Abstract
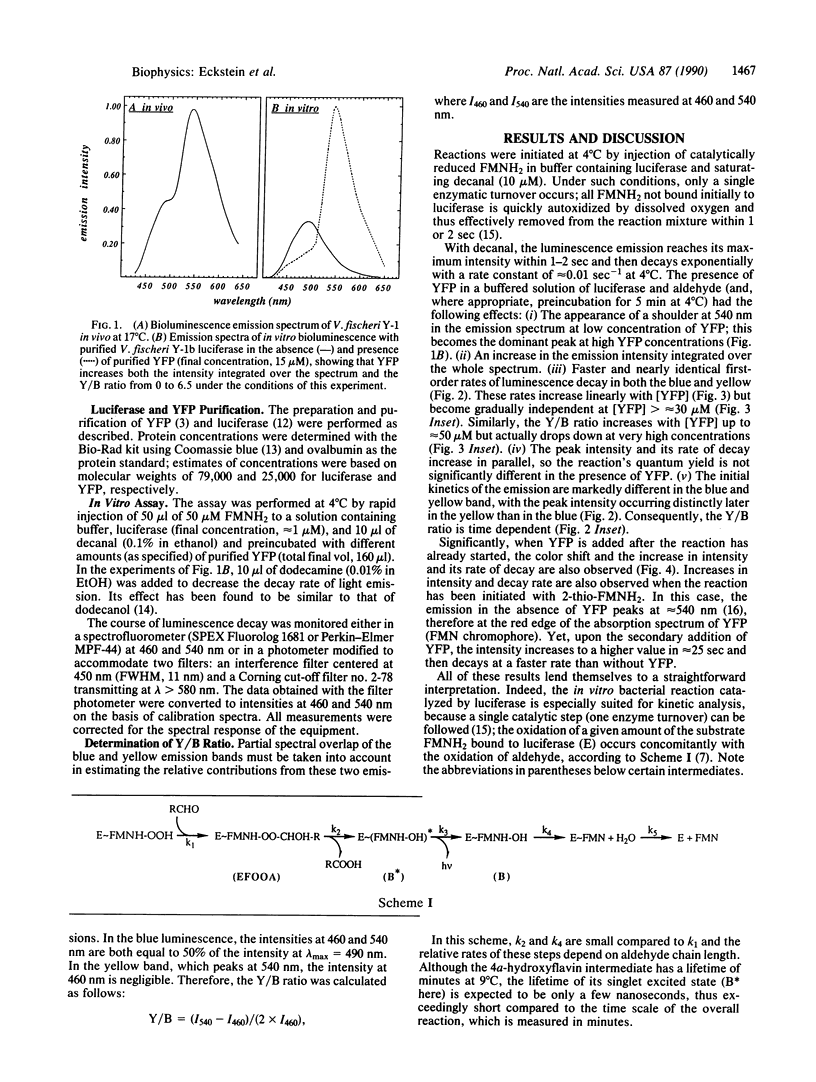
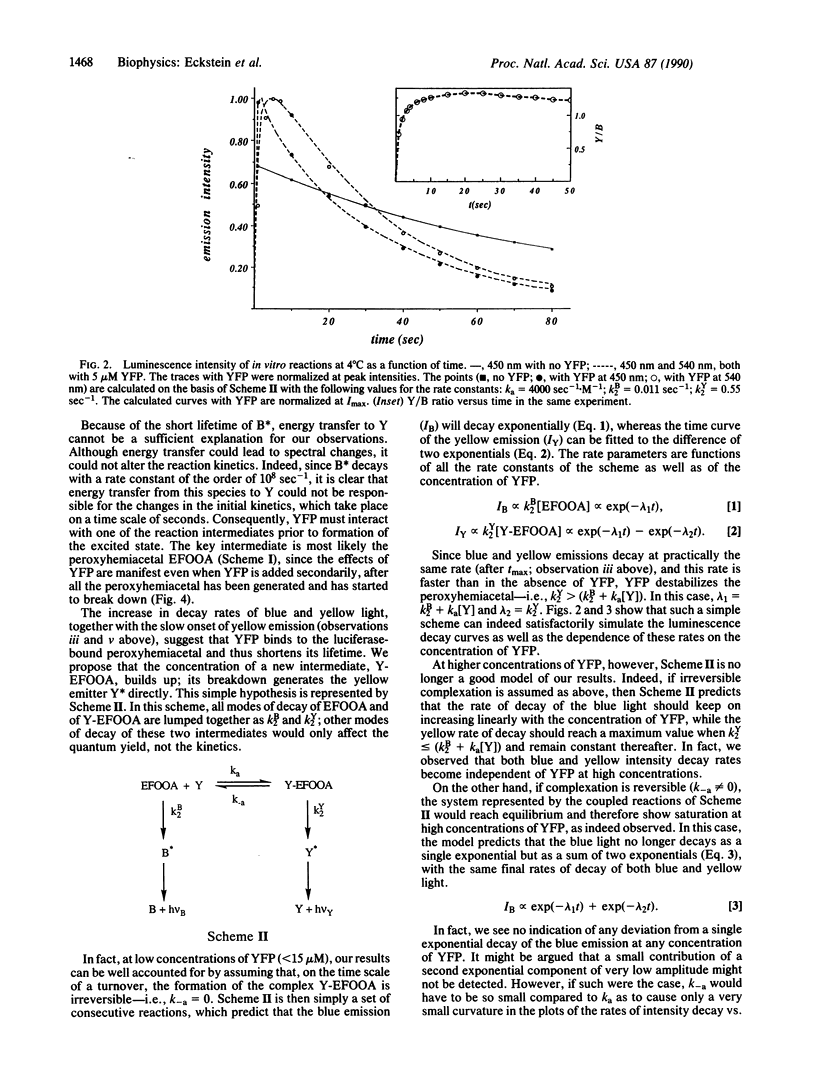
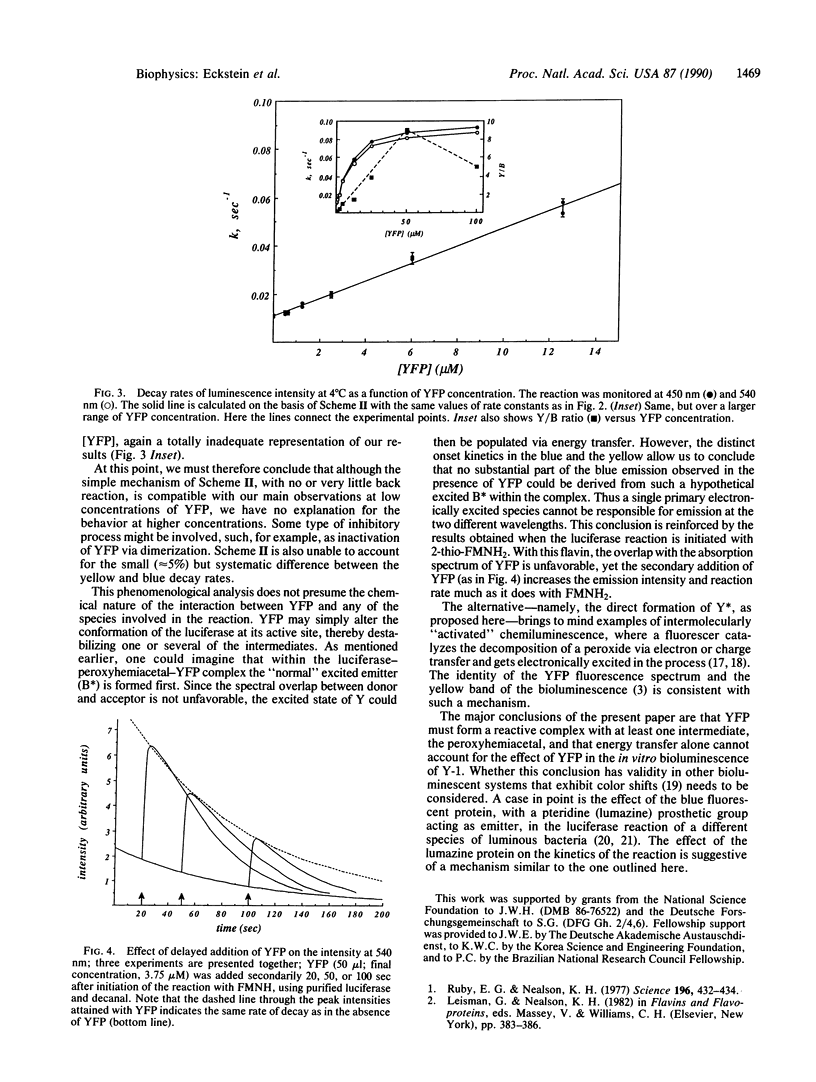
Yellow fluorescent protein (YFP), which has a bound FMN, was isolated from the marine bacterium Vibrio fischeri strain Y-1b. Its presence in a luciferase [alkanal monooxygenase (FMN-linked); alkanal, reduced-FMN:oxygen oxidoreductase (1-hydroxylating, luminescing), EC 1.14.14.3] reaction mixture causes a striking color change, and an increase in bioluminescence intensity, as well as a faster rate of intensity decay, so that the quantum yield is not changed. The emission spectrum shows two distinct color bands, one at 490 nm attributed to the unaltered emission of the luciferase system, the other peaking in the yellow around 540 nm due to YFP emission. The kinetics of the two color bands differ, so the spectrum changes with time. The yellow emission reaches its initial maximum intensity later than the blue, and then both blue and yellow emissions decay exponentially with nearly the same pseudo-first-order rate constants, linearly dependent on [YFP] (from 0.01 sec-1 with no YFP to a maximum of approximately 0.1 sec-1 at 4 degrees C) but exhibiting a saturation behavior. The data can be interpreted by assuming the interaction of YFP with the peroxyhemiacetal intermediate in the luciferase reaction to form an unstable new complex whose breakdown gives the yellow emitter in its excited state. This simple model fits well the data at [YFP] less than 15 microM. The results indicate that a single primary excited state cannot be responsible for the blue and the yellow emissions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daubner S. C., Astorga A. M., Leisman G. B., Baldwin T. O. Yellow light emission of Vibrio fischeri strain Y-1: purification and characterization of the energy-accepting yellow fluorescent protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8912–8916. doi: 10.1073/pnas.84.24.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubner S. C., Baldwin T. O. Interaction between luciferases from various species of bioluminescent bacteria and the yellow fluorescent protein of Vibrio fischeri strain Y-1. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1191–1198. doi: 10.1016/0006-291x(89)91368-5. [DOI] [PubMed] [Google Scholar]
- Hastings J. W. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J Mol Evol. 1983;19(5):309–321. doi: 10.1007/BF02101634. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Potrikus C. J., Gupta S. C., Kurfürst M., Makemson J. C. Biochemistry and physiology of bioluminescent bacteria. Adv Microb Physiol. 1985;26:235–291. doi: 10.1016/s0065-2911(08)60398-7. [DOI] [PubMed] [Google Scholar]
- Holzman T. F., Baldwin T. O. Isolation of bacterial luciferases by affinity chromatography on 2,2-diphenylpropylamine-Sepharose: phosphate-mediated binding to an immobilized substrate analogue. Biochemistry. 1982 Nov 23;21(24):6194–6201. doi: 10.1021/bi00267a026. [DOI] [PubMed] [Google Scholar]
- Kurfürst M., Ghisla S., Hastings J. W. Characterization and postulated structure of the primary emitter in the bacterial luciferase reaction. Proc Natl Acad Sci U S A. 1984 May;81(10):2990–2994. doi: 10.1073/pnas.81.10.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., O'Kane D. J., Gibson B. G. Bioluminescence spectral and fluorescence dynamics study of the interaction of lumazine protein with the intermediates of bacterial luciferase bioluminescence. Biochemistry. 1989 May 16;28(10):4263–4271. doi: 10.1021/bi00436a022. [DOI] [PubMed] [Google Scholar]
- Macheroux P., Schmidt K. U., Steinerstauch P., Ghisla S., Colepicolo P., Buntic R., Hastings J. W. Purification of the yellow fluorescent protein from Vibrio fischeri and identity of the flavin chromophore. Biochem Biophys Res Commun. 1987 Jul 15;146(1):101–106. doi: 10.1016/0006-291x(87)90696-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Hastings J. W. The effect of flavin isomers and analogues upon the color of bacterial bioluminescence. J Biol Chem. 1969 May 25;244(10):2572–2576. [PubMed] [Google Scholar]
- O'Kane D. J., Lee J. Chemical characterization of lumazine protein from Photobacterium leiognathi: comparison with lumazine protein from Photobacterium phosphoreum. Biochemistry. 1985 Mar 12;24(6):1467–1475. doi: 10.1021/bi00327a027. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. A luminous bacterium that emits yellow light. Science. 1977 Apr 22;196(4288):432–434. doi: 10.1126/science.850787. [DOI] [PubMed] [Google Scholar]
- Tu S. C. Isolation and properties of bacterial luciferase-oxygenated flavin intermediate complexed with long-chain alcohols. Biochemistry. 1979 Dec 25;18(26):5940–5945. doi: 10.1021/bi00593a028. [DOI] [PubMed] [Google Scholar]



