Abstract
Leishmania donovani is the causative organism of the neglected human disease known as visceral leishmaniasis which is often fatal, if left untreated. The cysteine biosynthesis pathway of Leishmania may serve as a potential drug target because it is different from human host and regulates downstream components of redox metabolism of the parasites; essential for their survival, pathogenicity and drug resistance. However, despite the apparent dependency of redox metabolism of cysteine biosynthesis pathway, the role of L. donovani cysteine synthase (LdCS) in drug resistance and redox homeostasis has been unexplored. Herein, we report that over-expression of LdCS in Amphotericin B (Amp B) sensitive strain (S1-OE) modulates resistance towards oxidative stress and drug pressure. We observed that antioxidant enzyme activities were up-regulated in S1-OE parasites and these parasites alleviate intracellular reactive oxygen species (ROS) efficiently by maintaining the reduced thiol pool. In contrast to S1-OE parasites, Amp B sensitive strain (S1) showed higher levels of ROS which was positively correlated with the protein carbonylation levels and negatively correlated with cell viability. Moreover, further investigations showed that LdCS over-expression also augments the ROS-primed induction of LdCS-GFP as well as endogenous LdCS and thiol pathway proteins (LdTryS, LdTryR and LdcTXN) in L. donovani parasites; which probably aids in stress tolerance and drug resistance. In addition, the expression of LdCS was found to be up-regulated in Amp B resistant isolates and during infective stationary stages of growth and consistent with these observations, our ex vivo infectivity studies confirmed that LdCS over-expression enhances the infectivity of L. donovani parasites. Our results reveal a novel crosstalk between LdCS and thiol metabolic pathway proteins and demonstrate the crucial role of LdCS in drug resistance and redox homeostasis of Leishmania.
Abbreviations: Amp B, Amphotericin B; APx, ascorbate peroxidase; CS, cysteine synthase; DTNB, 5,5`-dithiobis-(2-nitrobenzoic acid); FBS, fetal bovine serum; γ-GCS, γ-glutamylcysteine synthetase; GSH, glutathione; GST, glutathione S-transferase; LdCS, Leishmania donovani CS; ODC, ornithine decarboxylase; ROS, reactive oxygen species; SOD, superoxide dismutase; TryS, trypanothione synthetase; TryR, trypanothione reductase; TXN, tryparedoxin; TXNPx, tryparedoxin peroxidase; VL, visceral leishmaniasis
Keywords: Leishmania, Cysteine synthase, Drug resistance, Thiol metabolism, Oxidative stress, Amphotericin B, Trypanothione
Highlights
-
•
Over-expression of CS in L. donovani modulates oxidative stress & Amp B resistance.
-
•
Over-expressing parasite possess higher thiol to counteract the oxidative stress.
-
•
Over-expressing parasites showed increased activity of TXNPx, GST, SOD, and APx.
-
•
Expression/activity of LdCS is up-regulated in Amp B resistant clinical isolates.
-
•
Ex vivo results confirm that LdCS over-expression enhance the parasites infectivity.
-
•
Over-expressing parasites survived long time under oxidative stress conditions.
1. Introduction
In any living organism, cellular redox homeostasis forms an important part of its defense mechanism. Instability in the normal redox state can cause toxic effects through the elevation in the formation of reactive oxygen species (ROS) such as superoxide anions, hydrogen peroxide, hydroxyl, peroxynitrite, alkoxy, and peroxy radicals. These free radicals species and peroxides damage major components of the cell, like nucleic acids, lipids, proteins and membranes [1]. In order to maintain reducing milieu, efficient and diverse cellular defense mechanisms has been evolved by aerobic organisms including protozoan parasites.
Leishmania donovani is a unicellular protozoan parasite which causes neglected human disease known as visceral leishmaniasis (VL). It has a high fatality rate and is ranked next to malaria as the most deadly protozoan disease [2]. These parasites successfully withstand the oxidative/nitrosative burst conditions faced during their digenetic life cycle alternating between motile promastigotes form in the sand fly (Phlebotomus spp.) vector and non-motile amastigotes form within the phagolysosomes of infected human macrophages [3]. Efficient adaptation during different environmental conditions imposed by their life cycle stages largely depends upon trypanothione (T(SH)2) mediated redox homeostasis. T(SH)2 is a low molecular mass dithiol compound and participates actively in thiol-disulfide exchange reactions [4], [5], [6]. As a precursor for the biosynthesis of glutathione (GSH) and trypanothione, cysteine may play a pivotal role in maintenance of redox homeostasis. Moreover, cysteine biosynthesis pathway of Leishmania is a potential drug target as it is different from human host and regulates downstream components of redox metabolism of the parasites essential for their survival, posing an effective approach to therapeutic intervention.
The first line drug for the treatment of leishmaniasis was pentavalent antimonials, but it is no more preferred for the treatment of VL patient in India due to wide spread drug resistance and relapse cases in Bihar; which contributes about 90% of the VL cases in India [7]. Current drug regimens are liposomal preparation of Amphotericin B (Amp B) and miltefosine, alone or in combination; but recent reports of Amp B resistance [8], [9] have raised serious concerns over the future of Amp B therapy against VL. Polyene antibiotic Amp B alters membrane lipid profile and generates reactive oxygen species; which proves to be deleterious for the Amp B sensitive parasites and causes apoptotic cell death. Amp B resistant isolates alleviates the deleterious effects of ROS by retooling/orchestrating their trypanothione dependent thiol cascade mediated antioxidant defense mechanism [8], [10].
L. donovani, possess a unique dithiol T(SH)2 based redox homeostasis system, in contrast to their mammalian host which rely on catalase, glutathione reductase and selenium dependent glutathione peroxidase enzymes [11], [12]. T(SH)2 is synthesized by ATP-dependent conjugation of two molecules of glutathione and one molecule of spermidine in the presence of trypanothione synthetase (TryS). On the other hand, oxidized T(SH)2 is converted to its reduced form by trypanothione reductase (TryR) which solely relies upon availability of NADPH as electron donor [13], [14]. ROS detoxification in Leishmania heavily rely on the cascade of electron donor (NADPH; supplied through pentose phosphate pathway) and electron carriers involving T(SH)2 and several low molecular mass dithiol redox proteins [13], [15], [16]. Among low molecular mass antioxidants proteins; tryparedoxin (TXN) and tryparedoxin peroxidase (TXNPx) proteins play a pivotal role in the regulation of metabolic reconfiguration against oxidative and nitrosative stress viz. reduction of hydrogen peroxide, hydroperoxides and peroxynitrite [17], [18]. Therefore, combined action of TryS, TryR, TXN and TXNPx helps to alleviate ROS and together they orchestrate redox metabolism of the parasites which is vital for their survival [19].
Previously, we reported up-regulation of LdTryS expression in Amp B resistant L. donovani clinical isolates at both transcriptional and translational levels [8], [20]. In T. brucei, dsRNAi studies of TryS resulted in declined T(SH)2 and glutathionylspermidine (Gsp) level, growth arrest, impaired antioxidant capacity and infectivity [21]. Conditional knock-out studies of TryR in T. brucei showed impaired parasite growth and viability with increased sensitivity to H2O2 and less virulence in mice [22]. Further, in T. brucei, cytosolic cTXN knock down by dsRNAi resulted in increased sensitivity to hydroperoxides, alteration in morphology and impaired cell growth [23], [24]. Furthermore, dsRNAi studies carried out in T. brucei demonstrated the essentiality of cTXNPx; reduction in enzyme activity associated with impaired growth and increased sensitivity to hydroperoxides was observed [24]. Over-expression studies of cTXNPx in T. cruzi [25], [26] and L. chagasi [27] resulted in enhanced resistance towards free radical species. Recently, we also reported up-regulation of cTXN at translational level accompanied by enhanced peroxidase activity in Amp B resistant L. donovani clinical isolates [18]. These findings suggest that TryS, TryR, TXN and TXNPx proteins have a pivotal role in drug resistance and redox homeostasis.
Cysteine forms the basic building block of all thiols and it is the precursor molecule for glutathione biosynthesis which in turn serves as the precursor for biosynthesis of trypanothione [28], [29]. Cysteine also serves as a precursor for the biosynthesis of important metabolites such as coenzyme A, enzyme cofactors and ubiquitous iron-sulfur clusters, which play various important roles in electron transfer, redox regulation, nitrogen fixation, and sensing for regulatory processes [28], [30], [31]. Therefore, cysteine is physiologically associated with cell viability as well as oxidative stress tolerance, the factors of paramount importance for survival and virulence of L. donovani. In Leishmania, cysteine can be synthesized either by de novo cysteine biosynthesis pathway (with help of cysteine synthase and serine O-acetyltransferase) or by reverse transsulfuration pathway mediated by cystathionine β-synthase and cystathionine γ-lyase [28], [32], [33]. Thus, the cysteine biosynthesis pathway plays a crucial role in maintaining a cysteine rich environment vital for detoxifying ROS. Moreover, an increased amount of cysteine, glutathione and trypanothione levels has been associated with antimonials resistance in Leishmania [34], [35]. But the potential role of cysteine biosynthesis pathway in drug resistance has never been investigated. Furthermore, cysteine biosynthesis pathway can potentially act as a checkpoint for downstream components of thiol based cellular redox metabolism of the parasites, which are essential for defense mechanism against oxidative stress and drug resistance [20], [34], [35], [36].
Being the precursor molecule in redox metabolism, an important role of cysteine synthase (CS) protein in regulation of redox homeostasis and acquisition of drug resistance is apprehended but remains to be proved. In the present study, we investigated the role of LdCS and downstream thiol proteins (LdTryS, LdTryR, LdcTXN) in the antioxidant defense system in response to oxidative stress by over-expressing cysteine synthase (LdCS) enzyme of cysteine biosynthesis pathway in Amp B sensitive strain of L. donovani. The results show that over-expression of LdCS in the parasites enhance their ability towards stress tolerance and is associated with up-regulated thiol pathway proteins and higher thiol content; envisaging crucial interplay between LdCS and thiol pathway proteins in regulating redox active thiol content of parasites and may be of fundamental importance for drug resistance mechanism. Further, we investigated the expression of LdCS protein in Amp B resistant field isolates including their different growth stages. We observed that LdCS is significantly up-regulated in Amp B resistant isolates and during stationary stages of growth, which is alike, other proteins of thiol metabolic pathway.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals of analytical grade or higher were purchased and used from Sigma-Aldrich, Amresco (USA), and USB (USA) unless otherwise stated. Gel extraction kit was purchased from Qiagen. Plasmids and restriction enzymes were purchased from Novagen and Fermentas, respectively.
2.2. Animal ethical statement
Female BALB/c mice 6–8 weeks old was used for infectivity assessment after prior approval and procedure used was reviewed and approved by the Institutional Animal Ethical Committee (IAEC), (INT-10/29-12-2015), Rajendra Memorial Research Institute of Medical Sciences, Patna, Indian Council of Medical Research, New Delhi regulated by CPCSEA, Government of India, New Delhi. The RMRIMS, ICMR follows “The Guide for the Care and Use of Laboratory Animals”, 8th edition by the Institute for Laboratory Animal Research.
2.3. Clinical isolates and parasites culture
Drug resistant clinical isolates of L. donovani were obtained from the splenic aspirates of VL patients unresponsive to Amphotericin B (Amp B) treatment, in the indoor ward facility of RMRIMS, Patna, Bihar, India and these isolates were described earlier [20]. The resistant isolates (henceforth designated, R1 & R2) as well as the reference sensitive strains (henceforth designated S1 and S2) were finally maintained in M199 medium supplemented with 10% fetal bovine serum, 25 mM HEPES buffer (pH 7.2), 100 units/ml penicillin and 100 µg/ml streptomycin. The culture was initiated at 1×105 parasites/ml and grown at 24±1 °C in BOD incubator for 4–5 days before sub-culturing (late log phase).
2.4. Isolation of DNA
Total DNA was isolated using standard protocols [37] from 1×108 L. donovani promastigotes using phenol/chloroform/isoamyl-alcohol method (25:24:1 v/v) followed by ethanol precipitation. The quality and quantity of DNA were assessed by agarose gel electrophoresis and spectrophotometer (Hitachi, Japan), respectively.
2.5. PCR amplification and cloning of LdCS in pXG-GFP+
Based on the nucleotide sequence of the protein-encoding region of the putative Leishmania cysteine synthase gene, primers were designed to clone LdCS in pXG-GFP+ [38] vector to express LdCS as fusion proteins with a C-terminal GFP tag. The LdCS ORF was amplified by PCR from genomic DNA of L. donovani strain Ag83 using sense (5`-ATAGGATCCATGGCGGCACCGTTCGA-3`) and an antisense (5`- TAAGATATCGTCCTGCAGCTCCG-3`) primers; the added BamHI and EcoRV restriction sites are underlined, and the translation initiation codon is shown in bold. PCR was performed in a 50 μl reaction mixture containing 0.2 mM each dNTPs, 2.0 mM MgCl2, 1.0 µM each primer, 1 µg of L. donovani (Ag83) genomic DNA and 1.0 U DNA polymerase with Taq buffer (+(NH4)2SO4). The conditions used to amplify the LdCS gene were hot start at 95 °C for 5 min, followed by the 30 cycles of denaturation at 94 °C for 1 min, annealing at 58 °C for 45 s and elongation at 72 °C for 1.0 min and a final extension at 72 °C for 10 min. The ~1.0 kb PCR product of LdCS was observed, on 1.0% agarose gel electrophoresis. LdCS amplified PCR products was double digested with BamHI and EcoRV, purified with gel extraction kit (Qiagen), and cloned into BamHI and EcoRV double digested pXG-GFP+ vector. The ligation mixture was transformed in competent Escherichia coli DH5α cells (Novagen) which produced the LdCS-GFP construct. The insert and ORF orientation of construct was confirmed by colony PCR. Construct plasmid was isolated using Qiagen Miniprep kit, according to manufacturer's instructions.
2.6. Transfection and characterization of LdCS-GFP
The construct LdCS-GFP was transfected by electroporation [39] in the L. donovani-sensitive strain (S1) promastigotes and transformants selection was achieved with increasing concentrations of G418, upto a final concentration of 200 µg/ml. The culture was finally maintained in 150 µg/ml of G418. The parasites were characterized by immunoblot analysis with polyclonal anti-LdCS sera (1:3000) and LdCS enzymatic assay of LdCS-GFP over-expressor strain (henceforth designated, S1-OE).
2.7. In vitro drug sensitivity assay
In vitro drug sensitivity of wild type (S1) and over-expressor (S1-OE) strains of L. donovani was determined by incubating 1×106 cells/ml parasites in M199 medium (supplemented with 10% FBS) with different concentrations of Amp B and Sodium stibogluconate (SAG) [20]. Viable cells were counted in a hemocytometer (Rohem) by the Trypan blue exclusion method, after culturing for 24 h and inhibitory concentration (IC50) values were determined for both the wild type (S1) and S1-OE parasites. Results are expressed as means±SEM of three independent experiments.
2.8. MTT assay
MTT assay is a colorimetric assay for the measurement of metabolically active cells and was used for determining IC50 value of L. donovani treated with oxidative stress inducers (H2O2 and menadione). Briefly, Leishmania promastigotes (1×106 cells/ml) were cultured in 24 well plates and treated with increasing concentrations of H2O2 and menadione. At regular 3 h intervals, cells were harvested and MTT assay was performed as described previously by our group [20], [40]. The percentage of cell viability was determined by comparing with untreated L. donovani parasites. The experiment was repeated thrice and results are expressed as means±SEM.
2.9. Measurement of protein carbonyl by immunoblotting and spectrophotometric assay
Proteins are the most liable targets of free radical species accumulated in the cells due to redox imbalance. Oxidative modification of the proteins has been reported to generate carbonyl groups on amino acid residues [41], [42]. We monitored the protein carbonyl content of total cell lysates by derivatization of the carbonyl groups with 2,4-dinitrophenylhydrazine (DNPH) and subsequently detected using an anti-DNPH antibody as described previously [42], [43], [44]. Briefly, 30 µg proteins were incubated with DNPH (1 mM) in 2 M HCl for 20 min at room temperature to form the carbonyl derivative dinitrophenylhydrazone. Carbonylated proteins were separated on a 10% SDS-PAGE and immunoblotted on to a precharged (in 100% methanol) PVDF membrane. The membrane was probed with primary antibody anti-2, 4-dinitrophenol antibody (1:200 dilution) raised in rabbit. Alkaline phosphatase conjugated goat anti-rabbit IgG (Santa Cruz, 1:2000) was used as secondary antibody and blot developed with BCIP/NBT solution (Santa Cruz), as per manufacturer's instructions. Quantitative measurements of protein carbonylation were performed by spectrophotometric assay as described previously [41], [42], with minor modifications. Briefly, carbonylated proteins were precipitated with the addition of 30% trichloroacetic acid (w/v) and centrifuged at 12000×g for 5 min. Supernatant was discarded and the pellet was washed with ice cold ethanol and ethyl acetate solution (1:1 v/v). Finally the pellet was dissolved in 1 ml of 6 M guanidine HCl and the absorbance was measured at 360 nm. Protein carbonylation content was calculated using absorption coefficient of 22000 M−1 cm−1 and results were expressed as nmoles of carbonyl formed/mg of protein.
2.10. Quantitation of reactive oxygen species (ROS)
Intracellular ROS of each strain, at different stress conditions, was quantified using 2,7,-dichlorodihydrofluorescein diacetate (H2DCFDA, Sigma) as described previously [9], [44]. Briefly, 2×106 cells/well were incubated with H2DCFH-DA (20 mM, dissolved in DMSO) at a final concentration of 20 µM for 30 min. The emission intensities of DCF (dichlorofluorescein) (deesterified and oxidized metabolite of H2DCFH-DA) were recorded after 30 min of incubation using a Cary Eclipse Fluorescence Spectrofluorimeter (Agilent technologies); using an excitation wavelength of 504 nm and an emission wavelength of 529 nm. The fluorescence intensity is directly proportional to the accumulated ROS and expressed in relative fluorescence unit (RFU). The experiment was repeated thrice and results are expressed as means±SEM.
2.11. Determination of total intracellular reduced thiol content
The level of total intracellular thiol was measured in deproteinized cell extracts of each strain, as described previously [45]. Briefly, 1×108 cells were harvested, washed with PBS buffer (pH 7.2), and resuspended in 0.6 ml of 25% trichloroacetic acid. After 10 min incubation on ice, the denatured proteins and cell debris was removed by centrifugation at 13000×g for 10 min at 4 °C. The thiol content of the supernatant solution was determined with 0.6 mM 5, 5-dithio-bis (2-nitrobenzoic acid) (DTNB, Ellman's reagent) in 0.2 M Na3PO4 buffer (pH 8.0). The concentration of DTNB derivatives of thiols was estimated spectrophotometrically at 412 nm. Results are expressed as means±SEM of three independent experiments.
2.12. Total lysate preparation and enzymatic assays
All the assays were performed with 50 µg total cell lysate proteins of each strain, with or without stress treatment, unless specified. Total lysates were prepared as described previously with some modifications [46]. Briefly, parasites were harvested, washed twice with PBS and cell pellet was resuspended in lysis buffer containing PBS (pH 7.2), 1X protease inhibitor cocktail (Calbiochem) and 0.5 mg/ml digitonin. The suspension was subjected to 3–4 freeze thaw cycles and 2–3 brief pulses of sonication before centrifugation at 12000×g for 10 min. The amount of protein in the resulting supernatant was estimated by a dye-binding method [47] using BSA as the standard protein. All the enzymatic assays were repeated thrice and results are expressed as means±SEM.
2.13. Ascorbate peroxidase (APx) assay
The assay was carried out as described previously [48]. The rate of decomposition of ascorbic acid was measured for 5 min at 290 nm using a spectrophotometer (U3900, Hitachi, Japan) following addition of H2O2 and cell lysate to the reaction mixture. The rate of decomposition of ascorbic acid in the absence of enzyme source was taken as control. The enzyme activity was calculated as µmoles of ascorbate decomposed/min/mg protein.
2.14. Cysteine synthase assay
Cysteine synthase (CS) activity was assayed by measuring the production of L-cysteine at 560 nm using UV–visible spectrophotometer (U3900, Hitachi, Japan), as described previously [33], [49]. Briefly, the reaction mixture containing 50 mM Tris-HCl (pH 7.5), 30.0 mM O-acetylserine, 3.0 mM sodium sulfide, 0.1 mM EDTA, and cell lysate was incubated at 37 °C for 30 min and then the reaction was quenched by addition of 50 μl of concentrated acetic acid. Freshly prepared 50 μl of ninhydrin reagent was added to the reaction mixtures and further incubated at 95 °C for 10 min. Finally, the reaction mixtures were cooled down on ice, diluted with 200 μl of ethanol and production of L-cysteine was measured at 560 nm using spectrophotometer. The enzyme activity was calculated as µmoles of cysteine produced/min/mg protein.
2.15. Glutathione S-transferase (GST) assay
The GST assay was carried out as described previously [44]. Reaction was initiated by the addition of 5 μl of 1-chloro-2,4-dinitrobenzene (100 mM stock) at a final concentration of 1 mM to the reaction mixture containing 100 mM phosphate buffered saline (pH 7.4), 5 μl of reduced glutathione (200 mM) and cell lysate. The rate of increase in the absorption of GS-DNB (glutathione-dinitrobenzene) conjugate following addition of cell lysate and 1-chloro-2,4-dinitrobenzene to the reaction mixture was recorded for 5 min at 340 nm using a spectrophotometer (U3900, Hitachi, Japan). The rate of increase in the absorption of the conjugate in the absence of the cell lysate was used as the reaction control. The enzyme activity was calculated as µmoles of GS-DNB conjugate formed/min/mg protein.
2.16. Superoxide dismutase (SOD) assay
The assay was carried out as described previously [50]. The reaction was initiated by the addition of 50 μl of NH2OH.HCl (1 mM) at a final concentration of 0.1 mM to the reaction mixture containing sodium carbonate (50 mM), EDTA (0.1 mM), nitro blue tetrazolium (NBT) (24 μM), Triton X-100 (0.03%) and cell lysate. The rate of reduction of NBT following addition of NH2OH·HCl was recorded for 5 min at 560 nm using a spectrophotometer (U3900, Hitachi, Japan). The rate of reduction of NBT in the absence of the cell lysate was used as control. The enzyme activity was calculated as the percentage of inhibition in the reduction of NBT.
2.17. Trypanothione dependent peroxidase assay
T(SH)2 dependent peroxidase activity assay was carried out as described previously [18], [51]. The rate of oxidation of NADPH was measured for 5 min at 340 nm using a spectrophotometer (U3900, Hitachi, Japan) followed by the addition of H2O2 and cell lysate to the reaction mixture. The rate of oxidation of NADPH in the absence of enzyme source was taken as control. The enzyme activity was calculated as nmoles of H2O2 consumed/min/mg protein.
2.18. Analysis of LdCS and thiol pathway proteins expression under oxidative stress by immunoblot analysis
L. donovani promastigotes (S1 and S1-OE) in late log phase were treated with different concentration of H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h. Cells were harvested and total lysates was prepared as described previously by our group [9]. The membrane was probed with polyclonal α-LdCS sera (1:3000), α-LdTryS sera (1:3000), α-LdTryR sera (1:3000) and α-LdcTXN (cytosolic) sera (1:3000). α-LdActin (1:2000) antibody was used as a loading control. Alkaline phosphatase conjugated goat anti-rabbit IgG (Santa Cruz, 1:4000) was used as secondary antibody and blots were developed with BCIP/NBT solution (Santa Cruz), as per manufacturer's instructions. Densitometry analyses of the immunoblots were performed using Image J software (http://imagej.nih.gov/ij) and relative band intensity was plotted.
2.19. Expression level variation between Amp B sensitive and resistant isolates during logarithmic and stationary phases
Promastigotes of each strain were seeded at 1×105 cells/ml in M199 media supplemented with 10% FBS and allowed to grow in a B.O.D. incubator at 24 °C. The parasites were harvested during logarithmic (3rd day) and stationary stage (6th day) of growth, washed twice with PBS; and used for enzymatic assay and immunoblot analysis with total lysates prepared as described previously [9]. The membrane was probed with polyclonal anti-LdCS sera (1:3000) raised in rabbit. Alkaline phosphatase conjugated goat anti-rabbit IgG (Santa Cruz, 1:4000) was used as secondary antibody and blot developed with BCIP/NBT solution (Santa Cruz), as per manufacturer's instructions. Densitometry analysis of the immunoblots was performed using Image J software (http://imagej.nih.gov/ij) and relative band intensity was plotted. Anti-LdActin antibody, used as a loading control, was a kind gift from Dr. Amogh A. Sahasrabuddhe, CDRI, Lucknow, India.
2.20. Ex vivo infectivity assessment
Peritoneal macrophages were isolated from starch induced BALB/c mice, harvested and seeded on cover-slips at a density of 1×106 cells/ml in a 6-well flat bottom culture plates (Nunc, USA) in RPMI 1640 medium with 10% FBS overnight at 37 °C in 5% CO2 incubator [52]. Then, all wells were washed twice with PBS buffer (pH 7.2) or serum free medium to remove non-adherent cells and then infection was performed with L. donovani promastigotes at a ratio of 10:1 (parasite: macrophages). Non-internalized parasites were washed with RPMI 1640 medium only after 2 h of co-incubation. Infected macrophages were further incubated for 12 & 24 h at 37 °C in 5% CO2 incubator. The culture medium was removed and cells were washed twice with sterile PBS buffer (pH 7.2) after 12 and 24 h of infection. Finally, cover-slips were fixed with methanol, stained with Giemsa and visualized with light microscopy using an Olympus BX41 optical microscope at 100X. The numbers of infected and non-infected cells were determined >300 macrophages per cover-slip and 100 macrophages per cover-slip counted to identify the percentage of infectivity. Further, Giemsa stained amastigotes per 100 infected macrophages were also counted after 12 and 24 h of infection. The experiment was repeated thrice and percentage of infection is expressed as means±SEM.
2.21. Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). Student's t-test was used to estimate the statistical significance of the differences between groups. Differences between groups were considered statistically significant when p value was less than 0.05 (*p<0.05; **p<0.01; ***p<0.001).
3. Results
3.1. Over-expression of LdCS in L. donovani parasites
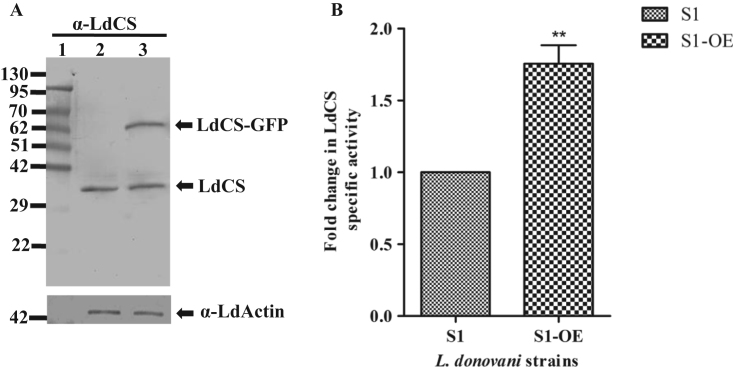
To elucidate the role of LdCS in imparting stress tolerance and drug resistance, we cloned LdCS gene into pXG-GFP+ vector and transfected the construct in sensitive L. donovani strain (henceforth designated, S1-OE). The presence of the LdCS gene in transfected parasites was confirmed by immunoblot analysis with polyclonal anti-LdCS sera which recognized a band of ~62 kDa in transfected parasites (S1-OE) corresponding to the predicted size of LdCS (~35 kDa) plus additional 26 kDa GFP tag (Fig. 1A, lane 3). The specific band of ~35 kDa corresponding to the endogenous LdCS was also observed (Fig. 1A, lane 2 and 3) in the lysate fractions of wild type strain (S1) and over-expressed strain (S1-OE), respectively. As expected, S1-OE exhibited ~1.8 fold higher LdCS enzyme activity as compared to the S1 strain (Fig. 1B) which confirmed the over-expression of active LdCS enzyme in the transfected parasites.
Fig. 1.
Characterization of LdCS over-expressor in Amp B sensitive strain of L. donovani. (A) Immunoblot analysis of LdCS in total lysates of sensitive (S1) and LdCS over-expressor (S1-OE) strains. Lane 1, protein marker; Lane 2 and 3, total lysates of S1 and S1-OE strains. α-LdActin was used as a protein loading control. (B) Fold change in cysteine synthase activity of S1-OE strain as compared to S1 strain. **, p<0.01; by Student's t-test using GraphPad Prism 5.0.
3.2. Over-expression of LdCS confers Amp B and SAG resistance to the sensitive L. donovani parasites
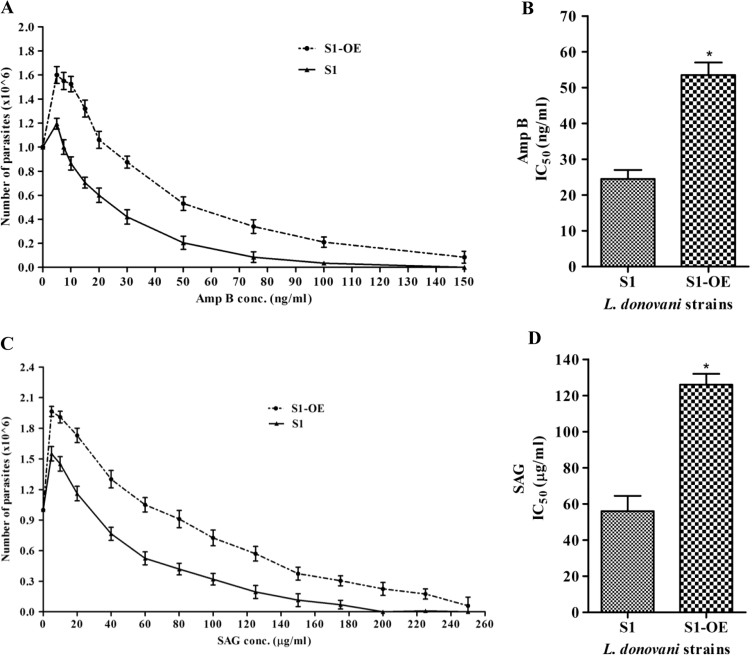
As reported earlier, an increased content of thiol moieties (cysteine, glutathione and trypanothione) have been associated with drug resistance [34], [35], [36]. To investigate the role of cysteine biosynthesis pathway in drug resistance, the sensitivity (IC50) of LdCS over-expressor (S1-OE) and wild type (S1) parasites to Amp B and SAG were determined. It was found that S1-OE parasites showed better survival rate under drug pressure (Fig. 2A and C) with an IC50 value of ~55 ng/ml and ~120 µg/ml for Amp B (Fig. 2B) and SAG (Fig. 2D), respectively, as compared to S1 parasites which showed IC50 of ~24 ng/ml and ~56 µg/ml for Amp B (Fig. 2B) and SAG (Fig. 2D), respectively. Thus, S1-OE parasites exhibit ~2 fold higher IC50 for both Amp B and SAG as compared to S1 parasites. In summary, antileishmanial drugs (Amp B and SAG) showed a higher IC50 against S1-OE parasites as compared to wild type; suggesting that S1-OE parasites are better tolerant towards drug pressure. Thus, over-expression of LdCS has a crucial role in modulating/regulating thiol mediated drug resistance in L. donovani parasites.
Fig. 2.
Drug sensitivity profile of wild type (S1) and (S1-OE) parasites. 1×106 parasites were subjected to increasing concentration of drugs (Amphotericin B and Sodium stibogluconate) for 24 h and cell viability determined by trypan blue exclusion method. (A) Amphotericin B (Amp B) concentrations ranging from 0 to 150 ng/ml was used for determination of cell viability for both wild type (S1) and (S1-OE) parasites. (B) Amp B IC50 values of wild type (S1) and (S1-OE) parasites. (C) Sodium stibogluconate (SAG) concentrations ranging from 0 to 260 µg/ml was used for determination of cell viability for both wild type (S1) and (S1-OE) parasites. (D) SAG IC50 values of wild type (S1) and (S1-OE) parasites. Results are expressed as means±SEM of three independent experiments. *, p<0.05; by Student's t-test using GraphPad Prism 5.0.
3.3. LdCS over-expression enhances the tolerance to oxidative stress inducers
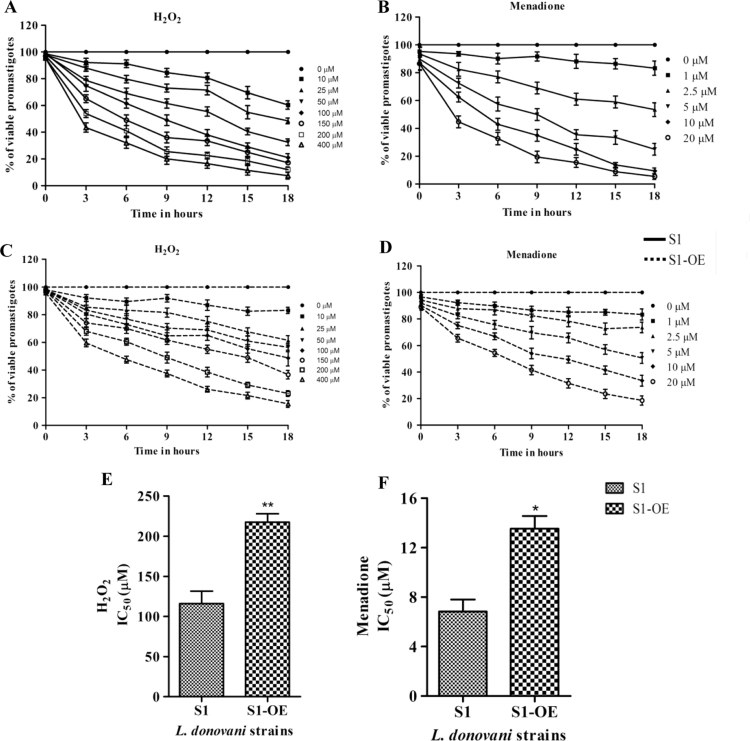
Both Amp B and SAG have been shown to induce ROS-mediated antileishmanial activity [6], [53], [54] whereas tolerance to oxidants, such as H2O2 and menadione, have been associated with drug resistance in Leishmania [35], [43]. Moreover, up-regulation of several proteins of thiol pathway has been implicated in imparting stress tolerance and acquisition of drug resistance by the parasites [18], [20], [40]. To explore a potentially similar mechanism of acquisition of drug resistance by S1-OE parasites, the stress tolerance sensitivity of S1 and S1-OE towards different oxidants were determined by comparing their cell viability at regular intervals of 3 h after treatment with increasing concentrations of oxidants. Although, the cell viability of both S1-OE and S1 parasites decreased in a time and concentration dependent manner, S1-OE parasites consistently displayed higher cell viability for all the tested doses and time durations as compared to S1 parasites. For example, addition of 100 µM H2O2 and 5 µM menadione to S1- OE parasites for 9 h resulted in ~75% viable cells as compared to ~50% in wild type (S1) parasites (Fig. 3A, B, C and D). Even after treatment with high doses of 200–400 µM H2O2 and 10–20 µM menadione, S1-OE parasites displayed ~30% cell viability as compared to ~5% in S1 parasites at the end of 18 h (Fig. 3A, B, C and D). The deduced IC50 values of S1-OE towards H2O2 and menadione was found to be 217±8.7 µM and 13.5±2.2 µM, respectively, which was ~1.87 and ~1.95 fold higher than S1 parasites (Fig. 3E and F). Thus, the results showed that over-expression of LdCS enhance the oxidative stress tolerance of the parasites indicating its role in redox homeostasis.
Fig. 3.
Analysis of cell viability of wild type (S1) and LdCS over-expressor (S1-OE) strains using MTT based assay exposed to lethal doses of ROS inducers H2O2 and menadione. (A and C) Percentage of viable wild type (S1) and S1-OE strain promastigotes after exposure to increasing concentrations of H2O2 (10–400 µM) for a period of 18 h, respectively. (B and D) Percentage of viable wild type (S1) and S1-OE strain promastigotes after exposure to increasing concentrations of menadione (1–20 µM) for a period of 18 h respectively. (E and F) IC50 values of H2O2 and menadione were determined between wild type (S1) and S1-OE parasites after a period of 9 h. Untreated parasites were used as a control in the experiment. Results are expressed as means±SEM of three independent experiments. *, p<0.05; **, p<0.01; by Student's t-test using GraphPad Prism 5.0.
3.4. Over-expression of LdCS prevents oxidative stress induced carbonylation of proteins
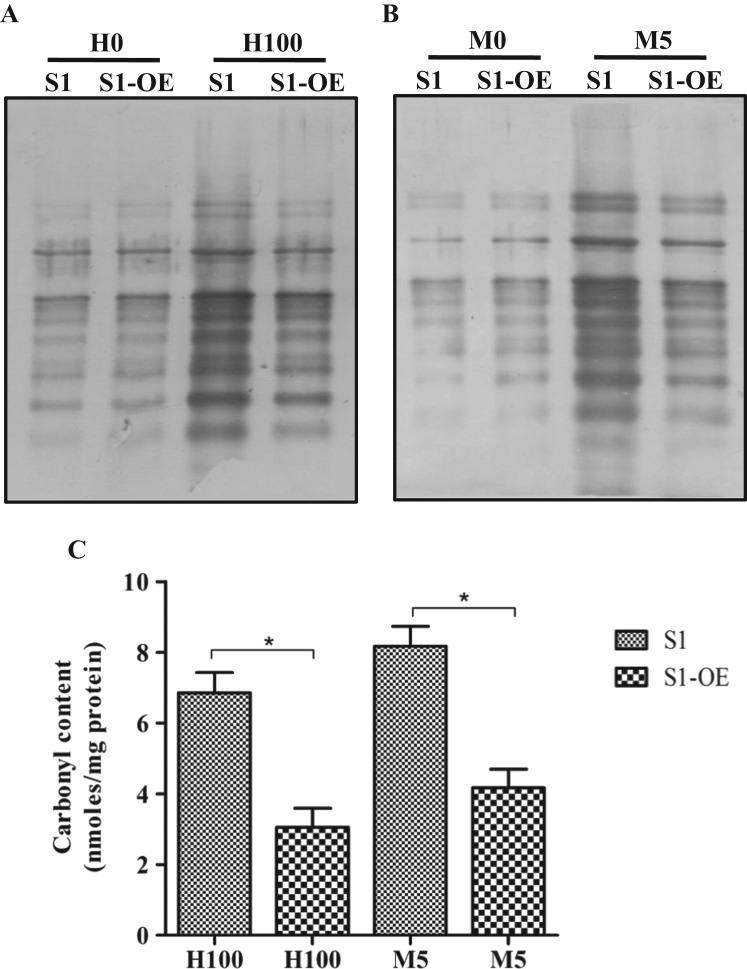
Oxidative stress induces the formation of carbonyl groups on proteins and has been widely considered as the biomarker of protein oxidation within the cells [41], [42]. To confirm the stress tolerance induced by LdCS over-expression, both S1 and S1-OE parasites were treated with lethal doses of H2O2 (100 µM) and menadione (5 µM) for 6 h; the carbonyl groups on amino acid residues derivatized using DNPH and subsequently immunoblotted using anti-DNPH antibody. Western blot analysis showed that in the case of the S1 parasites, higher oxidative stress resulted in a higher level of protein carbonylation and greater oxidative damage to intracellular proteins (Fig. 4A and B) as compared to S1-OE parasites. No significant differences were observed in the carbonylation pattern of the proteins in the untreated parasites for both the strains (S1 and S1-OE) (Fig. 4A and B). Also, quantitative measurements of protein carbonylation by spectrophotometric assay revealed that carbonyl content of proteins in S1 parasites after treatment with H2O2 (100 µM) and menadione (5 µM) for 6 h was ~2.3 and ~2.1 folds higher as compared to S1-OE parasites, respectively (Fig. 4C). Again, no significant differences were observed in the carbonyl content of the proteins in the untreated parasites for both the strains (S1 and S1-OE) (data not shown). Altogether, the results provided conclusive evidence that over-expression of LdCS protects Leishmania parasites against oxidative damage of proteins caused by ROS.
Fig. 4.
Oxidative damage to proteins. (A and B) Immunoblotting using an anti-DNPH antibody to detect oxidative damage to intracellular proteins in wild type (S1) and (S1-OE) parasites untreated and after exposure to H2O2 (100 µM) and menadione (5 µM) for 6 h, respectively. Lanes S1 and S1-OE represent 30 µg total cell lysate proteins of respective strains of L. donovani loaded in each well. H0 and M0 represent untreated controls. H100 and M5 represent total cell lysates treated with H2O2 (100 µM) and menadione (5 µM) for 6 h respectively. (C) Spectrophotometric quantitation of the protein carbonyls in total cell lysates of wild type (S1) and (S1-OE) parasites treated with H2O2 (100 µM) and menadione (5 µM) for 6 h. Results are expressed as means±SEM of three independent experiments. *, p<0.05; by Student's t-test using GraphPad Prism 5.0.
3.5. LdCS over-expressing (S1-OE) parasites alleviates the lethal increase in intracellular ROS levels by efficiently up-regulating the thiol pool
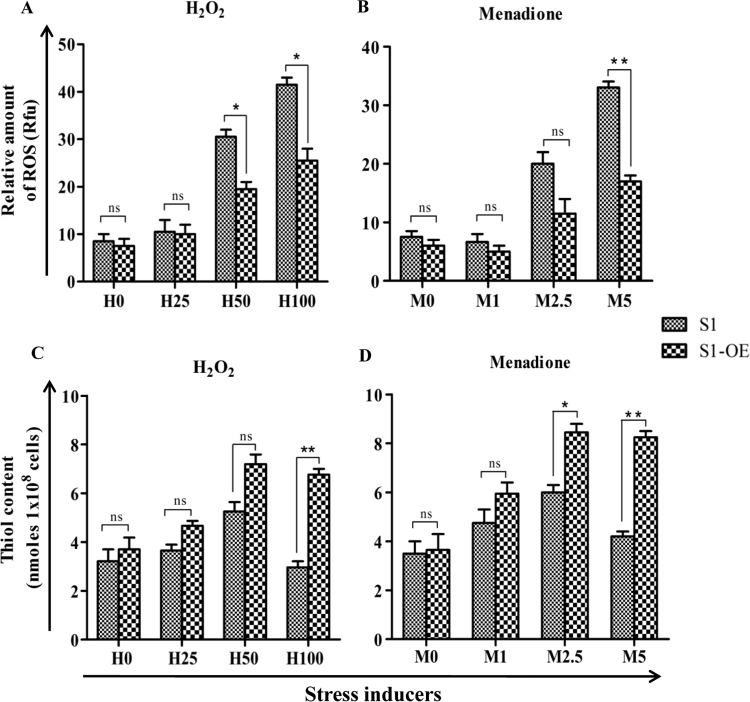
To investigate the impact of LdCS over-expression on the thiol content and/or its regulation in response to ROS, total intracellular reduced thiol content and ROS were measured in S1-OE and S1 parasites after treatment with increasing concentrations of H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM). It was observed that on treatment with 100 µM H2O2 and 5 µM menadione, S1-OE parasites accumulated ~1.6 and ~2.1 fold lesser intracellular ROS (Fig. 5A and B) and ~2.2 and ~2.0 fold higher thiol content (Fig. 5C and D), respectively, after 6 h treatment as compared to S1 cells. Thus, our results showed that S1-OE parasites exhibit more efficient thiol-based ROS quenching machinery indicating the role of up-regulated LdCS in modulation of redox homeostasis machinery of Leishmania culminating into enhanced stress tolerance and drug resistance.
Fig. 5.
Determination of intracellular ROS and thiol content of S1 and S1-OE parasites exposed to ROS inducers (H2O2 and menadione). (A and B) Wild type (S1) & (S1-OE) parasites were exposed to lethal doses of H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h and intracellular ROS were measured using Spectrofluorimeter. S1-OE parasites accumulated lesser intracellular ROS than wild type (S1) parasites upon exposure to lethal doses of oxidants. (C and D) Measurement of reduced intracellular thiol levels in wild type (S1) and S1-OE parasites after treatment with H2O2 and menadione for a period of 6 h. Results are expressed as means±SEM of three independent experiments. *, p<0.05; **, p<0.01; by Student's t-test using GraphPad Prism 5.0.
3.6. LdCS over-expression augments the ROS-primed up-regulation of antioxidant enzyme activities
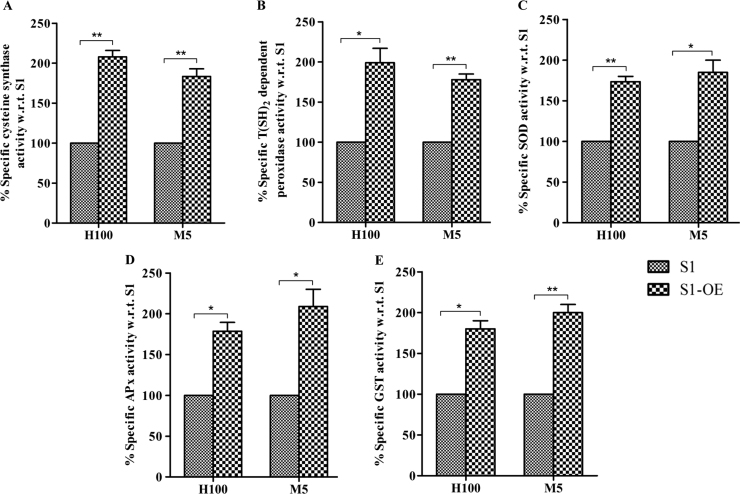
Apart from thiol content, antioxidant enzymes also play a vital role in inducing tolerance to oxidative stress. In order to ascertain the impact of LdCS over-expression on the stress induced modulation of antioxidant enzymes activities of CS, T(SH)2-dependent peroxidase, superoxide dismutase (SOD), glutathione-S-transferase (GST) and ascorbate peroxidase (APx) enzymes were measured in the cell lysates of S1 and S1-OE parasites exposed to lethal doses of H2O2 (100 µM) and menadione (5 µM) for 6 h. On treatment with ROS inducers (H2O2 and menadione), S1 parasites showed a reduction in antioxidant enzyme activities, whereas, similar treatment to S1-OE parasites elicited strong and significant up-regulation of antioxidant enzyme activities (Fig. 6). Notably, the enzyme activities of LdCS (Fig. 6A), T(SH)2-dependent peroxidase (Fig. 6B), SOD (Fig. 6C), GST (Fig. 6D) and APx (Fig. 6E) on treatment with H2O2 (100 µM) and menadione (5 µM) were found to be up-regulated by ~2.1, ~1.9, ~1.7, ~1.8, ~1.8 and ~1.8, ~1.7, ~1.9, ~2, ~2.1 fold, respectively, in S1-OE parasites as compared to S1 parasites. Conspicuously, the observed antioxidant enzyme activities were found to be inversely correlated with the build-up of ROS (Fig. 5A and B) and protein carbonylation (Fig. 4). Thus, the results show that LdCS over-expression induces the ROS-primed up-regulation of antioxidant activity in Leishmania indicating the impact of LdCS up-regulation on redox homeostasis efficiency of Leishmania and its potential role in imparting attributes favorable for drug resistance in the parasite.
Fig. 6.
Assay of LdCS activity and antioxidant enzymes in wild type (S1) and over-expressor (S1-OE) parasites. Wild type (S1) and (S1-OE) parasites were exposed to lethal dose of H2O2 (100 µM) and menadione (5 µM) for 6 h and total lysates were prepared and enzyme activities were measures as described in materials and methods section. (A) Measurement of cysteine synthase activity. (B) Measurement of trypanothione dependent peroxidase activity. (C) Measurement of SOD activity. (D) Measurement of ascorbate peroxidase activity. (E) Measurement of GST activity. Results are expressed as means±SEM of three independent experiments. *, p<0.05; **, p<0.01; by Student's t-test using GraphPad Prism 5.0.
3.7. ROS directly induces the up-regulation of LdCS accompanied by thiol pathway proteins
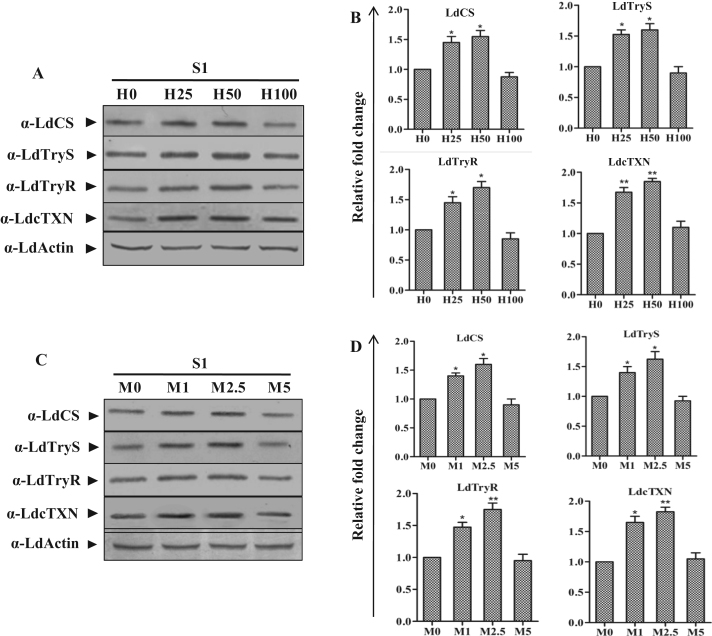
Although ROS is gaining widespread recognition as a signaling molecule in higher eukaryotes but the same is not yet established in pathogenic Leishmania parasites. Given the ROS-perplexed life cycle of Leishmania, we were interested in deciphering the ROS driven metabolic reconfiguration of LdCS and thiol machinery proteins in Leishmania. To gain insight into the regulatory network of LdCS in corroboration with thiol pathway because thiol pathway plays a pivotal role in redox homeostasis of Leishmania. Thus, S1 parasites were exposed to various doses of H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h and subjected to immunoblot analysis (Fig. 7A and C). A dose-dependent up-regulation of LdCS and different thiol metabolic pathway proteins viz. LdTryS, LdTryR and LdcTXN were observed after treatment with H2O2 as well as menadione, except at lethal doses (Fig. 7B and D). The coercive and cohesive up-regulation of these proteins by ROS inducers indicates the role of ROS in regulating the expression levels of these proteins; probably, in an effort to alleviate the lethal effects of ROS. Moreover, the results also indicate the putative association between LdCS and thiol pathway proteins in sustaining the oxidative stress conditions by the parasite.
Fig. 7.
ROS inducers (H2O2 and menadione) induce dose-dependent induction of LdCS and thiol pathway proteins (LdTryS, LdTryR and LdcTXN) expression in L. donovani. (A and C) Total cell lysates prepared from S1 parasites exposed to lethal doses of H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h were resolved on 10% SDS-PAGE and subjected to western blot analysis using α-LdCS, α-LdTryS, α-LdTryR, α-LdcTXN and α-LdActin antibodies, respectively. (B and D) Relative band intensities were determined by densitometric analysis and fold change in the expression of LdCS, LdTryS, and LdTryR & LdcTXN in S1 parasites exposed to lethal doses of H2O2 and menadione were graphically represented as means±SEM of three independent sets of experiments. Untreated parasites were used as control in the experiment. *, p<0.05; **, p<0.01; by Student's t-test using GraphPad Prism 5.0.
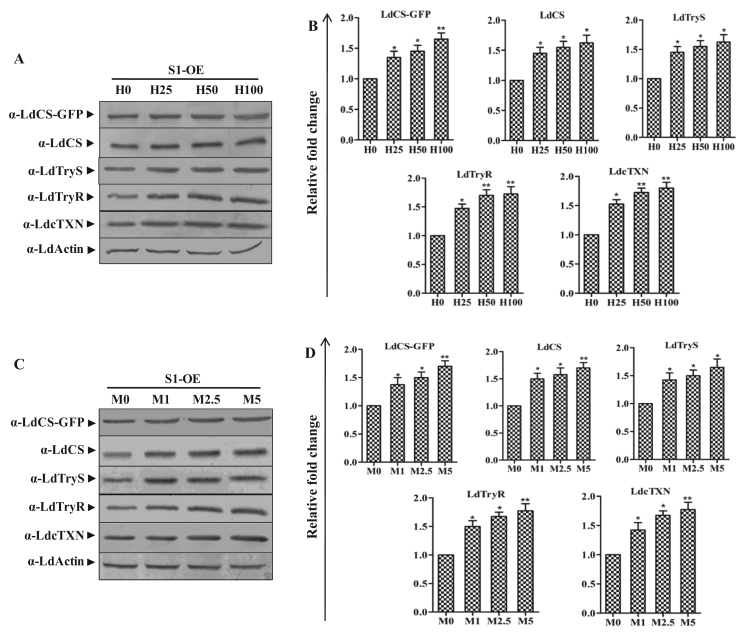
3.8. LdCS over-expression enhances the tolerance under oxidative stress due to higher expression of endogenous LdCS as well as thiol pathway proteins
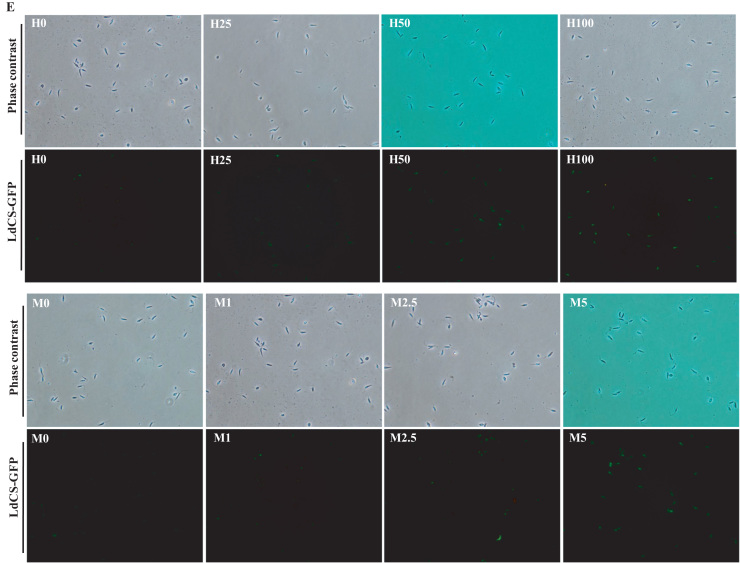
Gaining insight from the observed up-regulation of LdCS and thiol pathway proteins on treatment with ROS inducers, we further investigated the retrospective impact of LdCS over-expression on stress tolerance and thiol pathway proteins. Thus, we utilized the S1-OE strain over-expressing LdCS protein by subjecting them to immunoblot analysis with or without treatment with H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h (Fig. 8A and C). On ROS treatment, a further induction in the expression of endogenous LdCS, LdCS-GFP as well as thiol pathway proteins was observed. Interestingly, we observed a significant up-regulation of LdCS-GFP, LdCS, LdTryS, LdTryR and LdcTXN proteins of thiol pathway in the S1-OE parasites even at the lethal doses (Fig. 8B and D). Also, fluorescence microscopy analysis of S1-OE parasites (Fig. 8E) showed a visible dose dependent induction of LdCS-GFP after treatment with H2O2 and menadione. Notably, the magnitude of LdCS-GFP fluorescence was highest at maximum tested doses of H2O2 (100 µM) and menadione (5 µM) (Fig. 8E). Precisely, the results indicate two points: 1) LdCS serves a regulatory role for downstream thiol proteins and can independently modulate the thiol pathway. 2) LdCS over-expression (up-regulation) armors the parasite with enhanced ROS-primed up-regulation of thiol proteins as well as LdCS; which emphasize the potential role of CS in stress tolerance and drug resistance mechanism viz. up-regulated thiol metabolic pathway proteins associated with higher thiol content.
Fig. 8.
LdCS over-expression primed modulation of endogenous LdCS as well as thiol pathway proteins (LdTryS, LdTryR and LdcTXN) in L. donovani under oxidative stress. (A and C) Total cell lysates prepared from S1-OE parasites exposed to lethal doses of H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h were separated on 10% SDS-PAGE and subjected to western blot analysis using α-LdCS, α-LdTryS, α-LdTryR, α-LdcTXN and α-LdActin antibodies, respectively. (B and D) Relative band intensities were determined by densitometric analysis and fold change in the expression of LdCS-GFP, LdCS, LdTryS, LdTryR and LdcTXN in S1-OE parasites exposed to lethal doses of H2O2 and menadione were graphically represented as means±SEM of three independent sets of experiments. Untreated parasites were used as a control in the experiment. *, p<0.05; **, p<0.01; by Student's t-test using GraphPad Prism 5.0. (E) Fluorescence microscopy image of S1-OE parasites over-expressing LdCS-GFP protein treated with H2O2 (25 µM, 50 µM and 100 µM) and menadione (1 µM, 2.5 µM and 5 µM) for 6 h. Untreated parasites were used as a control. ,.
3.9. LdCS is up-regulated in Amp B resistant isolates as compared to sensitive strains of L. donovani
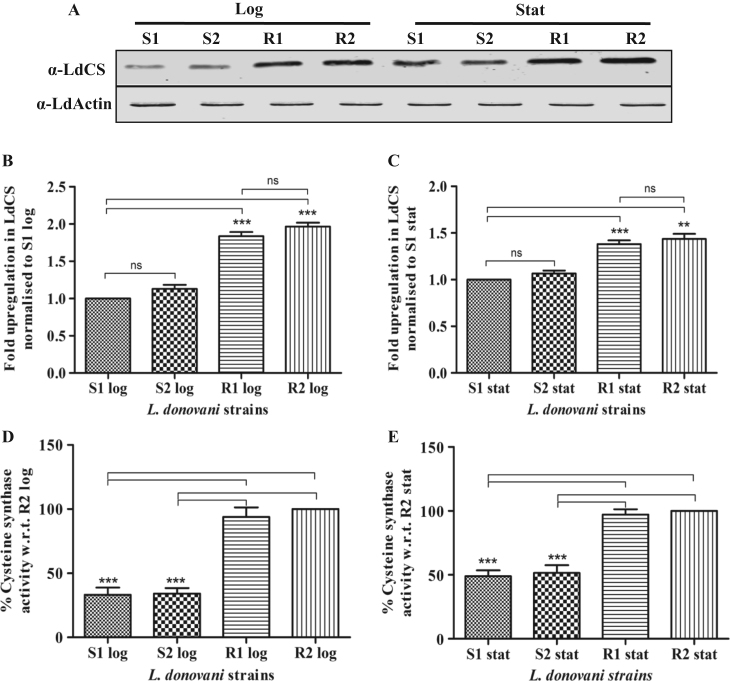
To cross validate our observations regarding the role of LdCS protein in drug resistance, we used clinical isolates of Amp B resistant parasites available in our laboratory and investigated the expression of LdCS protein in Amp B sensitive (S1 and S2) vs. resistant isolates (R1 and R2) in their logarithmic (log) vs. stationary stages (stat) by immunoblotting followed by densitometric analysis. As shown in Fig. 9A, the expression of LdCS protein was observed to be significantly higher in the resistant isolates as compared to the sensitive strains during both stages of growth indicating an apparent association between LdCS and drug resistance. The densitometric analysis showed ~1.8 fold up-regulation of LdCS expression in resistant isolates as compare to sensitive strains in the log phase (Fig. 9B) and ~1.4 fold up-regulation in the stat stage (Fig. 9C). Thus, the results indicate that LdCS expression is up-regulated in resistant isolates and during stationary stage of growth. An up-regulation of LdCS protein is expected to be manifested by a proportional increase in CS activity. To validate the qualitative as well as quantitative nature of up-regulation, we investigated LdCS enzyme activity in sensitive vs. resistant isolates during log and stat stages of growth. As shown in Fig. 9D, both the resistant isolates, R1 & R2, displayed a significant 65–70% higher activity as compared to either of the sensitive strains, S1 and S2 (P<0.001), during log stage and 50–55% higher activity in stationary stages (Fig. 9E). Thus, stationary stages invariably displayed a significantly higher CS activity as compared to logarithmic stages of growth; ~2 fold in sensitive strains and ~ 1.5 fold in resistant isolates. These results show that Amp B resistant clinical isolates have up-regulated LdCS expression and activity which further support our observations of enhanced drug resistance in S1-OE parasites.
Fig. 9.
Expression level of LdCS during logarithmic (log) and stationary (stat) stages of growth. (A) Immunoblot analysis of LdCS in total lysates of sensitive strains (S1 and S2) and resistant isolates (R1 and R2) during logarithmic and stationary growth stages. Lanes S1, S2, R1 and R2 represent 30 µg total cell lysate proteins of respective strains/isolates of L. donovani loaded into each well. α-LdActin was used as a protein loading control. (B and C) Densitometry analysis of immunoblot after normalization showing fold up-regulation of LdCS expression in L. donovani strains during logarithmic and stationary stages. (D) Densitometry analysis of immunoblot after normalization showing fold change of LdCS expression in stationary as compared to logarithmic stage in L. donovani strains. The fold change in LdCS expression during logarithmic vs. stationary stage of growth is higher in sensitive strains (S1 and S2) as compare to the resistant isolates (R1 and R2). The percentage change in LdCS activity during logarithmic (E) and stationary (F) stages of growth in sensitive strains (S1 and S2) and resistant isolates (R1 and R2) is represented. Sensitive strains show significant decrease in LdCS activity as compared to resistant isolates. (G) The fold change in cysteine synthase activity in logarithmic vs. stationary stage of growth is higher in sensitive strains (S1 and S2) as compare to the resistant isolates (R1 and R2). Results are expressed as means±SEM of three independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001; ns, non-significant by Student's t-test using GraphPad Prism 5.0.
3.10. Higher intracellular reduced thiol levels is present in Amp B resistant isolates of L. donovani
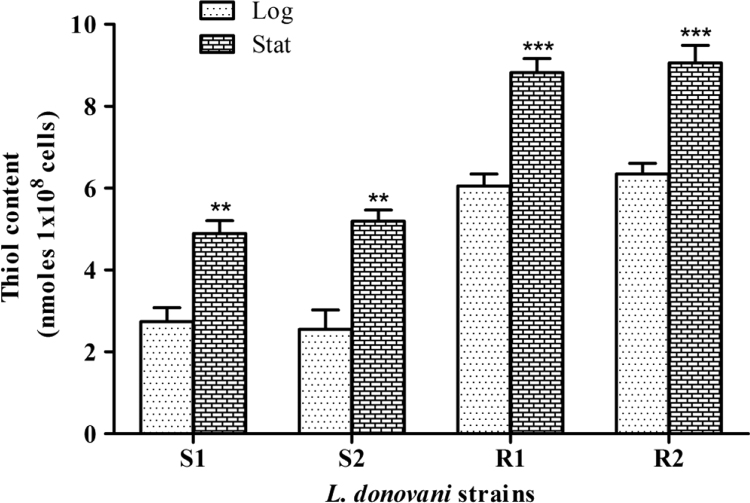
An up-regulated CS activity is expected to be associated with a higher level of downstream products and thiol content is one such vital and prominent end product whose biosynthesis may potentially depend on CS activity. To elucidate the correlation between CS activity and thiol level, total intracellular reduced thiol content during logarithmic and stationary stages of sensitive and resistant isolates was measured. As shown in Fig. 10, the thiol content was observed to be significantly higher in the resistant isolates R1 & R2, as compared to the sensitive strains S1 & S2, during both stages of growth, ~2 fold up-regulation in the log phase and ~1.5 fold up-regulation in the stat stage. Stage-wise, the thiol content was ~2 fold higher in sensitive strains of stat stage and ~1.5 fold higher in resistant isolates of stat stage as compared to their respective log stage. Importantly, a good positive correlation was observed between intracellular thiol levels and CS protein expression/activity, irrespective of growth stage and strain sensitivity. Previously, up-regulation of thiol pathway proteins [8], [20], [36] as well as thiol content [8], [34], [35] have been associated with drug resistance and virulence. Our results on physiologically relevant models suggests a potential role of LdCS in regulating thiol biosynthesis; which is a paramount factor for acquisition of drug resistance and hence, redox homeostasis in Leishmania.
Fig. 10.
Analysis of reduced intracellular thiol levels during logarithmic (log) and stationary (stat) stages of growth. The fold changes in thiol content of sensitive strains (S1 and S2) and clinical Amp B resistant isolates (R1 and R2) during logarithmic vs. stationary stage of growth. Results are expressed as means±SEM of three independent experiments. **, p<0.01; ***, p<0.001; by Student's t-test using GraphPad Prism 5.0.
3.11. Over-expression of LdCS in L. donovani increases infectivity
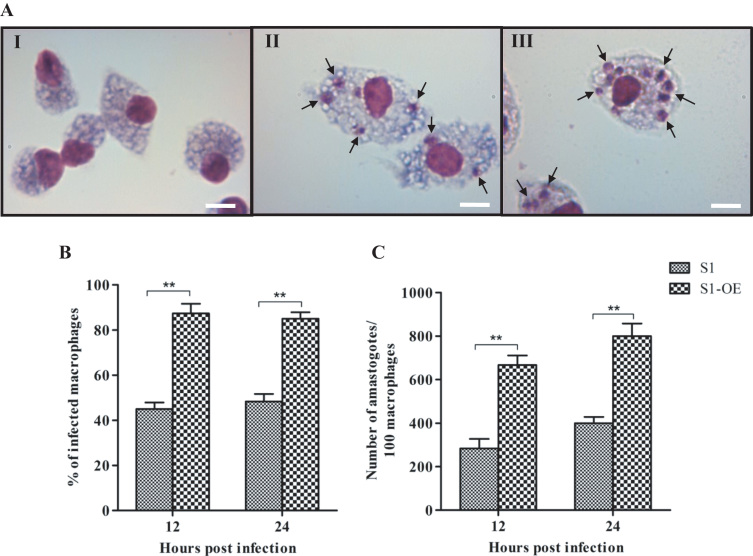
To elucidate the role of LdCS in counteracting the hostile conditions inside the macrophage during infection, we used an ex vivo model to compare the macrophage infectivity of S1 and S1-OE parasites. Peritoneal macrophages isolated from starch induced BALB/c mice were infected with S1 and S1-OE parasites. To check the integrity of the isolated macrophages, we also kept a control set of uninfected macrophages (Fig. 11). Giemsa-stained microscopic pictures of uninfected macrophages (I), macrophages infected with S1 (II) and S1-OE (III) parasites (Fig. 11A) showed that S1-OE parasites exhibit more infection as well as number of amastigotes inside the macrophages as compare to wild type (S1) parasites. To identify the differences in infectivity and rate of infection of both types of parasites, we counted infected and non-infected macrophages. S1-OE parasites showed significantly higher (~2.0 fold, p<0.01) percentage of macrophages infectivity (~90%) as compared to S1 parasites (~48%) (Fig. 11B). This significant difference in macrophages infectivity was also evident by the increase in the number of amastigotes per infected macrophage. After 12 h of infection with S1 and S1-OE parasites, numbers of amastigotes per 100 infected macrophages were found to be 279±34 and 651±39, respectively. Similarly, after 24 h of infection with S1 and S1-OE parasites, numbers of amastigotes per 100 infected macrophages were found to be 381±37 and 760±54, respectively (Fig. 11C). Thus, the number of amastigotes per infected macrophage was ~2.2 fold (p<0.01) higher with S1-OE parasites when compared to S1 parasites which is highly significant. Moreover, our results show that from 12 h to 24 h time period, the parasites did not show any significant change except 10–15% increase in number of amastigotes. Taken together, these results demonstrate that LdCS over-expression increases the infectivity of L. donovani parasites suggesting its potential role in survival under oxidative stress conditions inside macrophages.
Fig. 11.
Ex vivo infectivity assessment of S1 and S1-OE parasites inside the macrophages. Mouse peritoneal macrophages were infected with S1 and S1-OE parasites. (A) Giemsa-stained microscopic pictures of uninfected macrophages (I), macrophages infected with S1 (II) and S1-OE (III) parasites, respectively; black arrows pointing towards amastigotes. (B) Giemsa-stained infected macrophages were counted after 12 & 24 h of infection and the results are represented as the percentage of infected macrophages. (C) Giemsa-stained amastigotes per 100 infected macrophages were counted after 12 and 24 h under a light microscopy using an Olympus BX41 optical microscope at 100X. Results are expressed as means±SEM of three independent experiments. **, p<0.01; by Student's t-test using GraphPad Prism 5.0.
4. Discussion
Thiol metabolic pathway proteins orchestrate the antioxidant machinery in trypanosomatids by maintaining a reduced intracellular milieu. They protect against oxidative damage, maintain intracellular redox homeostasis and are also responsible for the maintenance of a reduced trypanothione pool in trypanosomes; essential for their survival, pathogenicity and drug resistance [22], [55], [56]. Leishmania parasites successfully acclimate to oxidative and nitrosative burst conditions encountered during host pathogen interaction; necessary for the establishment of infection [16], [57]. Cysteine is the building block of all thiols and is the precursor molecule for glutathione and trypanothione biosynthesis. Therefore, availability of sufficient cysteine is of paramount importance for maintenance of highly efficient arsenal to combat with the deleterious effects of ROS. In the present study, we elucidated the role of LdCS in the acquisition of drug resistance and redox homeostasis of Leishmania which is largely mediated by thiol pathway proteins. First, we demonstrated that over-expression of LdCS imparts stress tolerance and drug resistance to sensitive parasites by modulating the thiol pathway and antioxidant enzyme machinery. Later, we validated up-regulation of LdCS in clinical resistant isolates and infective stationary stages of growth.
CS roles in survival and growth under oxidative stress conditions have been studied in plants [45], [58] and Entamoeba histolytica [59]. Till date only a single study in L. braziliensis has been carried out to emphasize the crucial role of CS in oxidative stress tolerance [60]. To elucidate the role of CS in thiol mediated drug resistance in L. donovani parasite at molecular level, we transfected the episomal construct (pXG-LdCS-GFP+) in the Amp B sensitive L. donovani strain. Our results revealed that LdCS over-expressor (S1-OE) parasites conferred resistance to Amp B and SAG (Fig. 2). Amp B and SAG induces ROS mediated killing of parasites which decreases dose dependent viability of both S1-OE and S1 parasites but S1-OE parasites are better stress tolerant as compared to S1 parasites. Thus LdCS over-expression may play a pivotal role in drug unresponsiveness which may be due to the up-regulated thiol machinery; accompanied by higher thiol content. Similarly, increased thiol content has been reported earlier is implicated with drug resistance mechanisms in Leishmania parasites [34], [35], [61].
Further, we found that S1-OE parasites were less susceptible towards ROS inducers and successfully withstand/nullified the deleterious effects of oxidative challenge during H2O2 and menadione treatment (Fig. 3). Similar observation was previously reported in the protozoan parasite E. histolytica where over-expression of CS resulted in increased resistance towards H2O2 [59]. Likewise, over-expression of CS in Arabidopsis thaliana resulted in increased tolerance towards heavy metals and oxidative stress, accompanied by increased cysteine availability [62]. Moreover, the over-expression of bacterial gene encoding for CS in tobacco plants also resulted in elevated level of thiols; accompanied with tolerance towards stress conditions [58], [63], [64] indicating the conserved role of CS in imparting stress tolerance. An up-regulated LdCS and thiol pathway proteins activity is expected to be associated with a higher level of downstream product i.e. total reduced thiol content. Previously, over-expression of CS in E. histolytica showed that the transfected cell lines had a higher level of CS activity associated with increased total thiol content [59]. Likewise, we observed that S1-OE parasites exhibited reduced ROS levels and enhanced thiol content which indicates that these parasites alleviate intracellular ROS efficiently by maintaining the reduced thiol pool (Fig. 5).
In Trypanosomes including Leishmania, antioxidant enzymes help in maintaining the redox potential of the cell; thus forming an important part of parasite defense mechanism against oxidative stress. The major metabolic proteins which play pleiotropic role in redox homeostasis and drug resistance are the T(SH)2-dependent peroxidase, SOD, APx and GST [18], [40], [65]. As reported, T(SH)2-dependent peroxidase and tryparedoxin couple constitutes efficient peroxide detoxification machinery for the parasites; aptly associated with drug resistance [13], [18], [66]. SODs are metalloproteins which detoxifies superoxide anions (O2-) by converting them to molecular O2 or H2O2 [67]. Over-expression of FeSODA has been reported to enhance the response of L. donovani parasites to stress generated by peroxides; however significant changes in the expression levels of two other superoxide dismutase's (SODB1 and SODB2) were not observed [40]. APx is involved in the detoxification of H2O2 using ascorbate as a substrate. As reported, over-expression of APx in L. major resulted in enhanced tolerance towards oxidative stress induced apoptosis and protein damage [68]. Similarly, up-regulation of APx in L. donovani during oxidative/nitrosative stress have been reported by our group [40]. GST catalyzes the conjugation of reduced GSH to peroxides and xenobiotics for detoxification. Previous studies carried out in T. cruzi [69] and L. donovani [40] have highlighted the importance of GST enzyme in the survival of the parasites under oxidative stress conditions. Over-expression of CS in L. donovani augmented the ROS-primed up-regulation of antioxidant enzyme activities of LdCS, T(SH)2-dependent peroxidase, SOD, APx and GST on exposure to lethal stress doses (Fig. 6). The lower antioxidant enzyme activities in the S1 parasites under stress conditions may be due to stress induced error in global translation machinery; leading to the synthesis of enzymes in a functionally inactive form. This leads to accumulation of excess ROS in S1 parasites as compared to S1-OE parasites (Fig. 5), which proves to be lethal for the parasites, as observed in the viability assay (Fig. 3). In conclusion, LdCS over-expression modulates antioxidant enzymes activities in S1-OE parasites which may aids in more efficient quenching of ROS generated after oxidative stress treatment in the S1-OE parasites as compared to the S1 parasites; leading to enhanced survival.
Exposure to oxidative stress conditions cause changes in gene expression levels which form an important part of cellular defense mechanism and is mainly achieved at post transcriptional level in Leishmania [70]. Previously, up-regulation of thiol pathways proteins viz. TryS, TryR, γ-glutamylcysteine synthetase (γ-GCS), ornithine decarboxylase (ODC), cTXN and cTXNPx in L. donovani [20], [71], [72], L. braziliensis [73], [74], L. infantum [73], [74] and L. tarentolae [36], [75] have been reported to play a major role in ROS scavenging mediated stress tolerance and drug resistance. Recently, it was reported that expression of mTXN and mTXNPx proteins was also 12 and 4 fold higher in antimony resistant L. infantum canine isolates but differences in the expression of cytosolic isoforms of TXN and TXNPx were not observed in this study [76]. This suggests that either these isolates are different or canine isolated parasites differ from human isolates because antimony resistant isolates reported so far showed up-regulation of cytosolic isoforms of TXN and TXNPx [77], [78], [79]. In addition, a previous report describing kinetics of T(SH)2 biosynthesis in Trypanosoma cruzi [80] also supports the role of γ-GCS and TryS proteins in counteracting oxidative stress conditions. This study suggested that main control flux is regulated by γ-GCS and TryS proteins, followed by spermidine transporter, TryR and T(SH)2-dependent thiol pathway enzymes [80]. CS may also play a crucial role in regulating the T(SH)2 biosynthesis pathway because it provides cysteine for the biosynthesis of γ-glutamylcysteine and glutathione by γ-GCS and glutathione synthetase enzymes, respectively [6]. In this context, the observed up-regulation of thiol pathway proteins in S1 (Fig. 7) and S1-OE (Fig. 8) parasites on treatment with ROS inducers support the established role of these proteins in stress tolerance. Moreover, the significantly higher dose tolerance of ROS inducers in S1-OE parasites suggests a potent role of LdCS in stress tolerance. The cohesive up-regulation of these thiol pathway proteins in conjunction with LdCS up-regulation may contribute synergistically to enhance stress tolerance and acquisition of drug resistance.
So far, to the best of our knowledge, the link between T(SH)2 and cysteine biosynthesis pathways has been unexplored. Supplementation with methionine in culture medium significantly increases the concentration of both cysteine and trypanothione whereas extracellular addition of cysteine does not cause a similar increase in the trypanothione and glutathione levels in Leishmania [32]. Thus, this study suggests essential role of cysteine biosynthesis pathway for trypanothione biosynthesis and highlights the reason for retaining this pathway in protozoan parasites including Leishmania.
Logarithmic and stationary stages are the two major growth stages of Leishmania promastigotes life cycle, each stage having distinct metabolic and phenotypic features. During logarithmic stage, majority of the metabolic machineries are routed towards cell division, whereas, during stationary stage, differentiation from promastigotes to infective metacyclic occurs. As reported, stationary stage parasites are also linked with a shift towards up-regulated tricarboxylic acid cycle, β-oxidation and oxidative phosphorylation [81] which could result in increased bioavailability of acetyl Co-A and may favor de novo cysteine biosynthesis, although this remains to be proved experimentally. Majority of the variations in the stationary stages are aimed at either increasing the survival during infection or increasing the virulence of parasites. This metabolic shift involves alteration in the expression level of many proteins and their downstream metabolites, as exemplified by previously reported up-regulation of LdTryS protein of thiol metabolic pathway [20]. In the present study, we identified a similar up-regulation of LdCS expression/activity during stationary stages (Fig. 9) and, importantly, accompanied by an increase in total intracellular reduced thiol content (Fig. 10) which justifies the up-regulation of both LdTryS as well as LdCS enzymes, the former responsible for biosynthesis of trypanothione and the later responsible for supplying the precursor moiety of trypanothione i.e. L-cysteine. Thus, two different pathways cooperatively interact with each other to enhance trypanothione biosynthesis during stationary stages and hence, redox homeostasis. An increase in trypanothione biosynthesis would be an imperative imposition on stationary stage parasites because being the preparative phase before infection, the parasites need to armor themselves with efficient redox detoxification machinery to promptly counteract the oxidative/nitrosative burst and hostile conditions inside the macrophage during infection. In this context, our ex vivo infectivity studies of S1 and S1-OE parasites (Fig. 11) demonstrated that percentage of infectivity and number of amastigotes per infected macrophage was significantly higher with S1-OE parasites as compared to S1 parasites. LdCS over-expression enhances the infectivity of L. donovani parasites. Stress tolerant parasites are known to exhibit higher viability and/or infectivity as shown in several previous studies on CS [59], [60], cTXN [82], cTXNPx [72] and mTXNPx [83] proteins. As LdCS over-expression augments the level of these proteins (Fig. 8) and enhances the stress tolerance (Fig. 3) of parasites, the higher infectivity of S1-OE may be attributed to the cumulative effect of these proteins and strongly suggests the predominant effect of LdCS up-regulation on the redox homeostasis and infectivity of parasites.
Leishmania has two pathways for generating cysteine whereas many organisms including pathogenic parasites cope very well with just a single source. T. rangeli and T. brucei possess only the reverse transsulfuration pathway for cysteine biosynthesis [45], [84]; whereas Entamoeba solely possesses de novo or assimilatory pathway [28], [32], [33]. The redundancy to have these two alternate routes for cysteine synthesis in Leishmania spp. may be related to the availability of exogenous nutrients, which differs considerably between the invertebrate sand fly and mammalian hosts [32]; promastigotes reside in a glucose rich, amino acids scarce, slightly alkaline environment in the sand fly vectors, whereas, amastigotes have to cope with an acidic environment inside human macrophages where glucose is deficient and amino acids are abundant [85]. Indeed, axenic amastigote stage of L. donovani, L. panamensis and L. braziliensis possess higher expression levels of CS protein as compared to their non-infective stage (promastigote) [33], [60], [86]. These previous studies and our ex vivo infectivity results suggest a general mechanism of up-regulation of CS while approaching the infective stage (amastigote); aptly to armor with a highly equipped arsenal of intracellular thiol pool.
In conclusion, our study provides evidences that the over-expression of LdCS armors the pathogen with efficient thiol proteins dependent redox detoxification machinery; culminating into enhanced drug unresponsiveness. Stage and strain dependent differentially regulated LdCS expression could be due to the metabolic requirements of the parasite in order to maintain efficient thiol pool for cellular redox homeostasis to counteract hostile environment. Previously, many proteins of thiol metabolism have been explored as drug targets [14], [87] but the potential role of cysteine biosynthesis pathway components in Leishmania as drug target has remained to be investigated. Since the cysteine forms the basic building block of all thiols and the mammalian host lacks the pathway for de novo cysteine biosynthesis, CS may serve as a promising drug target worth future investigation for drug development against leishmaniasis.
Conflict of interest
The authors declared no conflicts of interest.
Author contributions
Kuljit Singh and Vahab Ali conceived and designed the experiments. Kuljit Singh carried out most of the experiments. Parool Gupta, Shashi S. Suman, Ayan K. Ghosh, Sanjiva Bimal, Krishna Pandey and Pradeep Das contributed reagents/materials/analysis tools. Kuljit Singh, Krishn Pratap Singh and Vahab Ali wrote the paper.
Acknowledgements
The pXG-GFP+ vector was received as a gift from Prof. S. M. Beverley, Department of Molecular Microbiology, Washington University School of Medicine. We also thank Dr. C. M. Gupta and Dr. Amogh A. Sahasrabuddhe, Laboratory of Structural biology, CDRI, Lucknow, India for kind gift of anti-LdActin antibody. We also acknowledge Mr. Sudarshan Prasad for technical assistance. Kuljit Singh (NIPER-Hajipur, SRF) and Krishn P. Singh (SERB- National-Post doctoral fellow), acknowledged the financial assistance in the form of fellowship support from Ministry of chemicals and fertilizers and Department of Science and Technology DST (No. DST/INT/JSPS/P-117-2011) New Delhi, India.
References
- 1.Avery S.V. Molecular targets of oxidative stress. Biochem J. 2011;434:201–210. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- 2.W.H.O, World health organization control of the leishmaniasis World Health Organ. Tech. Rep. Ser. Xii–Xiii 2010 pp. 1–186. [PubMed]
- 3.Bates P.A., Rogers M.E. New insights into the developmental biology and transmission mechanisms of Leishmania. Curr. Mol. Med. 2004;4:601–609. doi: 10.2174/1566524043360285. [DOI] [PubMed] [Google Scholar]
- 4.Fairlamb A.H., Blackburn P., Ulrich P., Chait B.T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985;227:1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert H.F. Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzym. Relat. Areas Mol. Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 6.Singh K., Garg G., Ali V. Current Therapeutics, Their Problems and Thiol Metabolism as Potential Drug Targets in Leishmaniasis. Curr. Drug Metab. 2016;17:897–919. doi: 10.2174/1389200217666160819161444. [DOI] [PubMed] [Google Scholar]
- 7.Sinha P.K., Ranjan A., Singh V.P., Das V.N., Pandey K., Kumar N., Verma N., Lal C.S., Sur D., Manna B., Bhattacharya S.K. Visceral leishmaniasis (kala-azar)--the Bihar (India) perspective. J. Infect. 2006;53:60–64. doi: 10.1016/j.jinf.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Purkait B., Kumar A., Nandi N., Sardar A.H., Das S., Kumar S., Pandey K., Ravidas V., Kumar M., De T., Singh D., Das P. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012;56:1031–1041. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pratap Singh K., Zaidi A., Anwar S., Bimal S., Das P., Ali V. Reactive oxygen species regulates expression of iron-sulfur cluster assembly protein IscS of Leishmania donovani. Free Radic. Biol. Med. 2014;75:195–209. doi: 10.1016/j.freeradbiomed.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Moreira W., Leprohon P., Ouellette M. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2011;2:e201. doi: 10.1038/cddis.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairlamb A.H., Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev. Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 12.Flohe L., Hecht H.J., Steinert P. Glutathione and trypanothione in parasitic hydroperoxide metabolism. Free Radic. Biol. Med. 1999;27:966–984. doi: 10.1016/s0891-5849(99)00172-0. [DOI] [PubMed] [Google Scholar]
- 13.Krauth-Siegel R.L., Comini M.A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim. Biophys. Acta. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Manta B., Comini M., Medeiros A., Hugo M., Trujillo M., Radi R. Trypanothione: a unique bis-glutathionyl derivative in trypanosomatids. Biochim. Biophys. Acta. 2013;1830:3199–3216. doi: 10.1016/j.bbagen.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Rhee S.G., Chae H.Z., Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Van Assche T., Deschacht M., da Luz R.A., Maes L., Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Radic. Biol. Med. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Bryk R., Griffin P., Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 18.Suman S.S., Equbal A., Zaidi A., Ansari M.Y., Singh K.P., Singh K., Purkait B., Sahoo G.C., Bimal S., Das P., Ali V. Up-regulation of cytosolic tryparedoxin in amp B resistant isolates of Leishmania donovani and its interaction with cytosolic tryparedoxin peroxidase. Biochimie. 2016;121:312–325. doi: 10.1016/j.biochi.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Brotherton M.C., Bourassa S., Legare D., Poirier G.G., Droit A., Ouellette M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014;4:126–132. doi: 10.1016/j.ijpddr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Equbal A., Suman S.S., Anwar S., Singh K.P., Zaidi A., Sardar A.H., Das P., Ali V. Stage-dependent expression and up-regulation of trypanothione synthetase in amphotericin B resistant Leishmania donovani. PLoS One. 2014;9:e97600. doi: 10.1371/journal.pone.0097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariyanayagam M.R., Oza S.L., Guther M.L., Fairlamb A.H. Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome. Biochem. J. 2005;391:425–432. doi: 10.1042/BJ20050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger S., Schwarz W., Ariyanayagam M.R., Fairlamb A.H., Krauth-Siegel R.L., Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- 23.Comini M.A., Krauth-Siegel R.L., Flohe L. Depletion of the thioredoxin homologue tryparedoxin impairs antioxidative defence in African trypanosomes. Biochem. J. 2007;402:43–49. doi: 10.1042/BJ20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson S.R., Horn D., Prathalingam S.R., Kelly J.M. RNA interference identifies two hydroperoxide metabolizing enzymes that are essential to the bloodstream form of the african trypanosome. J. Biol. Chem. 2003;278:31640–31646. doi: 10.1074/jbc.M303035200. [DOI] [PubMed] [Google Scholar]
- 25.Piacenza L., Peluffo G., Alvarez M.N., Kelly J.M., Wilkinson S.R., Radi R. Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem. J. 2008;410:359–368. doi: 10.1042/BJ20071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson S.R., Temperton N.J., Mondragon A., Kelly J.M. Distinct mitochondrial and cytosolic enzymes mediate trypanothione-dependent peroxide metabolism in Trypanosoma cruzi. J. Biol. Chem. 2000;275:8220–8225. doi: 10.1074/jbc.275.11.8220. [DOI] [PubMed] [Google Scholar]
- 27.Barr S.D., Gedamu L. Role of peroxidoxins in Leishmania chagasi survival. Evidence of an enzymatic defense against nitrosative stress. J. Biol. Chem. 2003;278:10816–10823. doi: 10.1074/jbc.M212990200. [DOI] [PubMed] [Google Scholar]
- 28.Nozaki T., Ali V., Tokoro M. Sulfur-containing amino acid metabolism in parasitic protozoa. Adv. Parasitol. 2005;60:1–99. doi: 10.1016/S0065-308X(05)60001-2. [DOI] [PubMed] [Google Scholar]
- 29.Krauth-Siegel R.L., Leroux A.E. Low-molecular-mass antioxidants in parasites. Antioxid. Redox Signal. 2012;17:583–607. doi: 10.1089/ars.2011.4392. [DOI] [PubMed] [Google Scholar]
- 30.Ali V., Nozaki T. Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by "amitochondriate" protozoan parasites. Clin. Microbiol. Rev. 2007;20:164–187. doi: 10.1128/CMR.00019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali V., Nozaki T. Iron-sulphur clusters, their biosynthesis, and biological functions in protozoan parasites. Adv. Parasitol. 2013;83:1–92. doi: 10.1016/B978-0-12-407705-8.00001-X. [DOI] [PubMed] [Google Scholar]
- 32.Williams R.A., Westrop G.D., Coombs G.H. Two pathways for cysteine biosynthesis in Leishmania major. Biochem. J. 2009;420:451–462. doi: 10.1042/BJ20082441. [DOI] [PubMed] [Google Scholar]
- 33.Singh K., Singh K.P., Equbal A., Suman S.S., Zaidi A., Garg G., Pandey K., Das P., Ali V. Interaction between cysteine synthase and serine O-acetyltransferase proteins and their stage specific expression in Leishmania donovani. Biochimie. 2016;131:29–44. doi: 10.1016/j.biochi.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee A., Padmanabhan P.K., Singh S., Roy G., Girard I., Chatterjee M., Ouellette M., Madhubala R. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 2007;59:204–211. doi: 10.1093/jac/dkl494. [DOI] [PubMed] [Google Scholar]
- 35.Mandal G., Wyllie S., Singh N., Sundar S., Fairlamb A.H., Chatterjee M. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology. 2007;134:1679–1687. doi: 10.1017/S0031182007003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haimeur A., Guimond C., Pilote S., Mukhopadhyay R., Rosen B.P., Poulin R., Ouellette M. Elevated levels of polyamines and trypanothione resulting from overexpression of the ornithine decarboxylase gene in arsenite-resistant Leishmania. Mol. Microbiol. 1999;34:726–735. doi: 10.1046/j.1365-2958.1999.01634.x. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2001. Molecular Cloning: a Laboratory Manual. [Google Scholar]
- 38.Ha D.S., Schwarz J.K., Turco S.J., Beverley S.M. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 39.Beverley S.M., Clayton C.E. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol. Biol. 1993;21:333–348. doi: 10.1385/0-89603-239-6:333. [DOI] [PubMed] [Google Scholar]
- 40.Sardar A.H., Kumar S., Kumar A., Purkait B., Das S., Sen A., Kumar M., Sinha K.K., Singh D., Equbal A., Ali V., Das P. Proteome changes associated with Leishmania donovani promastigote adaptation to oxidative and nitrosative stresses. J. Proteom. 2013;81:185–199. doi: 10.1016/j.jprot.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Weber D., Davies M.J., Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol. 2015;5:367–380. doi: 10.1016/j.redox.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Augustyniak E., Adam A., Wojdyla K., Rogowska-Wrzesinska A., Willetts R., Korkmaz A., Atalay M., Weber D., Grune T., Borsa C., Gradinaru D., Chand Bollineni R., Fedorova M., Griffiths H.R. Validation of protein carbonyl measurement: a multi-centre study. Redox Biol. 2015;4:149–157. doi: 10.1016/j.redox.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh A.K., Sardar A.H., Mandal A., Saini S., Abhishek K., Kumar A., Purkait B., Singh R., Das S., Mukhopadhyay R., Roy S., Das P. Metabolic reconfiguration of the central glucose metabolism: a crucial strategy of Leishmania donovani for its survival during oxidative stress. FASEB J. 2015;29:2081–2098. doi: 10.1096/fj.14-258624. [DOI] [PubMed] [Google Scholar]
- 44.Singh K., Saleh A.A., Bhadra A.K., Roy I. Hsp104 as a key modulator of prion-mediated oxidative stress in Saccharomyces cerevisiae. Biochem. J. 2013;454:217–225. doi: 10.1042/BJ20121806. [DOI] [PubMed] [Google Scholar]
- 45.Romero I., TÃllez J., Yamanaka L.E., Steindel M., Romanha A.J., Grisard E.C. Transsulfuration is an active pathway for cysteine biosynthesis in Trypanosoma rangeli. Parasit. Vectors. 2014;7:197. doi: 10.1186/1756-3305-7-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overath P., Czichos J., Haas C. The effect of citrate/cis-aconitate on oxidative metabolism during transformation of Trypanosoma brucei. Eur. J. Biochem. 1986;160:175–182. doi: 10.1111/j.1432-1033.1986.tb09955.x. [DOI] [PubMed] [Google Scholar]
- 47.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 48.Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 49.Gaitonde M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967;104:627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 51.Henderson G.B., Fairlamb A.H., Cerami A. Trypanothione dependent peroxide metabolism in Crithidia fasciculata and Trypanosoma brucei. Mol. Biochem. Parasitol. 1987;24:39–45. doi: 10.1016/0166-6851(87)90113-7. [DOI] [PubMed] [Google Scholar]
- 52.Ray A., Dittel B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010;35:E1488. doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brajtburg J., Powderly W.G., Kobayashi G.S., Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob. Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.No J.H. Visceral leishmaniasis: revisiting current treatments and approaches for future discoveries. Acta Trop. 2016;155:113–123. doi: 10.1016/j.actatropica.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Comini M.A., Guerrero S.A., Haile S., Menge U., Lunsdorf H., Flohe L. Validation of Trypanosoma brucei trypanothione synthetase as drug target. Free Radic. Biol. Med. 2004;36:1289–1302. doi: 10.1016/j.freeradbiomed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Dumas C., Ouellette M., Tovar J., Cunningham M.L., Fairlamb A.H., Tamar S., Olivier M., Papadopoulou B. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997;16:2590–2598. doi: 10.1093/emboj/16.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deschacht M., Van Assche T., Hendrickx S., Bult H., Maes L., Cos P. Role of oxidative stress and apoptosis in the cellular response of murine macrophages upon Leishmania infection. Parasitology. 2012;139:1429–1437. doi: 10.1017/S003118201200073X. [DOI] [PubMed] [Google Scholar]
- 58.Sirko A., Blaszczyk A., Liszewska F. Overproduction of SAT and/or OASTL in transgenic plants: a survey of effects. J. Exp. Bot. 2004;55:1881–1888. doi: 10.1093/jxb/erh151. [DOI] [PubMed] [Google Scholar]
- 59.Nozaki T., Asai T., Sanchez L.B., Kobayashi S., Nakazawa M., Takeuchi T. Characterization of the gene encoding serine acetyltransferase, a regulated enzyme of cysteine biosynthesis from the protist parasites Entamoeba histolytica and Entamoeba dispar. regulation and possible function of the cysteine biosynthetic pathway in Entamoeba. J. Biol. Chem. 1999;274:32445–32452. doi: 10.1074/jbc.274.45.32445. [DOI] [PubMed] [Google Scholar]
- 60.Romero I., Tellez J., Romanha A.J., Steindel M., Grisard E.C. Upregulation of cysteine synthase and cystathionine beta-Synthase contributes to Leishmania braziliensis survival under oxidative stress. Antimicrob. Agents Chemother. 2015;59:4770–4781. doi: 10.1128/AAC.04880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukhopadhyay R., Dey S., Xu N., Gage D., Lightbody J., Ouellette M., Rosen B.P. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA. 1996;93:10383–10387. doi: 10.1073/pnas.93.19.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dominguez-Solis J.R., Lopez-Martin M.C., Ager F.J., Ynsa M.D., Romero L.C., Gotor C. Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnol. J. 2004;2:469–476. doi: 10.1111/j.1467-7652.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 63.Noji M., Saito M., Nakamura M., Aono M., Saji H., Saito K. Cysteine synthase overexpression in tobacco confers tolerance to sulfur-containing environmental pollutants. Plant Physiol. 2001;126:973–980. doi: 10.1104/pp.126.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liszewska F., Blaszczyk A., Sirko A. Modification of non-protein thiols contents in transgenic tobacco plants producing bacterial enzymes of cysteine biosynthesis pathway. Acta Biochim. Pol. 2001;48:647–656. [PubMed] [Google Scholar]
- 65.Pal S., Dolai S., Yadav R.K., Adak S. Ascorbate peroxidase from Leishmania major controls the virulence of infective stage of promastigotes by regulating oxidative stress. PLoS One. 2010;5:e11271. doi: 10.1371/journal.pone.0011271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flohe L., Budde H., Bruns K., Castro H., Clos J., Hofmann B., Kansal-Kalavar S., Krumme D., Menge U., Plank-Schumacher K., Sztajer H., Wissing J., Wylegalla C., Hecht H.J. Tryparedoxin peroxidase of Leishmania donovani: molecular cloning, heterologous expression, specificity, and catalytic mechanism. Arch. Biochem. Biophys. 2002;397:324–335. doi: 10.1006/abbi.2001.2688. [DOI] [PubMed] [Google Scholar]
- 67.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 68.Dolai S., Yadav R.K., Pal S., Adak S. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot. Cell. 2009;8:1721–1731. doi: 10.1128/EC.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoneck R., Plumas-Marty B., Taibi A., Billaut-Mulot O., Loyens M., Gras-Masse H., Capron A., Ouaissi A. Trypanosoma cruzi cDNA encodes a tandemly repeated domain structure characteristic of small stress proteins and glutathione S-transferases. Biol. Cell. 1994;80:1–10. doi: 10.1016/0248-4900(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 70.Vanhamme L., Pays E. Control of gene expression in trypanosomes. Microbiol. Rev. 1995;59:223–240. doi: 10.1128/mr.59.2.223-240.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyllie S., Mandal G., Singh N., Sundar S., Fairlamb A.H., Chatterjee M. Elevated levels of tryparedoxin peroxidase in antimony unresponsive Leishmania donovani field isolates. Mol. Biochem. Parasitol. 2010;173:162–164. doi: 10.1016/j.molbiopara.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iyer J.P., Kaprakkaden A., Choudhary M.L., Shaha C. Crucial role of cytosolic tryparedoxin peroxidase in Leishmania donovani survival, drug response and virulence. Mol. Microbiol. 2008;68:372–391. doi: 10.1111/j.1365-2958.2008.06154.x. [DOI] [PubMed] [Google Scholar]
- 73.Andrade J.M., Murta S.M. Functional analysis of cytosolic tryparedoxin peroxidase in antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum lines. Parasit. Vectors. 2014;7:406. doi: 10.1186/1756-3305-7-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matrangolo F.S., Liarte D.B., Andrade L.C., de Melo M.F., Andrade J.M., Ferreira R.F., Santiago A.S., Pirovani C.P., Silva-Pereira R.A., Murta S.M. Comparative proteomic analysis of antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol. Biochem. Parasitol. 2013;190:63–75. doi: 10.1016/j.molbiopara.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Grondin K., Haimeur A., Mukhopadhyay R., Rosen B.P., Ouellette M. Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 1997;16:3057–3065. doi: 10.1093/emboj/16.11.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez V.G., Hernandez R.G., Lopez V.C., Tomas A.M., Sanchez J.M., Castanys S., Gamarro F. Decreased antimony uptake and overexpression of genes of thiol metabolism are associated with drug resistance in a canine isolate of Leishmania infantum. Int. J. Parasitol.: Drugs Drug Resist. 2016;6:133–139. doi: 10.1016/j.ijpddr.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Y.C., Hsu J.Y., Chiang S.C., Lee S.T. Distinct overexpression of cytosolic and mitochondrial tryparedoxin peroxidases results in preferential detoxification of different oxidants in arsenite-resistant Leishmania amazonensis with and without DNA amplification. Mol. Biochem. Parasitol. 2005;142:66–75. doi: 10.1016/j.molbiopara.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Wyllie S., Vickers T.J., Fairlamb A.H. Roles of trypanothione S-transferase and tryparedoxin peroxidase in resistance to antimonials. Antimicrob. Agents Chemother. 2008;52:1359–1365. doi: 10.1128/AAC.01563-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu J.Y., Lin Y.C., Chiang S.C., Lee S.T. Divergence of trypanothione-dependent tryparedoxin cascade into cytosolic and mitochondrial pathways in arsenite-resistant variants of Leishmania amazonensis. Mol. Biochem. Parasitol. 2008;157:193–204. doi: 10.1016/j.molbiopara.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Olin-Sandoval V., Gonzalez-Chavez Z., Berzunza-Cruz M., Martinez I., Jasso-Chavez R., Becker I., Espinoza B., Moreno-Sanchez R., Saavedra E. Drug target validation of the trypanothione pathway enzymes through metabolic modelling. FEBS J. 2012;279:1811–1833. doi: 10.1111/j.1742-4658.2012.08557.x. [DOI] [PubMed] [Google Scholar]
- 81.Berg M., Vanaerschot M., Jankevics A., Cuypers B., Maes I., Mukherjee S., Khanal B., Rijal S., Roy S., Opperdoes F., Breitling R., Dujardin J.C. Metabolic adaptations of Leishmania donovani in relation to differentiation, drug resistance, and drug pressure. Mol. Microbiol. 2013;90:428–442. doi: 10.1111/mmi.12374. [DOI] [PubMed] [Google Scholar]
- 82.Romao S., Castro H., Sousa C., Carvalho S., Tomas A.M. The cytosolic tryparedoxin of Leishmania infantum is essential for parasite survival. Int. J. Parasitol. 2009;39:703–711. doi: 10.1016/j.ijpara.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Castro H., Teixeira F., Romao S., Santos M., Cruz T., Florido M., Appelberg R., Oliveira P., Ferreira-da-Silva F., Tomas A.M. Leishmania mitochondrial peroxiredoxin plays a crucial peroxidase-unrelated role during infection: insight into its novel chaperone activity. PLoS Pathog. 2011;7:e1002325. doi: 10.1371/journal.ppat.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okalang U., Nanteza A., Matovu E., Lubega G.W. Identification of coding sequences from a freshly prepared Trypanosoma brucei brucei expression library by polymerase chain reaction. Int. J. Biochem. Mol. Biol. 2013;4:73–82. [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenzweig D., Smith D., Opperdoes F., Stern S., Olafson R.W., Zilberstein D. Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J. 2008;22:590–602. doi: 10.1096/fj.07-9254com. [DOI] [PubMed] [Google Scholar]
- 86.Walker J., Vasquez J.J., Gomez M.A., Drummelsmith J., Burchmore R., Girard I., Ouellette M. Identification of developmentally-regulated proteins in Leishmania panamensis by proteome profiling of promastigotes and axenic amastigotes. Mol. Biochem. Parasitol. 2006;147:64–73. doi: 10.1016/j.molbiopara.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Leopold F. The trypanothione system and the opportunities it offers to create drugs for the neglected kinetoplast diseases. Biotechnol. Adv. 2012;30:294–301. doi: 10.1016/j.biotechadv.2011.05.012. [DOI] [PubMed] [Google Scholar]