Abstract
Dental amalgam residues are probably the most important chemical residues generated from clinical dental practice because of the presence of heavy metals among its constituents, mainly mercury and silver.
Objective
The purpose of this study was to develop an alternative method for the recovery of silver residues from dental amalgam.
Material and Methods
The residue generated after vacuum distillation of dental amalgam for the separation of mercury was initially diluted with 32.5% HNO3, followed by precipitation with 20% NaCl. Sequentially, under constant heating and agitation with NaOH and sucrose, the sample was reduced to metallic silver. However, the processing time was too long, which turned this procedure not viable. In another sequence of experiments, the dilution was accomplished with concentrated HNO3 at 90ºC, followed by precipitation with 20% NaCl. After washing, the pellet was diluted with concentrated NH4OH, water and more NaCl in order to facilitate the reaction with the reducer.
Results
Ascorbic acid was efficiently used as reducer, allowing a fast reduction, thus making the procedure viable.
Conclusion
The proposed methodology is of easy application and does not require sophisticated equipment or expensive reagents.
Keywords: Dental amalgam, Silver, Solid wastes, Environment
INTRODUCTION
The unbridled development of the population and industry has led to an increase in the generation of residues2. The natural environment is not able to support the increasing exposure to chemical products caused by this development6. The acknowledgement that human intervention has been contributing to the deterioration of the natural environment has led several countries to search for alternatives for its restructuring7.
Regarding the residues generated in dental practice, the most concerning ones are those deriving from dental amalgam8,9,14,16 because this metal alloy has, among its constituents, mercury, silver, tin and copper1,12. The use of amalgam separators has been recommended to physically remove dental amalgam from waste water in dental clinics thus reducing the mercury emissions9,18.
The most abundant metal in dental amalgam is mercury1, and its recovery from dental amalgam has been done using vacuum distillation10,11,13. After recovery of mercury, however, other hazardous metals are still left1,5, among which, silver is the most abundant1. This metal is very dangerous both to aquatic and terrestrial organisms4,6,15,21. In humans, silver is metabolized and deposited in subcutaneous fat, and its excessive ingestion generates argyria, a cosmetic disorder3. In addition, silver is commonly used in the industry and its recovery could allow some financial return3,8.
Despite some studies have described processes to recover mercury from dental amalgam10,13, only one study focused on the recovery of silver using sucrose as a reducing agent11. In the present study, the purpose was to develop an alternative method for the recovery of silver residues from dental amalgam.
MATERIAL AND METHODS
Dental amalgam residues were received from the dental clinics of Bauru Dental School and Hospital for Rehabilitation of Craniofacial Anomalies (HRAC), University of São Paulo, Bauru, SP, Brazil. The amalgam was initially processed by vacuum distillation for mercury removal10,13, generating an amount of 5,516.93 g.
Process of silver recovery using sucrose as a reducing agent
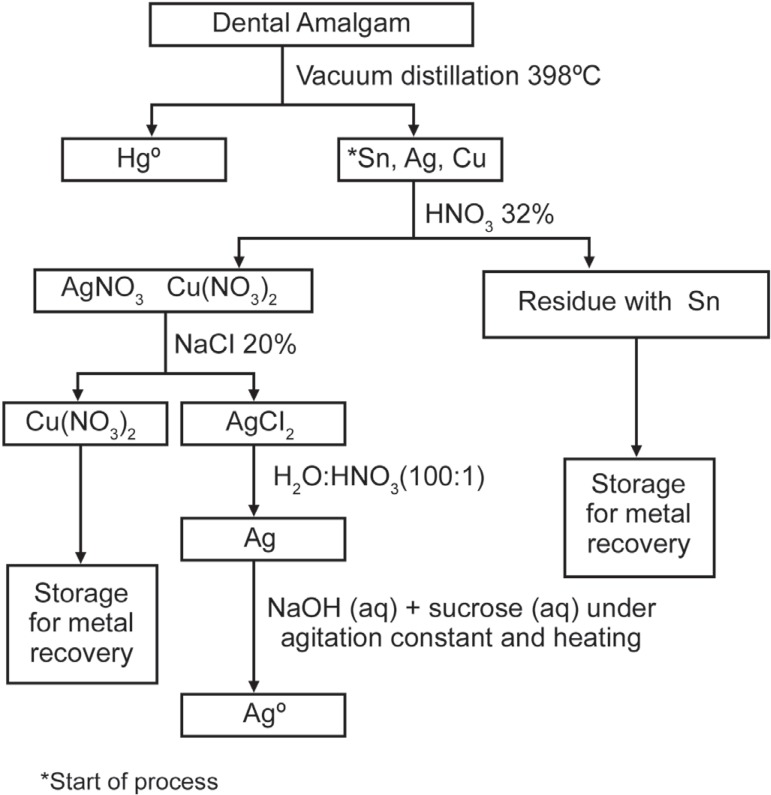
This process was adapted from the methodology proposed by Lee and Fung11 (1980). The amount of samples (residues of dental amalgam after recovering mercury) used ranged between 25 and 150 g. For residue dilution, 32.5% nitric acid was used for the formation of AgNO3 (aq) (Table 1). The solution containing AgNO3 was collected in another vial, where 20% NaCl was added to promote the precipitation of silver as AgCl2 (solid). This pellet was washed with nitric acid and deionized water (1:100 v/v) until the supernatant became colorless. In sequence, a solution of sucrose (concentrations ranging between 2.5 and 20%) and NaOH (concentrations ranging between 10 and 40%) was added to the pellet (Table 1) and, under agitation and heating, the AgCl2 was reduced to Ag0 (Figure 1).
Table 1. Procedure of silver recovery from dental amalgam residues using sucrose as reducing agent.
| Dilution | Precipitation | Reduction | ||||
|---|---|---|---|---|---|---|
| Samplemass (g) | 32.5% nitric acid volume at room temperature (mL)/ilution | 20% NaClvolume (mL) | Na OH concentration (%) | Na OH volume (mL) | Sucrose concentration (%) | Sucrose volume (mL) |
| 25.0 | 30.0/1 | 60.0 | 10.0 | 80.0 | 2.5 | 400.0 |
| 50.0 | 50.0/1 | 100.0 | 40.0 | 80.0 | 20.0 | 400.0 |
| 50.0 | 60.0/1 | 120.0 | 20.0 | 80.0 | 5.0 | 400.0 |
| 50.0 | 100.0/2 | 100.0 | 40.0 | 80.0 | 20.0 | 400.0 |
| 110.7 | 300.0/3 | 500.0 | 20.0 | 300.0 | 20.0 | 700.0 |
| 120.0 | 300.0/3 | 500.0 | 20.0 | 750.0 | 20.0 | 1600.0 |
| 134.4 | 300.0/3 | 550.0 | 20.0 | 400.0 | 20.0 | 440.0 |
| 150.0 | 300.0/3 | 500.0 | 20.0 | 750.0 | 20.0 | 2400.0 |
| 150.0 | 350.0/4 | 1200.0 | 20.0 | 800.0 | 20.0 | 1600.0 |
| 150.0 | 300.0/3 | 650.0 | 20.0 | 500.0 | 17.5 | 1100.0 |
| 150.0 | 300.0/3 | 800.0 | 20.0 | 400.0 | 20.0 | 2100.0 |
Figure 1.
Flow chart of silver recovery using sucrose
Process of silver recovery using ascorbic acid as a reducing agent
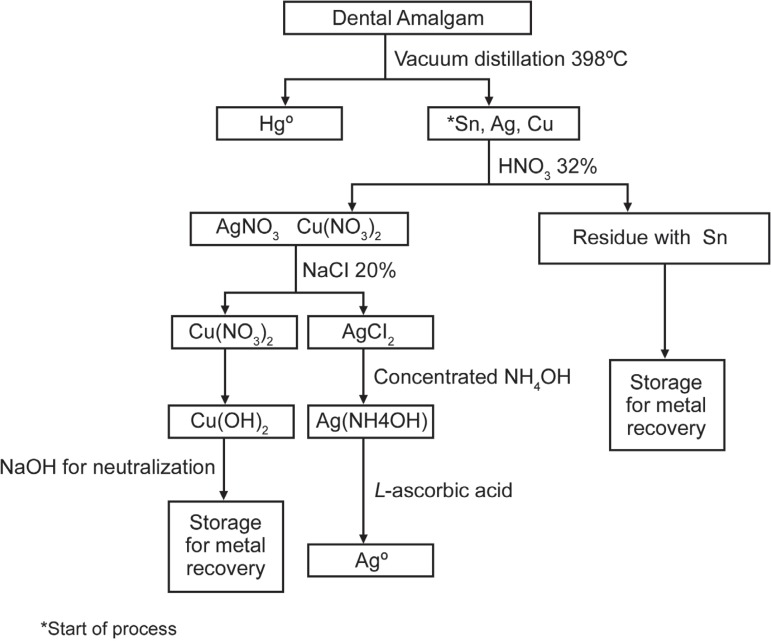
The amount of samples used ranged between 150 and 200 g. The tests were done in duplicates, as displayed in Table 2. For dilution, concentrated nitric acid at 90°C was used. The silver nitrate formed was collected in another vial. The precipitation with 20% NaCl was then accomplished as described above. After washing with nitric acid and deionized water (1:100 v/v), NH3*H2O, water and additional 20% NaCl were added, in order to solubilize the pellet, thus making easier the reaction with ascorbic acid. Ascorbic acid (75-100 g, according to the amount of sample) was then added under agitation and silver was immediately reduced to Ag0 (Figure 2).
Table 2. Procedure of silver recovery from dental amalgam residues using ascorbic acid as reducing agent.
| Dilution | Precipitation | Solubilization | Reduction | |||
|---|---|---|---|---|---|---|
| Sample mass (g) | Concentrated nitric acid volume at 90°C (mL) / dilution | 20% NaCl volume (mL) | Concentrated NH4OH volume (mL) | Deionized water volume (mL) | 20% NaCl volume (mL) | Amount of ascorbic acid (g) |
| A1150.0 | 150.0/1 | 300.0 | 500.0 | 1000.0 | - | 75.0 |
| A2150.0 | 150.0/1 | 300.0 | 500.0 | 1000.0 | - | 75.0 |
| B1150.0 | 500.0/5 | 750.0 | 200.0 | 2000.0 | 1000.0 | 75.0 |
| B2150.0 | 500.0/5 | 750.0 | 200.0 | 2000.0 | 1000.0 | 75.0 |
| C1200.0 | 400.0/3 | 1200.0 | 300.0 | 2000.0 | 1000.0 | 100.0 |
| C2200.0 | 400.0/3 | 1200.0 | 300.0 | 2000.0 | 1000.0 | 100.0 |
| D1200.0 | 500.0/5 | 1200.0 | 300.0 | 2000.0 | 1000.0 | 100.0 |
| D2200.0 | 500.0/5 | 1200.0 | 300.0 | 2000.0 | 1000.0 | 100.0 |
| E1200.0 | 400.0/3 | 1400.0 | 300.0 | 2000.0 | 1500.0 | 100.0 |
| E2200.0 | 400.0/3 | 1400.0 | 300.0 | 2000.0 | 1500.0 | 100.0 |
| F1200.0 | 500.0/5 | 1400.0 | 300.0 | 2000.0 | 1500.0 | 100.0 |
| F2200.0 | 500.0/5 | 1400.0 | 300.0 | 2000.0 | 1500.0 | 100.0 |
Figure 2.
Flow chart of silver recovery using ascorbic acid
Analysis of recovery efficiency
In order to assess the efficiency of recovery, Volhard method was used19. Initially, the recovered sample was weighed (±0.01 mg) and then diluted in concentrated nitric acid at 90°C. The resulting solution was titrated with potassium thiocyanate, in the presence of Fe2+, that was added as a saturated solution of ammonium ferrous sulfate in 20% nitric acid. In contact with thiocyanate, silver precipitates as AgSCN, which has a very low solubility. A slight excess of thiocyanate is identified by the formation of a soluble ferric complex [FeSCN]2+, which is intensively stained in red. The titration error in the Volhard method is small as the indicator is extremely sensitive to the thiocyanate ions. The reaction occurs as described below:
1- AgNO3(aq) + KSCN-(aq)→ AgSCN(s) KNO3(aq)
2- Fe3+(aq) + SCN-(aq) → [FeSCN]2+(aq) (dark red ferric complex)
RESULTS
Table 3 shows the procedures done for silver recovery using sucrose as reducing agent. The recovery (considering that the % of silver in dental amalgam is 32.5%1) ranged between 23.5 and 61.6% for the different conditions tested. However, the total time required in the reduction process ranged between 303 and 600 min. The other selected reducing agent was ascorbic acid. The procedures done in the study are described in Table 4. In this case, the reduction occurred immediately, differently from what was seen when the sucrose was used. In addition, the recovery in this case was higher (40% to 95.5%) when compared to the use of sucrose as reducing agent.
Table 3. Silver recovery from dental amalgam residues using sucrose as reducing agent.
| Sample mass (g) | Total time (min) | Amount of Agº produced (g) | Purity (%) | % recovery ª (32.5% of amalgam) |
|---|---|---|---|---|
| 25.0 | 303 | 3.9 | 99.9 | 24.4 |
| 50.0 | 430 | 15.5 | 49.3 | 48.4 |
| 50.0 | 360 | 9.2 | 94.2 | 28.8 |
| 50.0 | 373 | 23.0 | 87.1 | 71.9 |
| 110.7 | 390 | 41.5 | 52.6 | 58.6 |
| 120.0 | 330 | 61.1 | 44.1 | 79.6 |
| 134.4 | 455 | 54.0 | 19.6 | 62.8 |
| 150.0 | 600 | 59.1 | 84.1 | 61.6 |
| 150.0 | 440 | 64.5 | 86.0 | 67.2 |
| 150.0 | 365 | 62.1 | 52.9 | 64.7 |
| 150.0 | 565 | 87.9 | 37.0 | 91.6 |
Based on the American Dental Association (ADA) 1 , which considers that the percentage of silver in dental amalgam is 32.5%.
Table 4. Procedure of silver recovery from dental amalgam residues using ascorbic acid as reducing.
| Sample mass (g) | Amount of Agº produced (g) | Purity (%) | % recovery ª (32.5% of amalgam) | Real standard deviation (%) |
|---|---|---|---|---|
| A1150.0 | 38.4 | 99.6 | 40.0 | 2.4 |
| A2150.0 | 40.9 | 97.0 | 42.6 | |
| B1150.0 | 83.8 | 78.0 | 87.3 | 3.9 |
| B2150.0 | 76.7 | 80.5 | 79.9 | |
| C1200.0 | 108.0 | 91.1 | 84.4 | 11.9 |
| C2200.0 | 122.2 | 67.1 | 95.5 | |
| D1200.0 | 57.8 | 99.9 | 45.2 | 2.4 |
| D2200.0 | 106.7 | 52.0 | 83.4 | |
| E1200.0 | 111.9 | 72.9 | 87.4 | 7.9 |
| E2200.0 | 104.6 | 69.8 | 81.7 | |
| F1200.0 | 90.2 | 82.7 | 70.5 | 7.4 |
| F2200.0 | 92.7 | 89.8 | 72.4 |
Based on the American Dental Association (ADA) 1 , which considers that the percentage of silver in dental amalgam is 32.5%.
DISCUSSION
This is the first study that used ascorbic acid as a reducing agent for recovering silver from dental amalgam. In this study, two methods were tested to recover the silver that is left after the mercury is removed from dental amalgam: one using sucrose as a reducing agent, and the one using ascorbic acid as a reducing agent. The only study in the literature that attempted to recover silver from dental amalgam residues used sucrose as a reducing agent11. However, when sucrose was tested as a reducing agent in the present study, the total time required in the reduction process was too long (ranging between 303 and 600 min, depending on the conditions used). Due to this long processing time, a different reducing agent was used in attempt to speed the processing time. The selected reducing agent was ascorbic acid, since it has been reported as a strong reducing agent for metals17. In this case, the reduction occurred immediately, differently from what was seen when the sucrose was used. In addition, the recovery was slightly higher when compared to the use of sucrose as reducing agent.
It must be highlighted that some modifications done in the processing technique when the ascorbic acid was used instead of sucrose may have facilitated the reduction process, such as the use of concentrated nitric acid at 90°C, which allowed a faster and more efficient dilution. In the case of sucrose, dilution was performed by the use of 32.5% nitric acid at room temperature. Also the use of NaOH in the case of sucrose leads to the formation of a complex of AgO2, which has a very low solubility20. Thus, it was necessary to heat this mixture in order to solubilize it, thus demanding a long time for completion of the reducing process (Table 3). In the case of ascorbic acid, NH4OH was used instead of NaOH. Thus, a soluble complex is formed and there is more Ag+ available to be reduced20.
It should also be noted that the procedure of silver recovery using ascorbic acid does not require too much practice, specific knowledge or sophisticated equipments. Thus, its use seems to be viable, due to the high market value of the recovered silver. In addition, the reagents used are not expensive, since for every 150 g of treated silver residues, the cost is approximately around US$ 12.00. The amount of recovered silver is around 65 g, and the cost to buy 1 g of Ag (99% purity) is as high as US$ 2.40. Thus, the recovery of silver using the proposed technique with reduction by ascorbic acid, in addition to reducing the environmental impact caused by the discharge of this metal into the ecosystem, can also provide additional resources to the laboratory that can be used in other investigations.
CONCLUSIONS
The developed methodology was shown to be viable, since it does not require sophisticated equipments or specialized personnel, and is a cost-effective alternative.
ACKNOWLEDGEMENTS
The authors thank FAPESP (#2006/03164-0) for the concession of a scholarship to the first author and ODONTOPREV (Dental Services) for the financial support to this study.
REFERENCES
- 1.American Dental Association . Guide to dental materials. 2nd ed. Chicago: ADA; 1964. Council on dental materials and devices. American Dental Association. Specification no. 1 for alloy for dental amalgam; p. 85. [Google Scholar]
- 2.Boyd AS, Seger D, Vannucci S, Langley M, Abraham JL, King LE Jr. Mercury exposure and cutaneous disease. Pt 1J Am Acad Dermatol. 2000;43(1):81–90. doi: 10.1067/mjd.2000.106360. [DOI] [PubMed] [Google Scholar]
- 3.Ckelman MJE, Graedel TE. Silver emissions and their environmental impacts: a multilevel assessment. Environ Sci Technol. 2007;41(17):6283–6289. doi: 10.1021/es062970d. [DOI] [PubMed] [Google Scholar]
- 4.Drake PL, Hazelwood KJ. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005;49(7):575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 5.Edlich RF, Greene JA, Cochran AA, Kelley AR, Gubler KD, Olson BM, et al. Need for informed consent for dentists who use mercury amalgam restorative material as well as technical considerations in removal of dental amalgam restorations. J Environ Pathol Toxicol Oncol. 2007;26(4):305–322. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i4.70. [DOI] [PubMed] [Google Scholar]
- 6.Gorsuch JW, Klaine SJ. Toxicity and fate of silver in the environment. Environ Toxicol Chem. 1998;17(4):537–538. [Google Scholar]
- 7.Harjula H. Hazardous waste: recognition of the problem and response. Ann N Y Acad Sci. 2006;1076:462–477. doi: 10.1196/annals.1371.062. [DOI] [PubMed] [Google Scholar]
- 8.Hiltz M. The environmental impact of dentistry. J Can Dent Assoc. 2007;73(1):59–62. [PubMed] [Google Scholar]
- 9.Hylander LD, Lindvall A, Uhrberg R, Gahnberg L, Lindh U. Mercury recovery in situ of four different dental amalgam separators. Sci Total Environ. 2006;366(1):320–336. doi: 10.1016/j.scitotenv.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Iano FG, Sobrinho OS, Silva TL, Pereira MA, Figueiredo PJM, Alberguini PJMF, et al. Optimizing the procedure for mercury recovery from dental amalgam. Braz Oral Res. 2008;22(2):119–124. doi: 10.1590/s1806-83242008000200005. [DOI] [PubMed] [Google Scholar]
- 11.Lee CW, Fung KW. Recovery of silver and mercury from dental amalgam waste. Resource Recovery and Conservation. 1981;5:363–371. [Google Scholar]
- 12.Cenci MS, Pereira-Cenci T, Donassollo TA, Sommer L, Strapasson A, Demarco FF. Influence of thermal stress on marginal integrity of restorative materials. J Appl Oral Sci. 2008;16(2):106–110. doi: 10.1590/S1678-77572008000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pécora JD, Silva RS, Souza RA, Guimarães LFL, Shuhama T. Recycling of dental amalgam residues for the recovery of mercury and silver. Rev Fola/Oral. 1998;4(14):234–237. [Google Scholar]
- 14.Pichay TJ. Dental amalgam: regulating its use and disposal. CDA Journal. 2004;32(7):580–582. [PubMed] [Google Scholar]
- 15.Purcell TW, Peters JJ. Sources of silver in the environmentl. Environ Toxicol Chem. 1998;17(4):539–546. [Google Scholar]
- 16.Richards D. Amalgam, risk, benefits and the precautionary principle. Evid Based Dent. 2008;9(1):2. doi: 10.1038/sj.ebd.6400556. [DOI] [PubMed] [Google Scholar]
- 17.Roig MG, Rivera ZS, Kennedy JF. L-ascorbic acid: an overview. Int J Food Sci Nutr. 1993;44:59–72. doi: 10.3109/09637489509012538. [DOI] [PubMed] [Google Scholar]
- 18.Stone ME, Cohen ME, Berry DL, Ragain JC., Jr Design and evaluation of a filter-based chairside amalgam separation system. Sci Total Environ. 2008;396(1):28–33. doi: 10.1016/j.scitotenv.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 19.Triebold HO. Quantitative analysis. Read books: New York; 2007. [Google Scholar]
- 20.Vogel AL. Vogel's qualitative inorganic analysis. 7th ed. London: Longmans; 1996. [Google Scholar]
- 21.Webb NA, Shaw JR, Morgan J, Hogstrand C, Wood CM. Acute and chronic physiological effects of silver exposure in three marine teleosts. Aquatic Toxicol. 2001;54(3-4):161–178. doi: 10.1016/s0166-445x(01)00150-3. [DOI] [PubMed] [Google Scholar]