Abstract
Background:
From 1990-2005 at Ochsner Medical Center in New Orleans, LA, cardiopulmonary bypass (CPB) was used only when necessary during lung transplantation surgeries. Ochsner's lung transplant program was closed for more than 4 years after Hurricane Katrina, and since the program's reestablishment in 2010, the majority of lung transplantation surgeries have been performed with the patient on CPB and with a median sternotomy incision. The purpose of this study was to compare the outcomes of the CPB and non-CPB groups.
Methods:
After institutional review board approval, we conducted a retrospective review of the entire program using the Ochsner lung transplant database to identify patients in the non-CPB group from 1990-2005 and in the CPB group from 2010-2014. We calculated 1- and 3-year survival rates for each patient and reviewed medical records for evidence of stroke, the need for operative reexploration, and venous stenosis. We also performed a subgroup analysis of the first 20 consecutive patients undergoing lung transplantation on CPB with median sternotomy from February 2010 through April 2011 to examine intraoperative blood product use, the quantity of blood products administered, CPB cannulation and pump complications, ischemic time, and primary graft dysfunction.
Results:
Of the 208 patients in the non-CPB group, 74% had 1-year graft survival and 55% had 3-year survival following transplantation. After February 2010, 79 patients underwent lung transplantation on CPB with median sternotomy, and 90% of those patients had 1-year graft survival. Of the 46 patients available for 3-year follow-up, 59% were alive with functional grafts. The difference in 1-year survival rates between the 2 cohorts was statistically significant. Two deaths, 3 strokes, and 5 reexplorations of the chest for bleeding occurred during the perioperative time period in the CPB group, but no mortality was associated with these perioperative events. One patient who had perioperative complications died within the first year; the death was attributable to gastric perforation.
Conclusion:
Patients' early outcomes appear to have improved with the use of CPB and median sternotomy; however, 3-year survival is similar to the non-CPB group. Technical benefits of CPB with median sternotomy include decreased warm ischemia time during graft implantation, controlled hemodynamics and reperfusion, avoidance of single-lung ventilation of a freshly implanted graft, and the option to open the left atrium for implantation of a venous cuff without using a clamp. The surgical exposure facilitated by CPB with median sternotomy for lung transplantation appears to be a safe and feasible approach for lung transplantations.
Keywords: Cardiopulmonary bypass, intraoperative complications, lung transplantation, sternotomy, thoracic surgery
INTRODUCTION
The first human lung transplant was performed by Dr James Hardy at the University of Mississippi in 1967.1 The operation was a technical success, as the patient's PaO2 normalized; however, the patient did not survive his hospitalization. The first successful lung transplant was performed in 1981 by Reitz and colleagues as a combined heart-lung transplantation.2 Cooper and colleagues reported a single lung transplant in 1987,3 followed by a report of a bilateral sequential lung transplant in 1990.4 Overall survival rates of 88% at 1 year and 55% at 5 years are expected nationwide for patients receiving lung transplants, in part because of improved technique, immunosuppressive protocols, and updates to the lung allocation score.5
Today, the commonly used surgical approach for bilateral sequential lung transplantation is the transverse thoracosternotomy—the clamshell incision—the technique reported by Pasque, Cooper, and colleagues in 1990.4 The technique was developed to provide excellent exposure to both pleural cavities and mediastinal structures and to eliminate the need for cardiopulmonary bypass (CPB). Concerns about the use of CPB include complications related to cannulation, its effect on the body, and graft function.6 The clamshell incision consists of transverse sternal division, transverse division of the pectoralis major muscles, division of the serratus muscles laterally, and division of the intercostal muscles posteriorly. Despite the advantages of the clamshell incision, Macchiarini and colleagues reported significantly more sternal overriding and chronic postoperative pain in patients who had a clamshell compared to patients who had a median sternotomy with procedures performed on CPB.7 Median sternotomy has been the standard incision for cardiac surgery and has been adopted as the surgical approach for lung transplantation in some centers.
The lung transplant program at Ochsner Medical Center was voluntarily deactivated following Hurricane Katrina in 2005 and then reestablished in October 2009. At that time, our group decided to use a median sternotomy with CPB as our approach of choice for both single and bilateral sequential lung transplantations. Prior to the lung transplant program's reestablishment in 2009, we used CPB only when necessary during lung transplantation surgeries.
The purpose of this study was to compare the outcomes of the CPB and non-CPB groups. In addition to presenting our preliminary results, we discuss our surgical technique.
METHODS
After institutional review board approval, we conducted a retrospective review of the entire program using the lung transplantation database to identify patients. Patients who received a lung transplant from 1990-2005 comprised the non-CPB group. The patients in the CPB group received a lung transplant from 2010-2014. We calculated 1- and 3-year survival rates for each patient and analyzed the data using a Kaplan-Meier curve. We reviewed the patients' medical records to evaluate for stroke, the need for operative reexploration, and venous stenosis in the CPB group.
We also performed a subgroup analysis of the initial 20 consecutive patients who underwent lung transplantation on CPB with median sternotomy between February 2010 and April 2011. This group of patients was analyzed for intraoperative blood product use, the quantity of blood products administered, CPB cannulation and pump complications, ischemic time, and primary graft dysfunction.
RESULTS
Of the 208 patients in the non-CPB group, 153 patients (74%) had 1-year survival, and 115 (55%) had 3-year survival following transplantation. Of the 80 patients who received a lung transplant between 2010-2014, 79 patients underwent transplantation on CPB. Of those, 71 patients (90%) had 1-year survival. Of the 46 patients in the CPB group who were available for 3-year follow-up, 27 (59%) were alive with functional grafts. The difference in 1-year survival rates between the 2 cohorts was statistically significant, but no difference was seen between the groups in 3-year survival (Figure and Table 1). No retransplants were performed in either group.
Figure.
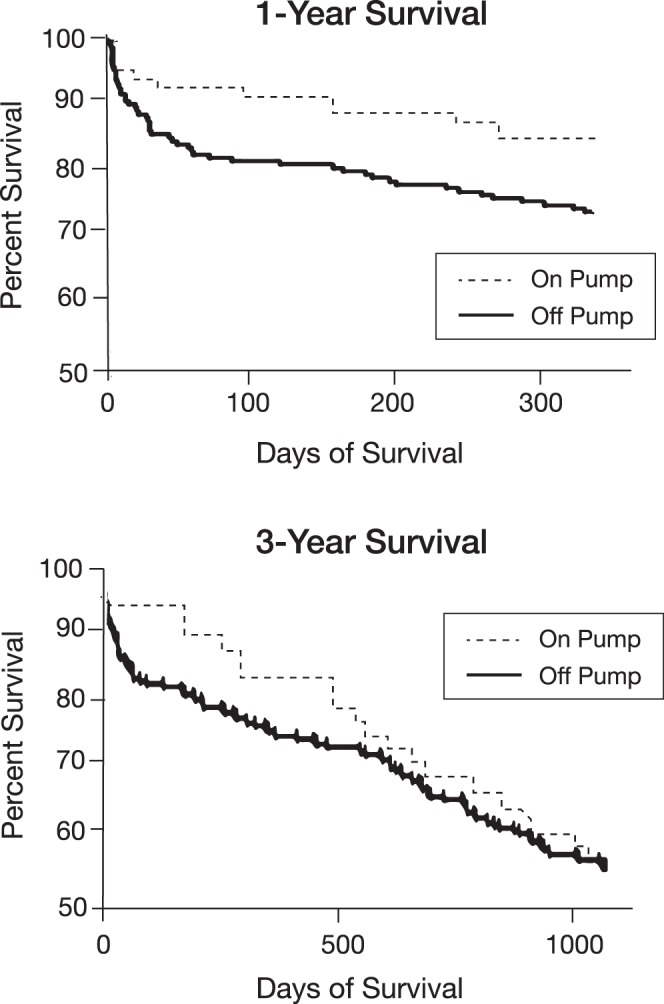
Survival at 1 and 3 years in the cardiopulmonary bypass (on pump) and non-cardiopulmonary bypass (off pump) groups.
Table 1.
Survival at 1 and 3 Years for Patients Receiving Lung Transplantation

The perioperative period was defined as 30 days from transplantation and was used to evaluate procedural outcomes in the CPB group. Two deaths occurred during the initial perioperative period: one attributable to primary graft failure and one to right ventricular failure. Three strokes occurred, 1 of which was hemorrhagic. One stroke likely occurred intraoperatively, 1 occurred on postoperative day (POD) 10, and the third occurred more than 2 months postoperatively and therefore was likely unrelated to the surgery or the use of CPB. Reexploration of the chest for bleeding occurred on 5 occasions, but none of the patients had bleeding from their cannulation sites. One anastomotic bleed occurred, but none of the other 4 patients had a specific bleeding site noted. No mortality was associated with these perioperative events. Of the patients who had perioperative complications, 1 patient was not alive at 1 year, with death attributable to gastric perforation.
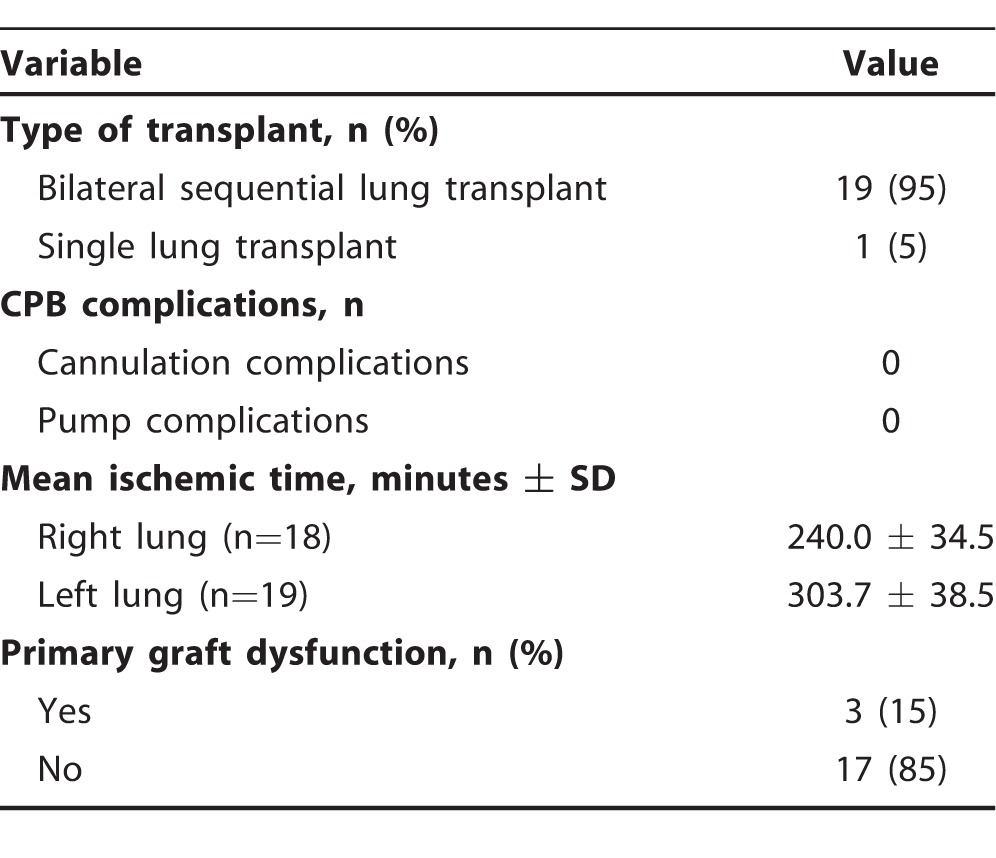
The first 20 patients in the CPB group included 19 bilateral sequential lung transplants and 1 single left lung transplant. Surgical details are presented in Table 2. In 18 right lungs, transplanted ischemic time was 240.0 ± 34.5 minutes. In 19 left lungs, ischemic time was 303.7 ± 38.5 minutes. In general, ischemic time on the lung allograft should be limited to <360 minutes.
Table 2.
Surgical Details for the First 20 Cardiopulmonary Bypass (CPB) Patients
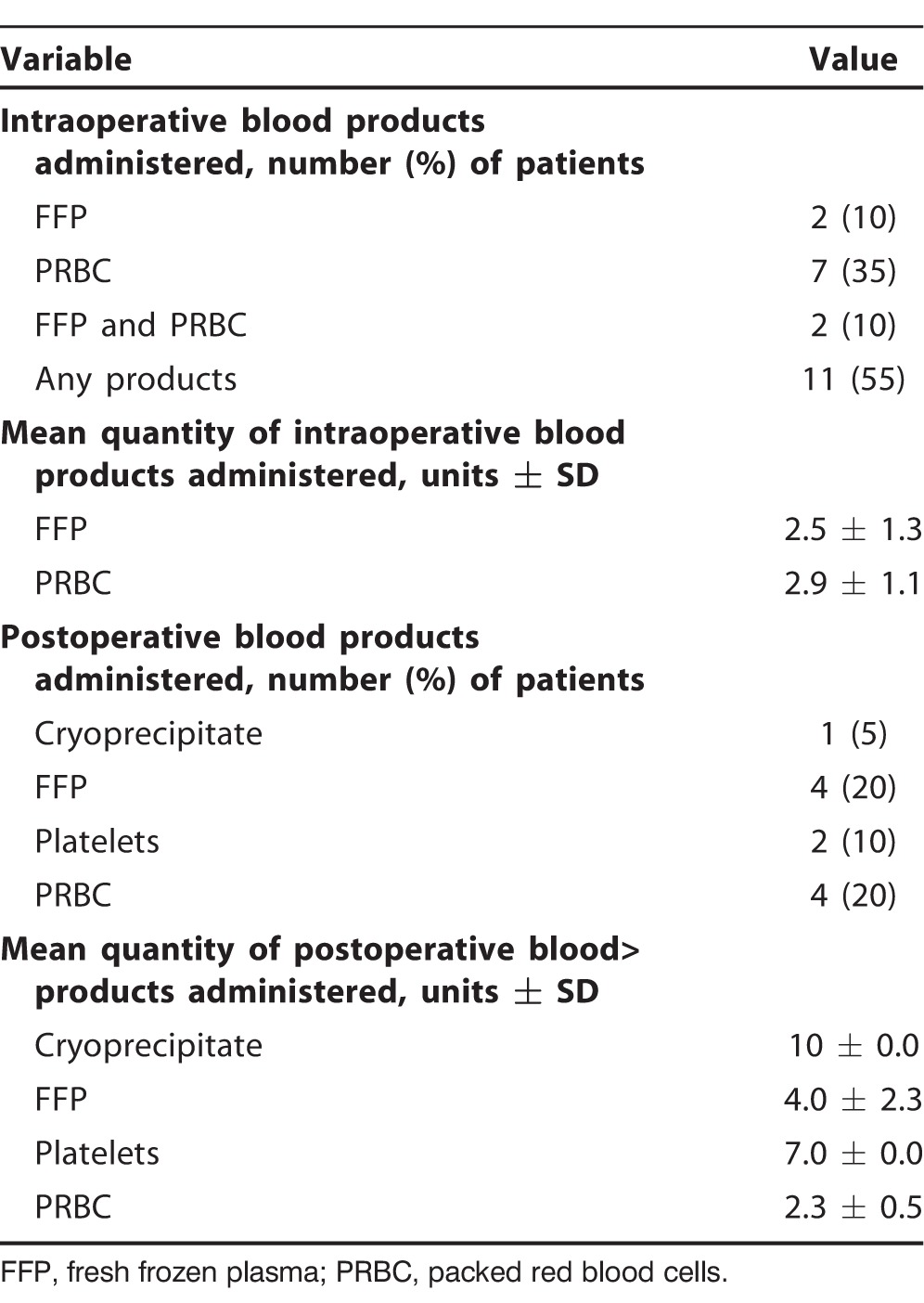
Transfusion requirements for this initial cohort of 20 CPB patients are listed in Table 3. These data were collected to define and review our center's use of blood products specific to this surgical technique. Eleven patients (55%) received an intraoperative transfusion of blood products that were administered by both perfusion and anesthesia (2 patients received both fresh frozen plasma [FFP] and packed red blood cell [PRBC] transfusions intraoperatively). Four patients (20%) received blood products within 24 hours postoperatively. One patient was on warfarin (Coumadin) at the time of the operation. This patient received 4 units of FFP intraoperatively and required 2 units of PRBCs and 2 units of FFP within 24 hours of postoperative care. This patient is also the only patient who required a reexploration secondary to a small intrapericardial thrombus compressing the right atrium and causing cardiac tamponade on POD 3.
Table 3.
Blood Products Used in the First 20 Cardiopulmonary Bypass Patients
No technical complications were directly related to CPB, and 3 patients developed primary graft dysfunction (PGD) (grade 3). The 3 patients identified with PGD all survived for 3 years. No pulmonary vein stenosis was identified intraoperatively or postoperatively in any patients.
DISCUSSION
Our operative technique involves the use of a median sternotomy incision and CPB in most cases. The patient is intubated with a single lumen tube. Transesophageal echocardiography is performed by the cardiac anesthesia team during the procedure. A median sternotomy is performed, and cannulas are placed in the ascending aorta for arterial drainage and in the superior vena cava and the right atrial appendage for dual venous drainage. Tricuspid repair and other cardiac procedures can be performed at this time. The pulmonary artery may be actively vented to avoid right ventricular distention, and the heart is kept beating and perfused during implantation.
The patient is placed on CPB. The left and right pleural spaces are opened, the hilum of the right lung is dissected around the vascular structures, and a right pneumonectomy is performed. The pericardium is opened around the pulmonary veins well away from the phrenic nerve. The pulmonary artery is dissected circumferentially from underneath the vena cava and from the dome of the left atrium. The bronchus is divided with a stapler at the pericardial reflection, and the soft tissue around the bronchus is preserved. The left pneumonectomy is performed in a similar fashion. Implantation of the donor lungs is performed sequentially. The airway anastomosis is performed first with PDS suture (Ethicon US, LLC). The venous cuff is anastomosed to the atrium, and the arterial anastomosis is performed. The lung is then reperfused, deaired, and gently ventilated with room air. Bronchoscopy can easily be performed prior to separating from CPB. Once the lungs exhibit good function, the patient is separated from CPB. Venous and arterial cannulas are removed, and protamine is administered to the patient.
The use of CPB offers several advantages. The exposure through a median sternotomy incision is greatly facilitated and expedites the pneumonectomy, especially in patients with difficult pleural spaces. The implant of the donor lungs is also greatly facilitated. The typical time to implant each lung is 20-25 minutes. Following implantation of each allograft, CPB offers the ability to control the hemodynamics of reperfusion and avoid single-lung ventilation of a freshly implanted graft while the second is implanted. Our technique has evolved to allow for an open anastomosis of the venous cuff. By employing techniques routine in cardiac surgery—venting of the ascending aorta and using carbon dioxide in the surgical field—the left atrium can be opened, allowing a direct cuff-to-cuff anastomosis. This technique allows us to maintain technical excellence even with the most challenging anatomy, with almost no risk of venous stenosis. These techniques are also applied to the challenging arterial anastomosis when required.
The possible complications of using CPB include the risk of bleeding, stroke, and problems related to cannulation. One perioperative stroke may have been related to this technique, but none of our patients had bleeding from the cannulation site or other cannulation complications. CPB has also been associated with the development of PGD. Risk for development of PGD is multifactorial and depends on risk factors from the donor and recipient and from operative factors.8 The incidence of PGD ranges from 10%-25%, with an associated 50% 30-day mortality.8 Three patients (15%) in our CPB cohort developed severe PGD; all 3 patients were treated for the condition with favorable results. Given our small numbers, it is unclear whether we can associate the development of PGD in our population solely to the use of CPB.
Reviewing the entire cohort of patients who received CPB would help clarify these initial findings. A limitation of this study is that both cohorts should be studied further to make additional meaningful comparisons. Aside from the routine use of CPB, many factors potentially could contribute to the outcomes in these 2 groups. These groups are from 2 separate time periods in the lung transplant program's history with potentially significant differences in patient selection, donor criteria, and clinical management. The 2 groups should also be analyzed further for characteristics such as age, sex, pulmonary disease, and comorbid conditions.
While not measured in this study, lower postoperative pain and improved participation with pulmonary toilet and ambulation have been our experience and may be an area of future study.
CONCLUSION
Early outcomes and 1-year survival in our lung transplant program appear to have improved with our use of CPB and median sternotomy; however, 3-year survival remains similar to that of patients who did not receive CPB. Technical benefits of CPB include decreased warm ischemia time during graft implantation, controlled hemodynamics and reperfusion, avoidance of single-lung ventilation of a freshly implanted graft, and the option to open the left atrium for implantation of a venous cuff without using a clamp. The surgical exposure facilitated by CPB with median sternotomy for lung transplantation appears to be a safe and feasible approach for lung transplantations.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, Systems-Based Practice, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Hardy JD, Webb WR, Dalton ML Jr, Walker GR Jr. . Lung homotransplantation in man. JAMA. 1963. December 21; 186: 1065- 1074. [DOI] [PubMed] [Google Scholar]
- 2. Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982. March 11; 306 10: 557- 564. [DOI] [PubMed] [Google Scholar]
- 3. Cooper JD, Pearson FG, Patterson GA, et al. Technique of successful lung transplantation in humans. J Thorac Cardiovasc Surg. 1987. February; 93 2: 173- 181. [PubMed] [Google Scholar]
- 4. Pasque MK, Cooper JD, Kaiser LR, Haydock DA, Triantafillou A, Trulock EP. . Improved technique for bilateral lung transplantation: rationale and initial clinical experience. Ann Thorac Surg. 1990. May; 49 5: 785- 791. [DOI] [PubMed] [Google Scholar]
- 5. Valapour M, Skeans MA, Smith JM, et al. Lung. Am J Transplant. 2016. January; 16 Suppl 2: 141- 168. 10.1111/ajt.13671. [DOI] [PubMed] [Google Scholar]
- 6. Ailawadi G, Zacour RK: . Cardiopulmonary bypass/extracorporeal membrane oxygenation/left heart bypass: indications, techniques, and complications. Surg Clin North Am. 2009. August; 89 4: 781- 796, vii-viii. 10.1016/j.suc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 7. Macchiarini P, Ladurie FL, Cerrina J, Fadel E, Chapelier A, Dartevelle P. . Clamshell or sternotomy for double lung or heart-lung transplantation? Eur J Cardiothorac Surg. 1999. March; 15 3: 333- 339. [DOI] [PubMed] [Google Scholar]
- 8. Lee JC, Christie JD. . Primary graft dysfunction. Proc Am Thorac Soc. 2009. January 15; 6 1: 39- 46. 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]


