Abstract
Objectives:
Our objective was to describe and determine the factors contributing to a recent drug-resistant tuberculosis (TB) outbreak in Georgia.
Methods:
We defined an outbreak case as TB diagnosed from March 2008 through December 2015 in a person residing in Georgia at the time of diagnosis and for whom (1) the genotype of the Mycobacterium tuberculosis isolate was consistent with the outbreak strain or (2) TB was diagnosed clinically without a genotyped isolate available and connections were established to another outbreak-associated patient. To determine factors contributing to transmission, we interviewed patients and reviewed health records, homeless facility overnight rosters, and local jail booking records. We also assessed infection control measures in the 6 homeless facilities involved in the outbreak.
Results:
Of 110 outbreak cases in Georgia, 86 (78%) were culture confirmed and isoniazid resistant, 41 (37%) occurred in people with human immunodeficiency virus coinfection (8 of whom were receiving antiretroviral treatment at the time of TB diagnosis), and 10 (9%) resulted in TB-related deaths. All but 8 outbreak-associated patients had stayed overnight or volunteered extensively in a homeless facility; all these facilities lacked infection control measures. At least 9 and up to 36 TB cases outside Georgia could be linked to this outbreak.
Conclusions:
This article highlights the ongoing potential for long-lasting and far-reaching TB outbreaks, particularly among populations with untreated human immunodeficiency virus infection, mental illness, substance abuse, and homelessness. To prevent and control TB outbreaks, health departments should work with overnight homeless facilities to implement infection control measures and maintain searchable overnight rosters.
Keywords: tuberculosis, disease outbreaks, homeless people, drug resistance, infection control
Although only about 6% of tuberculosis (TB) patients in the United States are homeless during the year before their diagnosis,1 outbreaks of TB among homeless people challenge TB control efforts.2–10 In the past, the Advisory Council for the Elimination of Tuberculosis has recommended that homeless facilities implement infection control measures and maintain lists of people staying there overnight to enable health department follow-up.11 Despite these national recommendations, it has been up to local authorities to determine whether to mandate them, and few have done so. This lack of a mandate may be leaving many homeless facilities susceptible to TB outbreaks.
Our objectives were to describe the details of a recent (2008-2015) outbreak of drug-resistant TB among homeless people in Georgia, to determine the factors that may have contributed to the outbreak, to report on some of the consequences of the outbreak, and to discuss the need for infection control measures and searchable overnight rosters in homeless facilities to prevent and control future TB outbreaks.
Methods
We conducted an outbreak investigation using clinical and public health records; overnight rosters provided by homeless facilities (eg, searchable paper-based or electronic records); booking and release date information available to the public on local jail websites; and interviews with patients or their proxies, health department staff members with knowledge of the outbreak, and staff members of 6 homeless facilities where patients involved in the outbreak had stayed overnight. The Fulton County Department of Health and Wellness (FCDHW), the Georgia Department of Public Health, and the Centers for Disease Control and Prevention (CDC) determined that this outbreak investigation did not constitute human subjects research and therefore waived institutional review board review.
We defined an outbreak case as TB diagnosed from March 2008 through December 2015 in a person residing in Georgia at the time of his or her diagnosis and for whom (1) the genotype of the Mycobacterium tuberculosis isolate was consistent with the outbreak strain or (2) TB was diagnosed clinically without a genotyped isolate available and there were established connections to another outbreak-associated patient (either directly or via an Atlanta-area homeless facility during the source patient’s infectious period). We identified the genotype of the outbreak strain on the basis of spacer oligonucleotide typing (spoligotyping) and 24-locus mycobacterial interspersed repetitive unit-variable number tandem repeat typing (MIRU-VNTR) results. Since 2004, CDC has offered isolate genotyping for every culture-confirmed TB case in the United States, using these 2 standard methods.12–15
We systematically reviewed each outbreak case to examine patient demographic information, clinical characteristics, and treatment outcomes, as well as to estimate infectious periods for pulmonary TB cases using the method recommended by CDC and the National Tuberculosis Controllers Association,16 establish connections among cases, determine potential transmission sites, and identify support given to facilitate isolation and treatment. The National Tuberculosis Surveillance System collects information about each newly reported case of TB in the 50 US states, District of Columbia, and other jurisdictions. In the National Tuberculosis Surveillance System, homelessness is defined on the basis of the 12 months preceding the initial diagnostic evaluation for TB17; we also asked about any history of homelessness. Mental illness is linked to homelessness18 and contributes to TB transmission in other settings.8,19 Accordingly, we searched all medical records for documentation of Axis I conditions (eg, psychotic, mood, anxiety, and cognitive disorders) per the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders,20 other than substance use disorders (including alcohol, noninjection and injection drugs, and any substances).
Given our evaluation of the epidemic curve of outbreak cases, we conceptualized the outbreak in Georgia as having 3 epidemiologic phases: (1) the initial outbreak phase from March 2008 through December 2009, when only 1 homeless facility was involved; (2) the quiescent phase from January 2010 through December 2013, when the number of cases waned; and after record-low temperatures in January 2014,21,22 (3) the recrudescent phase from January 2014 through December 2015, when multiple homeless facilities were involved. We aggregated and then compared the case characteristics for each phase.
We defined a contact as a person who had been in the same place at the same time as an outbreak-associated patient with a pulmonary form of TB, because disease involving the lungs can be infectious via airborne transmission during that patient’s infectious period. To identify contacts, we used the traditional name-based identification method.16 Because this approach was rarely successful, we also identified contacts by reviewing the rosters of homeless facilities for the dates when outbreak-associated patients had stayed there and were in their infectious periods. However, until 2015, only 2 of the 6 overnight homeless facilities used by patients during this outbreak had well-maintained overnight rosters that enabled the systematic identification of contacts.
To evaluate contacts who had not been identified by the aforementioned methods, FCDHW offered facility-based screenings to any interested clients, staff members, and volunteers at homeless facilities where outbreak-associated patients had stayed. During the screenings by FCDHW, the clinical evaluation for contacts and other screened people included either a tuberculin skin test or an interferon gamma release assay. For all of those tested, FCDHW defined a positive tuberculin skin test result as any skin induration ≥5 mm.16,23
The FCDHW clinician offered additional evaluation (eg, chest radiography, human immunodeficiency virus [HIV] test, sputum examination, physical examination) to those with TB symptoms, HIV infection, a current or prior positive TB test result, or a known TB exposure within the past 2 months. If active TB disease was excluded, those with a positive TB test result were considered to have latent M tuberculosis infection (LTBI) and offered treatment with 4 months of daily rifampin.16,23,24
We interviewed staff members and toured the 6 overnight homeless facilities used by patients during their estimated infectious periods. During these tours, we recorded data about the usual overnight census, client characteristics, types of services provided, seasons and hours of operation, registration procedures, use of germicidal ultraviolet air disinfection, and use of any infection control measures. These measures included required TB testing for client entry, TB symptom screening at intake each night, nightly designation of a person to monitor occupants for a persistent or worsening cough, provision of isolation areas for people with respiratory symptoms, infection control training for staff and volunteers, or routine TB testing for staff and volunteers.11
We used the CDC TB Genotyping Information Management System14 to examine the spread of the outbreak after the first US case occurred in Georgia in March 2008 and to compare the case characteristics of the Georgia outbreak with those of the same genotype in other states. We also estimated the contribution of the outbreak to the overall TB burden in Fulton County by determining the proportion of all culture-positive TB cases that had the outbreak-related genotype.
Results
Georgia Outbreak Cases
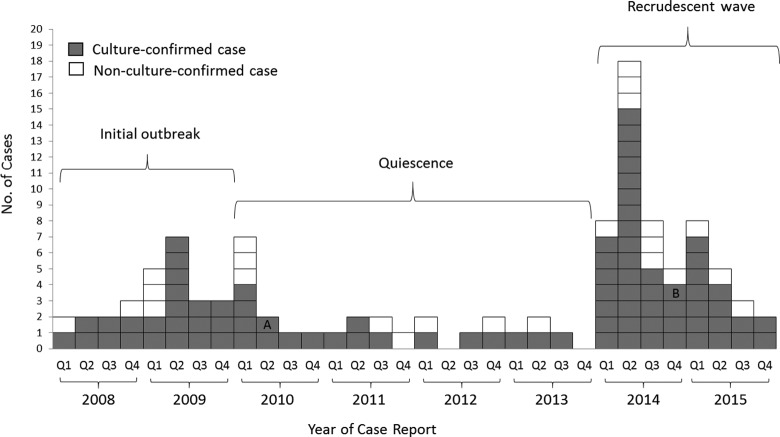
As of December 31, 2015, a total of 110 outbreak cases had been identified in Georgia. Of these 110 cases, 108 were unique patients; 2 patients had a TB recurrence >12 months after their last dose of treatment and, thus, according to National Tuberculosis Surveillance System instructions, were counted as having 2 separate TB cases. We identified 86 (78%) culture-confirmed cases, of which 84 (98%) were successfully genotyped (Figure 1). We also identified 2 (2%) culture-confirmed cases that were not successfully genotyped but involved patients who stayed overnight at Atlanta-area homeless facilities where outbreak-associated patients were present. All outbreak cases with drug susceptibility test results had TB that was resistant to isoniazid, which is typically part of first-line therapy for TB.24
Figure 1.
Distribution of 110 tuberculosis (TB) outbreak cases in Georgia, by year of case report, January 2008 to December 2015. The 110 cases include 84 genotyped TB outbreak-associated cases in Georgia and 26 epidemiologically linked TB cases in Georgia without genotype results. “A” indicates that the Mycobacterium tuberculosis isolate was not sent for genotyping for a patient with recurrent culture-confirmed TB and previous genotype-confirmed outbreak case. “B” indicates that the M tuberculosis isolate had unresolved and mixed genotyping results for a patient with culture-confirmed TB. Abbreviation: Q, quarter.
Of the 110 outbreak cases, 71 (65%) had TB detected when patients presented to health care providers for evaluation of TB symptoms, and 82 (75%) were hospitalized at the time of their diagnostic evaluation (Table 1). Only 2 (2%) patients had TB detected through FCDHW contact investigation activities, whereas 11 (10%) were identified through other TB screenings—for example, when a shelter implemented new TB testing requirements. The estimated median infectious period of cases during the initial outbreak phase was 136 days (interquartile range, 100-197); during the quiescent phase, 106 days (interquartile range, 97-122); and during the recrudescent phase, 142 days (interquartile range, 123-172; Table 1).
Table 1.
Characteristics of patients involved in 110 TB outbreak cases,a by outbreak phase, Georgia, March 2008 to December 2015
| Patient Characteristics | Phase, No. (%) or Median (IQR) | ||
|---|---|---|---|
| Initial Outbreak, 2008-2009 (n = 27) | Quiescent, 2010-2013 (n = 26) | Recrudescent, 2014-2015 (n = 57) | |
| Fulton County resident at TB diagnosis | 24 (89) | 19 (73) | 51 (89) |
| Sex | |||
| Male | 26 (96) | 24 (92) | 55 (96) |
| Female | 1 (4) | 1 (4) | 0 (0) |
| Transgender | 0 (0) | 1 (4) | 2 (4) |
| Age group, y | |||
| 15-24 | 2 (7) | 0 (0) | 1 (2) |
| 25-44 | 11 (41) | 4 (15) | 17 (30) |
| 45-64 | 14 (52) | 22 (85) | 37 (65) |
| ≥65 | 0 (0) | 0 (0) | 2 (4) |
| Race/ethnicity | |||
| Non-Hispanic black | 24 (89) | 25 (96) | 48 (84) |
| Non-Hispanic white | 1 (4) | 1 (4) | 8 (14) |
| Hispanic | 2 (7) | 0 (0) | 1 (2) |
| Born in the United States | 27 (100) | 25 (96) | 54 (95) |
| TB culture confirmed | 22 (81) | 18 (69) | 46 (81) |
| TB isoniazid resistant (of those with TB susceptibility results) | 22 (100) | 18 (100) | 46 (100) |
| Method of TB case detection | |||
| TB contact investigation | 2 (7) | 0 (0) | 0 (0) |
| Other TB screening | 2 (7) | 5 (19) | 4 (7) |
| Incidental CXR or laboratory finding | 3 (11) | 8 (31) | 15 (26) |
| Non–health department evaluation for TB-related symptoms | 20 (74) | 13 (50) | 38 (67) |
| Documented test for TB infection | 19 (71) | 16 (62) | 44 (77) |
| Location of diagnostic evaluation leading to TB diagnosis | |||
| Health department TB clinic | 5 (19) | 5 (19) | 7 (12) |
| Hospital | 19 (70) | 16 (62) | 47 (83) |
| Other | 3 (11) | 5 (19) | 3 (5) |
| Pulmonary TB | 25 (96) | 23 (88) | 54 (54) |
| Sputum AFB smear–positive disease or cavitary disease on CXR (of those with pulmonary TB) | 19 (76) | 13 (57) | 32 (59) |
| Contacts named (by those with pulmonary TB) | |||
| 0 | 16 (64) | 17 (74) | 27 (50) |
| Total (if named) | 2 (1-7) | 6 (1-7) | 2 (1-4) |
| Infectious period (of those with pulmonary TB), d | 136 (100-197) | 106 (97-122) | 142 (123-172) |
| Homeless | |||
| During year before TB diagnosis | 27 (100) | 19 (73) | 49 (86) |
| Ever | 27 (100) | 23 (89) | 52 (91) |
| Mental illnessb | 10 (37) | 6 (23) | 22 (39) |
| Substance use (during year before TB diagnosis) | |||
| Any substance use | 20 (74) | 11 (42) | 20 (35) |
| Excessive alcohol use | 13 (48) | 8 (31) | 11 (19) |
| Non–injection drug use | 17 (63) | 9 (35) | 12 (21) |
| Injection drug use | 1 (4) | 1 (4) | 0 (0) |
| In a prison or local jail | |||
| At time of diagnosis | 2 (7) | 5 (19) | 4 (7) |
| During year before TB diagnosis | 22 (81) | 11 (42) | 18 (32) |
| Prior to year before TB diagnosis | 21 (78) | 17 (65) | 36 (63) |
| Connection to outbreak (same TB genotype results) | |||
| Stayed overnight in homeless facility | 27 (100) | 21 (81) | 51 (89) |
| Volunteer, homeless facility | 0 (0) | 0 (0) | 3 (6) |
| Homeless, exposed in other location | 0 (0) | 1 (4) | 1 (2) |
| Undetermined | 0 (0) | 4 (15) | 2 (4) |
| HIV coinfection, total | 15 (56) | 9 (35) | 17 (30) |
| Newly recognized HIV infection (of total) | 1 (7) | 1 (11) | 1 (6) |
| HIV diagnosis before TB presentation | 14 (93) | 8 (89) | 16 (94) |
| Not receiving antiretroviral treatment (of those with known treatment status) | 13 (93) | 4 (67) | 11 (69) |
| Receiving antiretroviral treatment (of those with known treatment status) | 1 (7) | 2 (33) | 5 (31) |
| CD4+ count, cells/μL | 163 (94-417) | 148 (29-227) | 50 (35-132) |
| Housing support received during anti-TB treatment | 20 (74) | 12 (46) | 41 (72) |
| Hospitalizations | |||
| Hospitalized during diagnostic evaluation of or treatment for TB | 21 (78) | 19 (73) | 51 (89) |
| Hospitalized for unrelated reasons | 6 (24) | 2 (11) | 12 (24) |
| Duration of hospitalization, d | 15 (7-29) | 13 (8-45) | 12 (7-20) |
| Total duration of hospitalizations, person-days | 540 | 651 | 993 |
| Clinical outcomes as of October 24, 2016 | |||
| Completed anti-TB treatment | 23 (85) | 23 (88) | 50 (88) |
| Died before TB diagnosis recognized | 0 (0) | 1 (4) | 2 (4) |
| Died during anti-TB treatment | 3 (11) | 1 (4) | 5 (9) |
| Incomplete anti-TB treatment or status unknown | 1 (4) | 1 (4) | 0 (0) |
Abbreviations: AFB, acid-fast bacilli; CXR, chest x-ray; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
aPer National Tuberculosis Surveillance System instructions, recurrences in 2 patients 1 year after treatment counted as separate cases.
bMedical record documentation of American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (fourth edition, text revision) Axis I conditions (other than substance use disorders).20
Homelessness, substance use, and mental illness were common among patients in all 3 outbreak phases (Table 1). Of the 110 outbreak cases, 95 (86%) involved patients who had been homeless during the year before TB diagnosis, 51 (46%) involved patients who had used substances during the previous year, and 38 (35%) involved patients who had mental illness.
Ninety-nine (90%) outbreak cases were associated with overnight stays in ≥1 of the 6 overnight homeless facilities during the outbreak, whereas 3 (3%) cases occurred in nonhomeless people who had volunteered extensively in these homeless facilities and 2 (2%) cases occurred in homeless people who were exposed in locations other than a homeless facility (via work or social contacts; Table 1). Of the remaining 6 (5%) genotype-confirmed cases with an otherwise undetermined connection to the outbreak, 2 people had experienced homelessness during the year before TB diagnosis, and 1 had an earlier history of homelessness.
Of the 110 outbreak cases, 41 (37%) involved people living with HIV infection, of whom 8 were receiving antiretroviral treatment at the time of TB diagnosis (Table 1). In all patients with HIV coinfection, the median CD4+ count at the time of TB diagnosis during each outbreak phase was <200 cells/µL. Only 1 new diagnosis of HIV infection was made during each of the 3 outbreak phases.
Two-thirds of patients received local housing support (Table 1). The Georgia Department of Public Health estimated that its housing support from January 2014 through June 2015 (cost estimate available for only the recrudescent phase) was $390 000 (A. L. Latham, written communication, August 2015).
Twelve patients in the TB outbreak died, including 6 with HIV coinfection (Table 1). Of those 12 patients, 10 died from TB-related causes. Of the remaining 98 cases in which the patients were alive, all received TB medications through directly observed treatment: an adherence-enhancing strategy in which a health professional watches a patient swallow each dose of medication and records the dates that the administration was observed. As of October 24, 2016, all but 2 patients had completed at least 6 months of treatment; their whereabouts were unknown.
Contact Investigations
Across all 3 outbreak phases, 60 of the 102 (59%) outbreak-related patients with pulmonary TB were unable to name any contacts during their estimated infectious periods (Table 1). Complete data on contact investigations during the early outbreak and quiescent phases were not available. However, during the first 18 months of the recrudescent phase (January 2014 to June 2015), FCDHW evaluated 1360 homeless people through either facility-based or unrelated TB screenings. In addition, during this same period, FCDHW also evaluated 916 known contacts, some of whom had come to facility-based screenings and were retrospectively identified as contacts. As a result of these outbreak investigation activities, from this group of homeless people and contacts, 348 people were identified as having LTBI. As of June 30, 2015, only 58 (17%) of those with LTBI were receiving or had completed 4 months of rifampin.24
It was noted anecdotally that although outbreak-associated patients had often been present when screenings were offered in homeless facilities, they rarely participated in those voluntary screenings. However, only 2 of the 6 overnight homeless facilities involved in the outbreak had electronic registration or other well-maintained overnight rosters to corroborate patients’ self-reported shelter use and to aid in identifying contacts (Table 2).
Table 2.
Services, characteristics, and infection control measures of 6 overnight homeless facilities involved in a TB outbreak, by facility, Atlanta, Georgia, January 2014 to June 2015
| Facility | Primary Services Provided | Usual Overnight Census | Registration System for Clients (Before 2015) | TB Testing Requirement for Clients, Staff Members, and Volunteersa |
|---|---|---|---|---|
| A | Year-round emergency facility for men | 475 | Incomplete paper-based overnight roster, voluntary | No |
| B | Year-round facility for homeless men, transitional support services, substance abuse recovery program | 380 | Well-maintained electronic sign-in system | Voluntary TB testing was offered |
| C | Seasonal emergency facility for men | 110 | Incomplete paper-based system | No |
| D | Year-round facility for men and families, longer-term housing | 365 | Well-maintained electronic sign-in system | Voluntary TB testing was offered |
| E | Year-round facility for men, short-term housing | 179 | No information available, not electronic | No |
| F | Year-round facility for men, short-term housing | 76 | No information available, not electronic | No |
Abbreviation: TB, tuberculosis.
aSuch measures include TB symptom screening at intake each night, cough monitors, isolation area for clients with respiratory symptoms, and infection control training for staff members and volunteers.
Overnight Homeless Facilities as Suspected TB Transmission Sites
Patients who were part of the TB outbreak used various overnight facilities during infectious periods. Patient interviews and available facility records suggested that during the initial outbreak and quiescent phases (March 2008 to December 2013), all outbreak-associated patients stayed in facility A, which many described as their “shelter of choice,” where they typically resided for months at a time. However, starting with the recrudescent phase in January 2014, outbreak-associated patients slept in 3 other facilities (at least 21 at facility B, 5 at facility C, and 5 at facility D) and then still others (facilities E and F) by the end of 2015. Only facilities B and D had electronic registration rosters to aid in identifying contacts (Table 2). None of these overnight homeless facilities enforced infection control measures or used germicidal ultraviolet air disinfection during the 3 phases of the outbreak.
Contribution of Outbreak Cases to the Overall TB Burden in Fulton County
Of the 110 outbreak-associated cases in Georgia, 94 (85%) occurred in people residing in Fulton County at the time of TB diagnosis. During the initial outbreak phase, outbreak cases accounted for 51% (19 of 37) of Fulton County’s genotyped TB cases among homeless people and 25% (19 of 76) of its total genotyped TB cases (Table 3). During the recrudescent phase, outbreak cases accounted for 76% (35 of 46) of Fulton County’s genotyped TB cases among homeless people and 39% (40 of 103) of its total genotyped TB cases.
Table 3.
TB cases: total, among homeless patients,a and from outbreak cases, by outbreak phase, Fulton County, Georgia, March 2008 to December 2015b
| Phase | TB Cases, No. (%) | TB Cases Among Homeless Patients, No. (%) | ||
|---|---|---|---|---|
| Total | Outbreak | Total | Outbreak | |
| Initial outbreak, March 2008–December 2009 | 76 | 19 (25) | 37 | 19 (51) |
| Quiescent, January 2010–December 2013 | 149 | 11 (7) | 34 | 7 (21) |
| Recrudescent, January 2014–December 2015 | 103 | 40 (39) | 46 | 35 (76) |
Abbreviation: TB, tuberculosis.
aCases involving patients with any homelessness during the year before TB diagnosis.
bAll TB cases culture confirmed with genotype results.
Geographic Spread of Outbreak Beyond Georgia
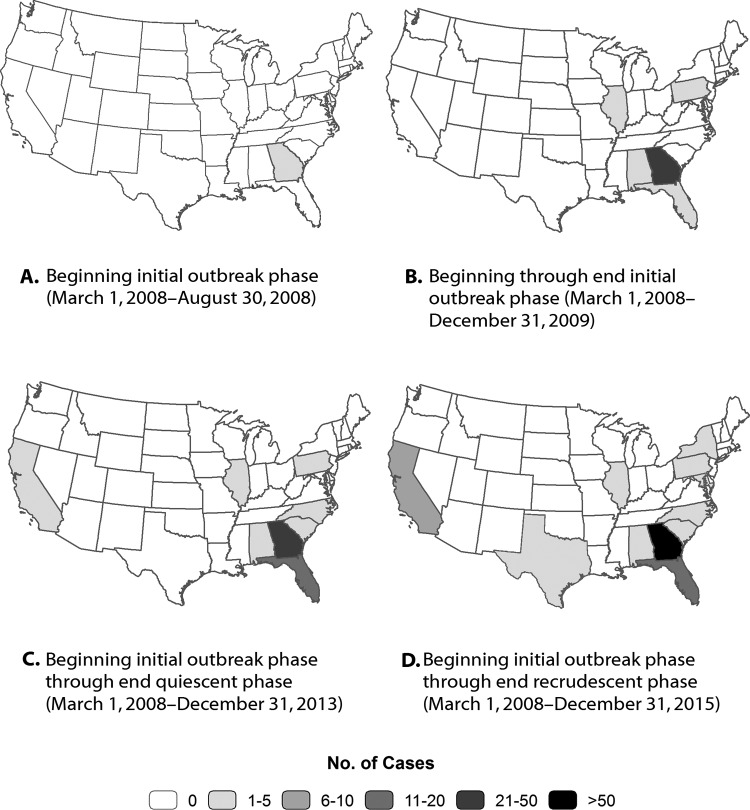
After the first 4 outbreak-associated cases were reported in Georgia starting in March 2008 (Figure 2A), the first case diagnosed outside Georgia was reported in September 2008 (Figure 2B). By the end of December 2015, a total of 120 genotyped TB cases consistent with the Georgia outbreak strain had been reported throughout the United States. Of these, 84 (70%) were in Georgia, and 36 (30%) were scattered across 9 other states (Figures 2C and 2D). Of the 36 non-Georgia cases, 9 (25%) were definitively linked to Atlanta facilities known to be involved in the outbreak, but 27 (75%) lacked the information to definitively determine linkage; also, 32 (89%) involved US-born patients, and 16 (44%) involved patients who had been homeless during the year before diagnosis.
Figure 2.
Geographic distribution of 120 tuberculosis (TB) cases with genotype consistent with the Georgia outbreak strain, United States, March 2008 to December 2015. The 120 cases include 84 genotyped TB outbreak-associated cases in Georgia and 36 genotyped TB cases outside of Georgia in which isolate genotype matched that of the Georgia outbreak cases.
Discussion
We describe an outbreak of isoniazid-resistant TB with a unique genotype that began in Atlanta and spread nationally. Nearly all connections in this outbreak were established retrospectively. In addition to the 110 outbreak cases in Georgia during 2008-2015, 36 cases with the same isolate genotype were identified in other areas of the United States, and at least one-quarter of these were definitively linked to Georgia. We also observed that a high proportion of TB cases in this outbreak were in patients who were homeless and living with HIV infection. This outbreak demonstrates that even when the incidence of TB in the United States was at its lowest level ever,20 under certain conditions, TB outbreaks could and did emerge or reemerge quickly.
Multiple factors may have contributed to this outbreak of TB in Georgia. First, 37% of the outbreak-associated patients had HIV coinfection, and most of these patients had low CD4+ counts, suggesting severe immunosuppression. HIV infection is one of the strongest risk factors for TB.16,23,24 In addition, despite having known HIV infection before their TB diagnosis, few of these patients were receiving antiretroviral treatment, which reduces the risk of TB.25,26 Second, other comorbidities or risk factors, such as mental illness and substance use, may have contributed to higher transmission rates in this setting. Both mental illness8,19 and substance use1,27 can contribute to transmission by decreasing patients’ willingness or ability to name contacts or participate in TB screenings. Third, the long median infectious periods that we identified, ranging from 106 to 142 days, may have resulted in extended periods of TB exposure for residents of crowded overnight homeless facilities.
Accentuating the aforementioned challenges is our finding that only 17% of people found to have LTBI by FCDHW during its outbreak investigation activities completed the 4 months of rifampin that was recommended to avert future cases. According to CDC, curing LTBI is the second priority in TB control after case finding and treatment.28 However, implementing LTBI treatment can be difficult in a population in which mental illness and substance use are common. In addition, the shortest recommended LTBI treatment regimen—12 weekly doses of isoniazid and rifapentine—is not recommended when the patient’s TB has isoniazid resistance.29
We also identified several challenges for case finding during this outbreak. As in other TB contact investigations involving homeless people,30–32 traditional name-based contact tracing methods had limited utility for case finding. Four of the 6 overnight homeless facilities involved in the outbreak lacked well-maintained overnight rosters, so missing rosters, as well as problems with illegibility and noncompliance, hindered efforts to identify contacts. Instead, most cases in this outbreak were identified through passive means (ie, patients seeking care because of symptoms). Screenings were not mandatory in any of the facilities involved in the outbreak and had little impact on case finding.
Most of the TB cases in this outbreak in Georgia involved patients with a history of homelessness. Even during the quiescent period, the proportion of TB cases in Fulton County that were associated with homelessness was 3 times the national average (∼20% vs 6%), suggesting ongoing risk for M tuberculosis exposures and transmission in the county during that time. Indeed, the unusually cold weather that hit Atlanta21,22 in early 2014 led to a surge in overnight homeless facility usage, which coincided with the recrudescent phase of the outbreak.
Given that homelessness and TB transmission are so strongly associated, the Advisory Council for the Elimination of Tuberculosis has made the following recommendations to homeless facilities: Maintain overnight rosters to assist with TB case finding; provide TB educational materials to guests, staff members, and volunteers; ensure that people with persistent cough are referred quickly to health care providers; optimize ventilation to reduce transmission; and consider use of germicidal ultraviolet air disinfection.11 A routine TB testing requirement for homeless clients can also decrease TB transmission.33 Despite these recommendations, the use of infection control measures in overnight homeless facilities is uncommon. Indeed, until 2015, there were no legal mandates requiring homeless facilities in Atlanta to have these measures in place; thus, no facilities had them. Had such measures been in place before 2015, TB transmission and its consequences might have been minimized. Notwithstanding these recommendations, implementing these measures is a substantial challenge for facilities, because as the incidence of TB has declined in the United States, resources for TB infection control and outbreak response have diminished.34,35
The consequences of this outbreak were substantial and far-reaching. First, 10 outbreak-associated patients had TB-related deaths. Although people who are homeless1 or who have HIV coinfection36,37 are at increased risk for TB-related death, death from TB is considered preventable.28 Second, the cost of local housing support to ensure respiratory isolation for contagious patients was >$20 000 per month during the recrudescent phase of the outbreak, and that cost excluded all of the other direct and indirect outbreak-related costs borne by the health department, hospitals, homeless service providers, and broader community.8,38 Third, the ongoing TB transmission associated with the outbreak resulted in the spread of TB to homeless facility volunteers and to people outside Georgia.
Limitations
This investigation had several limitations. First, although the proportion of culture-confirmed TB cases in Georgia that were successfully genotyped had improved from 85% in 2008 to >95% since 2010, some culture-confirmed cases were still not successfully genotyped. Second, inclusion of nongenotyped cases based on self-reported connections to Atlanta-area homeless facilities may have overestimated the number of outbreak cases. Third, because the TB genotype involved in this outbreak has been rare in the United States, we considered all genotype-matched cases to be connected to the outbreak, even in the absence of definitive epidemiologic linkages, but we cannot exclude the possibility that we overestimated the number of outbreak cases in Georgia and the scope of transmission outside of Georgia.
Conclusions
Despite a low incidence of TB in the United States, this investigation of a drug-resistant TB outbreak that began in Atlanta provides a warning about the ongoing potential for long-lasting and far-reaching outbreaks, particularly among high-risk populations with untreated HIV coinfection, mental illness, substance abuse, and homelessness. To prevent and control TB outbreaks, health departments should work with overnight homeless facilities to implement effective infection control measures and maintain searchable overnight rosters.
Acknowledgments
We thank the staff members and administration at Grady Memorial Hospital (Atlanta, Georgia), the TB clinic at FCDHW, Atlanta-area homeless facilities, and St Joseph’s Mercy Care Services (an Atlanta-area Health Care for the Homeless provider). We also thank Olivia Leach and Bryant Jones, from the Geospatial Research, Analysis and Services Program, a division of the Agency for Toxic Substances and Disease Registry at CDC, who provided assistance with developing the maps in Figure 2; Sandy Althomsons, an epidemiologist with the Division of TB Elimination at CDC, who provided assistance with data management and analysis; and C. Kay Smith, technical writer-editor with the National Center for HIV, Hepatitis, STD, and TB Prevention at CDC, who provided editorial assistance.
Footnotes
Authors’ Note: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC or other affiliated institutions.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by FCDHW, Georgia Department of Public Health, and CDC.
References
- 1. Bamrah S, Yelk Woodruff RS, Powell K, Ghosh S, Kammerer JS, Haddad MB. Tuberculosis among the homeless, United States, 1994-2010. Int J Tuberc Lung Dis. 2013;17(11):1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curtis AB, Ridzon R, Novick LF, et al. Analysis of Mycobacterium tuberculosis transmission patterns in a homeless shelter outbreak. Int J Tuberc Lung Dis. 2000;4(4):308–313. [PubMed] [Google Scholar]
- 3. Lathan M, Mukasa LN, Hooper N, et al. Cross-jurisdictional transmission of Mycobacterium tuberculosis in Maryland and Washington, DC, 1996-2000, linked to the homeless. Emerg Infect Dis. 2002;8(11):1249–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nickerson N, Linder J, Friou D, et al. Public health dispatch: tuberculosis outbreak in a homeless population—Portland, Maine, 2002-2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1184–1185. [PubMed] [Google Scholar]
- 5. McElroy PD, Southwick KL, Fortenberry ER, et al. Outbreak of tuberculosis among homeless persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2003;36(10):1305–1312. [DOI] [PubMed] [Google Scholar]
- 6. Hudson J, Van Zetta S, Brissette B, et al. Tuberculosis transmission in a homeless shelter population—New York, 2000-2003. MMWR Morb Mortal Wkly Rep. 2005;54(6):149–152. [PubMed] [Google Scholar]
- 7. Lofy KH, McElroy PD, Lake L, et al. Outbreak of tuberculosis in a homeless population involving multiple sites of transmission. Int J Tuberc Lung Dis. 2006;10(6):683–689. [PubMed] [Google Scholar]
- 8. Dobbins C, Marishta K, Kuehnert P, et al. Tuberculosis outbreak associated with a homeless shelter—Kane County, Illinois, 2007-2011. MMWR Morb Mortal Wkly Rep. 2012;61(11):186–189. [PubMed] [Google Scholar]
- 9. Samuel V, Benjamin C, Renwick O, et al. Notes from the field: tuberculosis cluster associated with homelessness—Duval County, Florida, 2004-2012. MMWR Morb Mortal Wkly Rep. 2012;61(28):539–540. [PubMed] [Google Scholar]
- 10. Knorr J, Sulivan Meissner J, Perri BR, et al. Notes from the field: outbreak of tuberculosis associated with a newly identified Mycobacterium tuberculosis genotype—New York City, 2010-2013. MMWR Morb Mortal Wkly Rep. 2013;62(45):904. [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Prevention and control of tuberculosis among homeless persons: recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1992;41(RR-5):13–23. [PubMed] [Google Scholar]
- 12. Oelemann MC, Diel R, Vatin V, et al. Assessment of an optimized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol. 2007;45(3):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Notice to readers: new CDC program for rapid genotyping of Mycobacterium tuberculosis isolates. MMWR Morb Mortal Wkly Rep. 2005;54(2):47. [Google Scholar]
- 14. Ghosh S, Moonan PK, Cowan L, et al. Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol. 2012;12(4):782–788. [DOI] [PubMed] [Google Scholar]
- 15. Grant J, Kammerer S, Baker B, et al. Tuberculosis genotyping—United States, 2004-2010. MMWR Morb Mortal Wkly Rep. 2012;61(36):723–725. [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54(RR-15):1–37. [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2013. Atlanta, GA: US Department of Health and Human Services; 2014. http://www.cdc.gov/tb/statistics/reports/2013/default.htm. Accessed March 4, 2016. [Google Scholar]
- 18. Fazel S, Khosla V, Doll H, et al. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5(12):e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cavanaugh JS, Powell K, Renwick OJ, et al. An outbreak of tuberculosis among adults with mental illness. Am J Psychiatry. 2012;169(6):569–575. [DOI] [PubMed] [Google Scholar]
- 20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- 21. National Climatic Data Center. State of the climate: national overview—January 2014. http://www.ncdc.noaa.gov/sotc/national/201401. Accessed April 4, 2016.
- 22. Jonsson P. Homeless in South “ran for their lives” as polar vortex bit hard. Christian Science Monitor. http://www.csmonitor.com/USA/Society/2014/0110/Homeless-in-South-ran-for-their-lives-as-polar-vortex-bit-hard. Published 2014. Accessed April 4, 2016.
- 23. Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49(RR-6):1–54. [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Treatment of tuberculosis: recommendations from the American Thoracic Society, CDC, and Infectious Diseases Society of America [published erratum appears in MMWR Morb Mortal Wkly Rep. 2005;53(51/52):1203]. MMWR Recomm Rep. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 25. Santoro-Lopes G, de Pinho AM, Harrison LH, et al. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34(4):543–546. [DOI] [PubMed] [Google Scholar]
- 26. Elzi L, Schlegel M, Weber R, et al. ; Swiss HIV Cohort Study. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin Infect Dis. 2007;44(1):94–102. [DOI] [PubMed] [Google Scholar]
- 27. Oeltmann JE, Kammerer JS, Pevzner ES, Moonan PK. Tuberculosis and substance abuse in the United States, 1997-1996. Arch Intern Med. 2009;169(2):189–197. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection [published erratum appears in MMWR Morb Mortal Wkly Rep. 2012;61(4):80]. MMWR Morb Mortal Wkly Rep. 2011;60(48):1650–1653. [PubMed] [Google Scholar]
- 30. Marks SM, Taylor Z, Qualls NL, et al. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000;162(6):2033–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reichler MR, Reves R, Bur S, et al. Evaluation of investigations conducted to detect and prevent transmission of tuberculosis. JAMA. 2002;287(8):991–995. [DOI] [PubMed] [Google Scholar]
- 32. Yun LW, Reves RR, Reichler MR, et al. Outcomes of contact investigation among homeless persons with infectious tuberculosis. Int J Tuberc Lung Dis. 2003;7(12)(suppl 3):S405–S411. [PubMed] [Google Scholar]
- 33. Kong PM, Tapy J, Calixto P, et al. Skin-test screening and tuberculosis transmission among the homeless. Emerg Infect Dis. 2002;8(11):1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jereb JA. Progressing toward tuberculosis elimination in low-incidence areas of the United States: recommendations of the Advisory Council for the Elimination of Tuberculosis [published erratum appears in MMWR Morb Mortal Wkly Rep. 2002;51(19):427]. MMWR Recomm Rep. 2002;51(RR-5):1–16. [PubMed] [Google Scholar]
- 35. Reichman LB. The U-shaped curve of concern. Am Rev Respir Dis. 1991;144(4):741–742. [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. Mortality among patients with tuberculosis and associations with HIV status—United States, 1993-2008 [published erratum appears in MMWR Morb Mortal Wkly Rep. 2011;60(4):115]. MMWR Morb Mortal Wkly Rep. 2010;59(46):1509–1513. [PubMed] [Google Scholar]
- 37. Marcy O, Laureillard D, Madec Y, et al. ; CAMELIA (ANRS 1295-CIPRA KH001) Study Team. Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis. 2014;59(3):435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marks SM, Taylor Z, Burrows NR, Qayad MG, Miller B. Hospitalization of homeless persons with tuberculosis. Am J Public Health. 2000;90(3):435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]