Abstract
Rationale
In order to improve understanding of the nature of drug-associated memory, the current studies addressed whether conditioned place preference (CPP) could develop under conditions in which there was a delay between presentation of context and drug exposure (i.e., retrograde or trace conditioning).
Objectives
The objective was to assess development of CPP when cocaine or methamphetamine was injected simultaneously with exposure to a salient context (S+), or after delays differing in length.
Methods
Dose response curves for conventional CPP were established using separate groups of Swiss Webster mice injected with cocaine or methamphetamine just prior to S+ exposure. To assess development of retrograde CPP, other groups received trace conditioning, where cocaine (15 mg/kg) or methamphetamine (0.5 mg/kg) was injected after a delay of 15, 60, 120, 180, 240, or 480 minutes following the end of the S+ session.
Results
Mice receiving conventional CPP with cocaine or methamphetamine during S+ showed significant place preference. None of the groups receiving delayed methamphetamine showed significant CPP; however, CPP was evident in mice receiving cocaine after delays of up to 4 hours following S+. In a separate study, delayed methamphetamine also did not result in significant place preference when presented in doses of 0.25 or 1 mg/kg.
Conclusions
These results suggest that psychostimulant drug taking may be broadly generalized to context through retrograde association with events in recent memory, a factor that may contribute to drug-seeking and relapse following abstinence.
Keywords: Conditioned place preference, memory, cocaine, methamphetamine, Pavlovian conditioning
Introduction
A major obstacle in treatment of long-term psychostimulant addiction and relapse is the profound desire for a drug that humans experience in the presence of a context previously associated with drug taking, or when drug-associated cues are encountered (Childress et al. 1999; Grant et al. 1996). The development of these associations is hypothesized to involve neuroplastic interactions among mesocorticolimbic circuits regulating natural reward and those responsible for contextual memory (Dong and Nestler 2014; Kelley 2004; van Huijstee and Mansvelder 2015), yet expression of memory for these associations is a stronger motivator of drug-seeking behavior than would be expected on the basis of natural reward (Crombag et al. 2008; Gipson et al. 2013). It has been argued that drug-related memories are more stable and long-lasting than those established through natural rewards (Dong and Nestler 2014), and these play a role in maintaining drug-seeking behavior and promoting relapse following abstinence (Berridge et al. 2009; Everitt 2014; Robinson and Berridge 2001; Saunders et al. 2013). The mechanisms underlying the neuroplasticity responsible for persistent drug-associated memories have been intensively studied with a view to providing effective approaches for treatment of psychostimulant addiction and prevention of relapse.
Animal models used in studies of drug-associated memory, such as conditioned place preference (CPP), are based on the assumption that positive associations involving context or specific cues during drug taking follow processes involved in Pavlovian conditioning. This assumption would suggest that significant temporal overlap of drug exposure with a salient context (a place designated as S+) would be necessary for conditioning to occur (Boakes and Costa 2014). Accordingly, during conventional CPP training, the drug is present simultaneously with exposure to S+ (Tzschentke 2007), and after one or more pairings of this type, the animal will exhibit a preference for S+ relative to a different context paired with a placebo treatment (S−). In order to improve understanding of the nature of persistent psychostimulant drug-associated memory, the current studies addressed the development of CPP under non-conventional conditions in which there was no temporal overlap between an S+ and drug exposure (i.e., trace conditioning). In these studies, development of CPP was assessed when cocaine or methamphetamine was presented after the end of exposure to S+ with delays of differing length from 15 to 480 min. Significant development of CPP under these conditions would not be predicted under conventional Pavlovian learning processes and would require retrograde association of drug exposure with recent memory for S+. However, in the current studies a retrograde conditioning of place preference was observed when exposure to cocaine occurred up to 4 hours following S+.
Materials and methods
Four hundred and sixty-five male Swiss-Webster mice (HSD: ND4), aged 2–3 mo, were obtained from Harlan (Indianapolis, IN) and housed four to a cage in the vivarium of the University of North Texas Health Science Center. Food and water were available ad libitum throughout the study, and the colony room was maintained at 23±1° C, on a 12-h light-dark cycle beginning at 0630 h.
The place preference apparatus consisted of clear acrylic test chambers (30.48 × 15.24 × 30.48 cms, lwh) (Custom Model 71-CFCPP, Omnitech Electronics, Inc., Columbus, OH) with four interchangeable grid and perforated hole floors (full, 30.48 × 15.24 cms, and split, 15.24 × 15.24 cms). This apparatus was equivalent to that described by Cunningham et al. (2006) and described by us previously (Gatch et al. 2016). In accordance with other reports (Tzschentke 2007) there was no overall preference for a particular floor type in this apparatus for the mice used in this study. The position of the mouse within the apparatus was recorded using a photocell-based system (Model 71-CPPX, Omnitech). The acrylic chambers were housed separately in sound-attenuating chambers (Model 71-ECC, Omnitech). Ambient noise within the chambers was 64 dB and testing took place under dim illumination (31.8±1.5 lux).
Place conditioning occurred over the course of 4 days. On the morning of day 1, all mice received a 30-min preference test (pretest) in the conditioning chambers prepared with split floors (half grid, half perforated). The floor on which a mouse spent the most time was labeled S− and the other labeled S+. On the mornings of days 2 and 3, all mice were administered a 0.9% saline injection immediately prior to being placed on the S− floor for a 30-min session. Mice were then returned to their home cages. Four h later, all mice were placed on the S+ floor for a 30-min session.
To assess conventional CPP under conditions of simultaneous exposure to drug and S+, different groups of mice received cocaine (10, 15, 20, and 40 mg/kg) or methamphetamine (0.25, 0.5, 1, 2, and 4 mg/kg) just prior to S+ exposure during the afternoon sessions. The lowest dose of each psychostimulant that produced maximum conditioned place preference was used for subsequent retrograde studies (cocaine 15 mg/kg and methamphetamine 0.5 mg/kg). In these studies, separate groups of mice received saline prior to S+, and injection of drug occurred in the home cage after a delay of 15, 60, 240, or 480 minutes from the end of the session. A follow-up study involved two additional groups injected in the home cage with 0.25 or 1 mg/kg methamphetamine after a delay of 15 min.
It should be noted that, based on the delay length, mice received cocaine or methamphetamine injections at different clock times. Mice assigned to the conventional conditioning (CC), 15, and 60-min delay groups were injected during their light cycle, whereas the 240 and 480-min delay groups were injected during the dark cycle. Separate groups of mice were also assigned to a no conditioning group (NC) that received only 0.9% saline during conditioning sessions. Mice did not receive matching home cage injections following the S− sessions, to avoid introducing variable degrees of temporal overlap with the afternoon S+ exposure.
A 30-min CPP test (test) was conducted on day 4 in which all mice were injected with saline and placed in the apparatus with split floors (half grid, half perforated), and the time spent on each floor was recorded. The times spent on the floor assigned to S+ during the pretest session and during the test session were compared, and a significant increase in time spent on the S+ floor during the test was considered to reflect a conditioned place preference. Locomotor activity was assessed as horizontal activity counts (photocell interruptions) during each S+ and S− session, to compare the locomotor stimulant effects of cocaine and methamphetamine during conventional CPP conditioning. Locomotor activity during the pretest was compared with activity on the test day to assess the effect of conventional and retrograde conditioning on this variable.
Statistical analyses
The effect of the two psychostimulants on CPP and locomotor activity were assessed using 2- and 3-way analyses of variance. Planned, within-subject comparisons (single degree of freedom F tests) between pretest and test were performed for each of the groups for assessment of CPP and locomotor activity effects. The alpha level was set at p< .05 for all analyses.
Results
Pretest phase
Analysis of the pretest data from all experiments failed to suggest a bias associated with floor type in the testing apparatus. When the time spent on either the grid or perforated floor was subjected to a 2-way analysis of variance, with experimental Group as a between-subjects factor and Floor (grid vs. perforated) as a within-subjects factor, there was no significant main effect or interaction (ps>.260).
During the pretest (prior to conditioning) the groups of mice tested in the conventional and delayed CPP experiments spent approximately equal time on the S+ floor (Tables-1, 2). In support of this observation, a separate 1-way analyses of variance for each experiment failed to indicate a significant effect (ps>.070).
Table – 1.
Conditioned Place Preference using the conventional paradigm
| Psychostimulant | Dose | Pretest S+ | Test S+ |
|---|---|---|---|
| Cocaine | 10 | 746 ± 34 | 931 ± 90* |
| 15 | 772 ± 18 | 998 ± 79* | |
| 20 | 680 ± 42 | 963 ± 104* | |
| 40 | 775 ± 34 | 701 ± 108 | |
| Methamphetamine | 0.25 | 747±22 | 876 ± 75 |
| 0.5 | 703 ± 25 | 1196 ± 85* | |
| 1 | 690 ± 30 | 1116 ± 66* | |
| 2 | 684 ± 32 | 988 ± 120* | |
| 4 | 777 ± 20 | 856 ± 108 | |
Each value represents the mean time spent on the S+ floor ± SE
significantly different from dose-matched pretest, p<0.05
Table – 2.
Conditioned Place Preference using retrograde (trace) paradigm
| Experiment | Delay | Pretest | Test |
|---|---|---|---|
| No Conditioning (NC) |
0 | 670±41 | 694±140 |
| Cocaine (15 mg/kg) |
0 | 772±18 | 1059±69*† |
| 15 | 702 ± 35 | 980 ±98* | |
| 60 | 720 ± 37 | 969 ± 115* | |
| 240 | 738 ± 22 | 929 ± 95* | |
| 480 | 764 ± 20 | 733 ± 114 | |
| Methamphetamine (0.5 mg/kg) |
0 | 703±25 | 1196±85*† |
| 15 | 644 ± 36 | 502 ± 96 | |
| 60 | 746 ± 26 | 954 ± 141 | |
| 240 | 700 ± 26 | 779 ± 117 | |
| 480 | 765±27 | 872 ± 135 | |
| Methamphetamine (0.25 mg/kg) |
0 | 751±26 | 950±137 |
| 15 | 716±52 | 499±99 | |
| Methamphetamine (0.1 mg/kg) |
0 | 644±48 | 974±127 |
| 15 | 658±41 | 848±118 | |
Each value represents the mean time spent on the S+ floor ± SE
significantly different from matched pretest, p<0.05
significantly different from NC, p<0.05
Conventional CPP
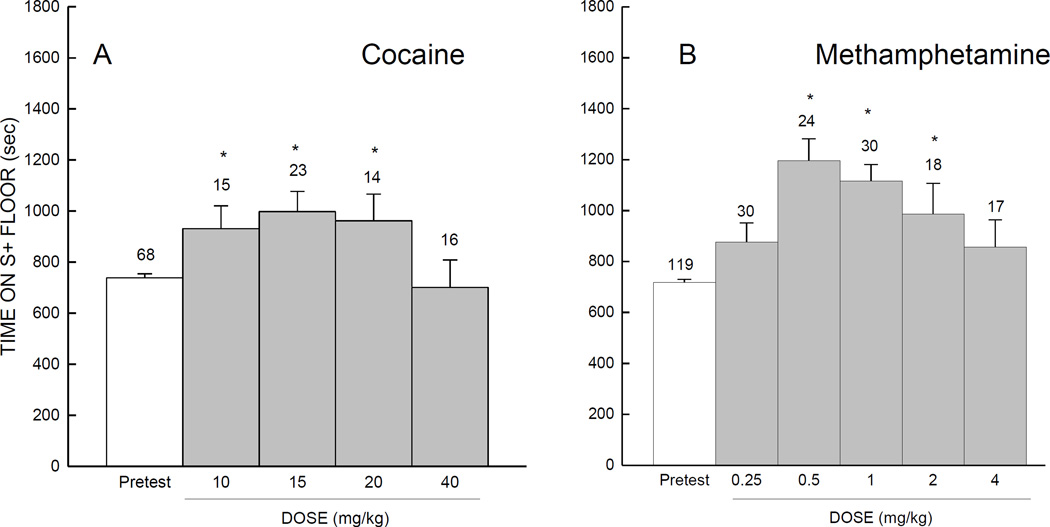
Dose finding studies performed for each psychostimulant using the conventional CPP paradigm yielded an inverted U-shaped dose-response. In Fig. 1A, groups of mice conditioned with cocaine at 10, 15, or 20 mg/kg spent more time on the S+ floor during the test session when compared with the pretest, whereas the 40 mg/kg cocaine group failed to do so. Conventional CPP was evident for conditioning with methamphetamine (Fig. 1B) at 0.5, 1, or 2 mg/kg, but not at 0.25 or 4 mg/kg of methamphetamine. In support of these observations, separate 2-way analyses of variance for cocaine and methamphetamine dose response, with Dose as a between-subjects factor and Conditioning (pretest vs. test) as a within-subjects factor, indicated a significant main effect of Conditioning [for cocaine, F (1,64) =13.94; for methamphetamine, F (1,114) >45.32; all ps<.001], an interaction of Dose with Conditioning, [for cocaine, F (3,64) =3.49; for methamphetamine,F (4,114) = 3.79; all ps<.006}, but no effect of Dose (ps>.112).
Fig. 1.
Mean number of seconds (±SE) spent on S+ floor during the pretest and test sessions. During conventional conditioning of place preference, mice were injected with different doses of either cocaine (A) or methamphetamine (B) * Indicates p<.05 in planned comparison with pretest
Comparison of the effects of cocaine and methamphetamine on conventional CPP, as shown in Fig. 1, suggested that methamphetamine had a comparatively larger effect. However, when time on the S+ floor was considered in an analysis of variance (with Psychostimulant and Conditioning as the factors), the effects of Psychostimulant (F(1,58)=0.32, p=.570), and the interaction with Conditioning (F(1,58)=3.80, p=.060), were not significant
Retrograde (Trace) CPP
Following the conventional CPP experiments, it was concluded that the lowest dose of psychostimulant that produced maximum CPP in groups of mice was 15 mg/kg for cocaine and 0.5 mg/kg for methamphetamine. These doses were used for the retrograde (trace) CPP studies.
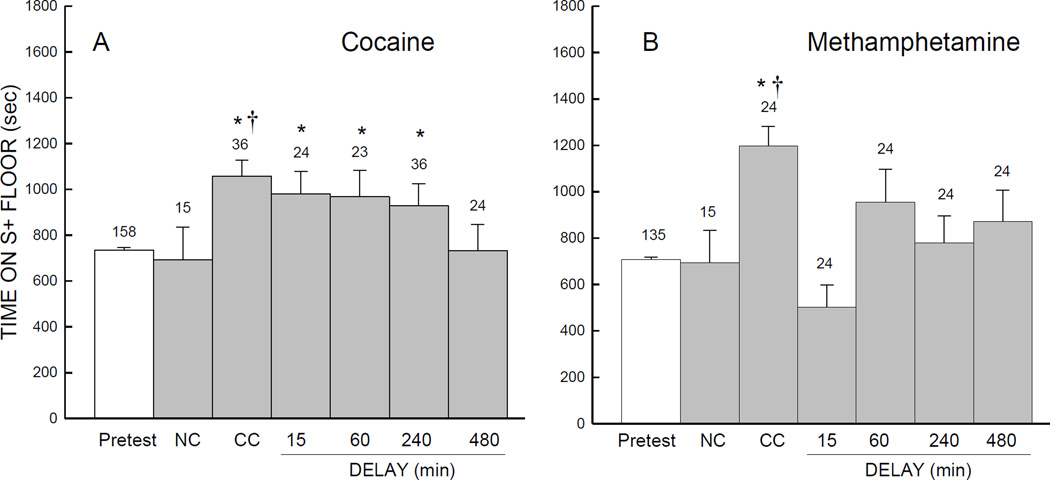
As shown in Fig. 2A, the group of mice that received conventional CPP training with cocaine (CC) spent more time on the S+ floor during the CPP test than during the pretest session. However, the groups of mice that received cocaine delayed by 15, 60, or 240 min following S+ exposure also spent more time on the S+ floor during the test, whereas only the 480-min delay group failed to do so. When the CPP data were subjected to a 2-way analysis of variance, with Delay (NC, CC, 15, 60, 240, or 480 min) as a between-subjects factor and Conditioning (pretest vs. test) as a within-subjects factor, there was a significant main effect of Conditioning (F(1,152)=15, p=.001), but no effect of Delay (F(5,152)=1.96, p=.088) or interaction of Delay with Conditioning, (F(5,152)=1.65, p=.15). A separate between-subjects analysis of variance on time spent on S+ floor during test session including the NC, CC, and delay groups in the cocaine experiment (Fig: 2A), indicated no significant main effect (F(5,152) =1.82, p=.111); however, planned comparisons of the treatment groups with the NC group indicated a significant difference for the CC group (Fig: 2A).
Fig. 2.
Mean number of seconds (±SE) spent on S+ floor during the pretest and test sessions. During conditioning, mice were injected with either 15 mg/kg cocaine (A) or 0.5 mg/kg methamphetamine (B). As described in the materials and methods, mice in the NC group received only saline injections, while mice in conventional conditioning (CC) groups received either cocaine (A) or methamphetamine (B) just before exposure to the CPP apparatus. Further, mice in the other exposure delay groups received psychostimulant (either cocaine or methamphetamine) injections in their home cage after exposure to the CPP apparatus. * Indicates p<.05 in planned comparison with pretest.. † Indicates p<.05 when compared to the NC group.
Conventional CPP training with methamphetamine also yielded an increase in time on the S+ floor during the CPP test (Fig.2B), though no significant increase in S+ time was evident in any of the delayed exposure groups. A separate 2-way analysis of variance on S+ time for methamphetamine indicated significant effects of Conditioning (F(1,129)=7.10, p<.009); Delay (F(5,129)= 4.30, p<.001), and a significant interaction of Delay with Conditioning (F(5,129)=3.50, p=.006). Further, a separate between-subjects analysis of variance on time spent on S+ floor during test session including the NC, CC, and delay groups in the methamphetamine experiment (Fig: 2B), indicated significant main effect (F(5,129) =4.03, p=.002). Planned comparisons of the treatment groups with the NC group indicated a significant difference for the CC group (Fig: 2B).
A follow-up experiment with different doses of methamphetamine (0.25 and 1 mg/kg) in the delayed (15-min) exposure groups, also did not yield significant CPP (Table 2). Planned individual comparisons of time spent on the S+ floor during the pretest vs. test for groups of mice injected with either the 0.25- or 1 mg/kg dose of methamphetamine following a 15-min delay, indicated that there was no significant change (all ps > .066).
Locomotor Activity
Conditioning Day
Mice receiving conventional place preference conditioning with cocaine or methamphetamine showed an increase in horizontal activity during the S+ session, relative to S−, for both conditioning days (data not shown). The magnitude of this increase in locomotion was larger for cocaine than methamphetamine. Analysis of variance (with Psychostimulant, Conditioning, and Day as the factors) confirmed this observation, yielding a significant main effect of Psychostimulant (F(1,58) =10.70, p=.002), as well an interaction of Psychostimulant with Day (F(1,58) =23.40, p<.001).
Test day
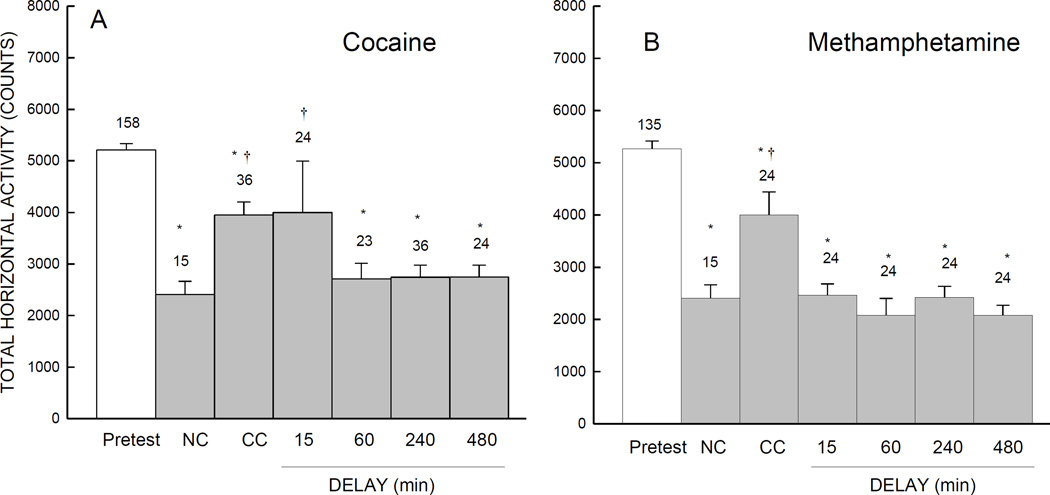
Locomotor activity on pretest and test day for CC, NC and delay groups for the two psychostimulants is shown in Fig. 3. Mice in all groups exhibited habituation as indicated by a reduction in total horizontal activity counts during test session in comparison to their own pretest. However, there was a difference in habituation between the experimental groups in comparison to the NC (saline) group. This difference in habituation was evident in groups of mice that were subjected to conventional CPP conditioning following both psychostimulants, and also in the 15-min delay cocaine group. Horizontal activity counts for NC, methamphetamine delay (15-, 60-, 240-, and 480-min), and cocaine delay (60-, 240-, and 480-min) groups were similar.
Fig. 3.
Mean number of total horizontal activity counts (±SE) during pretest and test sessions. During conditioning, mice were injected with either 15 mg/kg cocaine (A) or 0.5 mg/kg methamphetamine (B). As described in the materials and methods, mice in the no conditioning (NC) group received only saline injections, while mice in conventional conditioning (CC) groups, either received cocaine (A) or methamphetamine (B) just before exposure to the CPP apparatus. Further, mice in other exposure delay groups received psychostimulant (either cocaine or methamphetamine) injections in their home cage after exposure to the CPP apparatus. * Indicates p<.05 when compared to the total horizontal activity counts during pretest. † Indicates p<.05 when compared to the horizontal activity counts of the NC group.
These data for both cocaine and methamphetamine were subjected to separate 2-way, analyses of variance, with Delay (NC, CC, 15, 60, 240, or 480 min) as a between-subjects factor and Conditioning (pretest vs. test) as a within-subjects factor. For horizontal activity data of groups of mice injected with cocaine (Fig: 3A), there was a significant main effect of Conditioning (F (1,152) =103.11, p=.001), and an interaction of Delay with Conditioning, (F (5,152) =3.10, p=.01), but no effect of Delay (F (5,152) =1.35, p=.247). A separate between-subjects analysis of variance on total horizontal activity counts during test session for methamphetamine (Fig: 3B) indicated significant main effects of Conditioning (F(1,129) =305.90, p<.001), and Delay (F(5,129) =5.64, p<.001), and an interaction of Delay with Conditioning, (F(5,129)=4.10, p=.002).
Discussion
Results from the current study suggest that a salient context/cue (S+ floor) can become associated with the positive reinforcing effects of cocaine, even when the cocaine exposure is delayed until after exposure to S+ has ended and a new context is present. The retrograde associations spanned a period of up to 240 min, an effect that was not expected based on conventional Pavlovian conditioning, which should be optimal only when conditioning stimuli are presented with significant temporal overlap. The only other example of retrograde associative learning (long trace conditioning) with which we are familiar is taste-aversion learning, which occurs reliably even when malaise follows a novel taste by many hours (Etscorn and Stephens 1973; Garcia et al. 1955). The unusual retrograde effect observed with cocaine was notably absent when methamphetamine was tested under the same conditions. The 0.5 mg/kg dose of methamphetamine, which was optimal for conventional CPP, did not yield CPP when injected at any time after removal from the S+ floor. Moreover, neither higher (1 mg/kg) nor lower (0.25 mg/kg) doses of methamphetamine were effective in producing a retrograde effect when tested at 15 minutes following S+ exposure. These results were similar to those of earlier studies with nicotine, amphetamine, and ethanol in which the imposition of a delay longer than 5 min failed to yield any evidence of context-drug associations (Cunningham et al. 1997; Fudala and Iwamoto 1987; 1990).
It is unclear why the effects of delayed cocaine and methamphetamine differed so markedly in the current study. One possibility is that retrograde CPP depends on the dose and/or time course of the conditioning drug. The doses used for conditioning in this study were selected to provide optimal conventional CPP based on dose response studies reported, and the resulting conventional CPP effects for each drug were comparable. Moreover, these conditioning doses are commonly used for CPP studies in other laboratories (Prast et al. 2014; Shabani et al. 2012). However, the motor stimulant effects of 15 mg/kg of cocaine and 0.5 mg/kg of methamphetamine during conventional CPP conditioning were not well matched, with cocaine yielding a larger stimulant effect. Given that a higher dose of methamphetamine also failed to yield trace conditioning, the magnitude of locomotor stimulation would not appear to be the critical property leading to the retrograde CPP. However, independently of dosage, these two compounds also differ markedly in their time course, with cocaine having a comparatively more rapid onset and offset. These properties lead to different patterns of abuse for the two drugs (Gonzalez Castro et al. 2000; Simon et al. 2002), though it remains unclear how they might influence CPP expression in both conventional and retrograde conditioning paradigms.
Analysis of locomotor activity on the pretest versus the test day suggested a difference between the effects of methamphetamine and cocaine matching that for retrograde CPP. During the CPP test, mice receiving conventional conditioning with either of the psychostimulants were more active than their respective NC (saline) groups and most of the groups receiving retrograde conditioning. However, cocaine injected after a 15- minute delay had an effect that was similar to conventional conditioning, whereas no such effect was evident for methamphetamine or any of the other retrograde conditioning groups. These results suggest that conditioning of motor stimulation to context develops concurrently with CPP during conventional training with both psychostimulants, independently of floor type, and that such conditioning may also occur when exposure to cocaine is delayed. While the decay gradient for retrograde motor activity conditioning does not match that for retrograde CPP, expression of both phenomena may reflect the same property of cocaine.
While the rewarding effects of both cocaine and methamphetamine are thought to involve accumulation of dopamine (DA) at synapses in mesolimbic brain reward circuits, their differing ability to promote retrograde association in this study may reflect more subtle differences in mechanism of action. Cocaine may be more effective because at both high and low doses it yields a physiological signal-correlated accumulation of DA by inhibiting its reuptake, in contrast to methamphetamine which has a releasing effect and can inhibit firing of DA neurons (Branch and Beckstead 2012). While the effect of methamphetamine on DA signaling at low doses may be qualitatively more similar to cocaine, testing a lower dose of methamphetamine in the current studies yielded a diminished conventional CPP effect and did not promote retrograde association with S+ after delayed exposure. Thus, methamphetamine, in doses sufficient to generate robust conventional CPP, may interfere with neural processing necessary to generate association with events in recent memory.
The differences in ability of cocaine and methamphetamine to affect memory seem consistent with their ability to promote retrograde association. In humans, methamphetamine aided the recall of images when given prior to viewing (or encoding) the images, but not when given after encoding during the consolidation phase (Ballard et al. 2015). Memory enhancement by posttraining administration of amphetamine/methamphetamine appears to be limited to a very brief time window, findings that are supported by our data. In contrast, cocaine may have a longer effective critical period in which it can modulate memory. Administering a 2.5 mg/kg dose of cocaine to C57BL/6 mice within 2-h of training enhanced performance in a spatial task (Iniguez et al. 2012). However, posttraining administration of cocaine dose-dependently improved retention in C57BL/6 mice in an inhibitory avoidance task only when cocaine was injected immediately (but not 2 h) after training (Castellano et al. 1996). Thus, these reports suggest that cocaine’s ability to facilitate memory consolidation in a retrograde fashion is possibly time and task-dependent.
An attractive hypothesis consistent with the overall findings in these studies is that psychostimulant drug-taking may lead to broad generalization of a rewarding effect to different contexts by promoting unusually strong retrograde associations with events in labile recent memory. The ability to promote retrograde association could contribute significantly to the psychostimulant addiction cycle and the persistence of drug-related memory during abstinence.
Table – 3.
Total horizontal activity counts
| Experiment | Delay | Pretest | Test |
|---|---|---|---|
| No Conditioning (NC) |
0 | 4580±378 | 2407± 255* |
| Cocaine (15 mg/kg) |
0 | 5156±213 | 3953±250*† |
| 15 | 4925±308 | 3999 ± 999† | |
| 60 | 5392±324 | 2713 ± 302* | |
| 240 | 5303±274 | 2743± 238* | |
| 480 | 5681± 358 | 2745 ± 234* | |
| Methamphetamine (0.5 mg/kg) |
0 | 6131±344 | 3998 ± 443* |
| 15 | 4712±366 | 2465± 15*† | |
| 60 | 6062±292 | 2082±320* | |
| 240 | 4789±294 | 2421 ± 213* | |
| 480 | 5088±354 | 2081±186* | |
Each value represents the mean time ± SE
significantly different from matched pretest, p<0.05
significantly different from NC, p<0.05
Acknowledgments
Support for this research was provided by contract N01DA-13-8908 from the National Institute on Drug Abuse. NIDA had no further role in the design, analysis or publication of this report.
Footnotes
The authors declare no conflict of interest.
References
- Ballard ME, Weafer J, Gallo DA, de Wit H. Effects of acute methamphetamine on emotional memory formation in humans: encoding vs consolidation. PloS one. 2015;10:e0117062. doi: 10.1371/journal.pone.0117062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Current opinion in pharmacology. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA, Costa DS. Temporal contiguity in associative learning: Interference and decay from an historical perspective. Journal of experimental psychologyAnimal learning and cognition. 2014;40:381–400. doi: 10.1037/xan0000040. [DOI] [PubMed] [Google Scholar]
- Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. Journal of neurophysiology. 2012;108:802–809. doi: 10.1152/jn.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Zocchi A, Cabib S, Puglisi-Allegra S. Strain-dependent effects of cocaine on memory storage improvement induced by post-training physostigmine. Psychopharmacology. 1996;123:340–345. doi: 10.1007/BF02246644. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. The American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philosophical transactions of the Royal Society of LondonSeries B, Biological sciences. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Okorn DM, Howard CE. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Animal Learning & Behavior. 1997;25:31–42. [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends in pharmacological sciences. 2014;35:374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etscorn F, Stephens F. Establishment of conditioned taste aversions with a 24-hour CS-US interval. Physiological Psychology. 1973;1:251–253. [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories – indications for novel treatments of addiction. European Journal of Neuroscience. 2014;40 doi: 10.1111/ejn.12644. 2163–2182-2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with nicotine. Psychopharmacology. 1987;92:376–381. doi: 10.1007/BF00210847. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with amphetamine. Pharmacology, biochemistry, and behavior. 1990;35:89–92. doi: 10.1016/0091-3057(90)90209-z. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Science. Vol. 122. New York: NY; 1955. Conditioned aversion to saccharin resulting from exposure to gamma radiation; pp. 157–158. [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Locomotor, discriminative stimulus, and place conditioning effects of MDAI in rodents. Behav Pharmacol. 2016;27:497–505. doi: 10.1097/FBP.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Castro F, Barrington EH, Walton MA, Rawson RA. Cocaine and methamphetamine: differential addiction rates. Psychol Addict Behav. 2000;14:390–396. [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Charntikov S, Baella SA, Herbert MS, Bolanos-Guzman CA, Crawford CA. Post-training cocaine exposure facilitates spatial memory consolidation in C57BL/6 mice. Hippocampus. 2012;22:802–813. doi: 10.1002/hipo.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Prast JM, Schardl A, Sartori SB, Singewald N, Saria A, Zernig G. Increased conditioned place preference for cocaine in high anxiety related behavior (HAB) mice is associated with an increased activation in the accumbens corridor. Frontiers in behavioral neuroscience. 2014;8:441. doi: 10.3389/fnbeh.2014.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96 doi: 10.1046/j.1360-0443.2001.9611038.x. 103–114-103–114. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-Evoked Cocaine “Craving”: Role of Dopamine in the Accumbens Core. The Journal of Neuroscience. 2013;33 doi: 10.1523/JNEUROSCI.0450-13.2013. 13989–14000-13989–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Cunningham CL, Phillips TJ. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology. 2012;62:1134–1141. doi: 10.1016/j.neuropharm.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12 doi: 10.1111/j.1369-1600.2007.00070.x. 227–462-227–462. [DOI] [PubMed] [Google Scholar]
- van Huijstee AN, Mansvelder HD. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Frontiers in cellular neuroscience. 2015;8:466. doi: 10.3389/fncel.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]