
Genetic test utilization
Cost and accuracy.
Genetic testing is both cost-efficient and diagnostically precise. Nevertheless, it is important to establish that HD is present in the family via genetic test confirmation because some other illnesses may be misdiagnosed as HD. Unfortunately, in some instances there are no living relatives for whom such a test can be performed. The expense of the laboratory procedure for DNA testing is $250 to $300.
Confirmation of diagnosis.
Confirmation genetic testing is appropriate for persons with a suspected diagnosis of HD. Such confirmations are particularly helpful when there is no known family history because of the early death of a parent, adoption, non-paternity or a possible new mutation. Recent studies suggest that the frequency of new mutation to HD may be substantially higher than previously suspected.21 Almqvist et al.21 estimate that 24% of new diagnoses of HD represent individuals who have no family history of the illness. These studies suggest that the mutation rate may be as high as 6.9 per million,21 which is double previous estimates.22 Thus the use of HD testing is valuable in the absence of family history. Importantly, the confirmation of disease assists in establishing proper care of the individual and in revealing genetic risk for relatives. The recognition of de novo HD by genetic testing often brings with it implications for the children, siblings, and other relatives of the individual with the illness. It is important to arrange for this information to be imparted to those relatives now recognized to be at risk for the disease.
In some instances the “confirmation” genetic test does not apply. When the individual has an unequivocal family history of HD, but equivocal symptoms and a clinical diagnosis of HD cannot be made, the test is more appropriately considered “presymptomatic.” Although the genetic test can reveal whether or not a person carries the HD gene, it cannot establish the presence of symptoms of disease. Persons at risk for HD may develop other diseases, which should not be overlooked by a gene-positive test result.
Presymptomatic testing of persons at risk.
The presymptomatic direct genetic test includes counseling, neurological examination, and the DNA assay and may cost ∼$1000. Many persons choose to pay out of pocket to maintain a higher degree of confidentiality, particularly in light of concerns for access to health insurance, and potential for employment discrimination. Consequently, confidentiality is a primary concern for testing and many test programs implement additional procedure protocols for protection of medical records and storage of test results. In some instances individuals may seek testing anonymously or by using a pseudonym, with the view that confidentiality may be more highly guarded in this scenario.23 Anonymous tests raises additional concerns because there may be no means to contact the individual if a false address and telephone number are provided. Furthermore, no legal document of the test result exists and the individual may need to seek a second test under his or her legal name to document proof of a gene-negative result. Finally, next of kin cannot access the information if the individual dies, nor can a valid record be shared with medical professionals. The possible disadvantages of anonymous testing should be discussed with those who request it.
The primary consideration in presymptomatic test counseling is to increase the opportunity for the individual to make an informed choice concerning the risks, benefits and alternatives to testing. Individuals may seek testing with either an inflated or deflated view of their genetic risk. Some believe treatment options are more successful than they have proven to be to date. Some individuals are not informed about options other than testing. Finally, some individuals enter the testing protocol with the view that they are obliged to be tested or that if everyone at risk for HD were tested and all those proven to be gene-positive opted not to have children, the disease could be eliminated in a single generation. Unfortunately, the relatively substantial mutation rate to HD21 demonstrates that although testing may reduce the prevalence of the disease it will never eliminate it altogether.
The consensus among HD testing programs is that counseling information is delivered in two sessions, with the blood sample drawn on the second visit. This practice permits the individual to assimilate information about the test, and to fully consider the implications of testing before the decision to test is made. Once blood is drawn, there appears to be an increased commitment to following through with a test that has been ordered. Thus by drawing blood at the second visit, the individual is given the opportunity to change his or her mind. In our program, about one-third of those seen do not return for a second visit.
The individual is strongly encouraged to bring a friend or, if married, the spouse, to the counseling sessions. This companion offers a second perspective for interpreting the information about genetic risk, intermediate repeats and other complex factors important to making an informed choice. The companion gives the individual someone to talk to about this important decision. Further, this individual may provide an additional emotional support resource after the test.
Circumstances of concern for presymptomatic HD testing.
Some test circumstances are recognized to be associated with risk for adverse reactions. Occasionally, individuals request testing with a substantial investment in finding that they are not gene carriers. For example, an at-risk individual who seeks testing after becoming engaged to be married but who has not informed the fiancé of his or her risk status may not fully acknowledge the potential adverse consequences of a gene-positive test result. In these instances, there is a possibility for a heightened negative reaction from persons who are influenced by the results of the test, but may feel that critical information was withheld or hidden from them. We encourage individuals who are directly influenced by the test outcome to participate in test counseling.
Persons who have only recently learned that they are at risk for inheriting HD are a second special consideration in testing. This may occur when a parent is newly diagnosed in the absence of a family history, when an ancestor died young of other causes, or when divorce has caused the presence of the disease to be hidden from the family. Some at-risk individuals who grow up with a gradually increasing awareness of their risk develop an acceptance of the presence of the HD threat and testing can be scheduled at a time when other pressures are minimal. Adults who learn of their HD risk unexpectedly may find the sudden introduction of the threat of a severely disabling and ultimately fatal illness unbearable. The prospect of the illness can be overwhelmingly frightening and the desire to return to “the way things were” may compel them to seek testing immediately, regardless of the consequences or of concurrent events in their lives. This may be magnified when they already have children and the worry for their well-being can be paramount in the desire to remove the risk immediately. It is advisable that persons who have recently discovering their HD risk proceed cautiously when contemplating an HD test. When the desire to make the threat “go away” is so compelling that the individual cannot imagine or plan for the possible gene-positive result, he or she may be entering the test unprepared for its outcome. Because there is no medical urgency for intervention, it is possible for individuals to take advantage of the time it takes to adjust to and assimilate information in evaluating the available testing options, including the option to postpone the test or to not be tested.
Contraindications for genetic testing.
For adult-onset diseases such as HD, genetic testing of children is rarely considered appropriate, and some have argued that appropriate informed consent is not possible for minors.24 Many intelligent and insightful individuals at risk for HD opt never to be tested after a careful consideration of its risks and benefits. It is widely accepted that minors be given the opportunity to grow up, learn about the associated risks and benefits for themselves and make their own choice for or against testing. The testing of minors takes that choice away from them. Furthermore, the decision for testing today is in the context of limited treatment options. If the child is found to be a gene carrier, the emotional burden on the parent may also produce an adverse impact on the psychological well-being of the child. If a treatment is found in the next 10 or 20 years, minors who may not develop the disease for 30 or 40 years might unnecessarily experience life-changing and irreversible trauma. Testing for minors is to be avoided.
In some instances, persons may be in litigation for divorce, child custody, or criminal complaints. Although a gene-negative test result may be viewed as providing an advantageous position for these circumstances, it is important that the individual also consider the possible negative consequences of a gene-positive test. It may be necessary to assist adults who are in litigation and do not wish to be tested to prevent such tests from being mandated. Adults should not be tested against their wishes or if they (or appropriate family members) cannot provide consent due to psychiatric, cognitive, or other impairment.25,26
Prenatal testing.
Prenatal testing is not frequently requested for persons at risk for HD.18 About one-half of 1% of all HD tests involve a prenatal test. One apparent reason for the low frequency of prenatal testing is that many at-risk individuals seek predictive testing before becoming pregnant. The majority of those who test gene-positive opt not to have children rather than undergo prenatal testing. Prenatal test counseling follows a similar course to that defined for presymptomatic testing, but there are additional concerns for this procedure.
Unfortunately, many of those who seek prenatal testing are already pregnant when they contact the testing program. Because these individuals will not have undergone presymptomatic testing they request simultaneous presymptomatic and prenatal testing. When both the parent and the fetus are found to be HD gene carriers, the emotional impact is very profound. When an individual learns that he or she is a gene carrier and shortly thereafter learns that their unborn child is as well, the despair can challenge the emotional stability of both members of the couple. Intense grieving may occur with the loss of the anticipated healthy baby but also the loss of one’s prospect for a healthy life. Unfortunately, the time constraint imposed for prenatal testing means that there is little time to prepare couples for the potential adversities that they may encounter. Consequently, prenatal testing may be the most challenging HD test situation.
A second concern is raised when persons are uncertain about their views on the termination of a pregnancy. When a couple chooses not to terminate the pregnancy after learning that the fetus is an HD gene carrier, there may be significant implications for the unborn child later in life. These concerns may include emotional burdens already alluded to but also possible health insurance or career discrimination that is difficult to anticipate. Couples who are uncertain about terminating the pregnancy may want to consider whether prenatal testing is a wise choice.
Preimplantation genetic diagnosis.
The development of the technology to perform Preimplantation Genetic Diagnosis (PDG) offers a new option for couples seeking to have children who are known to be gene-negative and avoids ethical issues associated with terminating a pregnancy. Most often PGD tests are performed on single cells biopsied at the eight-cell embryo (day 3 of development). The genetic analysis for monogenic disorders such as HD takes advantage of PCR to amplify the DNA and for detection of the repeat sizes for each chromosome. The main concern for PCR in single-cell amplification is for allele drop-out or for one of the two chromosomes to not amplify.27 Eggs are harvested, fertilized in vitro, tested, and those testing gene-negative are implanted. The main impediments to PGD are its expense, which can run $14,000 and the low efficiency of in vitro fertilization (IVF), with only 20% to 30% of couples achieving pregnancy per IVF cycle. Pickering et al.28 report their experience in the first 100 PDG cycles performed at the Guy’s and St. Thomas’ Center in London. The overall pregnancy rate was 24% per cycle started, 29% per egg collection, 38% per transfer, and 40% per couple treated. Thus the success rate, even among experienced programs, does not lead to the majority of couples achieving their goal. Nevertheless, this option is known within the lay community and should be considered for some couples.
Some programs offer PDG for couples who do not wish to undergo HD presymptomatic testing.29 In this circumstance, parental gene status is not revealed to the parent during the protocol.
Genetic test protocols for Huntington’s disease
The goals of counseling are: (1) to inform the individual of his or her options about testing or other alternatives, depending upon personal circumstances, (2) to ensure that the individual is aware of the risks and possible adverse consequences of his or her specific testing circumstances, and (3) to inform the individual of the limitations and level of accuracy of the procedure. Counseling does not try to exclude or discourage persons but tries to insure that the individual is making an informed choice. The protocol used in our New England HD testing program includes telephone intake, two counseling visits, a neurological examination, and in-person delivery of test results.
Information about the family history of the person is gathered to confirm the presence of HD in the family and also to assess the current risk status of the individual. Cases that warrant the inclusion of other family members in the testing process should be identified and options for how to include those people should be discussed. This includes any tests which will reveal risk information about another individual. Occasionally individuals seek testing when the genetic status of their parent is unknown. This may happen when the parent died before the appearance of symptoms, or because the parent has not yet reached the age of disease onset. A positive result in this case would indicate that the parent is a gene carrier and would increase the risk for all descendants of this individual. If the parent is living, he or she must be informed before testing that a test is being done, and an understanding of how the result will be delivered should be determined. In both situations, the manner by which the siblings of the person being tested will be alerted to this information should be addressed before the test is performed. If an identical twin is seeking testing, implications for the co-twin must be considered and the co-twin must be counseled and involved in the decision to proceed with testing.
Neurological examination.
Many programs include a neurological evaluation as part of the pretest evaluation. Many at-risk persons seek testing in the context of concerns for having exhibited symptoms of HD, and the neurological evaluation will answer the question of whether the individual has symptoms of the illness. Not uncommonly, persons who learn that they do not have symptoms decide to postpone the genetic test, because their primary worry for the onset of the disease has been addressed. For those persons who may be symptomatic and who may be diagnosed in the course of the genetic test evaluation, it is important to recognize that the test is no longer presymptomatic and to initiate treatment associated with the onset of the illness.
With appropriate consideration for the associated risks, HD testing can be implemented in a variety of circumstances. The procedures to minimize risk for adverse consequences of the test are recognized and can be readily implemented in testing programs. By anticipating discussion and preparation for the unique concerns of the individual seeking testing, one can enhance the likelihood of a successful adjustment to the test result.
Acknowledgments
This work is partially supported by United States Public Health Service Grant P50NS016367 (Huntington’s Disease Center Without Walls), and grants from the Massachusetts Huntington’s Disease Society of America and the Jerry McDonald Huntington’s Disease Research Fund.
REFERENCES
- 1.Myers RH, Marans K, MacDonald ME. Huntington’s disease. In: Genetic instabilities and hereditary neurological diseases (Warren ST, Wells RT, eds), pp 301–323. New York: Academic Press, 1998.
- 2.Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306: 234–238, 1983. [DOI] [PubMed] [Google Scholar]
- 3.The Huntington’s Disease Research Collaborative Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72: 971–983, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Duyao MP, Ambrose CM, Myers RH, Novelletto A, Persichetti F, Frontali M et al. Trinucleotide repeat length: instability and age of onset in Huntington’s disease. Nat Genet 4: 387–392, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Chong SS, Almqvist E, Telenius H, LaTray L, Nichol K, Bourdelat-Parks B et al. Contribution of DNA sequence and CAG size to mutation frequencies of intermediate alleles for Huntington disease: evidence from single sperm analyses. Hum Mol Genet 6: 301–309, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Maat-Kievit A, Losekoot M, Van Den Boer-Van Den Berg H, Van Ommen GJ, Niermeijer M, Breuning M et al. New problems in testing for Huntington’s disease: the issue of intermediate and reduced penetrance alleles. J Med Genet 38: E12, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merritt AD, Conneally PM, Rahman NF, Drew AL. Juvenile Huntington’s chorea. In: Progress in neurogenetics. (Barbeau A, Brunette TR, eds), pp 645–650. Amsterdam: Excerpta Medica Foundation, 1969.
- 8.Zühlke C, Olaf R, Bockel B, Lange H, Thies U. Mitotic stability and meiotic variability of the (CAG)n repeat in the Huntington disease gene. Hum Mol Genet 2: 2063–2067, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Ranen NG, Stine CO, Abbott MH, Sherr M, Codori AM, Franz ML et al. Anticipation and instability of IT-15 (CAG)n repeats in parent-offspring pairs with Huntington’s disease. Am J Hum Genet 57: 593–602, 1995. [PMC free article] [PubMed] [Google Scholar]
- 10.Djoussé L, Knowlton B, Hayden M, Almqvist EW, Brinkman R, Ross C et al. Interaction of normal and expanded CAG repeat sizes influences age at onset of Huntington disease. Am J Med Genet 119a: 279–282, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Rosenblatt A, Brinkman RR, Liang KY, Almqvist EW, Margolis RL, Huang CY et al. Familial influence on age of onset among siblings with Huntington disease. Am J Med Genet 105: 399–403, 2001. [PubMed] [Google Scholar]
- 12.Li J-L, Hayden M, Almqvist EW, Brinkman R, Durr A, Dode C et al. A genome scan for modifiers of age at onset in Huntington’s disease: the HD MAPS Study. Am J Hum Genet 73: 682–687, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet 361: 1642–1644, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res 36: 455–460, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Ross CA. Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron 35: 819–822, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Almqvist EW, Bloch M, Brinkman R, Craufurd D, Hayden MR. A worldwide assessment of the frequency of suicide, suicide attempts, or psychiatric hospitalization after predictive testing for Huntington disease. Am J Hum Genet 64: 1293–1304, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor CA, Myers RH. Long-term psychological impact of Huntington’s disease linkage testing. Am J Med Genet 70: 365–370, 1997. [PubMed] [Google Scholar]
- 18.Nance MA, Myers RH, and US Huntington Disease Genetic Testing Group. Trends in predictive and prenatal testing for Huntington disease, 1993–1999. Am J Hum Genet 65: A406, 1999. [Google Scholar]
- 19.Farrer LA. Suicide and attempted suicide in Huntington disease: implications for preclinical testing of persons at risk. Am J Med Genet 24: 305–311, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld M, Myers RH, Cupples LA, Berkman B, Sax DS, Clark E. Increased rate of suicide among patients with Huntington’s disease. J Neurol Neurosurg Psychiatry 47: 1283–1287, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almqvist EW, Elterman DS, MacLeod PM, Hayden MR. High incidence rate and absent family histories in one quarter of patients newly diagnosed with Huntington disease in British Columbia. Clin Genet 60: 198–205, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Hayden MR. Huntington’s chorea. New York: Springer, 1981.
- 23.Visintainer CL, Matthias-Hagen V, Nance MA. U.S. Huntington Disease Genetic Testing Group. Anonymous predictive testing for Huntington’s disease in the United States. Genet Testing 5: 213–218, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Tyler A, Morris M, Lazarou L, Meredith L, Myring J, Harper P. Presymptomatic testing for Huntington’s disease in Wales 1987–1990. Br J Psychiatry 161: 481–488, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Hersch SM, Jones R, Koroshetz WJ, Quaid K. The neurogenetics genie: testing for the Huntington’s disease mutation. Neurology 44: 1369–1373, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines for the molecular genetics predictive testing Huntington’s disease. Neurology 44: 1533–1536, 1994. [PubMed] [Google Scholar]
- 27.Bui TH, Harper JC. Preimplantation genetic diagnosis. Clin Obstet Gynecol 45: 640–648, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Pickering S, Polidoropoulos N, Caller J, Scriven P, Ogilvie CM, Braude P. Preimplantation Genetic Diagnosis Study Group. Strategies and outcomes of the first 100 cycles of preimplantation genetic diagnosis at the Guy’s and St. Thomas’ Center. Fertil Steril 79: 81–90, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Stern HJ, Harton GL, Sisson ME, Jones SL, Fallon LA, Thorsell LP et al. Non-disclosing preimplantation genetic diagnosis for Huntington disease. Prenat Diagn 22: 503–507, 2002. [DOI] [PubMed] [Google Scholar]