Abstract
Summary: Huntington's disease is an autosomal dominant genetic disease, which results in progressive neuronal degeneration in the neostriatum and neocortex, and associated functional impairments in motor, cognitive, and psychiatric domains. Although the genetic mutation is identified, involving an abnormal CAG expansion within the htt gene on chromosome 4, the mechanism by which this leads to neuronal cell death and the question of why striatal neurones are targeted both remain unknown. Thus, in addition to the search for molecular and genetic strategies to inhibit development of the disease, we still need to identify effective strategies for cellular repair in affected individuals. Aspects of the human neuropathology can be well modeled by excitotoxic or metabolic lesions in experimental animals, and in transgenic mice carrying the htt mutation, providing the basis for testing alternative therapeutic strategies. The rationale and efficacy of alternative cell therapies are reviewed, including transplantation repair with embryonic striatal tissues, expansion and differentiation of striatal-like cells from stem cells, and in vivo and ex vivo gene therapy for delivery of neuroprotective growth factor molecules. Pilot and experimental clinical trials of several approaches are now also underway, and the alternative strategies are compared.
Keywords: Huntington's disease, cell therapy, neural transplantation, fetal tissues, stem cells, neuroprotection, trophic factors, ex vivo gene therapy
INTRODUCTION
Huntington's disease is an autosomal dominant neurodegenerative disorder associated with progressive cell loss and atrophy predominantly in the striatum and neocortex.1,2 Onset is typically in middle age (median age, 35-40 years) but can also occur less commonly in juveniles and in old age; the corticostriatal pathology is associated with a triad of cognitive, motor, and psychiatric symptoms leading to progressive disability and death within approximately 15-20 years. The disease is attributable to an inherited mutation of a polyglutamine expansion in a gene on chromosome 4, huntingtin (htt), the function of which still remains unknown 10 years after its identification.3 Although the mechanism of toxicity also remains unknown, the mutant protein exhibits abnormal aggregation and is toxic to cells in vitro. Moreover, abnormal huntingtin protein aggregates are seen to form within the cytoplasm and congregate within the nucleus of affected neurons both in postmortem brain tissues from patients4 and in transgenic animal models of the disease.5 Again, why these aggregates should concentrate in striatal neurones, and whether they are the cause of, or a marker of, the cell's response to degeneration remains unknown.
It can be anticipated that as we understand progressively more about the mechanisms of pathogenicity, new and more specific strategies for therapy will become available. In the meantime, therapeutic approaches can be considered along the following progression: from an early perspective of the disease as a devastating and untreatable progressive form of madness or dementia, through the recent decades during which a range of psychiatric drugs can alleviate some of the more overt disturbances of mood and hyperactivity but have no influence on the cognitive symptoms or progression of the disease, to a contemporary search for therapies at the cellular level to protect or slow cell death and to replace lost cells by transplantation. The approach to treating psychiatric symptoms is based on general pharmacological principles not specific to Huntington's disease, which are already well established in clinical practice, and this literature is well reviewed elsewhere.6,7 By contrast, the newer cell-based therapies, which are specifically targeted at the cellular pathology of Huntington's disease, is an area of active contemporary research that has only recently reached the stage of preliminary clinical trials and is the topic to which we restrict the present overview.
ANIMAL MODELS OF HUNTINGTON′S DISEASE
Excitotoxic amino acids
The development of cell-based therapies for Huntington's disease has over the last two decades been heavily dependent upon the models available for their investigation. Until recently, the most widely studied models have involved the use of excitotoxins and other cellular cytotoxins to induce patterns of cell loss in the brain of experimental animals that mimic the pathology of the human disease. The first such model involved stereotaxic injection of the prototypical excitotoxin kainic acid into the neostriatum of rats, inducing focal striatal cell loss and neurochemical depletions in dopamine, acetylcholine, and GABAergic markers of the intact striatum, whereas sparing fibers of passage.8–10 However, kainic acid has marked epileptogenic side effects causing remote nonspecific damage, and was soon replaced first by ibotenic acid and then quinolinic acid as the excitoxins of choice to produce less nonspecific toxicity and a profile of striatal cell loss that more closely mimics that seen in the human disease.10–12 These toxins target striatal neurones and consequently induce hyperkinesias, impaired motor skills, and deficits in spatial maze learning and executive function that are the equivalent in the rat of key motor and cognitive symptoms of human Huntington's disease.10,13
Metabolic toxins
A second class of model that has attracted increasing attention over the last decade is use of toxins such as 3-nitropropionic acid or malonate that disrupt mitochondrial energy metabolism, leading to striatal cell death and functional deficits14,15 by a mechanism that may reflect similar deficits in cellular metabolism observed in Huntington's disease.16 Some of the closest mimicry of the human disease has been provided by slow chronic administration in experimental primates.17 However, although metabolic toxins may provide a higher level of neuropathological validity than excitotoxic lesions, the interanimal variability and the incidence of gross nonspecific striatal damage are higher, it requires very slow chronic titration of delivery to achieve an acceptable level of specificity, and in practice these toxins are less suitable for exploring many cell therapies than the conventional excitotoxins.18
Transgenic mice
With the identification of the mutant gene, several groups have now generated transgenic mice carrying different forms of the mutant gene.19 The first reported strains involved insertion of an exon 1 fragment of the human gene carrying greatly expanded (115-150) CAG repeat lengths.20 Of the several lines generated, the R6/2 line has been the most studied, and exhibits the development of a behavioral phenotype involving ataxia, tremor, and a tendency to epilepsy from 8 weeks of age. The disease progresses rapidly with marked weight loss, and results in death by ∼13-15 weeks of age.20 Subsequent behavioral studies have indicated clear cognitive as well as motor impairments equivalent to those observed in human Huntington's disease.21,22 Although these mice show rather little overt cell death, at least until the very latest stage of the disease, it was in this strain that the intraneuronal nuclear inclusions were first demonstrated,5 suggesting that the phenotype is an expression of abnormal function of cells expressing the mutant protein rather than a result of cell death per se. This is supported by subsequent demonstration of impaired mitochondrial function in striatal neurons,23 abnormalities in the expression of glutamate, dopamine, and cholinergic receptors in the striatum,24 and abnormalities in synaptic plasticity seen in reduced long-term potentiation in hippocampal slices25 from transgenic animals.
Several other transgenic lines have subsequently become available, including transgenics containing different fragments of the huntingtin gene, insertion of the full-length gene in yeast artificial chromosomes, and knock-in of the human gene or mouse gene containing expanded CAG repeats into the normal gene locus. These each exhibit different profiles of aggregate expression, cellular pathology, and functional impairment, with significant differences in the time course of expression, disease, and lifespan.19 Of particular interest is the HD94 mouse line, containing an N-terminal fragment of the htt gene with 94 CAG repeats under the control of the Tet-Off conditional expression system.26 Untreated mice exhibit motor symptoms from ∼4-8 weeks of age, they express the mutant gene and develop inclusions when examined at 8 weeks of age, and show overt cell loss by 18 weeks of age, although disease is not so severe and lifespan is only moderately shortened. Switching off gene expression by administration of doxycycline from 18 weeks of age not only blocks further development of the disease but, more surprisingly, cellular inclusions retract and normal behavior is restored by 34 weeks of age.26 This provides further evidence not only that cellular dysfunction rather than cell death is the key cause of symptoms, at least early in the disease, but offers a particular stimulus for developing treatment modalities targeted at blocking the development of cellular dysfunction.19
PRIMARY FETAL CELLS FOR STRIATAL TRANSPLANTATION
Striatal transplantation in animals
Embryonic striatal tissues have been transplanted into the site of excitotoxic striatal lesions in mice, rats, and monkeys, where the grafts are seen to survive, express a wide range of cellular markers characteristic of the normal striatum, and function as demonstrated by alleviation of a range of behavioral deficits associated with the lesions. Thus, recovery has been described on motor tasks such as locomotor hyperactivity, rotational asymmetry, and use of the forepaws for skilled movements such as the reach, grasp, and retrieval of food pellets from narrow containers, and in a range of cognitive tasks, including passive avoidance, spatial learning, and delayed alternation, and in associative learning tasks in operant chambers.27–29 Correct performance of such cognitive tasks in particular requires the striatum to be integrated in a cortical–subcortical network, and recovery by grafts appears to require restoration of a functional circuitry in the brain.30 Indeed, under appropriate implantation conditions, striatal grafts develop rich afferent and efferent connections with appropriate targets in the host brain,31 which are shown to be functional both electrophysiologically32,33 and neurochemically.34–36 Therefore, there is now considerable evidence to indicate that striatal grafts can yield a functional repair of striatal cell loss that is similar to this aspect of the cellular pathology of Huntington's disease.
Striatal cell transplantation has developed almost entirely using excitotoxic lesion models, and there have been only two studies so far of cell transplantation in transgenic mice. In the first, we showed that striatal grafts survive readily in the striatum of R6/2 mice without the grafts themselves developing inclusion pathology, but the functional effects in alleviating hypokinesia were modest.37 In the second, cortical grafts in the anterior cingulate cortex of R6/1 transgenics also produced a modest delay in the development of motor symptoms.38
Both studies emphasize the need to consider the extent to which both striatal lesions and transgenic mouse models are predictive to the clinical situation. In the excitotoxic lesions, pathology has a restricted focus in the striatum and does not include the cortical pathology that develops later in the human disease. By contrast, in the transgenic mouse models, pathology can be very widely distributed throughout the brain,39 far more extensively than has typically been described in the adult human condition. Consequently, a more detailed consideration of graft placement will be essential in defining optimal modeling of potential clinical applications. As the disease progresses, the human pathology encompasses neocortical and other areas of the brain, but the striatal degeneration typically appears first; cell loss here is the most extensive, with the most widely affected cortical areas being the prefrontal and other areas of association cortex that project most heavily to the striatum.40,41 Thus, if the striatal pathology is primary, then striatal repair by cell transplantation may provide an effective reparative therapy. Conversely, others have argued that cortical and striatal pathology may be independent features of the disease42 and, because we require an intact circuit involving both cortical and subcortical components, focusing repair on just one, albeit major, component is unlikely to be effective.43 There remains no unequivocal neuropathological evidence that can convincingly discriminate between these hypotheses. Consequently, well conducted clinical trials are now required not only to determine whether clinical efficacy of neuronal grafts, similar to that which has been demonstrated in Parkinson's disease,44,45 can translate to Huntington's disease,46 but also to resolve the underlying dispute regarding the mechanisms of graft influences on disease progression and function.
Preclinical studies
The first steps in developing an effective clinical transplantation program in Huntington's disease requires the preclinical validation of the protocols and procedures necessary for translation of animal studies to man. This involves determination of the optimal parameters for collection and preparation of human fetal tissues for transplantation. Thus, a combination of anatomical studies of human development, growth of human striatal cells in culture, and xenotransplantation of human striatal tissues into experimental rats or mice, indicates that human fetal tissues at around 8-10 weeks of gestational age are likely to be suitable for harvesting striatal tissues for transplantation.47,48 Moreover, the xenotransplantation studies allow the accuracy of the striatal tissue identification and dissection to be validated so as to confirm that the striatal tissues collected do indeed differentiate into striatal neurones.49–51
The optimal dissection has been a particular issue of concern. Striatal grafts in rats typically have a “patchy” appearance comprising both striatal-like and nonstriatal-like compartments, depending on the morphology, neurochemical phenotypes, and receptors expressed by the cells.52 The striatum develops from the lateral and medial ridges of the ganglionic eminence (LGE and MGE, respectively) in the floor of the embryonic lateral ventricle.53 Traditionally, striatal grafts comprised the whole ganglionic eminence (i.e., containing both medial and lateral parts). However, Isacson and colleagues54,55 have shown that the grafts with the highest proportion of striatal-like cells, as determined by concentrations of acetylcholinesterase-positive neuropil, were obtained with a dissection restricted to the lateral compartment, and this has been confirmed using other measures such as DARPP-32 (dopamine- and adenosine 3′,5′-monophosphate-regulated phosphoprotein, Mr = 32,000) staining of medium spiny projection neurons.55–58 This would suggest that an LGE dissection would be optimal for maximizing specificity and functional recovery. Conversely, however, many striatal interneurons originate from the MGE,59 and it appears that a dissection that combines both compartments yields a higher total number of DARPP-32 cells and more convincing evidence for functional recovery.60 This suggests that cells from both compartments contribute to the mature striatal phenotype, and most clinical trials have selected a whole ganglionic eminence (WGE) dissection, at the expense of including a higher proportion of nonstriatal precursors, rather than one restricted to the LGE alone (Table 1).
TABLE 1.
Clinical Trials of Cell Transplants in Huntington's Disease
| Study | n | Donor Tissue/Side
|
Implant Tracks | Immune Treatment | Safety | Efficacy | Imaging | Anatomy | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Weeks | Dissect | ||||||||
| Cuba and Czech | 4 | 2–3 | ? | VM or WGE | 2–3 ? [B] | CyA | No pathological or immunological response137 | Not yet possible to determine | MRI-guided stereotaxy; no reported follow-up | |
| Mexico City | 2 | 1 | E12–13 | WGE | CN cavity | CyA + Pred | No surgical incidents or subsequent SEs138 | Slow progression of disease | Not reported | |
| Los Angeles | 14 | 5–8 | E8–10 | LGE | 1 CN + 4 Pu [B] | Not reported | Safe; no serious SEs74 | Benefit motor,75 limited neuropsych tests76 | MRI MRS78 and FDG PET75 | |
| Boston | 12 | 35–38 | Porcine | LGE | 2 CN + 4 Pu [U] | CyA or anti-MHC | Safe; no serious SEs86 | No change over 12 months86 | Not reported | |
| Tampa | 7 | 2–8 | E8–9 | LLGE | pcPu [B] | CyA 6 months | 1 death, 3 subdural hematomas83 | Modest (NS) changes in motor tests at 12 months83 | MRI and PET | 2 postmortem cases with good survival84 |
| Créteil | 5 | 2–4 | E7.5-9 | WGE | 2 CN + 3 Pu [B] | CyA 1 year | Procedure safe80 | Motor and electrophysiol improvements81 continue over 4 years | MRI and FDG PET; graft survival in 3 functional cases82 | |
| Mild psychiatric SEs | ||||||||||
| London | 2 | Possible psychiatric SE in one patient | Improvement in chorea in 1 of 2 patients | MRI and D2R PET; survival in PET | ||||||
| NEST-UK | 4 | 2–3 | E8–12 | WGE | 2 CN + 4 Pu [U] | Triple | Only SEs related to immunosuppression85 | Safety only, efficacy not reported | MRI; graft survival | |
[B] = bilateral implants; CN = caudate nucleus; CyA = cyclosporin A; E = weeks of embryonic age; LLGE = lateral aspect of the lateral ganglionic eminence; Pred = prednisolone; pcPU = postcommissural putamen; Pu = putamen; SEs = side effects; Triple = combined cyclosporin A, prednisolone, and azothiaprine; WGE = whole ganglionic eminence; [U] = unilateral implants; VM = ventral mesencephalon.
A second concern is that several early xenograft studies indicated that striatal grafts may occasionally “overgrow” to the extent of forming space occupying lesions or tumors.61 With a growing number of studies indicating no similar concerns with allografts in a variety of species, including primates, the observations of extensive growth in those early studies is now thought more likely to be attributable simply to the far greater growth capacity of human embryonic cells when implanted in the very much smaller rodent brain.
The third major area of preclinical development has been the need to develop and refine assessment protocols to be able to determine the longitudinal impact of grafts in small numbers of patients in a slowly progressive neurological disease. Although there is a rich cross-sectional literature of the functional pathology in Huntington's disease62,63 and the unified Huntington's disease rating scale (UHDRS) provides a useful index of the stage of the disease,64 until recently there has been very little careful analysis of the consistency of disease progression or determination of the most reliable tests in cognitive, motor, or psychiatric domains that can be used repeatedly to characterize this. As a result of the need for better assessment tools for transplantation studies, there have now been a number of detailed longitudinal studies published,65,66 alongside the development of a core assessment protocol to allow comparison of small numbers of patients in multiple centers under standardized conditions.67 In addition, both experimental and clinical studies have been employed to determine the efficacy and sensitivity of alternative imaging modalities to evaluate not only the extent of neurodegeneration in the disease, but also the survival of functional graft tissues in the host striatum.68–70
Clinical trials in Huntington's disease
On the basis of the experimental and preclinical studies, the first pilot clinical trials of neural transplantation in patients commenced in 1990 (Table 1). The early studies, from Cuba, Czechoslovakia, and Mexico City, each provided brief clinical accounts of implantation protocols and reported that the procedures were achieved without major complications or overt side effects. Each of these studies involved tissue implantation within 1-2 h of spontaneous abortion of the donated human fetal tissue, whereas most subsequent studies have been based on tissue donation from elective abortion. In either case, there are sensitive ethical and social issues associated with using human fetal tissues for transplantation, in particular for embryonic and fetal tissues collected from voluntary or spontaneous terminations of pregnancy, and it is imperative that such studies are undertaken only within a framework of strict ethical appraisal and informed consent.71 Moreover, the practical coordination of tissue donation and neurosurgery is greatly improved by the development of hibernation procedures that allow donated cells to be retained over several days rather than just a few hours without loss of viability.72,73
The first extensive series of implants has been undertaken by Kopyov and colleagues at the Good Samaritan hospital in Los Angeles. This group has reported on safety,74 and observed benefit in motor75 and neuropsychological76 tests in publications on small numbers of patients, and graft survival and neuronal differentiation by magnetic resonance imaging (MRI)77,78 in a total of 14 patients. Although setting the way for subsequent trials, and providing sophisticated imaging of graft survival and differentiation, this series has provided only a rather limited descriptive account of clinical outcomes, and has raised concerns about the lack of systematic assessment of the patients within a clear experimental trial design.
A second major study has been initiated in Créteil, France by Marc Peschanski and colleagues. This study is the first to be undertaken in accordance with the standardized core assessment protocol for intracerebral transplantation in Huntington's disease (CAPIT-HD),67 and detailed reports of the surgical methods, safety, functional efficacy, and imaging in the first five patients have now been published.79–82 In this series, three of the five patients showed a good clinical response in reduction of motor signs and improvement in UHDRS scores, which were associated with a positive graft signal in fluorodeoxyglucose—positron emission tomography and with restoration of lost sensory-evoked potentials in the electrophysiology tests.81,82 A fourth patient never showed any positive response and exhibited no sign of surviving grafts in the physiological and imaging assessments, whereas the fifth patient began to show signs of recovery until suffering a complete relapse immediately after an acute fever followed by change of MRI signal in the grafted region and loss of physiological markers of graft survival, raising the possibility of graft rejection. On the basis of these preliminary results, the French team is now embarking on a multicenter “French-speaking” trial to evaluate the ease of translating complex tissue handling and surgical protocols more widely among nonspecialist neurosurgical centers.
Two additional designed trials are now underway. The first open-label trial from Tampa, Florida, has recently reported on outcome in the first seven patients, of which six showed moderate improvement, whereas one showed significant deterioration resulting in an overall lack of significant change.83 However, three of the patients developed subdural hematomas after the surgery, which may be attributable to the fact that the stage of disease appears to have been more advanced in this series in comparison to other studies, suggesting that implanting tissue into the already heavily atrophied basal ganglia may involve significantly higher risk than operations in patients at an earlier stage of the disease. One patient in this series died at 18 months after transplantation, of causes unrelated to the surgery, and a detailed anatomical analysis of the postmortem brain has indicated healthy surviving grafts and good differentiation of the grafts into mature striatal-like tissue containing all striatal cell phenotypes examined.84
The other designed trial involves a multicenter collaboration among the six centers of the United Kingdom arm of the European network for striatal transplantation (“NEST-UK”). We have so far reported on neurosurgical safety in the first four patients over 6 months after unilateral transplantation, with the only complications being mild and reversible disturbance of routine hematological and biochemical indices associated with triple immunosuppression treatment, which has in all cases been controlled by titration of drug dose.85 On the basis of this pilot safety study, we have now proceeded to the preliminary efficacy stage of the trial according to the CAPIT-HD protocol in 10 patients after bilateral implantations.
One other study is worthy of note, involving implantation of striatal xenografts derived from porcine donors.86 Of the 12 patients in this study, half were treated with cyclosporin immunosuppression, and in the other six the fetal porcine tissues were treated with a monoclonal antibody directed against surface major histocompatibility complex I molecules.87 However, surviving grafts were not detectable on MRI, and the treatments had no functional benefit for the patients,86 which may not be surprising, because fully effective immunosuppression strategies for xenografts have still not been resolved and similar approaches to those used here have been seen to yield rather poor graft survival in experimental monkeys and in a postmortem from a patient with Parkinson's disease.88
On the basis of the preliminary trials, the two main European networks are collaborating on collecting further data, using comparable assessments to refine the preparation, implantation, and assessment methods to allow a more comprehensive analysis of efficacy in the cognitive and psychiatric as well as motor domains. However, the major progress in developing this program is the very limited reliable supply of suitable donor tissues. Some advances may be achieved with more accurate fetal tissue staging,48 adaptation of the collection methods (subject to appropriate ethical approval),89 and better storage and hibernation protocols,48,73 but donor tissues based on use of fresh primary fetal tissues, whether derived from elective or spontaneous abortion, can never achieve the levels of standardization and quality control required for a practical experimental medicine. Therefore, the existing trials must be considered “proof of principle” until a more reliable, standardizable, and quality-controlled source of tissue can be identified.
STEM CELLS FOR STRIATAL TRANSPLANTATION
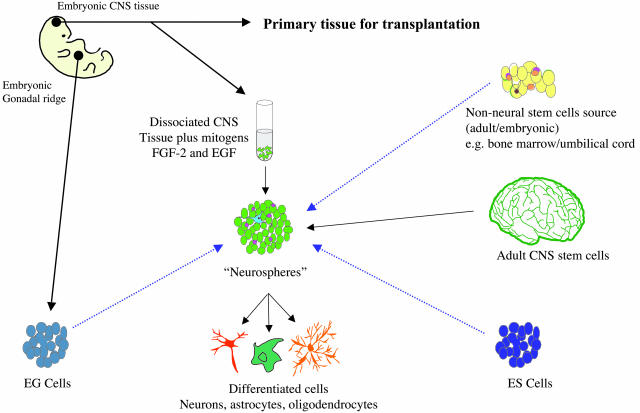
Currently, the most actively explored alternative source of tissues for neural transplantation in Huntington's disease are stem cells. There is discussion about the definition of a stem cell,90 but for present purposes, stem cells are precursor cells that can self-renew as well as differentiate down alternative lineages to generate some or all cells and tissues of the body. In addition, there are populations of dividing cells that may in fact consist largely of lineage-restricted progenitors with a limited division potential that could still be useful for clinical transplant purposes. Bearing these issues in mind, there are a number of potential stem/progenitor cells sources that are being actively considered for eventual transplant purposes (FIG. 1): 1) embryonic stem (ES) cells, which are derived from blastocysts and are believed to be totipotent, 2) pluripotent progenitor cells from the embryo, fetus, or neonate, which are already partially committed down a neural lineage, 3) similar progenitor cells isolated from the adult subventricular zone, 4) epidermal germ (EG) cells that are isolated from the gonadal ridge of the embryo and are normally destined to become the ovae and sperm, 5) umbilical blood that can be collected after birth, and 6) a range of other non-neuronal stem cells that may have the capacity to differentiate into neural cells given the appropriate signals.
FIG. 1.
Potential sources of stem cells for cell therapy in HD. This figure illustrates stem cell sources being considered as potential starting materials for neural transplantation. The blue dashed arrow denotes sources that require purification of neural precursors or application of methods to direct the cells toward a neural lineage.
We can ask three major questions of these proliferating cell systems: 1) Can they produce neurons? 2) Can they differentiate into striatal-like cells to be useful for neural transplantation studies? and 3) Can they survive transplantation into adult models of Huntington's disease and effect circuit reconstruction and restore lost function? As can be seen from the following brief discussion, for most cell sources, questions 1 and 2 are still the main focus of study, and question 3 remains to be addressed.
Fetal neural stem cells
Fetal neural stem cells are isolated from the fetal brain at various gestational periods and from multiple brain regions.91 They are produced by placing dissociated fetal brain cells into serum-free medium with mitogens such as FGF-2 and EGF. Under these conditions, differentiated cells die and more primitive cells proliferate as free-floating spheres of cells, the so-called “neurospheres”.92 The relative responsiveness of the cells to the different mitogens depends on the gestational age of the tissue and the length of time the tissue has been in culture.93 Such cells have been isolated from a number of species, including human,94,95 and can be expanded for prolonged periods in vitro. However, it seems that their neurogenic potential declines with increasing passage with the majority of progenitor cells differentiating to form glia,96–98 which may be taken as evidence of their progenitor status and may ultimately limit the amount of expansion that can be achieved. The fact that these cells are able to respond to signals in the developing CNS to produce a variety of different neuronal phenotypes is encouraging.99–101 In particular, after short-term expansion, neurospheres transplanted into the adult lesioned rodent brain demonstrate some evidence of striatal-like differentiation.102 However, after being expanded for longer periods of time, the cells appear to lose the differentiation potential of primary precursor cells.103 Considerable research effort is now being applied to methods to direct the differentiation of fetal neural stem cells into a striatal phenotype for transplantation, but this has not yet been reliably achieved and will require significant advances in our understanding of the developmental signals important for normal striatal differentiation.
Adult neural stem cells
The concept that there is no cell turnover in the adult CNS has been challenged over the last decade and superceded by the recognition that there is a continuous neurogenic turnover in limited areas. The clearest examples of areas in which neural stem cells appear to reside are the subventricular zone of the forebrain and the dentate gyrus of the hippocampus. In vitro these cells can produce neurons, astrocytes, and oligodendrocytes, offering up hope that it may eventually be possible to manipulate such cells in vivo. Neural stem cells have been identified in the subventricular zone of humans, although the precise nature of these cells and how they relate to their rodent counterparts remains uncertain.98
Embryonic stem cells
ES cells are isolated and expanded from the inner cell mass of the blastocyst stage embryo. A number of human lines have now been generated104,105 and in some countries banking of such cells is being developed. In the case of human ES cells, most currently available lines have been generated from surplus embryos following in vitro fertilization. As might be expected, these very primitive cells have the capacity to produce every cell type of the body. It seems that they are also capable of substantial expansion in culture, while remaining relatively stable in terms of the cell population characteristics, and they also have a substantial neurogenic potential.105,106 Human ES cells can be directed neurally using a variety of methods, including exposure to retinoic acid, placement of cells in serum-free medium with the mitogen FGF-2, and selection using cell-sorting methods. Studies on mouse ES cells have shown that specific neural progenitor subtypes can be derived by manipulating culture conditions to expose cells to a program of extrinsic signals that recapitulate the developmental events of neural patterning.107,108 Furthermore, it has been shown that cells specified this way in vitro retain their imposed cell-type specificities and go on to differentiate appropriately when reintroduced into the animal in vivo, at least for limited periods of time. To date, there has been no demonstration of differentiation into striatal cells. A potential concern with the use of these cells is whether methods for differentiation are 100% efficient, because even tiny numbers of undifferentiated cells may have the potential to form tumors.
Embryonic germ cells
Another potential human cell source, which has so far received rather little attention, is the EG cell. These are derived from primordial germ cells in the gonadal ridge of first-trimester embryos. So far, human EG (hEG) cells have been isolated and expanded by only two groups.109,110 These studies have shown that hEG cells can be derived from primordial germ cells, although the conversion efficiency has been low, proliferation has been limited to around 20 passages, and spontaneous differentiation is difficult to control. However, these problems may be surmounted by optimization of the culture conditions. EG cells, like ES cells, spontaneously form embryoid bodies (EBs), spherical structures in which cells begin to differentiate and which can form cell types from all three lineages of embryonic development. When dissociated, EB-derived cells can be vigorously and reliably expanded and efficiently cloned. The resulting cell lines predominantly express markers of the neural lineage, although markers of other lineages are also found. Their gene expression profiles appear to be relatively stable over multiple passages, although heterogeneous between individual lines.109 So far, EB-derived cells have been insufficiently characterized to properly assess their potential usefulness for neural transplantation. Although they appear to contain neural progenitors capable of robust, long-term expansion, it is not clear whether these are similar to the neural progenitors that can be derived from ES cells and whose subsequent differentiation can be controlled.
Non-neural stem cells
The possibility that easily harvested stem cell populations, such as adult bone marrow stem cells or umbilical cord cells, could have neurogeneic potential has generated a substantial amount of excitement and would open up the opportunity of autologous grafting as well as easy access to tissue for allografting. There have been reports of bone marrow stem cells having the capacity to differentiate into neurons after injection into the adult rodent host. However, this claim is contested and it may be that such results have been the result of cell fusion events rather than true transdifferentiation (for review, see Long and Yang111). The true capacity of other similar sources, including umbilical cord blood, is currently equally unclear.
Striatal differentiation
Directing the differentiation of various stem cells to acquire striatal phenotypes may require new insights into striatal development. The striatum comprises complex populations of projection neurons that largely derive from the LGE of the basal telencephalon, and interneurons that derive from progenitors in the MGE. Responses to extracellular signals are likely to be temporally controlled. A number of genes important in striatal development have already been identified (for review, see Jain and colleagues112). However, it may be necessary to identify a more complete set of genes involved in striatal differentiation and to understand more about their function to develop ways of directing primitive cells toward a striatal phenotype.
EX VIVO GENE TRANSFER FOR STRIATAL NEUROPROTECTION
As the availability of primary or stem cells for practical therapy remains problematic, an alternative approach has been to explore neuroprotective strategies for protecting host cells against the disease process itself.
CNTF delivery
The principle underlying neuroprotective gene therapy is that many classes of neurones can be protected against damage or loss, whether because of trauma or disease, by interfering with processes involved in cell death. This can be achieved at multiple levels, by blocking excitotoxicity, metabolic impairment, oxidative stress, or apoptotic processes, or by promoting survival, differentiation, and growth using a variety of molecules, such as trophic factors that guide and regulate neuronal development.113 In particular, a variety of growth factors, including the neurotrophins BDNF, neurotrophin (NT)-3, and NT-4/5, interleukins, FGF, GDNF, and CNTF have all been found to promote the survival of striatal neurones in culture.114–116 Many of these are also effective at protecting striatal neurons against excitotoxic117–119 or ischemic insult in vivo. Of the variety of molecules explored, CNTF is probably the most potent.120
Since trophic factors are large molecules and do not readily cross the blood–brain barrier, early studies required direct intracerebral delivery by cannulation. There has therefore been a search for improved delivery methods, including in vivo and ex vivo gene transfer. Thus, CNTF, GDNF, and other trophic factors have been incorporated into viral vectors, including adenovirus and lentivirus, for direct intracerebral infection of endogenous striatal neurons, and which can provide significant protection against excitoxic lesions.121,122 However, some of the problems associated with this approach are that it can be difficult to standardize and regulate intracerebral gene transfer, many of the vectors are toxic, and there are frequent difficulties in obtaining stable long-term gene expression.113 Consequently, an alternative approach has been to engineer cells to express the desired transgene, allowing full characterization and safety assessment in vitro, before transplantation for delivery of the gene product in vivo (so-called ex vivo gene therapy).123,124 Thus, for example, both NGF and FGF-secreting fibroblasts have been seen to protect striatal neurons against quinolinic acid toxicity.125,126
Although the neurosurgical delivery of cells that deliver trophic support is in principle entirely similar to that required for primary neuronal and stem cell grafts, the mechanisms by which the grafts are expected to act is quite different.127 Rather than the requirements to express specific neuronal phenotypes and integrate with host circuitry, trophic grafts are only required to secrete defined molecules at physiological levels, and preferably at regulatable rates, which may significantly ease the design demands. In an interesting development of this principle, Tan and Aebischer128 have developed the strategy of encapsulating engineered cells within semi-permeable polymer tubes which allow the passage of oxygen and nutrients into the grafts and the secreted growth factor back into the brain, but serve to isolate the cells of the graft from those of the host. This allows containment of cell lines that would otherwise be tumorigenic, and use of nonhuman cells that would otherwise be rejected by the host immune system, markedly enhancing both the practicality and the safety of the delivery system. Thus, intrastriatal implantation of baby hamster kidney cells engineered to express CNTF protected rodent striatal neurons against quinolinic acid toxicity129,130 and alleviated deficits on a range of both motor and cognitive tests.131 To provide preparation for clinical application, the strategy has been scaled up to primates, and found to be equally effective in providing functional neuroprotection against excitotoxic insult in the caudate and putamen.132,133
Preliminary clinical trials
The encapsulation strategy has already been used in pilot trials in several clinical conditions including terminal cancer pain,134 amyotrophic lateral sclerosis,135 and Parkinson's disease. A protocol for a clinical trial in Huntington's disease has been published136 and this trial is believed now to be underway, although no results are yet available.
FUTURE DEVELOPMENTS
It remains the case that there is as yet no effective therapy for repair, replacement, or protection of the neurodegeneration in Huntington's disease, or indeed of any similar neurogenetic disease. In the present account we have summarized the current state of play with cell replacement and neuroprotection strategies, which are in essence based on experimental strategies to repair the pathology or provide generalized cellular protection against nondisease-specific aspects of cell death. With the identification of the gene, there has been a proliferation of hypotheses about the pathogenic process and causation,2 although so far with little direct evidence that allows us to conclude the actual mechanism whereby the genetic mutation is translated into the slow, progressive, focal degeneration of striatal neurons. Nevertheless, new therapeutic strategies are suggested to block transcriptional dysregulation and downstream changes in cellular metabolism and response to stress,2 and it could be that these experimental studies identify novel treatments that prove effective in clinical trial, giving credence to the associated hypotheses of disease causation and process.
Until such time as we can actually halt or reverse the disease process, reparative therapies such as those described here will continue to play a major role. It is easy to challenge existing strategies with the charge that they might not work, because Huntington's disease might not be a purely striatal disease or that a trophic-mediated treatment might not impede the actual mechanism of cell death. However, with plausible hypotheses (but no certainty) about mechanistic processes, the only rational way forward when faced by such devastating disability is, in our view, to conduct informative well designed trials founded on high-quality experimental studies in vitro and in vivo. With the present rate of advance both in animal models and in the understanding of cellular and molecular processes in the disease, we can expect rapid advances in basic science leading quickly over the next decade to major advances in new strategies for therapy and improved prognosis for patients.
REFERENCES
- 1.Huntington's disease (Bates GP, Harper PS, Jones AL, eds). Oxford: Oxford University Press, 2002.
- 2.Leegwater-Kim J, Cha JHJ. The paradigm of Huntington's disease: therapeutic opportunities in neurodegeneration. NeuroRx 1: 128–138, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983, 1993. [DOI] [PubMed] [Google Scholar]
- 4.DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel J-P et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277: 1990–1993, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA et al. Formation of neuronal intranuclear inclusions (NII) underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90: 537–548, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Leroi I, Michalon M. Treatment of the psychiatric manifestations of Huntington's disease: a review of the literature. Can J Psychiatry 43: 933–940, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Nance MA, Westphal B. Comprehensive care in Huntington's disease. In: Huntington's disease (Bates GP, Harper PS, Jones AL, eds), pp 475–500. Oxford: Oxford University Press, 2002.
- 8.Coyle JT, Schwarcz R. Lesions of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature 263: 244–246, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Mason ST, Sanberg PR, Fibiger HC. Kainic acid lesions of the striatum dissociate amphetamine and apomorphine stereotypy: similarities to Huntington's chorea. Science 201: 352–355, 1978. [DOI] [PubMed] [Google Scholar]
- 10.Sanberg PR, Coyle JT. Scientific approaches to Huntington's disease. CRC Crit Rev Clin Neurobiol 1: 1–44, 1984. [PubMed] [Google Scholar]
- 11.Schwarcz R, Hökfelt T, Fuxe K, Jonsson G, Goldstein M, Terenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res 37: 199–216, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Schwarcz R, Whetsell WO, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science 219: 316–318, 1983. [DOI] [PubMed] [Google Scholar]
- 13.Brasted PJ, Döbrössy MD, Eagle DM, Nathwani F, Robbins TW, Dunnett SB. Operant analysis of striatal dysfunction. In: Innovative models of CNS diseases: from molecules to therapy (Emerich DW, Dean RL, Sanberg PR, eds), pp 249–273. Totowa, NJ: Humana, 2000.
- 14.Borlongan CV, Koutouzis TK, Sanberg PR. 3-Nitropropionic acid animal model and Huntington's disease. Neurosci Biobehav Rev 21: 289–293, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Beal MF, Brouillet EP, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem 61: 1147–1150, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AHV. Mitochondrial defect in Huntington's disease caudate nucleus. Ann Neurol 39: 385–389, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Palfi SP, Ferrante RJ, Brouillet E, Beal MF, Dolan R, Guyot MC et al. Chronic 3-nitropropionic acid treatment in baboons replicates the cognitive and motor deficits of Huntington's disease. J Neurosci 16: 3019–3025, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page KJ, Meldrum A, Dunnett SB. The 3-nitroproprionic acid (3NPA) model of Huntington's disease: do alterations in the expression of metabolic mRNAs predict the development of striatal pathology. In: Mitochondrial inhibitors as tools for neurobiology (Sanberg PR, Nishino H, Borlongan CV, eds), pp 141–156. Totowa, NJ: Humana, 1999.
- 19.Menalled LB, Chesselet MF. Mouse models of Huntington's disease. Trends Pharmacol Sci 23: 32–39, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493–506, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19: 3248–3257, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lione LA, Carter RJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation. J Neurosci 19: 10428–10437, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabrizi SJ, Workman J, Hart PE, Mangiarini L, Mahal A, Bates G et al. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann Neurol 47: 80–86, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Cha JH, Kosinski CM, Kerner JA, Alsdorf SA, Mangiarini L, Davies SW et al. Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human Huntington disease gene. Proc Natl Acad Sci USA 95: 6480–6485, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy KP, Carter RJ, Lione LA, Mangiarini L, Mahal A, Bates GP et al. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation. J Neurosci 20: 5115–5123, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 101: 57–66, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Dunnett SB, Nathwani F, Björklund A. The integration and function of striatal grafts. Prog Brain Res 127: 345–380, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Mayer E, Brown VJ, Dunnett SB, Robbins TW. Striatal graft-associated recovery of a lesion-induced performance deficit in the rat requires learning to use the transplant. Eur J Neurosci 4: 119–126, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Döbrössy MD, Dunnett SB. Striatal grafts alleviate deficits in response execution in a lateralised reaction time task. Brain Res Bull 47: 585–593, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Dunnett SB. Functional repair of striatal systems by neural transplants: evidence for circuit reconstruction. Behav Brain Res 66: 133–142, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Wictorin K. Anatomy and connectivity of intrastriatal striatal transplants. Prog Neurobiol 38: 611–639, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Xu ZC, Wilson CJ, Emson PC. Synaptic potentials evoked in spiny neurons in rat neostriatal grafts by cortical and thalamic stimulation. J Neurophysiol 65: 477–493, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Nakao N, Ogura M, Nakai K, Itakura T. Embryonic striatal grafts restore neuronal activity of the globus pallidus in a rodent model of Huntington's disease. Neuroscience 88: 469–477, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Campbell K, Kalén P, Wictorin K, Lundberg C, Mandel RJ, Björklund A. Characterization of GABA release from intrastriatal striatal transplants: dependence on host-derived afferents. Neuroscience 53: 403–415, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Sirinathsinghji DJS, Dunnett SB, Isacson O, Clarke DJ, Kendrick K, Björklund A. Striatal grafts in rats with unilateral neostriatal lesions. II. In vivo monitoring of GABA release in globus pallidus and substantia nigra. Neuroscience 24: 803–811, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Sirinathsinghji DJS, Heavens RP, Torres EM, Dunnett SB. Cholecystokinin-dependent regulation of host dopamine inputs to striatal grafts. Neuroscience 53: 651–663, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Dunnett SB, Carter RJ, Watts C, Torres EM, Mahal A, Mangiarini L et al. Striatal transplantation in a transgenic mouse model of Huntington's disease. Exp Neurol 154: 31–40, 1998. [DOI] [PubMed] [Google Scholar]
- 38.van Dellen A, Deacon R, York D, Blakemore C, Hannan AJ. Anterior cingulate cortical transplantation in transgenic Huntington's disease mice. Brain Res Bull 56: 313–318, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Morton AJ, Lagan M, Skepper JN, Dunnett SB. Progressive formation of inclusions in the brains of mice transgenic for the human Huntington's disease mutation parallels neurological decline. J Neurocytol 29: 679–702, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Vonsattel J-P, Myers RH, Stevens TJ. Neuropathologic classification of Huntington's disease. J Neuropathol Exp Neurol 44: 559–577, 1985. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald V, Halliday G. Pyramidal cell loss in motor cortices in Huntington's disease. Neurobiol Dis 10: 378–386, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Mazurek MF, Garside S, Beal MF. Cortical peptide changes in Huntington's disease may be independent of striatal degeneration. Ann Neurol 41: 540–547, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Greenamyre JT, Shoulson I. We need something better, and we need it now: fetal striatal transplantation in Huntington's disease? Neurology 58: 675–676, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Olanow CW, Kordower JH, Freeman TB. Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci 19: 102–109, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Dunnett SB, Björklund A, Lindvall O. Cell therapy in Parkinson's disease –stop or go? Nat Rev Neurosci 2: 365–369, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Peschanski M, C,saro P, Hantraye P. Rationale for intrastriatal grafting of striatal neuroblasts in patients with Huntington's disease. Neuroscience 68: 273–285, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Freeman TB, Sanberg PR, Isacson O. Development of the human striatum: implications for fetal striatal transplantation in the treatment of Huntington's disease. Cell Transplant 4: 539–545, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Rosser AE, Barker RA, Armstrong RA, Elneil S, Jain M, Hurelbrink CB et al. Staging and preparation of human fetal striatal tissue for UK study of neural transplantation in Huntington's disease. Cell Transplant 12: 679–686, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Pundt LL, Kondoh T, Conrad JA, Low WC. Transplantation of human striatal tissue into a rodent model of Huntington's disease: phenotypic expression of transplanted neurons and host-to-graft innervation. Brain Res Bull 39: 23–32, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Grasbon-Frodl EM, Nakao N, Lindvall O, Brundin P. Developmental features of human striatal tissue transplanted in a rat model of Huntington's disease. Neurobiol Dis 3: 299–311, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Hurelbrink CB, Armstrong RJE, Barker RA, Dunnett SB, Rosser AE. Hibernated human fetal striatal tissue: successful transplantation in a rat model of Huntington's disease. Cell Transplant 9: 743–749, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Graybiel AM, Liu FC, Dunnett SB. Intrastriatal grafts derived from fetal striatal primordia. 1. Phenotypy and modular organization. J Neurosci 9: 3250–3271, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smart IHM, Sturrock RR. Ontogeny of the neostriatum. In: The neostriatum (Divac I, Öberg RGE, eds), pp 127–146. New York: Pergamon, 1979.
- 54.Pakzaban P, Deacon TW, Burns LH, Isacson O. Increased proportion of acetylcholinesterase-rich zones and improved morphological integration in host striatum of fetal grafts derived from the lateral but not the medial ganglionic eminence. Exp Brain Res 97: 13–22, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Deacon TW, Pakzaban P, Isacson O. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: neural transplantation and developmental evidence. Brain Res 668: 211–219, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Nakao N, Odin P, Brundin P. Selective sub-dissection of the striatal primordium for cultures affects the yield of DARPP-32-containing neurones. NeuroReport 5: 1081–1084, 1994. [DOI] [PubMed] [Google Scholar]
- 57.Olsson M, Campbell K, Wictorin K, Björklund A. Projection neurons in fetal striatal transplants are predominantly derived from the lateral ganglionic eminence. Neuroscience 69: 1169–1182, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Naimi S, Jeny R, Hantraye P, Peschanski M, Riche D. Ontogeny of human striatal DARPP-32 neurons in fetuses and following xenografting to the adult rat brain. Exp Neurol 137: 15–25, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Marín O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci 20: 6063–6076, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watts C, Dunnett SB, Rosser AE. Effect of embryonic donor age and dissection on the DARPP-32 content of cell suspensions used for intrastriatal transplantation. Exp Neurol 148: 271–280, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Grasbon-Frodl EM, Nakao N, Lindvall O, Brundin P. Phenotypic development of the human embryonic striatal primordium: a study of cultured and grafted neurons from the lateral and medial ganglionic eminence. Neuroscience 73: 171–183, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Kremer B. Clinical neurology of Huntington's disease. In: Huntington's disease (Bates GP, Harper PS, Jones AL, eds), pp 28–61. Oxford: Oxford University Press, 2002.
- 63.Craufurd D, Snowden JS. Neuropsychological and neuropsychiatric aspects of Huntington's disease. In: Huntington's disease (Bates GP, Harper PS, Jones AL, eds), pp 62–94. Oxford: Oxford University Press, 2002.
- 64.Kieburtz K, Penney JB, Como P, Ranen N, Shoulson I, Feigin A et al. Unified Huntington's disease rating scale: reliability and consistency. Mov Disord 11: 136–142, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Bachoud-Lévi AC, Maison P, Bartolomeo P, Boiss, MF, Dalla Barba G, Ergis AM et al. Retest effects and cognitive decline in longitudinal follow-up of patients with early HD. Neurology 56: 1052–1058, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Ho AK, Brown R, Hodges JR, Ane MN, Snowden JS, Thompson J et al. Profile of cognitive progression in Huntington's disease. Neurology 61: 1702–1706, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Quinn NP, Brown R, Craufurd D, Goldman S, Hodges JR, Kieburtz K et al. Core assessment programme for intracerebral transplantation in Huntington's disease (CAPIT-HD). Mov Disord 11: 143–150, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Norman AB, Thomas SR, Pratt RG, Samaratunga RC, Sanberg PR. T1 and T2 weighted magnetic resonance imaging of excitotoxin lesions and neural transplants in rat brain in vivo. Exp Neurol 109: 164–170, 1990. [DOI] [PubMed] [Google Scholar]
- 69.Denys A, Leroy-Willig A, Riche D, Hantraye P. MR appearance of neural grafts in a primate model of Huntington disease. Am J Roentgenol 158: 215–216, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Torres EM, Fricker RA, Hume S, Myers R, Opacka-Juffry J, Ashworth S et al. Assessment of striatal graft viability in the rat in vivo using a small diameter PET scanner. NeuroReport 6: 2017–2021, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Boer GJ. Ethical guidelines for the use of human embryonic or fetal tissue for experimental and clinical neurotransplantation and research. J Neurol 242: 1–13, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Sauer H, Brundin P. Effects of cool storage on survival and function of intrastriatal ventral mesencephalic grafts. Rest Neurol Neurosci 2: 123–135, 1991. [DOI] [PubMed] [Google Scholar]
- 73.Hurelbrink CB, Tyers P, Armstrong RJE, Dunnett SB, Barker RA, Rosser AE. Long-term hibernation of human fetal striatal tissue does not adversely affect its differentiation in vitro or graft survival: implications for clinical trials in Huntington's disease. Cell Transplant 12: 687–695, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Kopyov OV, Jacques S, Lieberman A, Duma CM, Eagle KS. Safety of intrastriatal neurotransplantation for Huntington's disease patients. Exp Neurol 119: 97–108, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Kopyov OV, Jacques S, Kurth M, Philpott LM, Lee A, Patterson M et al. Fetal transplantation for Huntington's disease: clinical studies. In: Cell transplantation for neurological disorders (Freeman TB, Kordower JH, eds), pp 95–134. Totowa, NJ: Humana Press, 1998.
- 76.Philpott LM, Kopyov OV, Lee AJ, Jacques S, Duma CM, Caine S et al. Neuropsychological functioning following fetal striatal transplantation in Huntington's chorea: three case presentations. Cell Transplant 6: 203–212, 1997. [DOI] [PubMed] [Google Scholar]
- 77.Hoang TQ, Bluml S, Dubowitz DJ, Moats R, Kopyov O, Jacques D et al. Quantitative proton-decoupled 31P MRS and 1H MRS in the evaluation of Huntington's and Parkinson's diseases. Neurology 50: 1033–1040, 1998. [DOI] [PubMed] [Google Scholar]
- 78.Ross BD, Hoang TQ, Blüml S, Dubowitz DJ, Kopyov OV, Jacques DB et al. In vivo magnetic resonance spectroscopy of human fetal neural transplants. NMR Biomed 12: 221–236, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Palfi S, Nguyen JP, Brugières P, LeGuerinel C, Hantraye P, Rémy P et al. MRI-stereotactical approach for neural grafting in basal ganglia disorders. Exp Neurol 150: 272–281, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Bachoud-Lévi AC, Bourdet C, Brugières P, Nguyen JP, Grandmougin T, Haddad B et al. Safety and tolerability assessment of intrastriatal neural allografts in Huntington's disease patients. Exp Neurol 161: 194–202, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Bachoud-Lévi AC, R,my P, Nguyen JP, Brugières P, Lefaucheur JP, Bourdet C et al. Motor and cognitive improvements in patients with Huntington's disease after neural transplantation. Lancet 356: 1975–1979, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Gaura V, Bachoud-Lévi AC, Ribeiro MJ, Nguyen JP, Frouin V, Baudic S et al. Striatal neural grafting improves cortical metabolism in Huntington's disease patients. Brain 127: 65–72, 2004. [DOI] [PubMed] [Google Scholar]
- 83.Hauser RA, Furtado S, Cimino CR, Delgado H, Eichler S, Schwartz S et al. Bilateral human fetal striatal transplantation in Huntington's disease. Neurology 58: 687–695, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Freeman TB, Cicchetti F, Hauser RA, Deacon TW, Li XJ, Hersch SM et al. Transplanted fetal striatum in Huntington's disease: phenotypic development and lack of pathology. Proc Natl Acad Sci USA 97: 13877–13882, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosser AE, Barker RA, Guillard J, Harrower T, Watts C, Pickard J et al. Unilateral transplantation of human primary fetal tissue in four patients with Huntington's disease: NEST-UK safety report (ISRCTN no 36485475). J Neurol Neurosurg Psychiatry 73: 678–685, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fink JS, Schumacher JM, Ellias SA, Palmer EP, Saint-Hilaire M, Shannon K et al. Porcine xenografts in Parkinson's disease and Huntington's disease patients: preliminary results. Cell Transplant 9: 273–278, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Pakzaban P, Deacon TW, Burns LH, Dinsmore J, Isacson O. A novel mode of immunoprotection of neural xenotransplants: masking of donor major histocompatibility complex class I enhances transplant survival in the central nervous system. Neuroscience 65: 983–996, 1995. [DOI] [PubMed] [Google Scholar]
- 88.Deacon T, Schumacher J, Dinsmore J, Thomas C, Palmer P, Kott S et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson's disease. Nat Med 3: 350–353, 1997. [DOI] [PubMed] [Google Scholar]
- 89.Nauert GM, Freeman TB. Low-pressure aspiration abortion for obtaining embryonic and early gestational fetal tissue for research purposes. Cell Transplant 3: 147–151, 1994. [DOI] [PubMed] [Google Scholar]
- 90.Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci 26: 125–131, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Armstrong RJ, Svendsen CN. Neural stem cells: from cell biology to cell replacement. Cell Transplant 9: 139–152, 2000. [DOI] [PubMed] [Google Scholar]
- 92.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12: 4565–4574, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 208: 166–188, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Svendsen CN, ter Borg M, Armstrong RA, Rosser AE, Chandran S, Ostenfeld T et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Meth 85: 141–152, 1998. [DOI] [PubMed] [Google Scholar]
- 95.Carpenter MK. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol 158: 265–278, 1999. [DOI] [PubMed] [Google Scholar]
- 96.Jain M, Armstrong RJE, Tyers P, Barker RA, Rosser AE. GABAergic immunoreactivity is predominant in neurons derived from expanded human neural precursor cells in vitro. Exp Neurol 182: 113–123, 2003. [DOI] [PubMed] [Google Scholar]
- 97.Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28: 69–80, 2000. [DOI] [PubMed] [Google Scholar]
- 98.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427: 740–744, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Rosser AE, Tyers P, Dunnett SB. The morphological development of neurons derived from EGF- and FGF-2-driven human CNS precursors depends on their site of integration in the neonatal rat brain. Eur J Neurosci 12: 2405–2413, 2000. [DOI] [PubMed] [Google Scholar]
- 100.Fricker RA, Carpenter MK, Winkler C, Greco C, Gates MA, Björklund A. Site–specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci 19: 5990–6005, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Englund U, Björklund A, Wictorin K, Lindvall O, Kokaia M. Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proc Natl Acad Sci USA 99: 17089–17094, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Armstrong RJE, Watts C, Svendsen CN, Dunnett SB, Rosser AE. Survival, neuronal integration and fibre outgrowth of propagated human neural precursor grafts in an animal model of Huntington's disease. Cell Transplant 9: 55–64, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Skogh C, Parmar M, Campbell K. The differentiation potential of precursor cells from the mouse lateral ganglionic eminence is restricted by in vitro expansion. Neuroscience 120: 379–385, 2003. [DOI] [PubMed] [Google Scholar]
- 104.Thompson JA, Istkovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al. Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147, 1998. [DOI] [PubMed] [Google Scholar]
- 105.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18: 399–404, 2000. [DOI] [PubMed] [Google Scholar]
- 106.Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19: 1129–1133, 2001. [DOI] [PubMed] [Google Scholar]
- 107.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature 418: 50–56, 2002. [DOI] [PubMed] [Google Scholar]
- 108.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 110: 385–397, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA 95: 13726–13731, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Turnpenny L, Brickwood S, Spalluto CM, Piper K, Cameron IT, Wilson DI et al. Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells (Dayt) 21: 598–609, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Long Y, Yang KY. Bone marrow derived cells for brain repair: recent findings and current controversies. Curr Mol Med 3: 719–725, 2003. [DOI] [PubMed] [Google Scholar]
- 112.Jain M, Armstrong RJE, Barker RA, Rosser AE. Cellular and molecular aspects of striatal development. Brain Res Bull 55: 533–540, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Fawcett JW, Rosser AE, Dunnett SB. Brain damage, brain repair. Oxford: Oxford University Press, 2001.
- 114.Ventimiglia R, Mather PE, Jones BE, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci 7: 213–222, 1995. [DOI] [PubMed] [Google Scholar]
- 115.Nakao N, Odin P, Lindvall O, Brundin P. Differential trophic effects of basic fibroblast growth factor, insulin-like growth factor-1, and neurotrophin-3 on striatal neurons in culture. Exp Neurol 138: 144–157, 1996. [DOI] [PubMed] [Google Scholar]
- 116.Petersén Å, Brundin P. Effects of ciliary neurotrophic factor on excitotoxicity and calcium-ionophore A23187-induced cell death in cultured embryonic striatal neurons. Exp Neurol 160: 402–412, 1999. [DOI] [PubMed] [Google Scholar]
- 117.Alberch J, Pérez-Navarro E, Canals JM. Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull 57: 817–822, 2002. [DOI] [PubMed] [Google Scholar]
- 118.Perez-Navarro E, Canudas AM, Åkerud P, Alberch J, Arenas E. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 prevent the death of striatal projection neurons in a rodent model of Huntington's disease. J Neurochem 75: 2190–2199, 2000. [DOI] [PubMed] [Google Scholar]
- 119.Alexi T, Hughes PE, Faull RLM, Williams CE. 3-Nitropropionic acid's lethal triplet: cooperative pathways of neurodegeneration. NeuroReport 9: R57–R64, 1998. [DOI] [PubMed] [Google Scholar]
- 120.Anderson KD, Panayotatos N, Corcoran TL, Lindsay RM, Wiegand SJ. Ciliary neurotrophic factor protects striatal output neurons in an animal model of Huntington disease. Proc Natl Acad Sci USA 93: 7346–7351, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mittoux V, Ouary S, Monville C, Lisovoski F, Poyot T, Cond, F et al. Corticostriatopallidal neuroprotection by adenovirus-mediated ciliary neurotrophic factor gene transfer in a rat model of progressive striatal degeneration. J Neurosci 22: 4478–4486, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McBride JL, During MJ, Wuu J, Chen EY, Leurgans SE, Kordower JH. Structural and functional neuroprotection in a rat model of Huntington's disease by viral gene transfer of GDNF. Exp Neurol 181: 213–223, 2003. [DOI] [PubMed] [Google Scholar]
- 123.Raymon HK, Thode S, Gage FH. Application of ex vivo gene therapy in the treatment of Parkinson's disease. Exp Neurol 144: 82–91, 1997. [DOI] [PubMed] [Google Scholar]
- 124.Tuszynski MH. Growth-factor gene therapy for neurodegenerative disorders. Lancet Neurol 1: 51–57, 2002. [DOI] [PubMed] [Google Scholar]
- 125.Schumacher JM, Short MP, Hyman BT, Breakefield XO, Isacson O. Intracerebral implantation of nerve growth factor-producing fibroblasts protects striatum against neurotoxic levels of excitatory amino acids. Neuroscience 45: 561–570, 1991. [DOI] [PubMed] [Google Scholar]
- 126.Frim DM, Uhler TA, Short MP, Ezzedine ZD, Klagsbrun M, Breakefield XO et al. Effects of biologically delivered NGF, BDNF and bFGF on striatal excitotoxic lesions. NeuroReport 4: 367–370, 1993. [DOI] [PubMed] [Google Scholar]
- 127.Dunnett SB, Mayer E. Neural grafts, growth factors and trophic mechanisms of recovery. In: Neurodegeneration (Hunter AJ, Clarke M, eds), pp 183–217. New York: Academic Press, 1992.
- 128.Tan SA, Aebischer P. The problems of delivering neuroactive molecules to the CNS. Ciba Found Symp 196: 211–236, 1996. [DOI] [PubMed] [Google Scholar]
- 129.Emerich DF, Lindner MD, Winn SR, Chen EY, Frydel BR, Kordower JH. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington's disease. J Neurosci 16: 5168–5181, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Emerich DF, Bruhn SL, Chu Y, Kordower JH. Cellular delivery of CNTF but not NT-4/5 prevents degeneration of striatal neurons in a rodent model of Huntington's disease. Cell Transplant 7: 213–225, 1998. [DOI] [PubMed] [Google Scholar]
- 131.Emerich DF, Cain CK, Greco C, Saydoff JA, Hu ZY, Liu HJ et al. Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington's disease. Cell Transplant 6: 249–266, 1997. [DOI] [PubMed] [Google Scholar]
- 132.Emerich DF, Winn SR, Hantraye P, Peschanski M, Chen EY, Chu YP et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature 386: 395–399, 1997. [DOI] [PubMed] [Google Scholar]
- 133.Mittoux V, Joseph JM, Cond, F, Palfi S, Dautry C, Poyot T et al. Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington's disease. Hum Gene Ther 11: 1177–1187, 2000. [DOI] [PubMed] [Google Scholar]
- 134.Buchser E, Goddard M, Heyd B, Joseph JM, Favre J, Detribolet N et al. Immunoisolated xenogeneic chromaffin cell therapy for chronic pain. Initial clinical-experience. Anesthesiology 85: 1005–1012, 1996. [DOI] [PubMed] [Google Scholar]
- 135.Aebischer P, Schluep M, Déglon N, Joseph JM, Hirt L, Heyd B et al. Intrathecal delivery of CNTF using encapsulated genetically–modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med 2: 696–699, 1996. [DOI] [PubMed] [Google Scholar]
- 136.Bachoud-Lévi AC, D,glon N, Nguyen JP, Bloch J, Bourdet C, Winkel L et al. Neuroprotective gene therapy for Huntington's disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Hum Gene Ther 11: 1723–1729, 2000. [DOI] [PubMed] [Google Scholar]
- 137.Sramka M, Rattaj M, Molina H, Vojtassak J, Belan V, Ruzicky E. Stereotactic technique and pathophysiological mechanisms of neurotransplantation in Huntington's chorea. Stereotact Funct Neurosurg 58: 79–83, 1992. [DOI] [PubMed] [Google Scholar]
- 138.Madrazo I, Franco-Bourland RE, Castrejon H, Cuevas C, Ostrosky-Solis F. Fetal striatal homotransplantation for Huntington's disease: first two case reports. Neurol Res 17: 312–315, 1995. [DOI] [PubMed] [Google Scholar]