Abstract
The apolipoprotein E4 (E4) allele is present worldwide, despite its associations with higher risk of cardiovascular morbidity, accelerated cognitive decline during aging, and Alzheimer’s disease (AD). The E4 allele is especially prevalent in some tropical regions with a high parasite burden. Equatorial populations also face a potential dual burden of high E4 prevalence combined with parasitic infections that can also reduce cognitive performance. We examined the interactions of E4, parasite burden, and cognitive performance in a traditional, nonindustrialized population of Amazonian forager-horticulturalists (N = 372) to test whether E4 protects against cognitive decline in environments with a heavy pathogen burden. Contrary to observations in industrial populations, older adult E4 carriers with high parasite burdens either maintained or showed slight improvements in cognitive performance, whereas non-E4 carriers with a high parasite burden showed reduced cognitive performance. Being an E4 carrier is the strongest risk factor to date of AD and cognitive decline in industrial populations; it is associated with greater cognitive performance in individuals facing a high parasite and pathogen load, suggesting advantages to the E4 allele under certain environmental conditions. The current mismatch between postindustrial hygienic lifestyles and active parasite-rich environs may be critical for understanding genetic risk for cognitive aging.—Trumble, B. C., Stieglitz, J., Blackwell, A. D., Allayee, H., Beheim, B., Finch, C. E., Gurven, M., Kaplan, H. Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden.
Keywords: antagonistic pleiotropy, mismatch, cognitive decline, Tsimane, Alzheimer’s disease
The strongest reported genetic risk factor of Alzheimer’s disease (AD) is the apolipoprotein E4 allele (E4), which raises lifetime risk in carriers up to 4-fold over that of noncarriers (1–5). Women are particularly vulnerable, showing a >50% excess of AD over men, and greater levels of brain amyloid and other markers of neurodegeneration (6). Beyond increased AD risk, E4 carriers display a sharper rate of cognitive decline and of gray matter atrophy with synapse loss during normal adult aging than do noncarriers (7). In addition to cognitive decline and dementia risks, E4 is associated with higher total cholesterol and LDL, enhanced intestinal absorption of dietary cholesterol (8, 9), and increased cardiovascular disease (CVD) risk in industrialized populations (10–12). Since E4 contributes to extensive morbidity and mortality worldwide (13, 14); from an evolutionary perspective, the relatively high worldwide frequency of the E4 allele despite its costs to survivorship is somewhat puzzling. E4 is considered the ancestral allele in the genus Homo (15–18), and E4 frequencies show extensive geographical variation, from <5% to >45%, with higher frequencies at the equator and tropical latitudes and in northern Europe (15, 19). The Tsimane fall in the upper range, with 24–32% E4 across age groups (20).
Despite its strong association with AD risk in industrialized populations, the E4 allele may have conferred survival or reproductive benefits in response to parasite burdens in energy-limited, subsistence-level populations. In high-parasite-load environments, E4 appears to be protective against parasites by minimizing their deleterious impact by preventing or contributing to spontaneous clearance of some infections, including viral hepatitis C (21), Giardia and cryptosporidium in low-income urban areas (22–24), and cryptosporidium infection in a murine model with human APOE4 alleles (2). Infectious disease burden affects cognitive performance (25–27) through a variety of mechanisms including malabsorption of macro- and micronutrients from the parasite feeding directly on the host (and host gut contents), as well as increased host inflammatory burden and immune response to the parasitic infection (28). Although helminths and other parasites currently affect millions around the world, improved sanitation and public health campaigns have significantly reduced parasite burdens, particularly in industrialized populations, which are the best studied for cognitive aging. Modern lifestyles in such populations are an evolutionary novelty; >99% of human history occurred in small, mobile hunter–gatherer groups without access to sanitation or clean water. The current mismatch between modern hygienic lifestyles and active parasite-rich environs may be critical for understanding the evolution of genetic risks for cognitive aging.
Cognitive performance plays a crucial role in school achievement, job attainment, income, and social status, all of which contribute to reproductive fitness (29, 30). Cognitive ability has been critical to the success of the human evolutionary niche, which requires extractive foraging, skill-intensive hunting, and social intelligence (31, 32). Adaptations that benefit cognitive performance via resistance to parasites or pathogens could have important reproductive impacts in early or later life. The reproductive benefits of cognitive performance could occur during early reproductive life with an earlier age at first reproduction, or at later ages as postreproductive older adults enhance the reproductive success of descendants (33, 34). Given that the E4 allele is present in less than half the individuals in any one population, it is possible that there is a heterozygotic advantage in populations with a high infectious burden such that individuals with only one E4 allele have highest fitness.
Although 2 studies have shown positive associations between E4 and child cognitive development (35, 36), it is not known whether E4 confers cognitive protection to older adults with a high parasite load. Studies thus far have not taken a life-course perspective (either focusing only on children or examining only cognitive decline and AD in older adults), nor have there been adequate tests in populations more representative of human evolutionary past. From a mechanistic standpoint, the impact of E4 on cholesterol production and absorption and parasite/pathogen clearance should have the same impact on adults as children. In this study, we tested 2 hypotheses: hypothesis 1 (H1), the E4 allele interacts positively with parasite burden in predicting cognitive function in adults, vs. hypothesis 2 (H2), the positive early-life benefits of carrying E4, in interaction with parasite burden, with respect to brain development and cognitive performance is limited to childhood and adolescence (23, 35, 36), but could outweigh the purely deleterious consequences of E4 in causing more rapid decline in cognitive performance later in life, when the force of selection is reduced (37). H2 predicts that cognitive advantages from E4 are limited to children, H2 population 1 (H2:P1), whereas fitness-relevant disadvantages (poorer cognitive performance) should be observed among postreproductive adults, H2 population 2 (H2:P2). These hypotheses are tested among the Tsimane, a remote population of forager-horticulturalists (∼15,000 individuals) in the Bolivian Amazon who live a traditional lifestyle of small-scale horticulture, hunting, fishing, and gathering without access to sanitation, electricity, or running water (38). The Tsimane have a high rate of pathogen exposure, with more than two-thirds of adults showing active helminth infections (39, 40).
MATERIALS AND METHODS
The Tsimane participants (N = 372 from 28 villages) aged 6–88 yr (mean age, 37.2 yr; 51.6% male) participated in a series of cognitive tasks and a morning fasting blood collection as part of routine biomedical surveillance conducted annually by the Tsimane Health and Life History Project (Supplemental Fig. S1). Ages were estimated from a combination of known ages from written records, relative age lists, photocomparisons, and dated events. Spanish-speaking ability was self-reported on a 3-point scale (minimal to none, intermediate, and fluent), and years of schooling were also collected (41). All participants provided informed consent, and the procedures were approved by the institutional review boards at the University of California, Santa Barbara and University of New Mexico.
Biomarkers of parasitic burden
A manual leukocyte count and 5-part differential were collected immediately after a blood-collection, and erythrocyte sedimentation rate (ESR) was assessed by using the Westergren method (39). Eosinophil counts measured via manual 5-part differential were used as an index of parasitic infections, as they relate to both current and past history of multiple types of parasitic infections (42, 43). Although hookworm, which induces T-helper 2 response characterized by eosinophilia (43), is the most common helminth seen in 56% of Tsimane (39, 40), eosinophilia is provoked by parasitic infections of many different types. Instead of focusing on single species of parasite, eosinophils are used as a biomarker of parasitic infection more generally. Previous studies report that quantitative measures of helminth burden in fecal samples are only marginally associated with clinical symptoms (44). ESR and leukocyte counts were included as nonspecific measures of inflammation and infection, respectively.
Cognitive battery
Participants engaged in a 7-part cognitive battery assessing attention, psychomotor speed, verbal declarative memory, and semantic fluency, derived in part from the Mexican Healthy Aging Study (45, 46). The first task examined short- and long-term verbal memory via word recall; participants were asked to listen to and repeat a list of 8 Tsimane words immediately and again after 10 min. A second set of tasks assessed working memory with 3 digit-span tasks; participants were asked to repeat a series of digits in both the Tsimane and Spanish languages that increased in length until failure on 2 consecutive trials. Although not all Tsimane speak Spanish, all Tsimane understand Spanish numerals. In the third digit-span task (tactile), the interviewer touched a sequence of numbered boxes on a sheet of paper, and the participants were asked to touch the boxes in the same order. The next task examined semantic memory (category fluency) with 3 locally salient categories: animals, plants, and fish. Participants generated a list of items from memory that matched each category (for example, all animals) during a 2-min period. The final task was a visual scan where participants were shown a random array of symbols and asked to highlight all instances of a target symbol. All tests were conducted by a trained Tsimane translator at participants’ homes and in the Tsimane language, with the exception of the Spanish numbers in the digit-forward test. Twelve individuals who reported vision problems were removed from analyses for tasks requiring visual acuity (Supplemental Fig. S1).
Genotyping
Whole blood was frozen in liquid nitrogen before transfer on dry ice to the University of Southern California, where DNA was extracted by using standard protocols. Determination of the APOE2/E3/E4 alleles in the Tsimane derived from genotypes of 2 SNPs, rs429358, and rs7412 (20, 39). Genotyping was performed using the TaqMan Allelic Discrimination system (Thermo Fisher Scientific, Carlsbad, CA, USA).
Data analysis
Age, sex, schooling, and Spanish fluency are all associated with cognitive outcomes (45, 46), and are included as control variables in linear regression models. Community ID was used as a random effect in all analyses. All cognitive measures were transformed to z scores to facilitate effect size comparisons across the distinct cognitive tasks. Analyses are divided into 2 subsets based on the hypothesis of interest: 1) all individuals over age 30, to study cognitive aging [previous studies in industrialized populations and among Tsimane have shown cognitive decline beginning in the 20s (45), as specified by H1 and H2], and 2) the subset of the sample who are under age 18, and a second subset of postreproductive adults (aged 45+) to test H2.
RESULTS
E4 and biomarkers of infection
Among the study participants, the frequencies of APOE genotypes in the Tsimane were E3/E3 = 76.1%, E3/E4 = 21.3%, and E4/E4 = 2.6%, and the distribution of genotypes was in Hardy-Weinberg equilibrium (χ2 = 2.34; P = 0.31). Approximately one-quarter (23.9%) of the subjects carried at least 1 copy of E4 but, notably, the E2 allele is not present in the Tsimane. Given the low frequency of E4/E4 homozygotes, these individuals were grouped together with E4 heterozygotes for all genetic analyses.
For all adults aged 30+, eosinophil counts ranged from 61 cells/μl to 3400 cells/μl, with a mean count of 1282 cells/μl (sd±644); 85.9% of participants presented with eosinophilia (eosinophil count >600 cells/μl) (47). Of the adults aged 30+, E4 carriers had 20% lower eosinophil counts (β = −259 cells/μl; P = 0.006) and 9% lower leukocyte counts (β = −723 cells/μl; P = 0.031), after controlling for age and sex, with a random effect for community of residence. There was no association between E4 and ESR. By comparison, eosinophil counts among children under age 18 ranged from 110 cells/μl to 6604 cells/μl, with a mean ± sd count of 1676 ± 1164 cells/μl; 88.8% of children presented with eosinophilia. E4 status was not associated with eosinophil counts, leukocyte counts, or ESR in any age group.
Does E4 protect cognitive function in a high parasite environment? (H1)
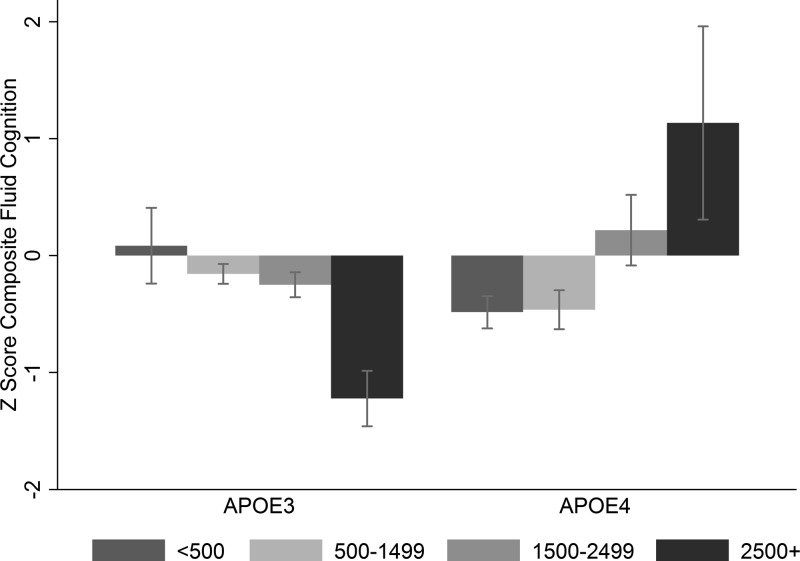
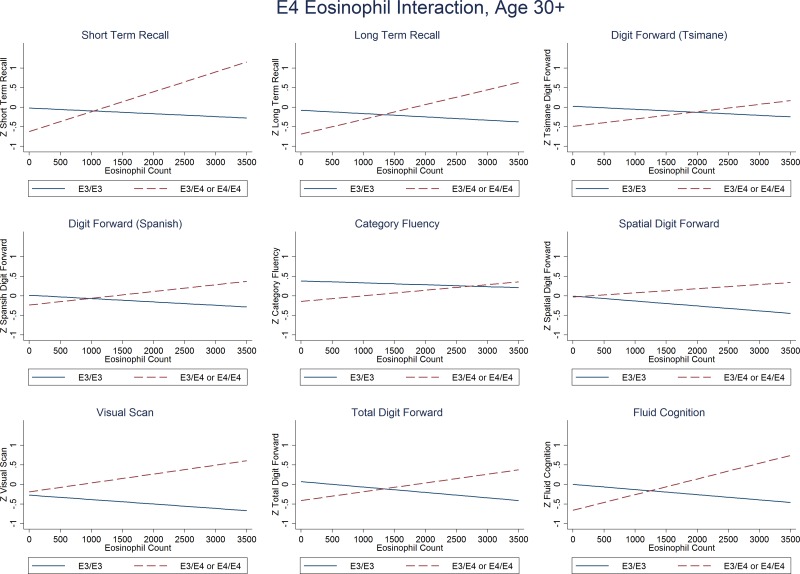
For adults aged 30+ (n = 242, mean age 49.3 yr, 50.8% male), E4 was associated with poorer cognitive performance on most measures of cognitive performance, especially for measures of fluid cognition (Table 1, Model 1 and Supplemental Table S1) after controlling for age, sex, education, Spanish language ability, and parasite burden (eosinophil count). However, there was a strong significant interaction between E4 and parasite burden (Fig. 1 and Table 1, Model 2 and Supplemental Table S1). Without including the interaction, the main effects of the E4 allele were weak and nonsignificant; after adding the interaction to the model, there was both a strong negative association between cognitive performance and the E4 allele and a strong positive interaction between E4 and eosinophils (Fig. 1). The interaction was strong enough that, with a high pathogen burden, E4 carriers were expected to outperform E3 carriers on cognitive measures (Figs. 1 and 2).
TABLE 1.
Mixed-effects linear regression
| Short-term recall | Long-term recall | Composite digit forward | Total composite fluid | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter |
Model 1 |
Model 2 |
Model 1 |
Model 2 |
Model 1 |
Model 2 |
Model 1 |
Model 2 |
| E4 | −0.018 | −0.666** | −0.069 | −0.656* | −0.089 | −0.525† | −0.137 | −0.760** |
| E4 × eosinophil count | - | 0.584*** | - | 0.517* | - | 0.415* | - | 0.555** |
| Age | −0.030*** | −0.030*** | −0.030*** | −0.029*** | −0.013** | −0.015** | −0.024*** | −0.024*** |
| Sex | 0.070 | 0.032 | −0.111 | −0.148 | 0.628*** | 0.608*** | 0.476*** | 0.438*** |
| Education | 0.086 | 0.091 | 0.012 | 0.016 | 0.147 | 0.189 | 0.138 | 0.149 |
| Spanish | −0.042 | −0.047 | 0.169*** | 0.163*** | 0.047 | 0.046 | 0.079 | 0.076 |
| Eosinophil count | 0.067 | −0.087 | 0.013 | −0.122 | −0.015 | −0.115 | 0.005 | −0.148 |
| AIC | 592.9 | 584.5 | 665.0 | 661.1 | 656.1 | 655.2 | 632.6 | 627.0 |
| Pseudo-R2 | 0.199 | 0.234 | 0.180 | 0.200 | 0.174 | 0.184 | 0.213 | 0.240 |
The analysis examined associations between E4, eosinophil count (eosinophils/μl ×1000), and cognitive performance z scores for fluid cognition tasks (n = 242 adults, aged 30+ yr). See Supplemental Table S1 for full results. Village ID is included as a random effect (not shown). AIC, Akaike’s information criterion. †P < 0.1; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Figure 1.
Predicted z scores for composite fluid cognitive performance (n = 242 adults aged 30+ yr), E4 status, and 4 levels of eosinophil count, controlling for age, sex, education, and Spanish-speaking ability, with community ID as a random effect. Categories were no eosinophilia, <500 eosinophils/μl; mild eosinophilia, <1500/μl; marked eosinophilia <2500/μl; and very marked eosinophilia ≥2500/μl (42).
Figure 2.
Each cognitive measure by eosinophil count in carriers of E4 (dashed line) and E3 (solid) in adults aged 30–88 yr (n = 242). Note that for nearly all measures of cognitive function E3/E3 genotypes perform better with nonclinical eosinophil counts (eosinophil count, <500 cells/μl) similar to what has been seen in industrial populations, whereas E4 carriers perform better in participants with higher eosinophil counts.
In the absence of eosinophilia, E4 carriers recalled 16% fewer words in short-term tasks (P = 0.004), 13% fewer words in long-term recall tasks (P = 0.047), and 12% fewer repeated numerals in the Tsimane digit forward task (P = 0.024). There were also nonsignificant trends among E4 carriers for recalling 10% fewer animals, plants, and trees in the category fluency task (P = 0.096), and 8% poorer performance on the composite digit span task (P = 0.068) and the combined Spanish, Tsimane, and spatial digit span tasks. Eosinophilia consistently had protective effects against E4, with significant interactions for short (P = 0.001) and long-term recall (P = 0.013), and trends toward an interaction for the Tsimane digit span (P = 0.097), a composite digit span (P = 0.051), and composite fluid task (P = 0.004); the other cognitive measures all had positive interactions between E4 and eosinophils but were not statistically significant (Fig. 2 and Supplemental Table S1). There was no significant interaction between sex and E4 status.
Is E4 associated with improved cognitive performance among children? (H2:P1)
Controlling for age, sex, education, and Spanish fluency, E4 was positively associated with visual scan (P = 0.037) and spatial forward tasks (P = 0.032), and positively (though not significantly) associated with 6/8 cognitive outcomes (Supplemental Table S2) in 124 children aged 6–18 (mean age 11.9 yr; 49.6% male). Height-for-age and weight-for-age z scores were positively, but not significantly associated with E4 (Supplemental Table S2). Unlike adults, there was no interaction between E4 and parasite load for cognitive outcomes (Supplemental Figs. S2A–F and S3).
Do postreproductive E4 carriers show reduced cognitive performance? (H2:P2)
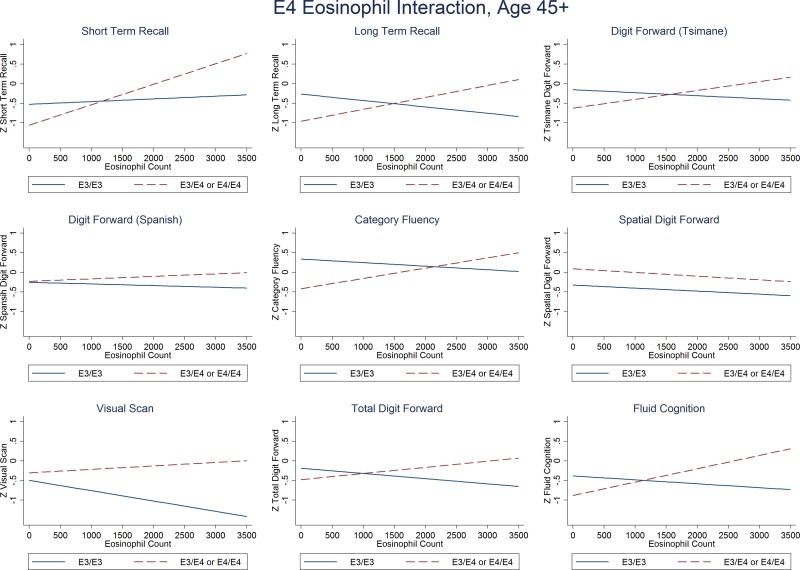
Limiting the sample to postreproductive adults aged 45–88 (n = 138; 51.5% male, mean age, 58.2 yr) produced results similar to those of all adults aged 30+. There was a consistent negative association between E4 and cognitive performance at lower eosinophil counts, whereas E4 was protective among eosinophilia cases (Fig. 3 and Supplemental Table S4).
Figure 3.
Each cognitive measure by eosinophil count in carriers of E4 (dashed line) and E3 (solid) in postreproductive adults aged 45–88 yr (n = 138).
DISCUSSION
Extensive evidence in human population samples and animal models has shown positive associations between parasite infections and cognitive impairment (25–27, 48). Genotypes that mitigate the negative impacts of parasite burden on cognitive performance should therefore have a selective advantage. We present evidence that the E4 allele, often associated with cognitive decline, AD, and CVD in industrialized populations with minimal parasite burden, was associated with higher cognitive function among Tsimane Amerindians, but only among individuals with evidence of high parasite burden. For homozygous E3/E3 carriers, higher eosinophil counts are associated with poorer performance on all cognitive measures (Supplemental Table S2). However, adults with high eosinophil counts, indicative of a high parasite burden, who carried at least 1 copy of the E4 allele (E3/E4 or E4/E4) showed better cognitive performance than did noncarriers. Although these eosinophil–cognitive function results differ from those in previous studies (25–27), they are consistent with studies showing a protective effect of E4 on cognition when exposed to environmental insults (35, 36). As a fatty acid transport protein, E4 plays critical roles in cholesterol metabolism and neurogenesis in the brain (49). E4 carriers also showed significantly lower eosinophil counts, suggesting potentially protective effects of E4 against parasitic infection or burden, consistent with previous reports (2, 21, 23). This result implies that E4 mitigates the effects of pathogen burden through at least 2 routes: by lowering the parasite load itself and by reducing its deleterious effects. Furthermore, our prior study of the Tsimane found that serum C-reactive protein, a general marker of inflammatory responses, was 60% higher in E3/E3 carriers than in E4 carriers (20), which is consistent with higher pathogen burden in E3 homozygotes. Thus, in a high parasite environment, the E4 allele may reduce parasite load, or alleviate the cognitive impacts of a high parasite load, possibly through differential cholesterol metabolism in the brain; the current study unfortunately cannot assess the mechanism by which cognitive function is maintained in a high pathogen environment.
Antagonistic pleiotropy, fluctuating selection, and heterozygotic advantage offer potential explanations for the worldwide prevalence of E4. There may be a fitness advantage to carrying E4 in some environments and at some life stages (24, 37) that are a trade-off against deleterious consequences (5, 9, 50). Although there may be some advantages (cognitive benefits and an increased ability to clear some infections) for E4 carriers during development when exposure risk to toxins (36) or disease is high (22, 24, 35), E4 is also associated with increased cardiovascular and AD risk (4, 10), as well as decreased longevity after age 65 (50–52) in industrial populations. However, it is not known whether E4 affects late-life mortality in populations with a high infectious burden.
Overall, our data do not support the antagonistic pleiotropy hypothesis with respect to early or late-life advantages. E4 was generally beneficial to individuals facing a high parasite load regardless of age (H1). Findings among Tsimane children are consistent with the better cognitive performance of E4 carrying children in Mexico City (36) and Brazilian favelas (low-income urban areas) (35). Among Tsimane children, nearly all measures of cognitive performance were positively associated with E4 status, although because of the relatively small sample size, a statistically significant difference was not detected. Rather, these gene-by-environment interactions are consistent with balancing selection in areas with endemic parasitism or fluctuating selection in environments with variable parasite exposure. Thus, E4 may be adaptive in some environments (with high parasite and pathogen load) and detrimental in others, such as industrialized areas with populations having a low parasite burden. Tropical regions rich in helminths have higher frequencies of the E4 allele (15), which may be protective against some parasites and infections (2, 23). In many industrialized populations, the much higher prevalence of elevated cholesterol, blood pressure, and obesity make vascular dementias a significant concern; in these populations, E4 may negatively impact cognitive performance and dementia risk via increased amyloid deposits in the brain (8, 9). Approximately 24% of Tsimane are carriers of E4, which is well within the range of what has been reported in Europe and North America, suggesting that our results are not related to outlier frequencies of APOE genotypes, as has been reported in some populations (15, 20).
In populations facing a diversity of endemic pathogens, E4 may be protective against age-related cognitive decline, and possibly even AD. A meta-analysis concluded that bacterial pathogens also increase risk of AD, with spirochetal bacteria being associated with a 10-fold higher risk and Chlamydophila pneumoniae with a 4-fold higher risk (53). Moreover, the human amyloid-β peptide has been shown to have protective antimicrobial benefits against cerebral bacterial infections in transgenic mouse models (54). More than two-thirds of adult Tsimane have intestinal helminths, and 50% of adults are anemic (38–40). Thus, in populations facing recurring pathogen exposure, the E4 allele may be adaptive by helping to clear infection and by decreasing the rate of cognitive decline during normal adult aging. In healthier populations, adults show progressive, slow attrition of cognitive processing and synapse loss (55), that is accelerated by E4 (7). These adult aging processes may involve different functions of the APOE protein than are protective for the cognitive development of children (23, 35, 36). In industrialized populations, sanitation, clean water, refrigeration, food safety, and medical advances have led to significant changes in the prevalence and incidence of parasitic and pathogenic infections, and thus may reduce the potential positive protective impacts of the E4 allele. In addition to vascular consequences of E4 that include higher risk of ischemic events, the potential benefit of the E4 allele for pathogen resistance may have less adaptive value for industrial populations with a lower pathogen burden from improved hygiene and modern medicine.
These results highlight the need for additional studies of the E4 gene by environmental interactions, especially in various nonindustrial environments, and for focusing on the neurobiology of middle age in addition to older age and the need to take a life-course perspective (55, 56). Other studies have found evidence of protective interactions between E4 alleles, cognitive function, and a host of other phenotypic characteristics, including testosterone (57), estrogen (58), and physical activity (59). Considered together, the evidence suggests that the impacts of the E4 allele are not always deleterious and may be adaptive and beneficial in some populations with a high infectious burden. We did not detect interactions between sex and E4 status in cognitive performance [for example, the female-E4 excess of cognitive deficits in AD (6)], perhaps because of the younger ages of most subjects or to the lower levels of circulating testosterone reported in male Tsimane (45).
Limitations
Although there is a strong association between eosinophil counts and chronic intestinal parasite load (42, 43), viral infections can also result in elevated eosinophil counts. That said, there was no interaction between E4 and other leukocyte subtypes (all P > 0.17; Supplemental Table S5), or ESR (P > 0.2), suggesting that current and past history of macroparasites drives this effect. In the current study, we used age-specific cutoffs to assess interactions between cognition, genotype, and parasite burden, but future studies with larger sample sizes and more statistical power will examine these interactions in finer detail. Children in this study ranged in age from 6–18 yr of age. Although this period encompasses significant cognitive development, the critical window for brain growth may be before this age range (60).
Given the overall small sample size of our study and of the number of E4/E4 homozygous individuals, our study does not have the power to test for a heterozygous advantage. However, we consider this possibility to be highly likely, given that none of the APOE alleles have gone to fixation among the Tsimane, as well as the context-dependent advantages and disadvantages of the E4 allele. Other versions of antagonistic pleiotropy in which the benefits and costs of allelic variants vary environmentally could also explain the existing pattern of diversity. Although our sample size is relatively small, our results are consistent with those reported in other South American populations with similar ancestry (22, 36).
CONCLUSIONS
Although being a carrier of the E4 allele is the single strongest risk factor for AD and cognitive decline in industrial populations, it is associated with greater cognitive performance in individuals who have a high parasite and pathogen load, suggesting that the E4 allele may have advantages in some environments. This finding may help explain the persistence and geographic distribution of such an otherwise deleterious allele.
ACKNOWLEDGMENTS
The authors thank the U.S. National Institutes of Health (NIH)/National Institute on Aging (NIA) for Grants R01AG024119 and R56AG024119, and the National Science Foundation (NSF) for Grant BCS-0422690. J.S. also thanks the Agence Nationale de la Recherche (ANR)–Labex IAST for financial support. The authors declare no conflicts of interest.
Glossary
- AD
Alzheimer’s disease
- CVD
cardiovascular disease
- E4
apolipoprotein E
- ESR
erythrocyte sedimentation rate
- H1
hypothesis 1
- H2
hypothesis 2
- H2:P1
hypothesis 2 population 1
- H2:P2
hypothesis 2 population 2
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
B. Trumble, M. Gurven, H. Kaplan, and C. Finch designed and performed the research; H. Allayee, B. Beheim, and A. Blackwell contributed new reagents or analytic tools; B. Trumble analyzed the data; and B. Trumble, M. Gurven, H. Kaplan, J. Stieglitz, and C. Finch wrote the paper.
REFERENCES
- 1.Small G. W., Ercoli L. M., Silverman D. H. S., Huang S.-C., Komo S., Bookheimer S. Y., Lavretsky H., Miller K., Siddarth P., Rasgon N. L., Mazziotta J. C., Saxena S., Wu H. M., Mega M. S., Cummings J. L., Saunders A. M., Pericak-Vance M. A., Roses A. D., Barrio J. R., Phelps M. E. (2000) Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 97, 6037–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azevedo O. G. R., Bolick D. T., Roche J. K., Pinkerton R. F., Lima A. A. M., Vitek M. P., Warren C. A., Oriá R. B., Guerrant R. L. (2014) Apolipoprotein E plays a key role against cryptosporidial infection in transgenic undernourished mice. PLoS One 9, e89562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Exel E., Eikelenboom P., Comijs H., Frölich M., Smit J. H., Stek M. L., Scheltens P., Eefsting J. E., Westendorp R. G. (2009) Vascular factors and markers of inflammation in offspring with a parental history of late-onset Alzheimer disease. Arch. Gen. Psychiatry 66, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509 [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Disease Neuroimaging Initiative Investigators (2014) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Disease Neuroimaging Initiative (2016) The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer’s disease of humans and mice. Neurobiol. Aging 37, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer’s Disease Neuroimaging Initiative (2015) 2014 Update of the Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 11, e1–e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahley R. W., Rall S. C. Jr (2000) Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537 [DOI] [PubMed] [Google Scholar]
- 9.Hagberg J. M., Wilund K. R., Ferrell R. E. (2000) APO E gene and gene-environment effects on plasma lipoprotein-lipid levels. Physiol. Genomics 4, 101–108 [DOI] [PubMed] [Google Scholar]
- 10.Eichner J. E., Dunn S. T., Perveen G., Thompson D. M., Stewart K. E., Stroehla B. C. (2002) Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am. J. Epidemiol. 155, 487–495 [DOI] [PubMed] [Google Scholar]
- 11.Song Y., Stampfer M. J., Liu S. (2004) Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann. Intern. Med. 141, 137–147 [DOI] [PubMed] [Google Scholar]
- 12.Bennet A. M., Di Angelantonio E., Ye Z., Wensley F., Dahlin A., Ahlbom A., Keavney B., Collins R., Wiman B., de Faire U., Danesh J. (2007) Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298, 1300–1311 [DOI] [PubMed] [Google Scholar]
- 13.American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2013) Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127, 143–152 [DOI] [PubMed] [Google Scholar]
- 14.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C. P. (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e2 [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg D. T. A., Kuzawa C. W., Hayes M. G. (2010) Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am. J. Phys. Anthropol. 143, 100–111 [DOI] [PubMed] [Google Scholar]
- 16.Finch C. E., Stanford C. B. (2004) Meat-adaptive genes and the evolution of slower aging in humans. Q. Rev. Biol. 79, 3–50 [DOI] [PubMed] [Google Scholar]
- 17.Fullerton S. M., Clark A. G., Weiss K. M., Nickerson D. A., Taylor S. L., Stengârd J. H., Salomaa V., Vartiainen E., Perola M., Boerwinkle E., Sing C. F. (2000) Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am. J. Hum. Genet. 67, 881–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P. P., Singh M., Mastana S. S. (2006) APOE distribution in world populations with new data from India and the UK. Ann. Hum. Biol. 33, 279–308 [DOI] [PubMed] [Google Scholar]
- 19.Ghasemian R. F., Borinskaya S., Yankovsky N. (2005) Worldwide distribution of apolipoprotein E gene alleles: is APOE* e4 allele a factor of adaptation to climate in humans? Iranian J. Public Health 34, 62–63 [Google Scholar]
- 20.Vasunilashorn S., Finch C. E., Crimmins E. M., Vikman S. A., Stieglitz J., Gurven M., Kaplan H., Allayee H. (2011) Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious load. Biodemogr. Soc. Biol. 57, 33–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller T., Fischer J., Gessner R., Rosendahl J., Böhm S., van Bömmel F., Knop V., Sarrazin C., Witt H., Mas Marques A., Kovacs P., Schleinitz D., Stumvoll M., Blüher M., Bugert P., Schott E., Berg T. (2016) Apolipoprotein E (APOE) allele frequencies in chronic and self-limited hepatitis C suggest a protective effect of APOE4 in the course of HCV infection. Liver Intl. 36, 1267–1274 [DOI] [PubMed] [Google Scholar]
- 22.Oriá R. B., Patrick P. D., Zhang H., Lorntz B., de Castro Costa C. M., Brito G. A., Barrett L. J., Lima A. A., Guerrant R. L. (2005) APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr. Res. 57, 310–316 [DOI] [PubMed] [Google Scholar]
- 23.Oriá R. B., Patrick P. D., Oriá M. O., Lorntz B., Thompson M. R., Azevedo O. G., Lobo R. N., Pinkerton R. F., Guerrant R. L., Lima A. A. (2010) ApoE polymorphisms and diarrheal outcomes in Brazilian shanty town children. Braz. J. Med. Biol. Res. 43, 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oriá R. B., Patrick P. D., Blackman J. A., Lima A. A. M., Guerrant R. L. (2007) Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med. Hypotheses 68, 1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale S. D., Erickson L. D., Berrett A., Brown B. L., Hedges D. W. (2016) Infectious disease burden and cognitive function in young to middle-aged adults. Brain Behav. Immun. 52, 161–168 [DOI] [PubMed] [Google Scholar]
- 26.Gale S. D., Erickson L. D., Brown B. L., Hedges D. W. (2015) Interaction between Helicobacter pylori and latent toxoplasmosis and demographic variables on cognitive function in young to middle-aged adults. PLoS One 10, e0116874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katan M., Moon Y. P., Paik M. C., Sacco R. L., Wright C. B., Elkind M. S. (2013) Infectious burden and cognitive function: the Northern Manhattan Study. Neurology 80, 1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall A., Hewitt G., Tuffrey V., de Silva N. (2008) A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern. Child Nutr. 4 (Suppl 1), 118–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowles S., Gintis H., Osborne M. (2001) Incentive-enhancing preferences: personality, behavior, and earnings. Am. Econ. Rev. 91, 155–158 [Google Scholar]
- 30.Strenze T. (2007) Intelligence and socioeconomic success: a meta-analytic review of longitudinal research. Intelligence 35, 401–426 [Google Scholar]
- 31.Kaplan H. S., Hooper P. L., Gurven M. (2009) The evolutionary and ecological roots of human social organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3289–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurven M., Kaplan H., Gutierrez M. (2006) How long does it take to become a proficient hunter? Implications for the evolution of extended development and long life span. J. Hum. Evol. 51, 454–470 [DOI] [PubMed] [Google Scholar]
- 33.Hooper P. L., Gurven M., Winking J., Kaplan H. S. (2015) Inclusive fitness and differential productivity across the life course determine intergenerational transfers in a small-scale human society. Proc. Biol. Sci. 282, 20142808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkes K., O’Connell J. F., Jones N. G., Alvarez H., Charnov E. L. (1998) Grandmothering, menopause, and the evolution of human life histories. Proc. Natl. Acad. Sci. USA 95, 1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitter S. S., Oriá R. B., Kvalsund M. P., Pamplona P., Joventino E. S., Mota R. M., Gonçalves D. C., Patrick P. D., Guerrant R. L., Lima A. A. (2012) Apolipoprotein E4 influences growth and cognitive responses to micronutrient supplementation in shantytown children from northeast Brazil. Clinics (Sao Paulo) 67, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright R. O., Hu H., Silverman E. K., Tsaih S. W., Schwartz J., Bellinger D., Palazuelos E., Weiss S. T., Hernandez-Avila M. (2003) Apolipoprotein E genotype predicts 24-month Bayley scales infant development score. Pediatr. Res. 54, 819–825 [DOI] [PubMed] [Google Scholar]
- 37.Williams G. C. (1957) Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 [Google Scholar]
- 38.Gurven M., Kaplan H., Winking J., Eid D., Vasunilashorn S., Kim J., Finch C., Crimmins E. (2009) Inflammation and infection do not promote arterial aging and cardiovascular disease among lean horticulturalists. PLoS One 4, e6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackwell A. D., Trumble B. C., Maldonado Suarez I., Stieglitz J., Beheim B., Snodgrass J. J., Kaplan H., Gurven M. (2016) Immune function in Amazonian horticulturalists. Ann. Hum. Biol. 43, 382–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackwell A. D., Tamayo M. A., Beheim B., Trumble B. C., Stieglitz J., Hooper P. L., Martin M., Kaplan H., Gurven M. (2015) Helminth infection, fecundity, and age of first pregnancy in women. Science 350, 970–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurven M., Kaplan H., Supa A. Z. (2007) Mortality experience of Tsimane Amerindians of Bolivia: regional variation and temporal trends. Am. J. Hum. Biol. 19, 376–398 [DOI] [PubMed] [Google Scholar]
- 42.O’Connell E. M., Nutman T. B. (2015) Eosinophilia in infectious diseases. Immunol. Allergy Clin. North Am. 35, 493–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair M. G., Herbert D. R. (2016) Immune polarization by hookworms: taking cues from T helper type 2, type 2 innate lymphoid cells and alternatively activated macrophages. Immunology 148, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocha M. O., Pedroso E. R., Greco D. B., Lambertucci J. R., Katz N., Rocha R. L., Rocha R. S., Rezende D. F., Neves J. (1996) Pathogenetic factors of acute schistosomiasis mansoni: correlation of worm burden, IgE, blood eosinophilia and intensity of clinical manifestations. Trop. Med. Int. Health 1, 213–220 [DOI] [PubMed] [Google Scholar]
- 45.Trumble B. C., Stieglitz J., Thompson M. E., Fuerstenberg E., Kaplan H., Gurven M. (2015) Am. J. Hum. Biol. 27, 582–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurven M., Fuerstenberg E., Trumble B., Stieglitz J., Beheim B., Davis H., Kaplan H. (2017) Cognitive performance across the life course among Bolivian forager-farmers with limited schooling. Dev. Psychol. 53, 160–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prieto R., Richter J. E. (2013) Eosinophilic esophagitis in adults: an update on medical management. Curr. Gastroenterol. Rep. 15, 324 [DOI] [PubMed] [Google Scholar]
- 48.Williamson L. L., McKenney E. A., Holzknecht Z. E., Belliveau C., Rawls J. F., Poulton S., Parker W., Bilbo S. D. (2016) Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav. Immun. 51, 14–28 [DOI] [PubMed] [Google Scholar]
- 49.Dietschy J. M., Turley S. D. (2001) Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12, 105–112 [DOI] [PubMed] [Google Scholar]
- 50.Ewbank D. C. (2004) The APOE gene and differences in life expectancy in Europe. J. Gerontol. A Biol. Sci. Med. Sci. 59, B16–B20 [DOI] [PubMed] [Google Scholar]
- 51.Kulminski A. M., Arbeev K. G., Culminskaya I., Arbeeva L., Ukraintseva S. V., Stallard E., Christensen K., Schupf N., Province M. A., Yashin A. I. (2014) Age, gender, and cancer but not neurodegenerative and cardiovascular diseases strongly modulate systemic effect of the apolipoprotein E4 allele on lifespan. PLoS Genet. 10, e1004141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soerensen M., Dato S., Tan Q., Thinggaard M., Kleindorp R., Beekman M., Suchiman H. E. D., Jacobsen R., McGue M., Stevnsner T., Bohr V. A., de Craen A. J., Westendorp R. G. J., Schreiber S., Slagboom P. E., Nebel A., Vaupel J. W., Christensen K., Christiansen L. (2013) Evidence from case-control and longitudinal studies supports associations of genetic variation in APOE, CETP, and IL6 with human longevity. Age (Dordr.) 35, 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maheshwari P., Eslick G. D. (2015) Bacterial infection and Alzheimer’s disease: a meta-analysis. J. Alzheimers Dis. 43, 957–966 [DOI] [PubMed] [Google Scholar]
- 54.Kumar D. K. V., Choi S. H., Washicosky K. J., Eimer W. A., Tucker S., Ghofrani J., Lefkowitz A., McColl G., Goldstein L. E., Tanzi R. E. (2016) Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 8, 340ra372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finch C. E. (2009) The neurobiology of middle-age has arrived. Neurobiol. Aging 30, 515–520, discussion 530–533 [DOI] [PubMed] [Google Scholar]
- 56.Finch C. E., Ruvkun G. (2001) The genetics of aging. Annu. Rev. Genomics Hum. Genet. 2, 435–462 [DOI] [PubMed] [Google Scholar]
- 57.Panizzon M. S., Hauger R., Xian H., Vuoksimaa E., Spoon K. M., Mendoza S. P., Jacobson K. C., Vasilopoulos T., Rana B. K., McKenzie R. (2014) Interaction of APOE genotype and testosterone on episodic memory in middle-aged men. Neurobiol Aging. 35, 1778.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaffe K., Haan M., Byers A., Tangen C., Kuller L. (2000) Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology 54, 1949–1954 [DOI] [PubMed] [Google Scholar]
- 59.Nichol K., Deeny S. P., Seif J., Camaclang K., Cotman C. W. (2009) Exercise improves cognition and hippocampal plasticity in APOE ε4 mice. Alzheimers Dement. 5, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuzawa C. W., Chugani H. T., Grossman L. I., Lipovich L., Muzik O., Hof P. R., Wildman D. E., Sherwood C. C., Leonard W. R., Lange N. (2014) Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. USA 111, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]