Abstract
Available evidence suggests that second-generation atypical antipsychotics are broadly similar to first-generation agents in terms of their efficacy, but may have a more favourable tolerability profile, primarily by being less likely to cause extrapyramidal symptoms. However, atypical antipsychotics are variably associated with disturbances in the cardiometabolic arena, including increased body weight and the development of metabolic syndrome, which may reflect differences in their receptor binding profiles. Effective management of schizophrenia must ensure that the physical health of patients is addressed together with their mental health. This should therefore involve consideration of the specific tolerability profiles of available agents and individualization of treatment to minimize the likelihood of adverse metabolic sequelae, thereby improving long-term adherence and optimizing overall treatment outcomes. Alongside this, modifiable risk factors (such as exercise, diet, obesity/body weight and smoking status) must be addressed, in order to optimize patients’ overall health and quality of life (QoL). In addition to antipsychotic-induced side effects, the clinical management of early nonresponders and psychopharmacological approaches for patients with treatment-resistant schizophrenia remain important unmet needs. Evidence suggests that antipsychotic response starts early in the course of treatment and that early nonresponse accurately predicts nonresponse over the longer term. Early nonresponse therefore represents an important modifiable risk factor for poor efficacy and effectiveness outcomes, since switching or augmenting antipsychotic treatment in patients showing early nonresponse has been shown to improve the likelihood of subsequent treatment outcomes. Recent evidence has also demonstrated that patients showing early nonresponse to treatment with lurasidone at 2 weeks may benefit from an increase in dose at this timepoint without compromising tolerability/safety. However, further research is required to determine whether these findings are generalizable to other antipsychotic agents.
Keywords: antipsychotic, cardiometabolic side effects, efficacy, nonresponse, schizophrenia, tolerability, treatment resistance
Introduction
The number of treatment options for schizophrenia has increased substantially over recent years with the development of novel atypical antipsychotics targeting different receptor subtypes. In parallel, we are improving our understanding of the potentially detrimental effects of antipsychotic treatment on patients’ physical health, and there has been an increasing focus on the importance of choosing the correct treatment for individual patients to ensure that both their mental and physical health needs are addressed [Kahn et al. 2015].
In the recent National Audit of Schizophrenia in the UK [Royal College of Psychiatrists, 2014], a number of concerns were raised about the current standard of care for patients with schizophrenia. In particular, it was found that expansion of early intervention programmes and improved continuity of care are warranted to optimize patient outcomes. Alongside the medical care received by people with schizophrenia, lifestyle factors play a significant role in the course of patients’ disease. Indeed, numerous reports in the literature have linked cannabis use with poorer outcomes in patients with psychosis compared with nonusers [Schoeler et al. 2016], and daily tobacco use is associated with an increased risk of psychosis and an earlier onset of psychotic illness [Gurillo et al. 2015]. Further examination of the causal relationships between extrinsic lifestyle factors and outcomes in schizophrenia is therefore needed.
We are now in a new era of treatment for schizophrenia, where we have a greater choice than ever of therapies for patients. Evidence suggests that although second-generation atypical antipsychotics have a similar efficacy to first-generation typical antipsychotic agents, they are more favourable in terms of tolerability, especially with regards to extrapyramidal symptoms. However, second-generation antipsychotics are not without side effects, and their impact on the endocrine, cardiovascular and metabolic systems requires close scrutiny and careful management, particularly since these agents vary considerably in terms of their cardiometabolic impact. In addition to the tolerability issues associated with some agents, poor response or resistance to atypical antipsychotic treatment may be seen in up to 30% of patients with schizophrenia, representing an important therapeutic challenge [Harvey and Rosenthal, 2016].
A holistic approach to the management of schizophrenia is key to successful patient outcomes. This should include: (1) ensuring appropriate patient lifestyle choices; (2) prescribing carefully selected treatments (based not only on efficacy but also on the side effect profile of the agent); (3) treatment interventions given to the right patient at the right time; and (4) treatment modulation and monitoring in patients showing treatment resistance or nonresponse to therapy. This article is based on a symposium, convened during the 29th Annual European College of Neuropsychopharma-cology Congress (September 2016, Vienna, Austria), which sought to discuss the relevant efficacy and safety data available for atypical antipsychotics in schizophrenia, with particular focus on cardiometabolic side effects, and how to optimize the treatment strategy in nonresponders and treatment-resistant patients.
Efficacy and tolerability of atypical antipsychotics: research trials and clinical reality
Christoph Correll
Hofstra Northwell School of Medicine, The Zucker Hillside Hospital, New York, USA
Antipsychotics are effective across a range of psychiatric disorders, including schizophrenia and mood disorders. Efficacy is defined by response to treatment in the acute phase, disease remission and eventual recovery over the longer term (at least 2 years) [Carbon and Correll, 2014].
Treatment of both acute and chronic schizophrenia should not only aim to improve symptoms but should also positively impact on patient functioning to ensure that patients remain stable over the longer term. However, even those patients who demonstrate a reduction in symptoms may remain unwell, and it has been observed that approximately half of chronically affected patients do not exhibit stable symptomatic remission for at least 6 months [Carbon and Correll, 2014]. Long-term follow up is conducted in patients who are receiving psychiatric care, and who are therefore often experiencing severe disease. It is these patients who have the greatest medical need and yet a meta-analysis conducted by Jääskeläinen and colleagues demonstrated only a 13.5% median recovery rate when pooling data from over 50 years of research in such patients, with first-episode patients also having low recovery rates, despite initially higher response and remission rates than chronic patients [Jääskeläinen et al. 2013]. Recovery is difficult to achieve, with approximately 80% of patients relapsing within 36 months once antipsychotic treatment is stopped [Carbon and Correll, 2014]. Relapse is the factor most associated with poor recovery, and patients who relapse represent a key therapeutic challenge, as they are at risk of symptom exacerbation and increased treatment resistance [Carbon and Correll, 2014].
Efficacy of antipsychotic agents in first-episode schizophrenia
Patients experiencing their first episode of schizophrenia are generally the most responsive to treatment and often require relatively low doses of antipsychotic agents [Zhang et al. 2013]. However, they are particularly sensitive to side effects and commonly relapse, often due to stopping treatment. Acute efficacy with regards to total and positive symptoms is similar between the older first-generation antipsychotics and the newer second-generation atypical antipsychotics [Zhang et al. 2013]. There are small advantages for atypical antipsychotics relating to depression and cognition; however, relapse and treatment discontinuation are generally higher with first-generation antipsychotics [Zhang et al. 2013].
When a person feels that their disease is no longer affecting them but that the side effects of treatment (e.g. sedation, weight gain) are impacting their life, they may choose to stop therapy altogether. Thus, balancing efficacy and safety is key to preventing relapse and ensuring patient adherence to treatment. Multidisciplinary interventions, focusing on patient engagement, treatment continuation, relapse prevention, physical health and functional recovery are paramount.
Efficacy of antipsychotic agents in multi-episode/chronic schizophrenia
Direct comparison of atypical antipsychotics is complex, since there have been few head-to-head trials. The network meta-analysis performed by Leucht and colleagues sought to create a hierarchy for 15 antipsychotic drugs based on evidence for their acute treatment efficacy and major side effects, collected from 212 randomized controlled trials [Leucht et al. 2013]. When considering the efficacy of antipsychotic agents as a factor of their ability to control symptoms, all agents were superior to placebo. The difference in antipsychotics given in the first-line setting was small, with the exception of clozapine. However, caution must be exercised when interpreting the results for clozapine as these were driven primarily by older and small studies versus placebo or first-generation antipsychotics. Furthermore, in a follow-up meta-analysis in refractory patients, no difference between clozapine and other atypical antipsychotics was observed [Samara et al. 2016], although this lack of superiority of clozapine may have been due to less severely ill patients entering randomized trials or inappropriately low clozapine doses [Kane and Correll, 2016].
While this network meta-analysis of acute anti-psychotic efficacy [Leucht et al. 2013] provides useful evidence-based information based on direct and mostly indirect comparisons of agents, it also involved the collation of data from studies spanning >20 years and there are a number of methodological issues that should be considered when evaluating its results. In particular, trial weighting was based on sample size rather than methodological or scientific rigour. For instance, differences in comparator drugs and doses, patient characteristics, trial design and the size of placebo response that has increased over time could not be fully accounted for [Correll and De Hert, 2013]. In addition, the effect size difference for efficacy was very small (median 0.11), where 0.2 is considered small, 0.5 a medium effect size and 0.8 a large effect size. Such issues preclude a valid comparison between agents, which can only be achieved in rigorously conducted head-to-head studies.
A notable finding from recent trials is the increasing placebo response observed. When considering the early studies of risperidone and olanzapine in the 1990s, the mean change from baseline in total Positive and Negative Syndrome Scale (PANSS) scores was minimal in the placebo groups. However, in studies of the newer agents, such as lurasidone, a much greater placebo effect has been observed (up to 16 PANSS points in some studies). Therefore, these agents had to demonstrate a large effect in order to achieve clinical superiority over placebo [Alphs et al. 2012]. Indeed, some of the placebo responses seen in recent trials are equivalent to the effect sizes seen in earlier trials with risperidone or olanzapine.
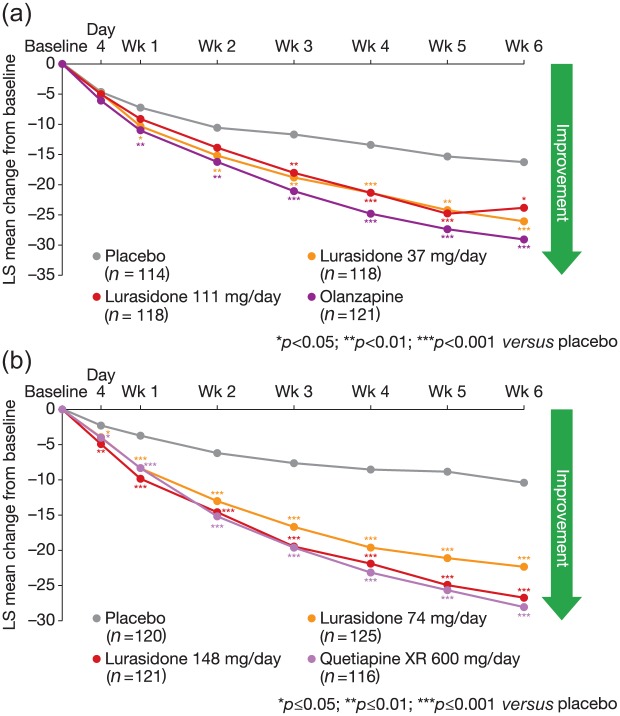
In well-controlled and rigorously conducted head-to-head studies, the small differences in effect size seen in the meta-analysis by Leucht and colleagues are not replicated. While lurasidone may have appeared to have a lower effect compared with some of the other atypical antipsychotics in the meta-analysis rankings, direct comparison of PANSS scores for lurasidone with olanzapine and quetiapine demonstrated no significant difference between the active comparators, although all agents resulted in significantly better scores than placebo (Figure 1) [Loebel et al. 2013; Meltzer et al. 2011].
Figure 1.
MMRM PANSS scores for lurasidone versus placebo or olanzapine in the PEARL2 trial (a), MMRM PANSS scores for lurasidone versus placebo or quetiapine in the PEARL3 trial (b) [Loebel et al. 2013; Meltzer et al. 2011]. Reprinted with permission from Elsevier and from the American Journal of Psychiatry (American Psychiatric Association), respectively.
LS, least square means; MMRM, mixed models repeated measures; PANSS, Positive and Negative Syndrome Scale.
Once an initial response has been achieved in schizophrenia, maintenance therapy becomes the most important aspect of ensuring that patients remain stable [Kishimoto et al. 2013; Leucht et al. 2012].
Differentiating atypical antipsychotics based on their side effect profile
While the difference in treatment effect may be small, the range and severity of side effects associated with atypical antipsychotics is broad and there is considerable heterogeneity across individual antipsychotics. Clinically relevant adverse effects include extrapyramidal symptoms, weight gain, metabolic effects, cardiovascular effects, sexual dysfunction, hyperprolactinaemia, antimuscarinic effects, blood dyscrasia and sedation, which not only increase physical morbidity and mortality risk, but can also impair quality of life (QoL), cause stigma and decrease medication adherence [Haddad and Sharma, 2007; Leucht et al. 2013]
Surveys of patients and relatives have demonstrated that the metabolic consequences of medication have a particularly detrimental impact in terms of morbidity, QoL and satisfaction with care [McIntyre, 2009]. Understandably, schizophrenia relapse is associated with poorer QoL; however, in patients with stable disease the metabolic side effects associated with treatment can profoundly impact their QoL and functioning [Briggs et al. 2008]. Thus, clinicians need to take into account the side effects in their own right, but also in order to enhance efficacy and functionality through improved treatment adherence, which is often impaired by adverse effects.
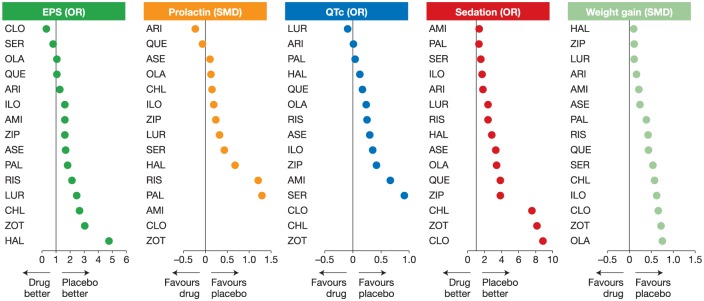
Assessment of the comparative tolerability of atypical antipsychotics is limited by a relatively limited number of direct head-to-head data from clinical trials and by difficulties in cross-study comparison, due to differences in study methodology and patient populations, as well as inconsistency in the reporting of adverse effects [Haddad and Sharma, 2007; Leucht et al. 2013]. Nevertheless, meta-analysis of data from clinical trials together with results from selected individual head-to-head trials have demonstrated clear differences between antipsychotic agents in their relative risk of particular side effects, including weight gain, hyperprolactinaemia, QTc prolongation and sedation [Leucht et al. 2013], with lurasidone and aripiprazole demonstrating the most favourable side effect profiles when counting the ranking of being closest to placebo (Figure 2). Disturbances in the metabolic axis, including increased weight and evidence of metabolic syndrome, are seen across the majority of antipsychotic agents. Patients starting treatment gain weight regardless of the antipsychotic prescribed [Correll et al. 2009]. However, some agents have a relatively low risk (such as aripiprazole, ziprasidone and lurasidone), while others carry a much more substantial risk (e.g. clozapine and olanzapine) [De Hert et al. 2011].
Figure 2.
Differing tolerability profiles of antipsychotics in a network meta-analysis. Adapted from Leucht et al. 2013 [Leucht et al. 2013]. Reprinted with permission from Elsevier.
AMI, amisulpride; ARI, aripiprazole; ASE, asenapine; CHL, chlorpromazine; CLO, clozapine; EPS, extrapyramidal symptoms; HAL, haloperidol; ILO, iloperidone; LUR, lurasidone; OLA, olanzapine; OR, odds ratio; PAL, paliperidone; QUE, quetiapine; RIS, risperidone; SER, sertindole; SMD, standardized mean difference; ZIP, ziprasidone; ZOT, zotepine.
When considering the management of medical risk associated with antipsychotics, agent selection should initially be based on efficacy in combination with the safety profile, individualizing treatment by selecting an agent with side effects that the patient is able or willing to tolerate. Second, clinicians should ensure that patients adopt a healthy lifestyle and reduce risk factors that can contribute to increased psychiatric morbidity. Third, clinicians need to be prepared to monitor and address the side effects that patients experience, either by switching to an alternative antipsychotic with a different safety profile, or by employing additional approaches to help manage the side effects, such as adding behavioural interventions or off-label adjunctive medications, such as metformin [Zheng et al. 2015] or topiramate [Zheng et al. 2016], to counter bothersome or potentially dangerous side effects.
Summary
Across multiple studies and taking into account study-specific characteristics, differences in the efficacy of antipsychotics appear to be relatively small and difficult to predict, whereas adverse effect differences are far larger and much easier to predict [Fornaro et al. 2016; Haddad and Sharma, 2007; Leucht et al. 2013; Taylor et al. 2014; Yildiz et al. 2015]. Such findings have important implications for clinical practice, including the need to foresee, monitor and manage side effects and metabolic parameters, individualize treatment choices, and appropriately educate and involve patients in making optimized treatment decisions.
Metabolic and cardiovascular risks associated with antipsychotics: review of the evidence
Gavin P. Reynolds
Biomolecular Sciences Research Centre, Sheffield Hallam University, Sheffield, UK
People with schizophrenia have an increased prevalence of many risk factors for the development of cardiovascular disease, including obesity, smoking, diabetes, hypertension, dyslipidaemia and metabolic syndrome [De Hert et al. 2009]. Consequently, life expectancy in patients with schizophrenia may be reduced by approximately 20 years, compared with the general population. Importantly, antipsychotic treatment has a complex association with cardiovascular morbidity and mortality in schizophrenia. Untreated psychotic illness appears to have cardiotoxic effects, which may be reduced by low or moderate doses of antipsychotics, while higher doses of antipsychotics may increase cardiovascular mortality, perhaps by the effects on metabolic morbidity [Tiihonen et al. 2009; Torniainen et al. 2015].
Other risk factors associated with an increased risk of metabolic and cardiovascular disease known to play a role in patients with schizophrenia include poor self-care (e.g. poor diet with increased fat and refined sugar, but less fibre, fruit and vegetables), sedentary lifestyle with less exercise and high stress as a response to psychotic symptoms, particularly in poorly responding or inadequately treated patients.
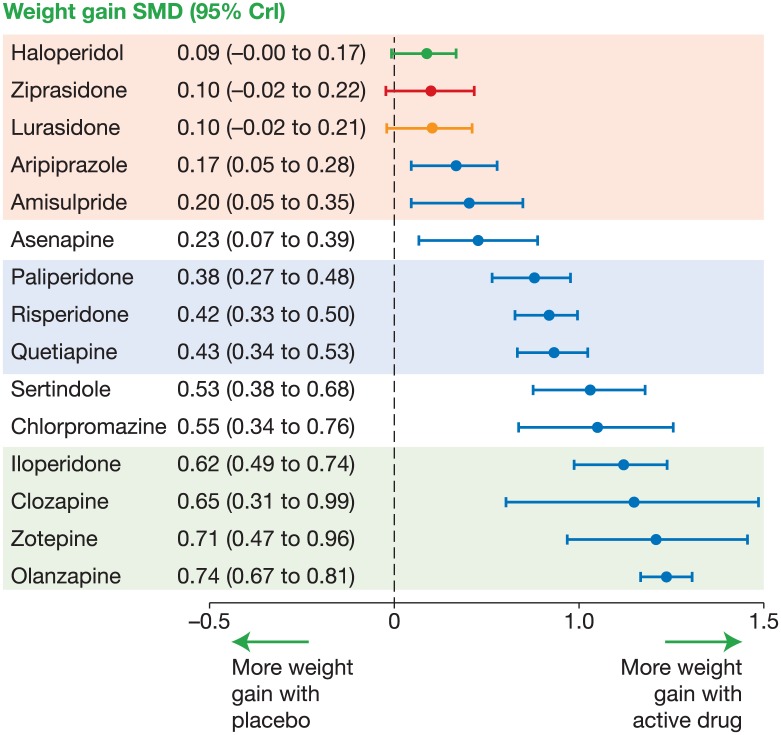
In young untreated patients with schizophrenia, studies have demonstrated no consistent trends towards increases in metabolic abnormalities, fat deposits or body mass index (BMI) compared with controls [Arranz et al. 2004; Fleischhacker et al. 2013; Zhang et al. 2004; Sengupta et al. 2008], despite some evidence for impaired glucose tolerance [Perry et al. 2016; Venkatasubramanian et al. 2007]. However, there is clear evidence demonstrating that antipsychotic treatment is associated with metabolic side effects. In the Leucht and colleagues 2013 meta-analysis, three distinct groups of agents emerged based on their effect on weight gain in patients with schizophrenia (Figure 3). Olanzapine had the greatest and most consistent effect on weight gain, with paliperidone, risperidone and quetiapine occupying a middle position with a moderate effect. Haloperidol, ziprasidone and lurasidone did not differ significantly from placebo in their effects on body weight [Leucht et al. 2013]. The patients included in many of the randomized controlled trials examined in this meta-analysis were inevitably pretreated, so may have already experienced metabolic disturbance and weight gain due to previous therapy; as such, these data may underestimate the true effects of antipsychotics on body weight.
Figure 3.
Meta-analysis of antipsychotic drug-induced weight gain [Leucht et al. 2013]. Reprinted with permission from Elsevier.
Crl, credible interval; SMD, standard mean difference.
In 41 drug-naïve, Chinese patients, treated for their first episode of schizophrenia, an increase in BMI of 7.7% was documented as early as 10 weeks after treatment initiation (risperidone or chlorpromazine) [Zhang et al. 2004]. In 87 Spanish patients, BMI increased by 10.1% after 3 months and by 17.1% after 9 months (risperidone or olanzapine) [Templeman et al. 2005]. In the recent EUFEST study in 498 patients (162 drug-naïve), the least squares (LS) mean change in body weight from baseline to the 52-week endpoint (last observation carried forward) was significantly greater for all agents compared with ziprasidone (+2.24 kg [standard error, 1.1], p < 0.05), with olanzapine demonstrating the largest amount of weight gained (+10.06 kg) [Fleischhacker et al. 2013]. In addition to weight gain, the consequences of antipsychotic treatment in drug-naïve patients included substantial deposition of both subcutaneous and intra-abdominal fat, elevated glucose and lipid markers, and elevated leptin secretion, all of which may have potential cardiometabolic effects [Zhang et al. 2004].
Leptin is a hormone released by adipose tissue into the bloodstream and sensed by the hypothalamus, its primary function being to control food intake. In experimental models, animals deficient in leptin and leptin receptors continually eat, eventually becoming obese. In patients who are taking antipsychotics, leptin levels rise as they gain weight; however, this does not suppress their food intake as it should, suggesting that antipsychotic agents may interfere with hypothalamic control of food intake [Reynolds and Kirk, 2010]. There are a number of receptor mechanisms that are implicated in the weight gain associated with antipsychotics, including serotonin 5-HT2C, histamine H1, alpha-adrenergic, dopamine D2 and muscarinic M3 receptors. Indeed, olanzapine-induced weight gain can be modelled by a combination of 5-HT2C (but not H1) antagonism and D2 antagonism in the rat [Kirk et al. 2009]. The action of antipsychotics at receptors in the hypothalamus (mediating hormonal control of food intake) and limbic system (controlling appetite and reward) warrant further examination.
Some antipsychotic agents may offer protection from weight gain. For example, addition of aripiprazole or ziprasidone to olanzapine in rats results in a significant decrease in food intake compared with olanzapine alone [Kirk et al. 2004; Snigdha et al. 2008], and other animal studies have demonstrated that lurasidone treatment can suppress the short-term weight gain associated with olanzapine [Reynolds et al. 2016]. Such models of feeding behaviour may, however, be subject to methodological issues and limitations (e.g. sex and species specificity) [Benarroch et al. 2016]. Nevertheless, in a meta-analysis of trials examining adjunctive treatment with aripiprazole, there was an observed amelioration in the weight gain associated with clozapine or olanzapine, with a significant decrease in glycated haemoglobin, total cholesterol and low-density lipoprotein cholesterol [Mizuno et al. 2014]. It may therefore be hypothesized that the relatively limited weight gain associated with some antipsychotics, including aripiprazole, ziprasidone and lurasidone, could be related to a protective mechanism, rather than simply the lack of a hyperphagic effect, which may, in part, be due to 5-HT1A receptor partial agonism [Reynolds and Kirk, 2010].
While the protective effects of certain antipsychotics against weight gain have been demonstrated, there are a number of considerations when using adjunctive therapy. First, it is important to consider the effect of combining treatment on a patient’s psychiatric symptoms, to ensure there is no exacerbation of existing symptoms or development of new ones. Studies of adjunctive aripiprazole have not revealed any evidence of unfavourable effects, with reported improvements in the Scale for the Assessment of Positive Symptoms and the Clinical Global Impression Scale [Fleischhacker et al. 2010; Muscatello et al. 2011]. Second, since the currently available evidence is from patients with weight gain associated with olanzapine and clozapine, further research is required to determine whether the protective effect of aripiprazole (or indeed ziprasidone or lurasidone) on olanzapine-induced weight gain is generalizable to other antipsychotic agents associated with weight gain. Finally, one must not overlook individual variation within the patient population, as some people may not benefit from this protective effect.
A further aspect of the metabolic and cardiovascular effects of treating schizophrenia is the emergence of diabetes. Increased risk of the development of diabetes is likely to be a consequence of weight gain, which increases insulin resistance. However, there may be other contributing factors, including the acute effect that some antipsychotic drugs, most notably clozapine and olanzapine [Newcomer, 2005; Rummel-Kluge et al. 2010; Vidarsdottir et al. 2010; Hahn et al. 2013; Chintoh et al. 2009; Houseknecht et al. 2007], can exhibit in disrupting glucose regulation. This can occur independently of adiposity or weight gain [Newcomer et al. 2002; Teff et al. 2013] and appears to be a direct induction of insulin resistance [Henderson et al. 2005], indicating the importance of monitoring plasma glucose even in the absence of changes in body weight.
| British Association for Psychopharmacology (BAP) guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment |
| In patients with schizophrenia who experience weight gain while taking an antipsychotic agent, there are a number of strategies that can be employed, which are supported by the recent BAP guidelines [Cooper et al. 2016]: |
| • Start initial therapy with an antipsychotic with a lower risk of associated weight gain (e.g. aripiprazole, ziprasidone, lurasidone, amisulpride) |
| • In cases where patients have gained weight on an antipsychotic agent, consider switching to a lower risk antipsychotic, as outlined above |
| • Consider adjunctive treatment with metformin or aripiprazole (if treated with olanzapine or clozapine) to address weight gain |
| • Ensure that lifestyle changes are implemented, including a healthy diet and exercise. |
Summary
There is little consistent evidence of metabolic or cardiovascular illness in people with first-episode psychosis before antipsychotic treatment; however, poorly treated schizophrenia may increase cardiovascular risk factors and mortality. In patients with well-managed and stable disease, weight gain is a common side effect with several atypical antipsychotics and can lead to further metabolic abnormalities. The exact mechanism for antipsychotic-induced weight gain is yet to be elucidated, but antagonism at 5-HT2C receptors and/or other receptors that interfere with the hormonal control of food intake has been implicated.
There are a number of factors that may influence the development of metabolic risk, including the choice of antipsychotic agent, with some antipsychotics able to reduce the impact of weight gain induced by other drugs. It is also vital that modifiable factors, such as exercise, diet, adjunctive pharmacotherapy and smoking, are addressed when assessing any metabolic or cardiovascular disturbance in patients with schizophrenia. Evidence-based guidelines have been developed for the management of metabolic and cardiovascular risk in people with schizophrenia, emphasizing the importance of physical health in the management of psychosis.
Optimizing care in schizophrenia: the challenge of treating early nonresponders and treatment-resistant patients
David Taylor
The Maudsley Hospital, London, UK
In addition to managing the side effects associated with the treatment of schizophrenia, the clinical management of early nonresponders and psychopharmacological approaches for patients with treatment-resistant schizophrenia remain key unmet needs. Studies indicate that response to antipsychotic therapy begins within the first weeks of treatment and escalates over time, with the greatest effect being observed within the first 2 weeks [Agid et al. 2003]. There are a number of clinical predictors of non-response to antipsychotic treatment, which can be categorized into modifiable and nonmodifiable factors (Table 1) [Carbon and Correll, 2014]. Early nonresponse (defined as <20% improvement on PANSS total score at 2 weeks) accurately predicts nonresponse over the longer term [Kinon et al. 2010].
Table 1.
Clinical predictors of antipsychotic nonresponse [Carbon and Correll, 2014].
| Fixed risk factors | Modifiable risk factors |
|---|---|
| Male sex | Longer duration of untreated psychosis |
| Younger age of illness onset | Nonadherence to treatment |
| Poor premorbid adjustment | Greater number of relapses |
| Longer illness duration | Comorbidities (e.g. addiction) |
| Greater illness severity | Early non-response at week 2 |
Patients who exhibit an early response to treatment experience better symptom improvement, have improved functioning and are more likely to adhere to therapy compared with early non-responders [Ascher-Svanum et al. 2008; Kinon et al. 2008; Liu-Seifert et al. 2005]. Indeed, patients who do not experience even minimal improvement after 2 weeks’ treatment are unlikely to respond at a later stage and may consequently benefit from a treatment change [Kinon et al. 2010; Samara et al. 2015]. In a study by Samara and colleagues, an early nonresponse [<20% reduction in PANSS or Brief Psychiatric Rating Scale (BPRS)] at 2 weeks had a specificity of 86% in predicting nonresponse over the longer term [Samara et al. 2015].
Examination of the trajectory of response to antipsychotic treatment over an 8-week period (irrespective of antipsychotic choice) revealed distinct subgroups of patients based on a 50% reduction in BPRS [Levine and Leucht, 2010]. A small proportion (8.2%) of patients are poor responders who experience little or no response; moderate response is observed in the majority of people (76.4%); and rapid responders (who have the greatest magnitude of response) represent only 15.4% of the patient population.
Switching or augmenting antipsychotic treatment may be beneficial in early nonresponders. In a study by Hatta and colleagues, 60 patients were treated with risperidone or olanzapine and assessed for response at 2 weeks. Early responders remained on the same treatment, and early nonresponders were either switched to the alternative drug, or a combination of both drugs [Hatta et al. 2014]. Irrespective of initial treatment, early responders were most likely to remain on treatment compared with early nonresponders (in any treatment arm). In early nonresponders, a ⩾40% improvement in PANSS total score was seen in only 8% of patients switching from risperidone to olanzapine, compared with 29% in the risperidone plus olanzapine combination arm. In early non-responders who received olanzapine as first-line therapy, a ⩾40% improvement in PANSS total score was seen in 25% of patients who switched to risperidone, compared with 50% of patients who switched to the combination therapy [Hatta et al. 2014]. In a larger study of 628 patients, early nonresponders to risperidone at 2 weeks were either kept on risperidone therapy or switched to olanzapine [Kinon et al. 2010]. Again, early responders fared better on the majority of clinical outcomes compared with early nonresponders, including those who switched to olanzapine. Switching early nonresponders to olanzapine at week 2 resulted in a small (though statistically significant) reduction in PANSS total score, compared with non-responders who remained on risperidone (p = 0.02). However, in a subanalysis of nonresponders who were still moderately-to-severely ill after 2 weeks, those who switched to olanzapine experienced a more marked improvement, compared with those who remained on risperidone (p < 0.05) [Kinon et al. 2010].
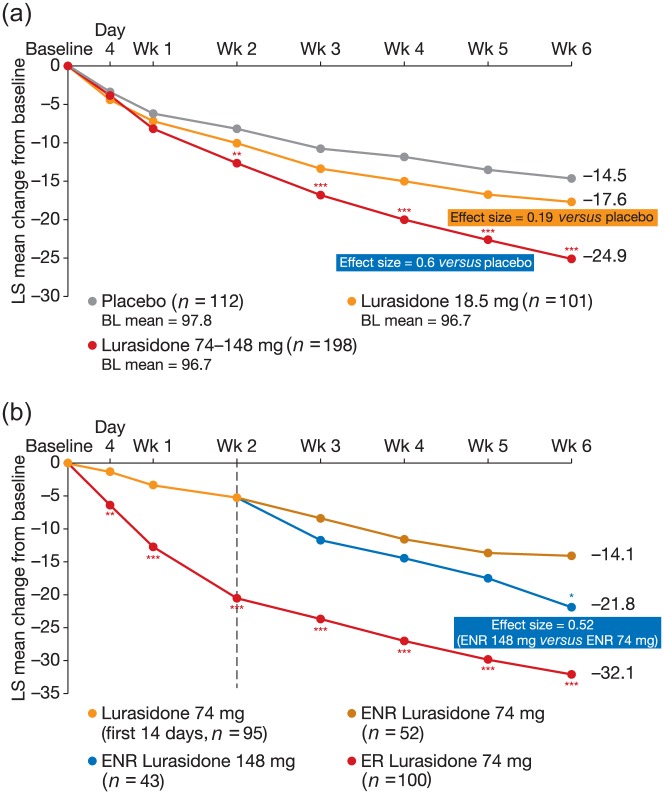
Another potential strategy to improve the outcome of psychopharmacotherapy in early nonresponders is antipsychotic dose adjustment. In a placebo-controlled study of lurasidone, patients were randomized to receive 18.5 mg lurasidone, 74 mg lurasidone or placebo. The lower dose of lurasidone was included to evaluate the efficacy of this dose, and this was the primary objective of the study [Loebel et al. 2016]. Early responders to 74 mg lurasidone remained on treatment (74 mg/day lurasidone), while early nonresponders were re-randomized to either remain on the same dose, or undergo dose escalation of lurasidone (148 mg/day). This secondary aim of the study was to assess whether doubling the known therapeutic dose in early nonresponders would result in a clinically meaningful response over 6 weeks of therapy. A sizeable placebo effect was observed, with a change in PANSS total score from baseline of 14.5 points. Low-dose lurasidone resulted in a 17.6-point change (Figure 4a). For patients who received lurasidone 74 mg or 148 mg, there was a significant improvement in the PANSS total score over placebo (24.9 points, Figure 4a). Early responders achieved a 32.1-point change in PANSS total score (Figure 4b); the early non-responders who remained on lurasidone 74 mg achieved a 14.1-point improvement, while those nonresponders who switched to 148 mg achieved a mean change in PANSS total score of 21.8 points (effect size of 0.52, Figure 4b), which was statistically significant compared with the change observed in early nonresponders who remained on lurasidone 74 mg [Loebel et al. 2016]. Importantly, doubling the dose of lurasidone did not have a significant impact on safety or tolerability. This study demonstrated that early nonresponders (at week 2) may benefit from an escalated dose of lurasidone up to 148 mg/day. It also demonstrated that low-dose lurasidone (18.5 mg/day) is not effective for the treatment of patients with schizophrenia, which is consistent with the recommended starting dose of 37 mg. This is the first rigorous demonstration of the clinical value of a dose increase in early antipsychotic non-responders, and may provide an additional avenue for treatment modulation (other than switching or combination strategies) in these patients. While these data are encouraging, more research is required, as it is still unclear whether a similar effect will be seen with other antipsychotic agents.
Figure 4.
(a) LS mean change in PANSS score from baseline in patients receiving placebo, low-dose lurasidone, or lurasidone 74 mg or 148 mg (combined data); (b) LS mean change in PANSS score in ER and ENR who remained on 74 mg lurasidone or switched to 148 mg lurasidone [Loebel et al. 2016]. Adapted with permission from Physicians Postgraduate Press, Inc.
BL, baseline; ENR, early nonresponders; ER, early responders; LS, least square means; PANSS, Positive and Negative Syndrome Scale.
Summary
Since insufficient efficacy data exist to differentiate between nonclozapine antipsychotics in treatment-resistant schizophrenia, rigorous, pragmatic, randomized, controlled trials are needed to address this uncertainty [Kane and Correll, 2016; Samara et al. 2016]. Psychopharmacological approaches to treatment resistance include identifying patients showing early nonresponse to anti-psychotic treatment and implementing a treatment change, either by switching to, or augmenting with, a new antipsychotic [Hatta et al. 2014; Kinon et al. 2010]. Recent data also indicate that patients showing early nonresponse to lurasidone treatment (at 2 weeks) may benefit from an increased dose at this timepoint [Loebel et al. 2016]. A variety of clinical strategies are being utilized to address the needs of patients with early nonresponse; however, it is important to remember that the available evidence is based on group mean values and that clinical decision-making must rely on a case-by-case assessment.
Conclusion
The adverse metabolic consequences of antipsychotic treatment and early treatment nonresponse or treatment resistance represent important challenges in the management of schizophrenia. However, these challenges are beginning to be addressed through the development of novel antipsychotic agents and by an increasing understanding of their underlying mechanisms of action, particularly in relation to receptor binding profiles. Although the primary aim of antipsychotic treatment is to effectively control the symptoms of the disease, it is now unequivocally recognized that the physical health of an individual living with schizophrenia is as important to their overall long-term outcomes and QoL as their mental health. Given the clear evidence demonstrating both the potential adverse metabolic effects of antipsychotic treatment and the wide variation in the side effect profiles of different agents, individualization of treatment is key to effective schizophrenia management. Each patient’s treatment should be matched to their unique clinical characteristics, while ensuring that lifestyle and other modifiable risk factors are concurrently addressed. Individualization of treatment not only involves careful consideration of the most appropriate initial treatment but also vigilant monitoring of the subsequent effects of treatment, in terms of both efficacy and side effects, and a willingness to adapt or change treatment, as required. If a patient does not show a response to treatment early on (within 2 weeks), it is important to consider either changing or augmenting the treatment, or (at least in the case of lurasidone) increasing the dose at this timepoint to optimize the likelihood of subsequent response. Furthermore, if a patient responds to treatment but shows signs of developing adverse metabolic side effects, it is equally important to consider changing or adapting treatment in order to mitigate such side effects and optimize the patient’s long-term physical health.
Acknowledgments
Medical writing assistance was provided by Lucy Clements of mXm Medical Communications, Tonbridge, UK.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The symposium was organized and funded by Sumitomo Dainippon Pharma, Tokyo, Japan and Sunovion Pharmaceuticals, New Jersey, USA. Medical writing assistance was funded by Sunovion Pharmaceuticals. This supplement was supported by an educational Grant from Sunovion Pharmaceuticals.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Robin Murray has received honoraria for lectures from Janssen, Lilly, Otsuka, Sunovion and Lundbeck.
Christoph Correll has received grant funding from Takeda and honoraria from Alkermes, Allergan, Bristol-Myers Squibb, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante, Medscape, Neurocrine, Otsuka, Pfizer, ProPhase, Sunovion, Takeda and Teva.
Gavin Reynolds has received travel support, research support, honoraria for lectures and advisory board attendance from Lundbeck, Johnson & Johnson, Otsuka, Sunovion and Janssen.
David Taylor is employed by the NHS, King’s College London, UK and King’s Health Partners, UK. He has been an advisory board member for Lundbeck and Servier, and has received honoraria and research funding from Sunovion, Janssen, Otsuka and Lundbeck. He has no shares or other interests. This supplement was supported by an educational Grant from Sunovion Pharmaceuticals Inc. Medical writing assistance for this article was funded by Sunovion Pharmaceuticals.
Contributor Information
Robin Murray, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, 16 De Crespigny Park, London SE5 8AF, UK.
Christoph U. Correll, Hofstra Northwell School of Medicine, The Zucker Hillside Hospital, New York, USA
Gavin P. Reynolds, Biomolecular Sciences Research Centre, Sheffield Hallam University, Sheffield, UK
David Taylor, The Maudsley Hospital, London, UK.
References
- Agid O., Kapur S., Arenovich T., Zipursky R. (2003) Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60: 1228–1235. [DOI] [PubMed] [Google Scholar]
- Alphs L., Benedetti F., Fleischhacker W., Kane J. (2012) Placebo-related effects in clinical trials in schizophrenia: what is driving this phenomenon and what can be done to minimize it? Int J Neuropsychopharmacol 15: 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz B., Rosel P., Ramirez N., Dueñas R., Fernández P., Sanchez J., et al. (2004) Insulin resistance and increased leptin concentrations in noncompliant schizophrenia patients but not in antipsychotic-naïve first-episode schizophrenia patients. J Clin Psychiatry 65: 1335–1342. [DOI] [PubMed] [Google Scholar]
- Ascher-Svanum H., Nyhuis A., Faries D., Kinon B., Baker R., Shekhar A. (2008) Clinical, functional, and economic ramifications of early nonresponse to antipsychotics in the naturalistic treatment of schizophrenia. Schizophr Bull 34: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch L., Kowalchuk C., Wilson V., Teo C., Guenette, M., Chintoh, A., et al. (2016) Atypical antipsychotics and effects on feeding: from mice to men. Psychopharmacology (Berl) 233: 2629–2653. [DOI] [PubMed] [Google Scholar]
- Briggs A., Wild D., Lees M., Reaney M., Dursun S., Parry D., et al. (2008) Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes 6: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M., Correll C. (2014) Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci 16: 505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintoh A., Mann S., Lam L., Giacca A., Fletcher P., Nobrega, J., et al. (2009) Insulin resistance and secretion in vivo: effects of different antipsychotics in an animal model. Schizophr Res 108: 127–133. [DOI] [PubMed] [Google Scholar]
- Cooper S., Reynolds G., Barnes T., England E., Haddad, P., Heald, A., et al. (2016) BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 30: 717–748. [DOI] [PubMed] [Google Scholar]
- Correll C., De Hert M. (2013) Antipsychotics for acute schizophrenia: making choices. Lancet 382: 919–920. [DOI] [PubMed] [Google Scholar]
- Correll C.U., Manu P., Olshanskiy V., Napolitano B., Kane J.M., Malhotra A.K. (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302: 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M., Dekker J., Wood D., Kahl K., Holt R., Möller H. (2009) Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 24: 412–424. [DOI] [PubMed] [Google Scholar]
- De Hert M., Detraux J., Van Winkel R., Yu W., Correll C. (2011) Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8: 114–126. [DOI] [PubMed] [Google Scholar]
- Fleischhacker W., Heikkinen M., Olié J., Landsberg W., Dewaele P., McQuade R., et al. (2010) Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 13: 1115–1125. [DOI] [PubMed] [Google Scholar]
- Fleischhacker W., Siu C., Bodén R., Pappadopulos E., Karayal O., Kahn R., et al. (2013) Metabolic risk factors in first-episode schizophrenia: baseline prevalence and course analysed from the European First-Episode Schizophrenia Trial. Int J Neuropsychopharmacol 16: 987–995. [DOI] [PubMed] [Google Scholar]
- Fornaro M., Stubbs B., De Berardis D., Perna G., Valchera A., Veronese N., et al. (2016) Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci 17: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurillo P., Jauhar S., Murray R., MacCabe J. (2015) Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry 2: 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad P., Sharma S. (2007) Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs 21: 911–936. [DOI] [PubMed] [Google Scholar]
- Hahn M., Wolever T., Arenovich T., Teo C., Giacca A., Powell V., et al. (2013) Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J Clin Psychopharmacol 33: 740–746. [DOI] [PubMed] [Google Scholar]
- Harvey P., Rosenthal J. (2016) Treatment resistant schizophrenia: course of brain structure and function. Prog Neuropsychopharmacol Biol Psychiatry 70: 111–116. [DOI] [PubMed] [Google Scholar]
- Hatta K., Otachi T., Fujita K., Morikawa F., Ito S., Tomiyama H., et al. (2014) Antipsychotic switching versus augmentation among early non-responders to risperidone or olanzapine in acute-phase schizophrenia. Schizophr Res 158: 213–222. [DOI] [PubMed] [Google Scholar]
- Henderson D., Cagliero E., Copeland P., Borba C., Evins E., Hayden D., et al. (2005) Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry 62: 19–28. [DOI] [PubMed] [Google Scholar]
- Houseknecht K., Robertson A., Zavadoski W., Gibbs E., Johnson D., Rollema H. (2007) Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology 32: 289–297. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen E., Juola P., Hirvonen N., McGrath J., Saha S., Isohanni M., et al. (2013) A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull 39: 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R., Sommer I., Murray R., Meyer-Lindenberg A., Weinberger D., Cannon T., et al. (2015) Schizophrenia. Nat Rev Dis Primers 1: 15067. [DOI] [PubMed] [Google Scholar]
- Kane J., Correll C. (2016) The role of clozapine in treatment-resistant schizophrenia. JAMA Psychiatry 73: 187–188. [DOI] [PubMed] [Google Scholar]
- Kinon B., Chen L., Ascher-Svanum H., Stauffer V., Kollack-Walker S., Sniadecki J., et al. (2008) Predicting response to atypical antipsychotics based on early response in the treatment of schizophrenia. Schizophr Res 102: 230–240. [DOI] [PubMed] [Google Scholar]
- Kinon B., Chen L., Ascher-Svanum H., Stauffer V., Kollack-Walker S., Zhou W., et al. (2010) Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 35: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk S., Glazebrook J., Grayson B., Neill J., Reynolds G. (2009) Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl) 207: 119–125. [DOI] [PubMed] [Google Scholar]
- Kirk S., Neill J., Jones D., Reynolds G. (2004) Ziprasidone suppresses olanzapine-induced increases in ingestive behaviour in the rat. Eur J Pharmacol 505: 253–254. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Agarwal V., Kishi T., Leucht S., Kane J., Correll C. (2013) Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry 18: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S., Cipriani A., Spineli L., Mavridis D., Orey D., Richter F., et al. (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382: 951–962. [DOI] [PubMed] [Google Scholar]
- Leucht S., Tardy M., Komossa K., Heres S., Kissling W., Salanti G., et al. (2012) Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379: 2063–2071. [DOI] [PubMed] [Google Scholar]
- Levine S., Leucht S. (2010) Elaboration on the early-onset hypothesis of antipsychotic drug action: treatment response trajectories. Biol Psychiatry 68: 86–92. [DOI] [PubMed] [Google Scholar]
- Liu-Seifert H., Adams D., Kinon B. (2005) Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs. BMC Med 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel A., Cucchiaro J., Sarma K., Xu L., Hsu C., Kalali A., et al. (2013) Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res 145: 101–109. [DOI] [PubMed] [Google Scholar]
- Loebel A., Silva R., Goldman R., Watabe K., Cucchiaro J., Citrome L., et al. (2016) Lurasidone dose escalation in early nonresponding patients with schizophrenia: a randomized, placebo-controlled study. J Clin Psychiatry 77: 1672–1680. [DOI] [PubMed] [Google Scholar]
- McIntyre R. (2009) Understanding needs, interactions, treatment, and expectations among individuals affected by bipolar disorder or schizophrenia: the UNITE global survey. J Clin Psychiatry 70(Suppl. 3): 5–11. [DOI] [PubMed] [Google Scholar]
- Meltzer H., Cucchiaro J., Silva R., Ogasa M., Phillips D., Xu J., et al. (2011) Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry 168: 957–967. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Suzuki T., Nakagawa A., Yoshida K., Mimura M., Fleischhacker W., et al. (2014) Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull 40: 1385–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatello M., Bruno A., Pandolfo G., Micò U., Scimeca G., Di Nardo F., et al. (2011) Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res 127: 93–99. [DOI] [PubMed] [Google Scholar]
- Newcomer J. (2005) Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19(Suppl. 1): 1–93. [DOI] [PubMed] [Google Scholar]
- Newcomer J., Haupt D., Fucetola R., Melson A., Schweiger J., Cooper B., et al. (2002) Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 59: 337–345. [DOI] [PubMed] [Google Scholar]
- Perry B., McIntosh G., Weich S., Singh S., Rees K. (2016) The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry 3: 1049–1058. [DOI] [PubMed] [Google Scholar]
- Reynolds G., Dalton C., Watrimez W., Jackson J., Harte M. (2016) Adjunctive lurasidone suppresses food intake and weight gain associated with olanzapine administration in rats. Poster presented at British Association for Psychopharmacology Summer Meeting Brighton, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G., Kirk S. (2010) Metabolic side effects of antipsychotic drug treatment–pharmacological mechanisms. Pharmacol Ther 125: 169–179. [DOI] [PubMed] [Google Scholar]
- Royal College of Psychiatrists. (2014) Report of the Second Round of the National Audit of Schizophrenia (NAS) 2014. London: Healthcare Quality Improvement Partnership. [Google Scholar]
- Rummel-Kluge C., Komossa K., Schwarz S., Hunger H., Schmid F., Lobos C., et al. (2010) Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 123: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara M., Dold M., Gianatsi M., Nikolakopoulou A., Helfer B., Salanti G., et al. (2016) Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry 73: 199–210. [DOI] [PubMed] [Google Scholar]
- Samara M., Leucht C., Leeflang M., Anghelescu I., Chung Y., Crespo-Facorro B., et al. (2015) Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry 172: 617–629. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Parrilla-Escobar M., Klink R., Fathalli F., Ying Kin Ng, Stip E., et al. (2008) Are metabolic indices different between drug-naïve first-episode psychosis patients and healthy controls? Schizophr Res 102: 329–336. [DOI] [PubMed] [Google Scholar]
- Schoeler T., Monk A., Sami M., Klamerus E., Foglia E., Brown R., et al. (2016) Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. Lancet Psychiatry 3: 215–225. [DOI] [PubMed] [Google Scholar]
- Snigdha S., Thumbi C., Reynolds G., Neill J. (2008) Ziprasidone and aripiprazole attenuate olanzapine-induced hyperphagia in rats. J Psychopharmacol 22: 567–571. [DOI] [PubMed] [Google Scholar]
- Taylor D., Cornelius V., Smith L., Young A. (2014) Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis. Acta Psychiatr Scand 130: 452–469. [DOI] [PubMed] [Google Scholar]
- Teff K., Rickels M., Grudziak J., Fuller C., Nguyen H., Rickels K. (2013) Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes 62: 3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman L., Reynolds G., Arranz B., San L. (2005) Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics 15: 195–200. [DOI] [PubMed] [Google Scholar]
- Tiihonen J., Lonnqvist J., Wahlbeck K., Klaukka T., Niskanen L., Tanskanen A., et al. (2009) 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 374: 620–627. [DOI] [PubMed] [Google Scholar]
- Torniainen M., Mittendorfer-Rutz E., Tanskanen A., Björkenstam C., Suvisaari J., Alexanderson K., et al. (2015) Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull 41: 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G., Chittiprol S., Neelakantachar N., Naveen M., Thirthall J., Gangadhar B., et al. (2007) Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naïve schizophrenia. Am J Psychiatry 164: 1557–1560. [DOI] [PubMed] [Google Scholar]
- Vidarsdottir S., De Leeuw van Weenen J., Frölich M., Roelfsema F., Romijn J., Pijl H. (2010) Effects of olanzapine and haloperidol on the metabolic status of healthy men. J Clin Endocrinol Metab 95: 118–125. [DOI] [PubMed] [Google Scholar]
- Yildiz A., Nikodem M., Vieta E., Correll C., Baldessarini R. (2015) A network meta-analysis on comparative efficacy and all-cause discontinuation of antimanic treatments in acute bipolar mania. Psychol Med 45: 299–317. [DOI] [PubMed] [Google Scholar]
- Zhang J., Gallego J., Robinson D., Malhotra A., Kane J., Correll C. (2013) Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 16: 1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yao Z., Liu W., Fang Q., Reynolds G. (2004) Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Magnetic resonance imaging study of previously untreated people with schizophrenia. Br J Psychiatry 184: 58–62. [DOI] [PubMed] [Google Scholar]
- Zheng W., Li X., Tang Y., Xiang Y., Wang C., de Leon J. (2015) Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol 35: 499–509. [DOI] [PubMed] [Google Scholar]
- Zheng W., Xiang Y., Xiang Y., Li X., Ungvari G., Chiu H., et al. (2016) Efficacy and safety of adjunctive topiramate for schizophrenia: a meta-analysis of randomized controlled trials. Acta Psychiatr Scand 134: 385–398. [DOI] [PubMed] [Google Scholar]