Abstract
Background:
The dendritic cell (DC)-based vaccine targeting the highly immunogenic tumor antigen, MUC1, has been promising for a cancer immunotherapy; however, predictive biomarkers for beneficial clinical responses of the vaccine remain to be determined.
Methods:
DCs loaded with MUC1-derived peptide were subcutaneously administered to patients with MUC1-positive non-small cell lung cancer (NSCLC) that was refractory to standard anticancer therapies, every 2 weeks. The effectiveness and tolerability of the vaccine were evaluated, and predictive biomarkers of clinical responses were explored.
Results:
Between August 2005 and May 2015, 40 patients received the vaccines. The median survival time (MST) after the initial vaccination was 7.4 months, and the 1-year survival rate was 25.0%. The MST for patients who received more than six vaccinations was 9.5 months, and the 1-year survival rate was 39.3%. In this cohort, patients who experienced immune-related adverse events, including skin reactions at the vaccination site and fever, had significantly longer survival times compared with patients without those immune-related adverse events (12.6 versus 6.7 months, p = 0.042). Longer survival times were also observed in patients whose peripheral white blood cells contained >20.0% lymphocytes (12.6 versus 4.5 months; p = 0.014). MUC1-specific cytotoxic immune responses were achieved in all of seven patients analyzed who received six vaccinations.
Conclusion:
The MUC1-targeted DC-based vaccine induced an antitumor immune response that promoted prolonged survival of patients with refractory NSCLC. The occurrence of immune-related adverse events and having a higher percentage of peripheral lymphocytes were predictive biomarkers of a beneficial clinical response during cancer immunotherapy for NSCLC.
Keywords: cancer immunotherapy, dendritic-cell-based vaccine, MUC1 tumor antigen, non-small cell lung cancer, predictive biomarker
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide [Torre et al. 2012], and patients who do not achieve a therapeutic benefit from first- or second-line chemotherapy have an extremely poor prognosis [Giroux Leprieur et al. 2013]. Clinical studies have previously reported on cases where a third line of chemotherapy or further is required; a monotherapy can achieve a median survival time (MST) of 6.8–10.4 months; however, grade 3–4 of hematological adverse events were observed in as many as 22% of patients [Matsubara et al. 2013; Miyoshi et al. 2014]. Resistance for targeted therapy, such as epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and anaplastic lymphoma kinase inhibitors, will inevitably develop through a variety of mechanisms [Cadranel et al. 2013; Steuer and Ramalingam, 2014]. Therefore, other therapeutic strategies are urgently required to improve the survival of patients with refractory NSCLC.
A previous paper reported that an MUC1-targeted dendritic cell (DC)-based vaccine was able to significantly prolong the survival of patients with MUC1-expressing breast and lung cancer [Kontani et al. 2003]. DCs, professional antigen-presenting cells [Steinman, 1991], can be generated from peripheral blood mononuclear cells (PBMCs) ex vivo, and loaded with tumor antigens for use in a DC-based vaccine. Tumor antigen-loaded DCs administered into patients will migrate into lymph nodes near the vaccination site and activate tumor antigen-specific cytotoxic T lymphocytes (CTLs) that can then attack cancer cells [Fong and Engleman, 2000].
For cancer vaccines, the selection of a target tumor antigen that enables an immune response to be generated against the tumor while avoiding unfavorable adverse events is crucial. The tumor antigen MUC1 has been reported to be abundantly expressed in many types of cancers, such as breast, lung and colon cancers [Lakshmanan et al. 2015; Yonezawa et al. 2011], and is strongly immunogenic [Quinlin et al. 2007; Wright et al. 2000; Kohlgraf et al. 2004; Kontani et al. 2001]. The tandem repeat domain in the core protein of MUC1 contains antigenic epitopes that are recognized by T cells in a major histocompatibility complex (MHC)-independent manner [Barnd et al. 1989; Wright et al. 2008]. These findings suggest that targeting MUC1 on cancer cells would be beneficial for cancer immunotherapy because it is widely applicable without consideration of patient MHC haplotypes.
In this study, we explored predictive biomarkers for clinical responses in the cancer vaccine, reporting that immune-related adverse events and having a higher percentage of peripheral lymphocytes were predictive biomarkers of beneficial clinical responses during the treatment of NSCLC with cancer immunotherapy.
Materials and methods
Patients
Patients were eligible for the vaccine when they met all of the following criteria: NSCLC with high expression of MUC1 (by more than 60% of cancer cells) as confirmed by immunohistochemistry; NSCLC was refractory to evidence-based standard anticancer treatments and an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2. Patients who required treatment with systemic administration of steroids or systemic chemotherapy were excluded because these drugs could suppress antitumor immunity.
Ex vivo generation of dendritic cells for the vaccine
After obtaining written informed consent from patients, PBMCs were harvested by apheresis, purified by gradient centrifugation, and preserved in liquid nitrogen until use. To culture DCs, nonadherent cells were removed from the PBMCs, and the adherent cells on plastic culture plates were cultured in the presence of granulocyte macrophage colony-stimulating factor (Primmune, Kobe, Japan) at 200 ng/ml and interleukin (IL)-4 (Primmune) at 100 ng/ml for 6 days. The DCs were freshly prepared from the preserved PBMCs each time for a vaccination. To mature the DCs and help them upregulate the expressions of costimulatory molecules, OK-432 (Chugai Phar-maceutical, Tokyo, Japan) was added into the culture medium at 0.1 KE/ml and 1 day later, the cells were harvested, and pulsed with MUC1 peptide (NH2-TRPAPGSTAP PAHGVTSAPDTR PAPGSTAP-COOH) [Kontani et al. 2003] overnight at room temperature. The following day, the cells were washed, and suspended in 2.0 ml of saline. The frequency of viable cells after cell processing was usually over 90%, and expression of CD80, CD86 and MHC class II on the ex vivo-generated DCs was confirmed by flow cytometry (data not shown).
Vaccine administration
Approximately 1 × 107 DCs loaded with MUC1 peptide were subcutaneously administered in the axilla or supraclavicular fossa of patients biweekly. The vaccination was discontinued if the disease rapidly progressed, if systemic treatment with steroids was required or upon patient request. The vaccine in our hospital was approved by the Ministry of Health, Labor and Welfare, Japan, as an advanced medical treatment for advanced or recurrent NSCLC.
Immunological monitoring assays
To evaluate the immunological responses in patients, immunological monitoring assays were performed on PBMC samples before and after six vaccinations. To examine a T cell recall response for MUC1-derived peptide, an interferon-gamma (IFN-γ) ELISPOT assay was performed. PBMCs were stimulated with MUC1 peptide in the presence of IL-2 for 2 weeks. The PBMCs were then cocultured overnight with TISI cells, HLA-A24+ lymphoblastoid cell line, as target cells loaded with MUC1 peptide were placed in 96-well ELISPOT plates (Merck Millipore, Darmstadt, Germany). The spots of IFN-γ were stained using human IFN-γ ELISPOT Ready-SET-Go (eBiosciences, San Diego, CA, USA). Positive spots were counted using a dissecting microscope. To detect regulatory T cells (Treg cells), PBMCs were stained with anti-CD4, anti-CD25, and anti-Foxp3 antibodies (eBioscience). The population of Treg cells in the PBMCs was analyzed on a FACScan flow cytometer. (BD Biosciences, San Jose, CA, USA), and data were presented as dot plots produced using CellQuest software (BD Biosciences).
Statistical analysis
The retrospective study and exploratory immunological research (UMIN clinical trial ID: 000021866) was approved by the Institutional Review Board of Shiga University of Medical Science. Assuming that a 1-year survival rate of 40% in eligible patients indicates potential usefulness, whereas a 1-year survival rate of 20% is the lower limit of interest, with an α error of 0.05 and a power of 0.7, a minimum of 25 assessable patients was necessary. In consideration of the scale of the study, a power of 0.7 was chosen in this study. Allowing for a patient ineligibility rate of 20%, we planned to enroll at least 31 patients in the study. Survival time was measured from the initial vaccination to the date of death. MST and 1-year survival rates were calculated using the Kaplan–Meier method. The survival between groups was compared using log-rank tests, and p values < 0.05 were considered statistically significant. All analyses were performed using SPSS statistics 22.0 software (IBM, Armonk, NY, USA).
Results
Patients’ characteristics
Between August 2005 and May 2015, 40 patients (24 males and 16 females) with a median age of 61, ranging 38–82 years, received the DC-based vaccine (Table 1). The patient ECOG performance scores were 0 for 26 patients, 1 for nine patients, and 2 for five patients. The NSCLCs were classified as adenocarcinomas in 33 patients, squamous cell carcinomas in five patients, large cell carcinoma in one patient, and pleomorphic carcinoma in one patient. MUC1 expression in 60% or more of the cancer cells was confirmed by immunohistochemistry. A total of 27 patients had advanced NSCLC with stage IIIb or IV at diagnosis, and 15 patients (53.6%) had distant metastases prior to vaccination. Of the patients, 13 had recurrent NSCLC after radical surgery, which included lobectomy or pneumonectomy.
Table 1.
Patients’ characteristics.
| Age (median) | 38–82 (61) |
| Gender, n (%) | |
| Male | 24 (60.0) |
| Female | 16 (40.0) |
| Pathology, n (%) | |
| Adenocarcinoma | 33 (82.5) |
| Squamous cell carcinoma | 5 (12.5) |
| Large cell carcinoma | 1 (2.5) |
| Pleomorphic carcinoma | 1 (2.5) |
| Performance status, n (%) | |
| 0 | 26 (65.0) |
| 1 | 9 (22.5) |
| 2 | 5 (12.5) |
| Cancer status, n (%) | |
| Advanced | 28 (70.0) |
| Recurrent | 12 (40.0) |
| Previous therapy, n | |
| Advanced (n = 28) | |
| 1st/2nd/3rd/4th lines | 2/9/8/9 |
| EGFR-TKI | 15 |
| Radiation | 5 |
| Recurrent (n = 12) | |
| EGFR-TKI | 5 |
| Radiation | 5 |
| Number of vaccinations (median) | 1–42 (6) |
EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
Prior to vaccination, all patients with advanced and recurrent NSCLC had received the evidence-based standard anticancer therapies, and their NSCLC was proved to persist. Among patients with advanced NSCLC, nine, eight and nine patients had received second lines, third lines and fourth or further lines of anticancer therapies, respectively. Because the remaining two patients with advanced NSCLC were over 80 years old, one had received gefitinib monotherapy, and the other had received radiation therapy alone. Furthermore, prior to vaccination, one or more types of EGFR-TKI had been administered to 15 patients with advanced NSCLC and to five patients with recurrent NSCLC.
Following vaccination, 19 patients received further anticancer therapies, whereas 21 patients did not. Among those that continued anticancer therapy, six received EGFR-TKIs, however, they had either NSCLC that had been refractory to those agents prior to vaccination or NSCLC with wild-type EGFR. Nine patients received chemotherapy, consisting of a single anticancer agent, and five patients received palliative radiation therapy.
Overall survival and adverse events
The MST from the initial vaccination was 7.4 months [95% confidence interval (CI), 4.2–10.6], and the 1-year survival rate was 25.0% (95% CI, 11.6–38.4) (Figure 1A). Concerning adverse events, elevation of body temperature above the patient’s own average temperature was observed in 16 patients (40.0%). Among them, five patients experienced a grade 1 fever. The event usually happened approximately 6 hours after vaccination and lasted for 2 days. Six patients (14.5%) experienced a skin reaction at the vaccination site, such as induration, redness, mild pain or swelling. These events usually occurred 1 day after vaccination and lasted for 7 days. Acute lung injury was observed in one patient after two vaccinations; however, we could not confirm the relationship between the event and the DC-based vaccine because the patient took alternative and complementary medicine in addition to the vaccine. Hematological adverse events were not observed in any patients.
Figure 1.
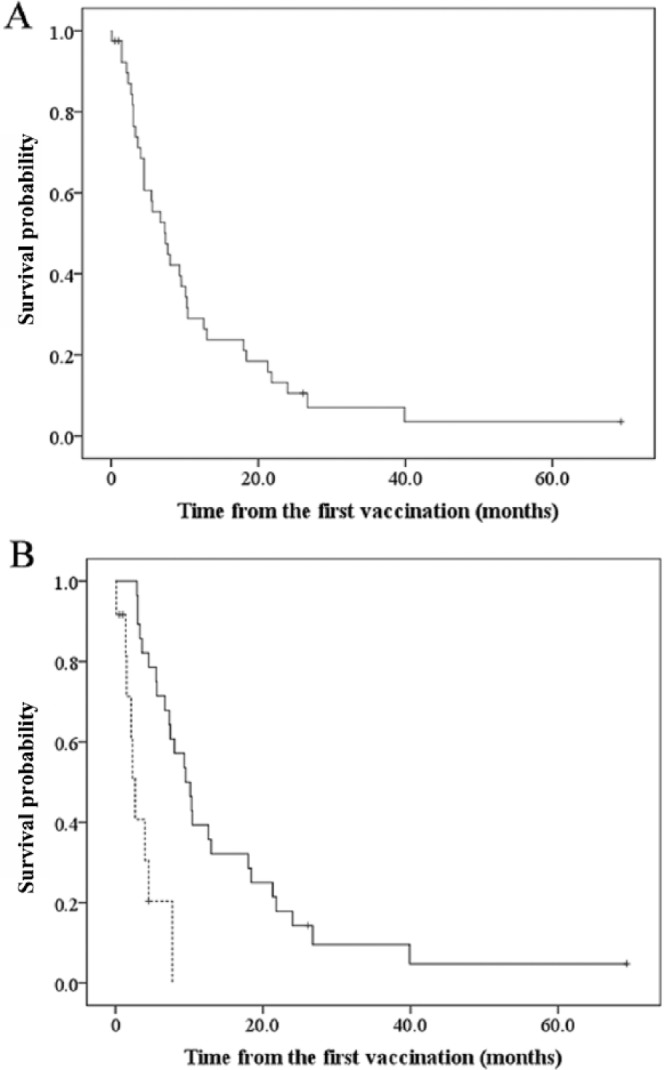
(A) The survival curve of patients with refractory non-small cell lung cancer who received MUC1-targeted dendritic-cell-based vaccines. (B) Patients’ survival and the number of vaccinations. Solid line: the group with the higher number of vaccinations; dotted line: the group with fewer vaccinations.
The number of vaccinations and the clinical response
Following vaccination, it is likely to take between 3–6 months for activation of an antitumor immune response [Hoos et al. 2010]. Based on this feature of cancer vaccines, we focused on the association between the number of vaccinations that patients had received and the clinical response induced (Table 2). One group of patients received five or fewer vaccinations (fewer vaccinations group, n = 12). This group consisted of seven male and five female patients with a median age of 62, ranging 43–81 years. The median number of vaccinations they received was two, and their ECOG performance status was 0 in five patients, 1 in four patients and 2 in three patients. The MST from the initial vaccination was 2.7 months (95% CI, 1.8–3.6). Another group of patients received six or more vaccinations (higher number of vaccinations group, n = 28). This group consisted of 17 male and 11 female patients with a median age of 61, ranging 38–82 years. The median number of vaccinations they received was 10 (range, 6–42), and their ECOG performance status was 0 in 21 patients, 1 in five patients and 2 in two patients. The MST from the initial vaccination in the group with a higher number of vaccinations was 9.5 months (95% CI, 6.5–12.5), showing a significantly longer survival than the fewer vaccinations group (p < 0.0001) (Figure 1B). In the group with a higher number of vaccinations, the 1-year survival rate was 39.3% (95% CI, 21.2–57.4). We evaluated the response of the patients’ tumors to treatment after six vaccinations according to the response evaluation criteria in solid tumors version 1.1 (RECIST) [Eisenhauer et al. 2009]. Stable disease was detected in 12 patients and progressive disease in 17, which gives a disease control rate of 42.9% (95% CI, 24.5–61.2).
Table 2.
Patients’ characteristics by number of vaccinations.
| Five or fewer vaccinations (less vaccinations group) n = 12 | Six or more vaccinations (group with higher number of vaccinations) n = 28 | |
|---|---|---|
| Age (median) | 43–81 (62) | 38–82 (61) |
| Gender, n | ||
| Male | 7 | 17 |
| Female | 5 | 11 |
| Pathology, n | ||
| Adenocarcinoma | 10 | 23 |
| Squamous cell carcinoma | 1 | 4 |
| Large cell carcinoma | 1 | 0 |
| Pleomorphic carcinoma | 0 | 1 |
| Performance status, n | ||
| 0 | 5 | 21 |
| 1 | 4 | 5 |
| 2 | 3 | 2 |
| Cancer status, n | ||
| Advanced | 10 | 18 |
| Recurrent | 2 | 10 |
| Number of vaccinations (median) | 1–5 (2) | 6–42 (10) |
Predictive biomarkers for a clinical response
Predictive biomarkers of a clinical response to the vaccines were investigated in patients in the group with a higher number of vaccinations because patients in the fewer vaccinations group may not have receive enough vaccinations to activate antitumor immune responses. We first focused on the occurrence of immune-related adverse events, including fevers and skin reactions at the vaccination site in the group with a higher number of vaccinations. The MST following the initial vaccination of patients who had experienced one of those adverse events (n = 14) was 12.6 months (95% CI, 0.0–27.3), demonstrating a significantly longer survival than patients who did not experience an immune-related adverse event (n = 14; 6.7 months; 95% CI, 3.4–10.0; p = 0.042) (Figure 2A). In addition, the percentage of lymphocytes, including B and T lymphocytes and other cell types of lymphocytes in peripheral white blood cells prior to the initial vaccination, was also analyzed. The MST from the initial vaccination in patients whose percentage of peripheral lymphocytes was above 20.0% prior to vaccination (n = 19) was 12.6 months (95% CI, 8.5–16.7), demonstrating a significantly longer survival than patients whose percentage of peripheral lymphocytes was below 20.0% (n = 8; 4.5 months; 95% CI, 0.0–11.0; p = 0.014) (Figure 2B). For 18 of 28 patients in the group with a higher number of vaccinations, we were able to evaluate the percentage of peripheral lymphocytes following six vaccinations. As a result, more than 20% of increase in the percentage of peripheral lymphocytes was observed in four patients (22.2%), and the significant change was not observed in 10 patients (55.6%) following six vaccinations. These data suggest that the occurrence of immune-related adverse events and a higher percentage of peripheral lymphocytes prior to vaccination are useful biomarkers for predicting the clinical response induced by the vaccine.
Figure 2.
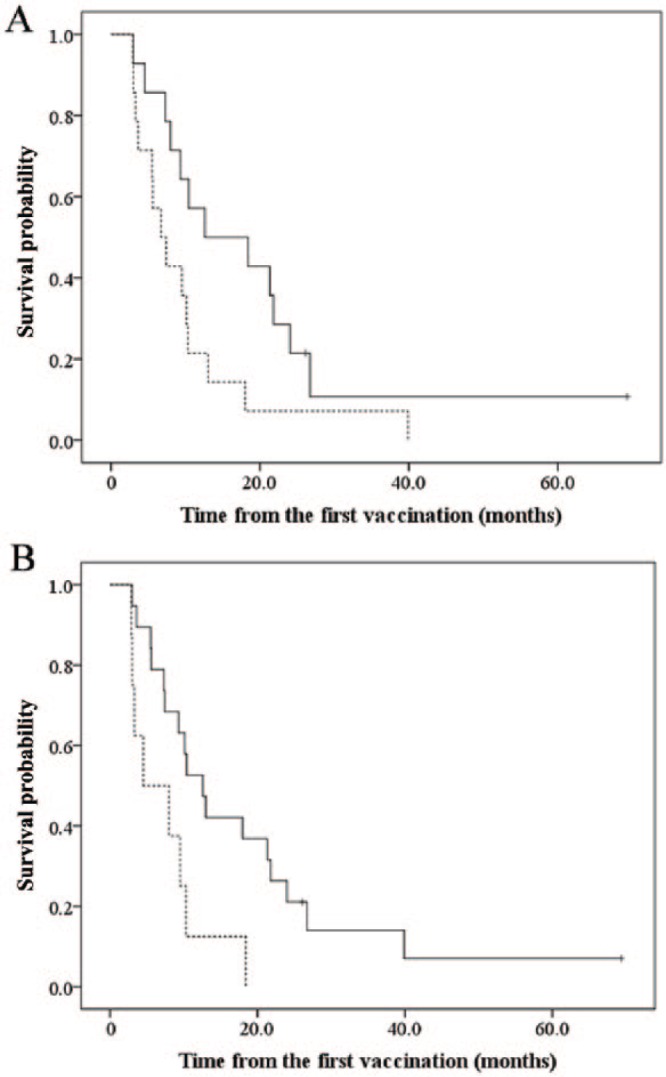
(A) Patients’ survival and occurrence of immune-related adverse events. Solid line: patients with immune-related adverse events; dotted line: patients without immune-related adverse events. (B) Patients’ survival and percentage of peripheral white blood cells. Solid line: patients with peripheral lymphocytes > 20.0%; dotted line: patients with peripheral lymphocytes < 20.0%.
Immunological monitoring
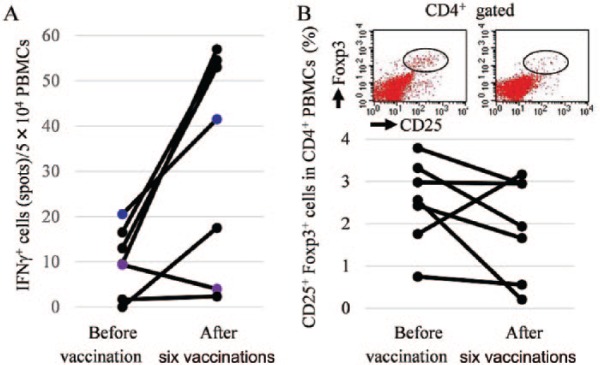
To evaluate whether antitumor immune responses were elicited in patients following the MUC1-targeted DC-based vaccines, immunological monitoring assays were performed [Nagorsen et al. 2004]. In an ELISPOT assay, we investigated the number of lymphocytes that secreted IFN-γ in response to a stimulation of MUC1 peptide. The data demonstrated that MUC1-specific T cell recall response was augmented by the vaccine in all patients whose PBMCs were acquired both before and after six vaccinations (n = 7) (Figure 3A), suggesting that MUC1-specific immune responses were successfully induced by the vaccine. Next, to determine if immune suppressor cells inhibited the vaccine-induced immune response, we examined the frequency of Treg cells, an immune suppressor cell type, in the PBMCs of patients in the group with a higher number of vaccinations. Using flow cytometry, Treg cells were determined as CD4+CD25+Foxp3+ cells, and the frequency of those cells was decreased after six vaccinations in all patients who were analyzed (n = 7) (Figure 3B). From these data, we concluded that the MUC1-specific DC-based vaccine induced an MUC1-specific CTL response and inhibited immune-suppressive mechanisms in the group with a higher number of vaccinations, which led to prolonged survival.
Figure 3.
(A) Immunological monitoring by ELISPOT assay. (B) The frequency of Treg cells in the peripheral blood mononuclear cells.
PBMCs, peripheral blood mononuclear cells; IFNγ+, interferon-gamma positive; CD25, CD4 and Foxp3, various Treg cells.
Discussion
In this study, we explored predictive biomarkers for clinical responses in the MUC1-targeted DC-based vaccine for patients with NSCLC that was refractory to standard anticancer therapies. The vaccine improved the survival of patients, and the occurrence of immune-related adverse events and having a higher percentage of peripheral lymphocytes prior to vaccination were predictive markers for a clinical response in cancer immunotherapy for NSCLC.
In terms of the time to onset of antitumor effects, cancer vaccines, such as peptide-based and DC-based vaccines, behave quite distinctively from other kinds of cancer immunotherapies and chemotherapies [Bilusic and Gulley, 2012]. This is because anticancer vaccines need to activate an antitumor immune response prior to the cancer cells being attacked, which causes a delay in antitumor effects [Schlom et al. 2007]. This suggests that the clinical response to cancer vaccines should be evaluated differentially to other cytotoxic anticancer therapies. In the present study, when we evaluated the antitumor response in the group with a higher number of vaccinations according to conventional RECIST grading, neither complete responses nor partial responses were observed, yielding a disease control rate of 42.9 % after six vaccinations. Despite no patients achieving a complete response or partial response, the MST in the group with a higher number of vaccinations was prolonged to 9.5 months. This result indicates that changes in tumor burden caused by the cancer vaccine may not reflect the clinical response induced. Given that cancer vaccines typically require 3–6 months to activate the antitumor immune system prior to the onset of a clinical response [Hoos et al. 2010], we also recommend that the clinical response achieved by cancer vaccines should be evaluated by patient survival time rather than by tumor shrinkage.
Evaluating tumor burden would be of importance in cancer vaccines for patients with severely advanced NSCLC. Because cancer vaccines exert a delayed antitumor effect, patients with an aggressively growing tumor are unlikely to be good candidates for cancer vaccines, and would gain little benefit from these therapies. Therefore, even within the initial 3–6 months following vaccination, when the cancer vaccines have not yet exerted an antitumor effect, the tumor burden should be evaluated to determine the ability of the vaccine to delay tumor growth over time.
In the present study, the participants included patients with either advanced or recurrent NSCLC that was refractory to standard anticancer therapies. In some cases, the patients had previously received EFGR-TKI when the NSCLC had the EGFR mutation, and in cases where patients had wild-type EGFR, secondary or further lines of chemotherapy were administered. This demonstrates that few anticancer therapies remain an option for these patients. For these patients, the MUC1-targeted DC-based vaccine exerted a reasonably good clinical response that provided patients with an MST of 7.4 months and a 1-year survival rate of 29.3%. For patients who had received more than six vaccinations, the MST was 9.5 months and 1-year survival rate was 39.3%. Previously, Yoshiyama and colleagues reported that a peptide-based vaccine for the treatment of patients with NSCLC that was refractory to standard anticancer therapies provided an MST of 304 days and 1-year survival rate of 42% [Yoshiyama et al. 2012]. In addition, clinical responses of nivolumab in patients who had previously received heavy anticancer therapies showed an MST of 9.9 months and 1-year survival rate of 42% [Gettinger et al. 2015]. Although we cannot directly compare the clinical responses between studies, the MUC1-targeted DC-based vaccine exerted an antitumor effect that induced similar outcomes to other types of immunotherapy.
Targeting MUC1 is a promising strategy to treat cancer because the antigen is highly immunogenic. In pancreatic cancer, the MUC1-targeted DC-based vaccine for an adjuvant therapy after surgery exerted a significant antitumor effect, demonstrating that 4 of 12 patients have survived without evidence of recurrence for around 5 years after the surgery [Lepisto et al. 2008]. In NSCLC, tecemotide (L-BLP25) is a liposome-conjugated vaccine containing MUC1-derived peptide [Sahgha and Butts, 2007] that when administered, is phagocytosed and digested by DCs. The MUC1-presenting DCs then activate T cells to elicit an MUC1-specific antitumor immune response. In a phase III clinical study treating unresectable stage III NSCLC with tecemotide (START study), tecemotide as a first-line anticancer therapy provided patients with an MST of 25.8 months, whereas the MST of patients in the placebo group was 22.4 months, demonstrating no therapeutic benefit of tecemotide (p = 0.111) [Butts et al. 2014; Mitchell et al. 2015]. The lack of response provided by tecemotide might be because it was administered to patients independently of MUC1 expression in their NSCLC cells. We consider that patient selection, namely selection of patients with high expression of target antigen on cancer cells, is critical for tumor-antigen-targeted cancer immunotherapies, and that MUC1-targeted vaccines are not effective against NSCLC with limited or no expression of MUC1. MUC1 is reported as highly expressed on adenocarcinoma cells [Yonezawa et al. 2011]; however, our MUC1 immunohistochemistry data demonstrate that the expression of MUC1 on more than 60% of adenocarcinoma cells occurs in only 40.3% of patients (data not shown). Accordingly, to avoid an ineffective anticancer therapy, we examined MUC1 expression on NSCLC cells by immunohistochemistry, and only patients with MUC1 expressed by more than 60% of cancer cells were eligible.
Biomarkers to predict the clinical response of patients receiving cancer immunotherapy are important to determine the benefit of an immunotherapy; however, specific biomarkers are yet to be established. In the present study, we explored biomarkers to predict the clinical response induced by the cancer immunotherapy. Patients who experienced immune-related adverse events, such as a skin reaction at the vaccination site or fever, showed significantly longer survival time than those without such events. Since fevers can be prevented in some patients who routinely take nonsteroidal anti-inflammatory drugs, this event is unlikely to be applicable to all patients as a predictive biomarker. However, a skin reaction at the vaccination site, such as rubor, swelling, or induration, may be an appropriate predictive biomarker that reflects the induction of an immune response by the vaccines. Previous papers reported a significant association between favorable clinical responses and a positive reaction for the skin delayed-type hypersensitivity (DTH) test [Aarntzen et al. 2009]. Patients who had a positive response for the skin DTH test using a tumor-antigen-derived peptide applied in the vaccine had significantly longer survival times than those who were negative for the skin DTH test [Lesterhuis et al. 2006; Koido et al. 2014]. This indicates that the DTH test could serve as a useful predictive biomarker for a DC-based vaccine.
The data in the present study suggest that the percentage of lymphocytes within peripheral white blood cells is another possible biomarker for predicting an immunotherapy-associated clinical response. Patients whose peripheral white blood cells consisted of more than 20.0% lymphocytes showed a significantly longer survival than those with a lower percentage of peripheral lymphocytes This suggests that patients with a higher percentage of peripheral lymphocytes are good candidates for anticancer vaccination. Advanced-stage cancer patients frequently have a low percentage of lymphocytes and a high percentage of neutrophils in the peripheral white blood cells. A high percentage of neutrophils in the peripheral white blood cells prior to chemotherapy was reported as associated with a poor prognosis of patients with advanced NSCLC [Teramukai et al. 2009]. This is probably because of exhaustion of the antitumor immune response, resulting in activation of tumor-associated neutrophils and suppression of antitumor-effector lymphocytes. Thus, a lower percentage of peripheral lymphocytes in cancer patients would reflect both exhaustion and suppression of antitumor immune responses. For those patients, passive cancer immunotherapies, such as cancer vaccines, are unlikely to restore the deteriorated antitumor immune response.
Still, some limitations should be pointed out in this study. First, this was a retrospective study; therefore, we need to plan a prospective study to evaluate the effectiveness of MUC1-targeted DC-based vaccine for refractory NSCLC. Furthermore, in a prospective study, we need to confirm the usefulness of predictive biomarkers that we explored in the present study. Second, in cancer immunotherapy, an immunological monitoring is paramount in the evaluation of immune responses elicited by the therapy [Nagorsen et al. 2004]. However, in the present study, the number of patients in whom we evaluated the immune response was limited. In a prospective study, a CTL response needs to be directly evaluated for all patients receiving the MUC1-targeted DC-based vaccine.
In conclusion, the MUC1-targeted DC-based vaccine has the potential to induce an antitumor immune response that can prolong the survival of patients with NSCLC refractory to standard anticancer therapies. The occurrence of immune-related adverse events and a higher percentage of peripheral lymphocytes were predictive biomarkers of a clinical response after administering an immunotherapy to NSCLC patients.
Acknowledgments
The authors thank Dr Takuya Fujita and Ms Miho Yamamoto from Shiga University of Medical Science for their technical assistance in the preparation of dendritic-cell-based vaccines.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Koji Teramoto, Department of Medical Oncology and Surgery, Shiga University of Medical Science, Seta-Tsukinowa, Otsu, Shiga 520-2192, Japan.
Yoshitomo Ozaki, Department of Surgery, Shiga University of Medical Science, Otsu, Shiga, Japan; Department of Thoracic Surgery, National Hospital Organization Higashi-Ohmi General Medical Center, Higashi-Ohmi, Shiga, Japan.
Jun Hanaoka, Department of Surgery, Shiga University of Medical Science, Otsu, Shiga, Japan.
Satoru Sawai, Departments of Surgery, Shiga University of Medical Science, Otsu, Shiga, Japan; Department of Thoracic Surgery, National Hospital Organization Kyoto Medical Center, Kyoto, Japan.
Noriaki Tezuka, Department of Surgery, Shiga University of Medical Science, Otsu, Shiga, Japan.
Shozo Fujino, Department of Surgery, Shiga University of Medical Science, Otsu, Shiga, Japan; Department of Surgery, University Hospital Mizonokuchi, Teikyo University School of Medicine, Kawasaki, Kanagawa, Japan.
Yataro Daigo, Department of Medical Oncology, Shiga University of Medical Science, Otsu, Shiga, Japan.
Keiichi Kontani, Department of Respiratory, Breast and Endocrine Surgery, Kagawa University Faculty of Medicine, Kita-gun, Kagawa, Japan.
References
- Aarntzen E., Figdor C., Adema G., Punt C., de Vries I. (2009) Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother 57: 1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnd D., Lan M., Metzgar R., Finn O. (1989) Specific, major histocompatibility complex—unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A 86: 7159–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilusic M., Gulley J. (2012) Endpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccines. Cancer Immunol Immunother 61: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C., Socinski M., Mitchell P., Thatcher N., Have L., Krzakowski M., et al. (2014) Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for non-small-cell lung cancer (START): a randomized, double-blind, phase III trial. Lancet Oncol 15: 59–68. [DOI] [PubMed] [Google Scholar]
- Cadranel J., Ruppert A., Beau-Faller M., Wislez M. (2013) Therapeutic strategy for advanced EGFR mutant non-small-cell lung carcinoma. Crit Rev Oncol Hematol 88: 477–493. [DOI] [PubMed] [Google Scholar]
- Eisenhauer E., Therasse P., Bogaerts J., Schwartz L., Sargent D., Ford R., et al. (2009) New response criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247. [DOI] [PubMed] [Google Scholar]
- Fong L., Engleman E. (2000) Dendritic cells in cancer immunotherapy. Annu Rev Immunol 18: 245–273. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux Leprieur E., Antoine M., Vieira T., Duruisseaux M., Poulot V., Rabbe N., et al. (2013) Clinical and molecular features in patients with advanced non-small-cell lung carcinoma refractory to first-line platinum-based chemotherapy. Lung Cancer 79: 167–172. [DOI] [PubMed] [Google Scholar]
- Hoos A., Eggermont A., Janetzki S., Hodi F., Ibrahim R., Anderson A., et al. (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102; 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlgraf K., Gawron A., Higashi M., van Lith M., Shen X., Caffrey T., et al. (2004) Tumor-specific immunity in MUC1. Tg mice induced by immunization with peptide vaccines from the cytoplasmic tail of CD227 (MUC1). Cancer Immunol Immunother 53: 1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koido S., Homma S., Okamoto M., Takakura K., Mori M., Yoshizaki S., et al. (2014) Treatment with chemotherapy and dendritic cells pulsed with multiple Wilm’s tumor 1 (WT-1)-specific HMC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res 20: 4228–4239. [DOI] [PubMed] [Google Scholar]
- Kontani K., Taguchi O., Narita T., Izawa M., Hiraiwa N., Zenita K., et al. (2001) Modulation of MUC1 mucin as an escape mechanism of breast cancer cells from autologous cytotoxic T-lymphocytes. Br J Cancer 84: 1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontani K., Taguchi O., Ozaki Y., Hanaoka J., Sawai S., Inoue S., et al. (2003) Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int J Mol Med 12: 493–502. [PubMed] [Google Scholar]
- Lakshmanan I., Ponnusmy M., Macha M., Haridas D., Majhi P., Kaur S., et al. (2015) Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J Thorac Oncol 10: 19–27. [DOI] [PubMed] [Google Scholar]
- Lepisto A., Moser A., Zeh H., Lee K., Bartlett D., McKolanis J., et al. (2008) A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 6: 955–964. [PMC free article] [PubMed] [Google Scholar]
- Lesterhuis W., de Vries I., Schuurhuis D., Boullart A., Jacobs J., de Boer A., et al. (2006) Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol 17: 974–980. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Maemondo M., Inoue A., Ishimoto O., Watanabe K., Sakakibara T., et al. (2013) Phase II study of irinotecan as a third- or fourth-line treatment for advanced non-small cell lung cancer: NJLCG0703. Respir Investig 51: 28–34. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Thatcher N., Socinski M., Wasilewska-Tesluk E., Horwood K., Szczesna A., et al. (2015) Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analysis. Ann Oncol 26: 1134–1142. [DOI] [PubMed] [Google Scholar]
- Miyoshi S., Ito R., Katayama H., Kadowaki T., Yano S., Watanabe S., et al. (2014) Phase II trial of S-1 as third-line or further chemotherapy in patients with advanced non-small-cell lung cancer. Int J Clin Oncol 19: 1005–1010. [DOI] [PubMed] [Google Scholar]
- Nagorsen D., Scheibenbogen C., Thiel E., Keilholz U. (2004) Immunological monitoring of cancer vaccine therapy. Expert Opin Biol Ther 4: 1677–1684. [DOI] [PubMed] [Google Scholar]
- Quinlin I., Burnside J., Dombrowski K., Phillips C., Dolby N., Wright S. (2007) Context of MUC1 epitope: immunogenicity. Oncol Rep 17: 453–456. [DOI] [PubMed] [Google Scholar]
- Sahgha R., Butts C. (2007) L-BLP25: a peptide vaccine strategy in non-small cell lung cancer. Clin Cancer Res 13: 4652–4654. [DOI] [PubMed] [Google Scholar]
- Schlom J., Arlen P., Gulley J. (2007) Cancer vaccines: moving beyond current paradigms. Clin Cancer Res 13: 3776–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. (1991) The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9: 271–296. [DOI] [PubMed] [Google Scholar]
- Steuer C., Ramalingam S. (2014) ALK-positive non-small cell lung cancer: mechanisms of resistance and emerging treatment options. Cancer 120: 2392–2402. [DOI] [PubMed] [Google Scholar]
- Teramukai S., Kitano T., Kishiba Y., Kawahara M., Kubota K., Komuta K., et al. (2009) Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organization LC00–03. Eur J Cancer 45: 1950–1958. [DOI] [PubMed] [Google Scholar]
- Torre L., Bray F., Siegel R., Ferlay J., Lortet-Tieulent J., Jemal A. (2012) Global cancer statistics. CA Cancer J Clin 2015. 65: 87–108. [DOI] [PubMed] [Google Scholar]
- Wright S., Kilinski L., Talib S., Lowe K., Burnside J., Wu J., et al. (2000) Cytotoxic T lymphocytes from humans with adenocarcinomas stimulated by native MUC1 mucin and a mucin peptide mutated at a glycosylation site. J Immunother 23: 2–10. [DOI] [PubMed] [Google Scholar]
- Wright S., Rewers-Felkins K., Quinlin I., Fogler W., Phillips C., Townsend M., et al. (2008) MHC-unrestricted lysis of MUC1-expressing cells by human peripheral blood mononuclear cells. Immunol Invest 37: 215–225. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Higashi M., Yamada N., Yokoyama S., Kitamoto S., Kitajima S., et al. (2011) Mucin in human neoplasm: clinical pathology, gene expression and diagnostic application. Pathol Int 61: 697–716. [DOI] [PubMed] [Google Scholar]
- Yoshiyama K., Terazaki Y., Matsueda S., Shichijo S., Noguchi M., Yamada A., et al. (2012) Personalized peptide vaccination in patients with refractory non-small cell lung cancer. Int J Oncol 40: 1492–1500. [DOI] [PubMed] [Google Scholar]