Abstract
Practitioners of ancient societies from the time of Hippocrates and earlier recognized and treated the signs of inflammation, heat, redness, swelling, and pain with agents that block or inhibit proinflammatory chemical mediators. More selective drugs are available today, but this therapeutic concept has not changed. Because the acute inflammatory response is host protective to contain foreign invaders, much of today’s pharmacopeia can cause serious unwanted side effects, such as immune suppression. Uncontrolled inflammation is now considered pathophysiologic and is associated with many widely occurring diseases such as cardiovascular disease, neurodegenerative diseases, diabetes, obesity, and asthma, as well as classic inflammatory diseases (e.g., arthritis and periodontal diseases). The inflammatory response, when self-limited, produces a superfamily of chemical mediators that stimulate resolution of the response. Specialized proresolving mediators (SPMs), identified in recent years, are endogenous mediators that include the n-3–derived families resolvins, protectins, and maresins, as well as arachidonic acid–derived (n-6) lipoxins, which promote resolution of inflammation, clearance of microbes, reduction of pain, and promotion of tissue regeneration via novel mechanisms. Aspirin and statins have a positive impact on these resolution pathways, producing epimeric forms of specific SPMs, whereas other drugs can disrupt timely resolution. In this article, evidence from recent human and preclinical animal studies is reviewed, indicating that SPMs are physiologic mediators and pharmacologic agonists that stimulate resolution of inflammation and infection. The findings suggest that it is time to challenge current treatment practices—namely, using inhibitors and antagonists alone—and to develop immunoresolvents as agonists to test resolution pharmacology and their role in catabasis for their therapeutic potential.—Serhan, C. N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms.
Keywords: omega-3 PUFA, leukocytes, resolvins, lipoxins, protectins, maresins
The innate immune system has evolved to recognize self from nonself, where phagocytes play a central role to protect the host from invading organisms and foreign objects. The repertoire and cell trafficking of the acute inflammatory response is protective, and the cardinal signs of inflammation—heat, redness, swelling, and eventual loss of function—were recognized by physicians of ancient civilizations (1). The therapeutic approach to treating excessive inflammation and the resulting collateral tissue damage has not changed significantly since ancient practitioners of folk medicine used willow bark as prescribed by Hippocrates (2). Therapeutic approaches to inflammation have focused on suppressing, blocking, or inhibiting proinflammatory mediators of inflammation. The compounds in willow bark, such as salicylate, provide the basis for aspirin, many nonsteroidal anti-inflammatory drugs (NSAIDs), and other drugs. Although many of these are effective, it is now clear that excessive or uncontrolled inflammation is associated with many widely occurring diseases and that new therapeutic interventions should be developed (3, 4).
The current treatment approaches relieve gross signs and symptoms but can give rise to severe immune suppression with opportunistic infections. The temporal events in self-limited acute inflammatory responses are known to resolve at the histologic level (Fig. 1) with the loss of inflammatory cells from the tissue and the return of function (5). The cellular steps and tissue histology of the stage were set as, for example, viewed clinically in the resolution of lung inflammation (6, 7). Yet the role and function of resolution phase mediators remained to be discovered. Focus on the fundamental mechanisms in the resolution response, via a modern systems approach, in my laboratories, have led to the isolation and complete structural elucidation of several novel families of proresolving mediators of inflammation that together constitute a superfamily of structurally distinct bioactive agents. These are biosynthesized from essential polyunsaturated fatty acid (PUFA) precursors to function as potent local-resolution agonists. We coined the term for the first of these new mediators, the “resolvins” (Rvs; resolution phase interaction products). They function in the repertoire of catabasis to restore the host’s tissues from their leukocyte-defensive position in the battlefields with microbial invaders (8–10, and reviewed in refs. 11, 12).
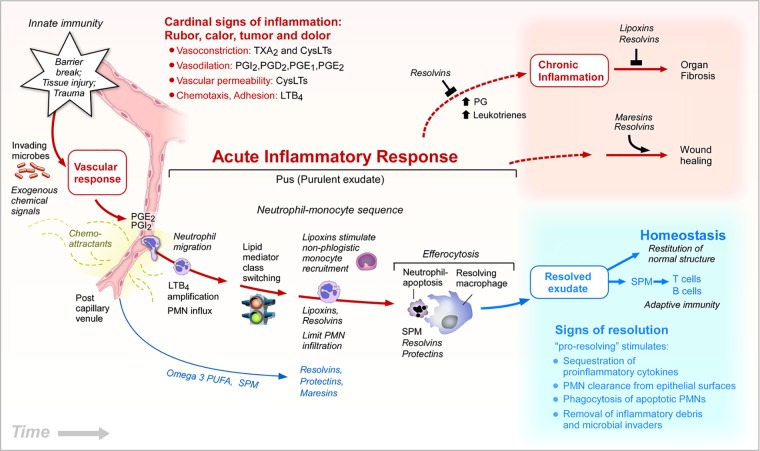
Figure 1.
Eicosanoids and proresolving mediators in the inflammatory response. Time-course illustration of the key roles of lipid mediators in the initiation and resolution of acute inflammation. Eicosanoids function and contribute to cardinal signs of inflammation. Prostaglandin E2 (PGE2) and PGI2 permit neutrophils to transmigrate from postcapillary venules across endothelial cells to migrate and chemotax along a gradient of leukotriene B4 (LTB4), a potent chemoattractant. Lipid mediator class switching occurs as neutrophils congregate in pus or purulent exudates (see text and ref. 34 for details). Lipoxins (LXs) stimulate nonphlogistic monocyte recruitment. LXs, resolvins (Rvs), and other specialized proresolving mediators (SPMs) are produced in pus to limit or stop further neutrophil tissue infiltration. SPMs, Rvs, maresins (MaRs), and protectins, each stimulate efferocytosis of apoptotic neutrophils and cellular debris by macrophages. Resolving macrophages and apoptotic neutrophils also produce SPMs (see ref. 135). Edema also brings circulating n-3 polyunsaturated fatty acids (PUFAs) [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] into exudates for temporal conversion to SPMs by exudate cells (85). SPMs stimulate the signs of resolution and resolve exudates (33). Rvs and SPMs block chronic inflammation and reduce fibrosis. MaRs and specific Rvs enhance wound healing and tissue regeneration.
The identified proresolving pathways and mediators that expedite resolution of inflammation have been rapidly embraced by colleagues studying immunopharmacology and have opened the potential for considering new therapeutic directions (13, 14). The cells, mediators, and mechanisms in the resolution of inflammation have been the recent subject of several in-depth reviews, which I suggest for interested readers (15–23). This overview addresses the emergence of new concepts and potential for endogenous mediators of resolution and tissue regeneration within the resolution terrain that give rise to a new discipline: resolution pharmacology. There has not been a conceptual change in direction of patient care for inflammatory diseases or infections for centuries, and new approaches are needed to minimize or eliminate unwanted side effects and immune suppression that can accompany prolonged use of traditional anti-inflammatory therapies [e.g., steroids, NSAIDs and cyclooxygenase (COX) inhibitors], as well as newer biologics focused on blocking cytokines, such as anti-TNF therapies. Those drugs currently available in the pharmacopeia for treatment consist mainly of inhibitors and receptor antagonists to manage excessive and infectious inflammation. The new terrain of natural resolution, their pathways and endogenous mediators uncovered give us new concepts and hope. These are fertile ground for treating inflammation in the 21st century by pharmacologic means that are in alignment with the self-limited inflammatory response and its natural timely resolution, with tissue regeneration and return of function.
ALPHA SIGNALS OMEGA IN THE CASCADE
Inflammation is certainly close to all of us. We are all affected daily by the acute inflammatory response protecting us from microbial invasions and tissue injury. My colleagues and I started to think about the molecular decision paths that can lead from acute inflammation to either chronicity or the ideal outcome of complete resolution (Fig. 1). Textbooks show that the resolution of acute inflammation has been thought to be a passive process, meaning that inflammatory mediators from the initiation of the acute response [e.g., chemoattractants, complement components, C5a, C3b, prostaglandins (PGs), chemokines, and cytokines] would simply dilute and dissipate (5, 24, 25) to stop the infiltration of leukocytes into the tissues. By studying self-limited acute inflammatory responses with inflammatory exudates formed in vivo in mice, an adaptation of the well-known rat air pouch model (26), we learned that, during self-limited responses in animal models devised with a systems approach, this process gives rise to active resolution where Rvs and other specialized proresolving mediators (SPMs)—a superfamily of proresolving mediators that include Rvs, protectins (PDs), maresins (MaRs) and lipoxins (LXs) (12)—take part to bring about a state of resolution and tissue function (8–10, 27). We first focused our efforts on periodontal disease (28) because it is a chronic inflammation and infection that has certainly been a public health concern in the United States and around the world (29, 30). Periodontal disease is a very good example of leukocyte-mediated tissue destruction, primarily by neutrophils that can amplify inflammation via debris produced around the ligaments of the tooth, and we therefore thought that this clinical scenario would be an ideal one for testing the principles of stimulating resolution to control the unwanted side effects of this form of infectious inflammation.
The main questions that interest me and my research team are as follows: What are the endogenous controllers of excessive inflammation and infection? How are these linked and what are the signaling molecules involved? The specialized proresolving mediators–each a separate family of bioactive mediators, the Rvs, PDs, and MaRs, with separate biosynthesis pathways and receptors (Fig. 2)—play a role in these processes (12). Recent findings introduced 3 novel pathways that stimulate within-tissue regeneration (31). The complete stereochemical assignment via total organic synthesis of each of these mediators has confirmed their original structural assignments, as well as the basis for resolution pharmacology.
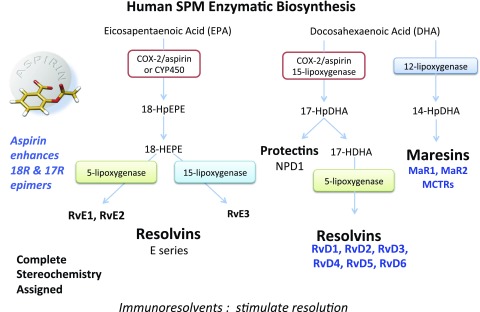
Figure 2.
Human SPM biosynthetic routes. Biosynthesis of E-series Rvs is initiated with molecular oxygen insertion at carbon-18 position of EPA, which is converted to bioactive E-series members RvE1–E3. The resolution metabolome also activates 17-lipoxygenation of DHA; 17S-HpDHA is converted to Rv-epoxide intermediates by the leukocyte 5-LOX. The intermediates are transformed to RvD1–D6, each of which carries potent actions. 17-HpDHA is also the precursor to the 16,17-epoxide-PD intermediate, which is converted to NPD1/PD1 and related PDs (see Fig. 6A). MaRs are produced by macrophages via initial lipoxygenation at the carbon-14 position by lipoxygenation and insertion of molecular oxygen, producing a 13S,14S-epoxide-MaR intermediate that is enzymatically converted to the MaR family members MaR1, MaR2, and MCTRs. The stereochemistry of each bioactive SPM has been established, and SPM biosynthesis in murine exudates and human tissues confirmed. (See refs. 58, 103, 163, 164 for original reports, total organic synthesis, and stereochemical assignments and the text for further details. For complete stereochemistry of individual SPMs, see refs. 72, 104, 107.) Low-dose aspirin triggers the 17R and 18R/S epimers of the Rvs (9, 53) and 17R-epimer PDs (165, 166).
The illustration in Fig. 1, composed according to results of our studies, emphasizes the resolution of inflammation as an active process with novel mediators and checkpoint endogenous controlled mechanisms. Samuelsson and colleagues of the Karolinska Institute (Stockholm, Sweden) contributed seminal discoveries of the biosynthetic pathways for the PGs and leukotrienes (LTs) (32), which are major mediators that evoke the classic cardinal signs of inflammation (5). PGs and LTB4 play critical roles at the beginning of the acute inflammatory response and, in a controlled laboratory setting (with a fixed time 0 of the initiation of the acute inflammatory response) we induced this temporal sequence of key cellular and molecular events, to study the signs of resolution (12, 33) that are regulated by the proresolving mediators (Table 1). A temporal lipid mediator class switch leads to the production of the LXs (34) and also involves phospholipases (35) and the Rvs, which give signals to macrophages and the nonphlogistic recruitment of monocytes (36, 37), as they eat and take up the apoptotic neutrophils via phagocytosis (38). When added back pharmacologically, Rvs and other SPMs, such as the PDs regulate inflammatory mediators by counterregulation [TNFα, platelet activating factor (PAF), PGs, LTs, chemokines, and cytokines] to head off inflammation in animal models (see Table 2 for recent preclinical models) as well as organ fibrosis (39–42). The MaRs and RvE1 were first recognized (43, 44) to stimulate resolution, tissue regeneration, and subsequent wound healing (21, 45). PG biosynthesis is critical to resolution, because PGE2 and PGD2 stimulate the induction of 15 type I lipoxygenases (LOXs) that are necessary for producing LXs and the specific SPMs, Rvs and PDs. Hence, inhibitors of prostanoid biosynthesis, such as the classic NSAIDs, disrupt timely resolution (20, 46–48).
TABLE 1.
Structural elucidation and biosynthesis of novel bioactive mediators
| Chemical and biochemical procedures used to deduce structures of bioactive SPMs |
|---|
| Precursor analysis, 14C label incorporation |
| MS-MS spectra and after deuterium incorporation |
| GC-MS of several derivatives, trapping intermediates |
| MS spectra of several derivatives |
| UV chromophore |
| Confirmation total organic synthesis, physical properties, and biologic function |
| Bioactions and function of proresolving mediators |
|---|
| Limit PMN tissue infiltration, cessation |
| Reduce collateral tissue damage by phagocytes |
| Shorten Ri resolution interval |
| Enhance macrophage phagocytosis, and efferocytosis |
| Counterregulate proinflammatory chemical mediators (PAF, LTs, PGs) |
| Increase anti-inflammatory mediators (IL-10 and others) |
| Increase microbial killing and clearance by innate immune cells |
| Enhance tissue regeneration |
TABLE 2.
SPMs in atherosclerosis, diabetes, and obesity (recent preclinical animal disease systems)
| SPM | Action | Reference |
|---|---|---|
| Diabetes | ||
| RvE1 | RvE1 regulates murine neutrophil phagocytosis in type 2 diabetes | 138 |
| RvD1 | Resolution of inflammation by RvD1 is essential for peroxisome proliferator-activated receptor-γ-mediated analgesia during postincisional pain in type 2 diabetes | 139 |
| Effect of enriching the diet with menhaden oil or daily treatment with RvD1 on neuropathy in type 2 diabetes | 140 | |
| Proresolution therapy for the treatment of delayed healing of diabetic wounds | 141 | |
| RvD1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice | 142 | |
| PD1 | NPD1/PD1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes | 143 |
| PDx | PDx alleviates insulin resistance by activating a glucoregulatory axis | 144 |
| Atherosclerosis | ||
| RvE1 | RvE1 attenuates atherosclerosis on top of atorvastatin | 145 |
| RvE1 attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis | 132 | |
| Aspirin-triggered LX and RvE1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis | 110 | |
| Atherosclerosis: evidence for impairment of resolution of vascular inflammation | 146 | |
| RvD2 and MaR1 | Prevent atheroprogression in mice | 147 |
| RvD1 | Reduced RvD1 in human carotid atherosclerotic plaques increases plaque instability | 112 |
| Decreases human pulmonary artery hyperreactivity | 148 | |
| Obesity and steatosis | ||
| RvDs | RvD1 primes resolution initiated by calorie restriction in obesity-induced steatohepatitis | 61 |
| RvD1 and RvD2 govern local inflammatory tone in obese fat | 149 | |
| RvD1 and its precursor promote resolution of adipose tissue inflammation by eliciting macrophage polarization | 150 | |
| PD1 | Obesity-induced insulin resistance and hepatic steatosis: a role for Rvs and PDs | 151 |
| Impaired local production of proresolving lipid mediators in obesity | 152 | |
The new concept from these results is that, at experimental time 0, the resolution process begins and is an active process (27, 49): alpha signals omega; the beginning signals the end or termination of the acute inflammatory response. The main physiologic responses that we used to structurally elucidate the stop signals of inflammation that proved to be proresolving mediators (Table 1), evoking cessation of neutrophilic infiltration (the reduction of diapedesis) to limit further neutrophilic infiltration to a site of inflammation, are the physiologic responses at the postcapillary venule that stimulate macrophage phagocytosis, as well. When added back pharmacologically, the effect is perceived in experimental animals as anti-inflammatory and is different from the proresolving action, which is actually composed of both of these, where the proresolving mediators increase the clearance and killing, efferocytosis, and phagocytosis of apoptotic polymorphonuclear neutrophils (PMNs), and therefore it took some time to discover that proresolution was not identical to anti-inflammation (Table 1). To be able to do this systematically and gain the evidence, we introduced resolution indices to be quantitative in experimental animal models (27) and this extended, to our surprise, to the uptake and killing of microbes (50, 51). Keep this in mind when thinking about the immune suppression of classic anti-inflammatory therapies—NSAIDs and steroids that are widely used (3) as in, for example, the treatment of arthritis or other widely occurring inflammatory conditions and diseases (e.g., neurodegenerative diseases, obesity, and metabolic syndrome).
During the resolution phase, we were able to systematically assign stereochemistries and biosynthesis of the E-series Rvs (Fig. 2). There are 3 of them: RvE1 (5S, 12R, 18R-trihydroxy-eicosa-6Z, 8E, 10E, 14Z, 16E-pentaenoic acid) (8, 52, 53); RvE2 (54); and the newest and third family member, RvE3 (55). Docosahexaenoic acid (DHA) is enriched in several human organs, including the brain, eye, breast milk, and testes (56, 57), and we found that it is also used by inflammatory exudates, or pus cells (Figs. 4 and 5). DHA is transformed into 6 separate and structurally distinct D-series Rvs, and we have carried out the complete stereochemical assignment of each (58) (Fig. 2). During this process, a hydroperoxide-containing intermediate is converted into an epoxide that is precursor to the PD family (22) and neuroprotectin D1 (NPD1; 10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid); we collaborated with Nicolas Bazan and his colleagues to introduce NPD1 (59). Later, within this inflammatory response, macrophages arrive in resolving exudates, and they convert DHA to the MaR family members (43) illustrated in Fig. 2.
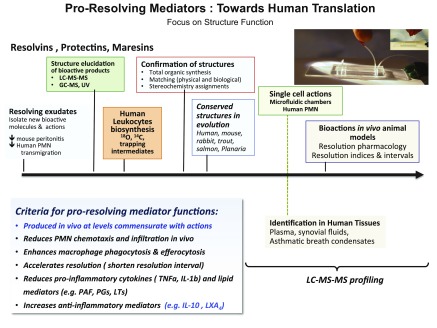
Figure 4.
Steps to human translation of SPMs. Timeline illustration of the key steps from identification in self-limited resolving exudates and function, structural elucidation, and biosynthesis, to single-cell actions and preclinical disease models with birth of resolution indices and resolution pharmacology for these endogenous autacoid mediators.
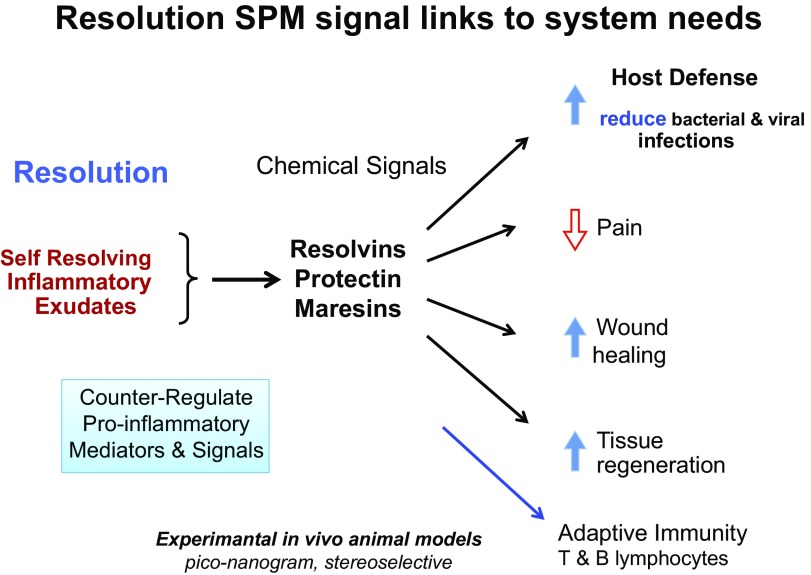
Figure 5.
Resolving exudates produce chemical signals to link system needs. Chemical signals produced by resolving exudate leukocytes act in host defense to reduce pain, enhance wound healing and tissue regeneration, and act on cells of the adaptive immune system.
The Rvs, LXs, PDs, and MaRs have been studied as SPMs by many independent laboratories (>2000 PubMed citations), and their potent actions in reducing inflammation have been documented, along with the development of a wide range of preclinical disease models using validated commercially available Rvs and other synthetic SPMs. In nanogram to microgram amounts, they are stereoselective and active, and they have a novel mechanism of action in the airway; in cardiovascular, ocular, and renal functions; and in the brain, periodontal arena, gastrointestinal disease (60), and the liver (61). Table 2 lists only a few of these potent and more recently discovered actions in, for example, animal disease models of atherosclerosis, diabetes, and obesity that illustrate the agonist actions of SPMs and their potential to control and treat excessive inflammation in human disease.
HOW DO SPMs WORK?
In the postcapillary venule (Fig. 1), results indicate that SPMs limit the further recruitment of PMNs, yet stimulate the nonphlogistic recruitment of mononuclear cells (Table 1). When macrophages encounter SPMs, they increase phagocytosis, resulting in the removal of apoptotic PMNs and microbes, and they clear PMNs from the sites. SPMs have proved not to be immunosuppressive in multiple in vivo experimental animal models (those cited in Table 2, for example). Biosynthesized SPMs counterregulate the early initiators of acute inflammation, the PGs; they regulate COX-2 expression, LTs, and PAF formation (Fig. 3); counterregulate the proinflammatory cytokines; and increase IL-10 (14).They regulate NF-κB gene products and ultimately lead to the regulation of edema (52).
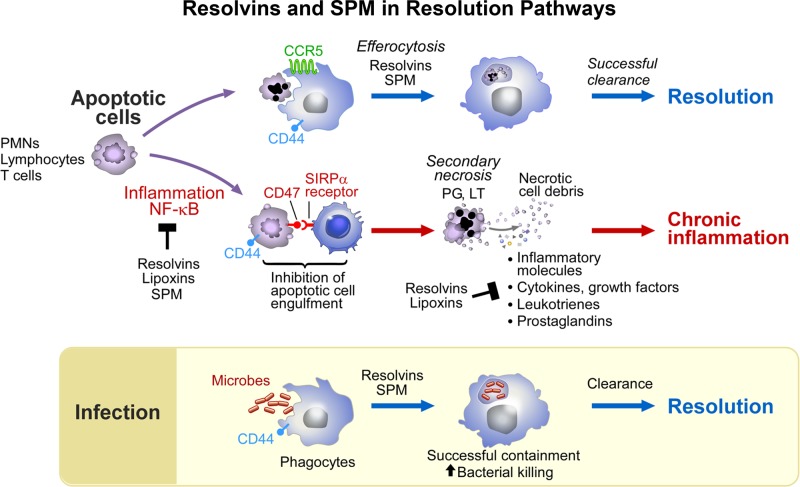
Figure 3.
Rvs and SPMs in the cellular resolution pathway. SPMs stimulate efferocytosis and the uptake of debris for successful clearance from tissues and resolution. SPMs block NF-κB; inhibition of containment of apoptotic cells leads to secondary necrosis and chronic inflammation (see text and ref. 167 for further details). SPMs counterregulate proinflammatory mediators and growth factors, cytokines, LTs, and PGs.
SPMs activate 5 separate GPCR receptors (62, 63). Prof. Mauro Perretti studied the LXA4 receptor, which can form heterodimers to evoke differential intracellular signaling (64, 65). This receptor is also shared by RvD1 (66) and has been confirmed in receptor-knockout mice (67). SPM receptors are expressed on different cell types, giving rise to tissue selectivity. This finding has been confirmed in knockout and transgenic mice (68), as has most recently the RvD2 receptor (63). Interested readers are directed to reviews, on which I have collaborated with Derek Gilroy and Christopher Buckley (20) and Chiang and Dalli (23, 62), for further detail. These SPM receptors counterregulate NFκB, and they increase heme-1-oxygenase in many target organs, to give organ protection (69–71).
PRORESOLVING LIPID MEDIATORS IN HUMAN TISSUES ENABLE PERSONALIZED, PRECISION MEDICINE IN THE RESOLUTION METABOLOME: FUNCTIONAL METABOLOMICS
To emphasize our operationalization of lipid mediator metabolomics (72), we routinely identify 6 diagnostic ions in each mediator, a key point for accurate and rigorous identification. We usually perform computational and cluster analyses of these pathway mediators. These are critical in organ and tissue profiles, such as those of blood and plasma (72, 73), which can also be useful in personalized and precision medicine in the near future. From this approach, we and others have now shown by mass spectrometry that many human tissues produce proresolving mediators (Table 3). SPMs are produced in human whole blood where they function to increase both phagocytosis and bacterial killing (72). Of interest are the recent results in a study of human breast milk (74) that have high levels of SPMs that are functional and stimulate resolution. This finding suggests that there are organs and tissues that produce SPMs in addition to the inflammatory exudate and innate immune system. Recently, synovial fluids were profiled by Norling et al. (75) and Barden et al. (76), who showed that increases in SPMs upon supplementation with RvE2 correlated with reduced pain scores. Some of the highest levels of the proresolving mediators are found in the human placenta (77), which is intriguing, and in the human and murine lymph nodes as well (72). SPMs and eicosanoids are present in patients who have sepsis and are admitted to intensive care units and who reflect the disease time course and dynamics (73).
TABLE 3.
Human tissue SPM identification and profiling via LC-MS-MS
| Study | Reference |
|---|---|
| Identification and signature profiles for proresolving and inflammatory lipid mediators in human tissue | 72 |
| n-6 and -3 PUFA mediators in human urine | 153 |
| RvD1, RvD2, and other mediators of self-limited resolution of inflammation in human blood | 154 |
| The human urine metabolome | 155 |
| Human inflammatory and resolving lipid mediator responses to resistance exercise | 156 |
| High levels of anti-inflammatory and proresolving lipid mediators LXs and Rvs in human milk | 157 |
| Human milk SPMs stimulate resolution of inflammation | 74 |
| Metabolomic profiling of lipid mediators in sputum from adult cystic fibrosis patients | 158 |
| Resolution of inflammation is altered in Alzheimer’s disease; brain and CSF reduced SPMs and their receptors | 159 |
| Plasma metabolomics in human pulmonary tuberculosis disease | 160 |
| Human arthritis, supplementation of n-3 fatty acids increases SPMs; RvE2 correlates with reduced pain | 76 |
| Human arthritis synovial exudates; RvD1 and RvD3 | 75 |
| Human arthritis synovial exudates | 161 |
| Intensive care unit sepsis patients: eicosanoids and SPMs | 73 |
| Randomized controlled trial in chronic kidney disease: increase in SPMs on n-3 fatty acid supplementation | 80 |
| D-series Rv precursor 17-HDHA in maternal and cord blood in pregnancy | 162 |
| Human carotid atherosclerotic plaques | 112 |
RvD1 (7S, 8R, 17S-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid), 17-epi-RvD1, and RvD2 (7S, 16R, 17S-trihydroxy-docosa-4Z, 8E, 10Z, 12E, 14E, 19Z-hexaenoic acid), as well as PD1, were each identified in 51 samples of human placentas. Supplementation with n-3 PUFAs increased the placental SPM precursors 17-hydroxydocosahexaenoic acid (HDHA) and 18-hydroxyeicosapentaenoic acid, whereas increases in placental Rvs and PD1 were not significantly increased by oral supplementation (77). The effect is different in rat placentas, where increased consumption of n-3 PUFAs increases pathway precursors (Fig. 2) and RvD1 and 17-epi-RvD1. PDs [e.g., PD1 and PDx (10S,17S-diHDHA)], also increase in late-gestation rat placenta, along with increased expression of ALOX15 mRNA (78). Hence, these placental studies demonstrate that the local organ control of Rvs and PDs is apparently tightly regulated in placentas from humans and rats, illustrating species differences in the relationship between oral consumption of n-3 PUFAs and local biosynthesis of SPMs in mammalian organs. In obese individuals supplemented with n-3 fatty acids, increases in peripheral blood RvD1 and RvD2 were essentially doubled within months to functional levels in these subjects (79).
These relationships and the governing principles between oral supplementation of n-3 fatty acids and local SPM production and organ levels require further detailed studies that are randomized and controlled to establish these relationships rigorously. These are under way by, for example, Trevor Mori, Anne Barden and colleagues (80, 81), and have already given compelling evidence for the relationship of n-3 PUFA supplementation to tissue Rvs and other SPMs. The further optimization of SPM profiling approaches via liquid chromatography tandem mass spectrometry (LC-MS-MS), along with available deuterium-labeled internal and synthetic SPM standards for calculating recoveries and amounts of specific SPM family members (33, 72, 82), will continue to permit rigorous interrogation of these relationships (73, 83). Such investigations have only recently commenced in human tissues to determine age, gender, and organ-dependent levels of healthy individuals vs. specific pathologic scenarios (Table 2).
SPMs IN HUMAN CLINICAL TRIALS: CAPITAL STARVATION
Our approach to human translation from the other direction—that is, without substrate supplementation (Figs. 4 and 5)—because there are SPM-specific GPCR receptors, was to prepare a series of SPM analogs that resisted local inactivation (58, 84, 85), hence using the body’s own stop signals of inflammation as agonists of resolution as templates (86) to make new proresolving therapeutics. We pursued this approach, for example, with RvD1, by devising receptor mimetics in animal models and elsewhere to advance the development of agonists for the proresolving actions (85). This approach was used with RvE1 and other SPMs because they are inactivated within tissues near the site of formation (58). In a demonstration of the resolution of inflammation with the use of a proresolving agonist in human translation with Thomas Van Dyke (Forsyth Institute, Cambridge, MA, USA), we are currently conducting an ongoing periodontal disease proof of concept with an oral month rinse containing an SPM analog to reduce inflammation by stimulating resolution. This trial is supported by the U.S. National Institutes of Health, National Institute of Dental and Craniofacial Research at the Forsyth Institute, should report their findings in 2017. Also, the World Health Organization awarded the name navamepent for RvE1 analog containing eye drops. The results of a phase 1 and 2 multicenter double-blind placebo trial have been reported. More than 232 patients were treated. The results of this trial, designed by Per Gjorstrup, the former chief medical officer of Resolvyx Pharmaceuticals (Cambridge, MA, USA), of which the author was the original scientific founder, gave the first demonstration of clinical efficacy of a proresolving therapeutic approach in humans with dry eye–associated inflammation (87, 88).
The phase 2 trial controlling inflammation in the eye with topical drops (87) was based on the physiologic roles (89–91) of SPMs, such as Rvs and LXs in the eye (92–95), and apparent gender differences, where females displayed delayed healing of wounded corneal tissue and lower levels of 15-LOX-derived SPMs, such as LXA4 (96, 97). Because 15-R/S-methyl LXA4, an analog of the aspirin-triggered form of LXA4, one of the first stop signals and proresolving mediators, was effective in infantile eczema in a double-blind, placebo-controlled study randomized at 2 centers (98) and RvD1, RvD2, and MaR1 act on T-cell responses (99), it is likely that SPMs have physiologic roles in human skin and barrier functions that remain to be studied and that their functions extend from the resolution phase into the adaptive immune response (100). It is hoped that results of these studies will open up new directions for treatment of a wide range of human diseases by controlling the resolution and catabasis mechanisms of the inflammatory response via resolution and regeneration agonists (Fig. 5). The availability of capital funds is the limiting step in testing this approach in which SPMs and their analogs would be used in human clinical trials to treat disease.
TISSUE REGENERATION FROM SIGNALING RESOLUTION IN RESPONSE TO MICROBIAL INVASION AND SURGICAL INTERVENTIONS
What about the relationship between the demands of the tissue on injury, resolution, and bacterial invasion? Are there signals from resolving infectious exudate that act in tissue regeneration? SPMs control the severity of infections in animal preclinical models, enhancing both phagocytosis and killing and reducing collateral tissue damage and proinflammatory mediators (ref. 50, and reviewed in ref. 101). To investigate, we used the mouse model of peritonitis, where very rapid neutrophilic infiltration occurs, followed by the resolution phase of self-limited infections (102), using fluorescence-activated cell-sorting and lipid mediators-metabolipidomics to interrogate the infectious pus that is produced. With Escherichia coli infections, we moved to another model, the planaria, a primordial organism (44, 45, 102), for surgical injury experiments. RvE1 and MaR1 each stimulated tissue regeneration in this system after surgical intervention where we introduce a quantitative index for tissue regeneration rates (45). In these, we can surgically remove their heads or tails that grow back in a very short period of time on the order of days. In these experiments, we systematically took extracts (Fig. 5) of the resolving exudates from E. coli infection to address this question [in Dalli et al. (102)]: Can mediators/signal molecules that are produced in the resolution phase of infections accelerate tissue regeneration?
In short, the answer was affirmative. In these two model systems, primordial chemical signals were produced that activate this evolutionally conserved tissue regeneration isolated from infectious inflammatory exudates. The first structures that were identified (102) through structural elucidation (Fig. 6) carried carbon 14 position alcohols, and we knew that this placed them into the family of MaRs, which have a conjugated double-bond system. The first was a glutathione adduct, a 13-glutathionyl-14-hydroxy, 13R-glutathionyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid, and we coined the term MaR conjugates in tissue regeneration (MCTR)1. The second is MCTR2 (13R-cysteinylglycinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid). These are both bioactive, and structural elucidation and biosynthesis studies permitted assembly of the pathway (102). We found novel bioactive structures in human milk, mouse exudate, and human macrophages that stimulated the regeneration of tissue in planaria, substantially shortening the time interval necessary for complete regeneration. This finding was exciting, as were the results of add-back experiments. In mice with E. coli-induced peritonitis, the infection takes about 20 h to resolve. When given at just 50 ng of MCTR per mouse, the resolution interval was reduced from ∼20 to 10 h, shortening by ∼50% the time before resolution (27). Whereas this might be considered to lead to some reduction of neutrophilic responses or immunosuppression, instead there was a statistically significant increase in E. coli phagocytosis and the killing of the bacteria by the neutrophils that were at the site of infection (102).
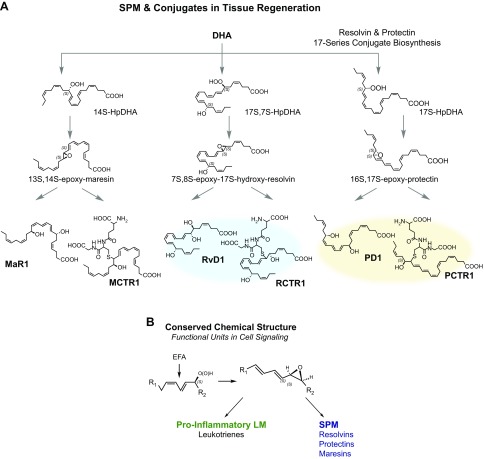
Figure 6.
A) Biosynthesis and structural elucidation of SPM conjugates in tissue regeneration. DHA is the substrate to production of epoxide intermediates in 3 separate pathways that are conjugated via glutathione-S-transferase to give novel mediators that are proresolving and potent mediators in tissue regeneration (see text for details). B) Conserved chemical structure of the epoxide intermediate. Results of the studies of tissue regeneration and resolution, when viewed together with those of earlier studies of LTs, indicate that the allylic epoxide depicted is produced via LOX reactions and abstraction of hydrogen from a hydroperoxide precursor made from 1,4-cis-penta-diene-containing essential fatty acids and a pivotal functional unit in cell signaling to produce proinflammatory LTs and the bronchoconstrictor SRS-A and SPMs that are temporally dissociated as well as cell-type specific in their function and actions.
Continued investigations along these lines permitted the elucidation of 3 of the pathways (31) depicted in Fig. 6A. Central to each pathway are epoxide intermediates that have been confirmed and prepared by total organic synthesis by Nicos Petasis (44) and Trond Hansen (103) and colleagues. The epoxide eMaR is the intermediate of MaR1, as well as the precursor of MCTR1 and MCTR2, each of which possess potent biologic actions. We also used a hind-limb ischemia model because it is an example of reflow injury to the lung and secondary injury to other organs that can be experienced in the operating room during an operation on the limbs. The resulting reflow injury is an example of acute leukocyte-mediated tissue damage of the delicate lung tissues. When treated with just 50 ng per mouse, which is a low dose compared with current anti-inflammatories (3, 5), each MCTR protects the organ from secondary injury (104).
In assigning the complete stereochemistry recently for MCTR1 and MCTR2, we identified a third member of this pathway, denoted MCTR3 (13R-cysteinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid) in collaboration with Bernd Spur and his group (105). From the original isolation, we were able to match the stereochemistry with NMR-identified material from the entire MCTR pathway (104, 105). LC-MS-MS chromatograms proved the match with synthetic compounds, an approach that we have taken for each of the SPMs. Synthetic MCTR1, MCTR2, and MCTR3 also helped to improve the isolation and LC-MS-MS chromatography and quantitation of the endogenous MCTRs. Levels produced in mouse exudates; in blood from patients with sepsis, where both MCTR2 and MCTR3 are present; and in isolated human macrophages were within those of their respective bioactions (31). We used synthetic materials to assign the double-bond geometries and the chirality of the alcohols of the bioactive products in these pathways (Fig. 6A). Investigations in planaria demonstrated that MCTR1, MCTR2, and MCTR3 are each bioactive, enhancing regeneration (105). MCTR3 possesses potent biologic actions. This rank order is reversed from that observed for the cysteinyl LT pathway, where with slow-reacting substance of anaphylaxis (SRS-A), there is a decrease in most of the biologic actions of cysteinyl LTs, with LTE4 showing the least activity in this LT series (32, 106).
What about expediting or shortening the resolution interval? MCTR1 added back at the peak of PMN infiltration in peritonitis changes the resolution interval from 12 to 10 h, MCTR2 from 12 to 8 h, and the synthetic MCTR3 from 12 to 9 h, so there is a substantial reduction in the resolution interval. With human macrophage and real-time imaging at 1 nM, MCTR3 was potent above E. coli alone or MCTR1 and MCTR2 (105). We used this system on infected mice and isolated their lymphoid tissue (e.g., spleen), and exudates from the peritonitis were isolated, and the PD conjugates (Fig. 6A), PCTR1, PCTR2, and PCTR3, were identified as bioactive (104, 105). These novel molecules enhanced tissue regeneration in planaria and stimulated macrophage phagocytosis of bacteria (105). Because the alcohol group is at the 17 position with a triene, we knew it was related to the PD family (31). These are the conjugates that are produced from the epoxide intermediate related to the PD biosynthetic family that also is involved in PD biosynthesis (Fig. 6A). This is the contributory pathway that accelerates both resolution and tissue regeneration—namely, the PD pathway. These molecules possess conjugated trienes, and the Rv pathway, which are conjugated tetraenes and are both formed from the carbon position 17-hydroperoxide intermediate. Rv conjugate in tissue regeneration (RCTR)1, RCTR2, and RCTR3 and PCTR1, PCTR2, and PCTR3 are each bioactive. Each stimulates bacterial killing via enhanced phagocytosis, stimulates efferocytosis and clearance, and promotes tissue regeneration (44, 104, 107, 108).
For an example of the biologic effect on human macrophages, PCTRs each dose dependently stimulate phagocytosis and regeneration (PCTR1, PCTR2, and PCTR3) in subnanomolar concentrations, compared with PD1. RCTR1, RCTR2, and RCTR3, in the biosynthetic pathways were the intermediates of this epoxide (Fig. 6). We proposed this intermediate from oxygen-18 incorporation studies and found that it is a precursor of PD1. The total organic synthesis confirmed that this 16,17-epoxide intermediate, 16,17-epoxy-PD, was successfully performed by Trond Hansen and his group (103). This epoxide is converted to PCTR1 by macrophages of the M2 phenotype (108).
The assigned stereochemistry of the PD pathway epoxide intermediate (Fig. 6A) is 16S,17S-epoxide and is in the trans-trans-cis double-bond geometry (22) that is converted to PCTR1 (108). The biologic actions of PCTR1 are very intriguing, in that it also stimulates E. coli clearance during infection in mouse exudates and in the spleen, enhancing macrophage biologic action in both organs, as well as the killing and clearance of E. coli. The resolution intervals with E. coli resolve with PCTR1 in this system, shortening from 21 to 9 h (>50% shortening), to accelerate resolution. This shortening of the interval is also the case for accelerated surgical wound healing—for example, in planaria with PCTR1. We tested agonist-induced activation of cytokine arrays for many cytokines and growth factors. With serum-treated zymosan as the stimulus of macrophages, PCTR (1 nM) substantially reduces TNF-α, IL-8, and IL-12 and stimulation by PCTR1 of other key regulators: leptin and RANTES, for example. The lion’s share of the M1 macrophages compared to SRS-A and LTC4, LTD4, and LTE4 and the summation of the 3 pathways just illustrated, the MCTR1, MCTR2, and MCTR3, and the conjugates from the PD and the Rv families. The M2 macrophage shows a dramatic shift where there is less LT production and a substantial increase up to ∼70% of the new proresolving conjugates (108). Three epoxide intermediates have been identified that are pivotal in producing proresolving mediators (i.e., MaR1, RvD1, PD1, and the other members of the SPM pathways; reviewed in ref. 22), as well as the new conjugates: MCTRs, RCTRs, and PCTRs (Fig. 6).
The SPMs function to actively keep the inflammatory response within physiologic boundaries to accelerate the return to tissue function. They do not block inflammation; there is no immune suppression. They facilitate the clearance of debris, and the conjugates directly promote tissue regeneration. What we have learned through these studies is that there is a conserved chemical structural unit that appears to be central as an epoxide intermediate (see Fig. 6B), derives from essential fatty acids, and is key in the biosynthesis of potent mediators across species. In the case of arachidonic acid, the proinflammatory mediators and smooth muscle contractors, the LTs, are produced from the LTA4 epoxide intermediate (32, 106). In biosynthesis from the n-3 essential fatty acids [eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and DHA], the proresolving mediators are produced (33), where each family (Rvs, PDs, and MaRs) is produced from its respective pivotal epoxide intermediates that possess this conserved chemical unit (Fig. 6B). In these recent studies, we identified the MaR conjugates, PD conjugates, and the Rv conjugates in tissue regeneration (see Figs. 4 and 5) and their main biologic functions. The stereochemistry of MCTR1, MCTR2, MCTR3, and PCTR1 (105, 108) have been established, and they are produced in mouse lymphoid tissues, in infectious inflammatory exudates, and in specific human tissues.
FAILED RESOLUTION IN HUMAN DISEASE
From the initial studies of the actions of Rvs and SPMs (8–10) and early results in LXs in humans (109), it is clear that reduced amounts of SPMs could contribute to disease pathologies. Failed mechanisms in resolution could arise from multiple factors. For example, reduced dietary intake of n-3 essential fatty acids (EPA, DHA), genetic polymorphisms in the enzymes involved in SPM biosynthesis or SPM receptors, dysfunctional SPM receptors or diminished expression, and abnormal intracellular post-SPM receptor signaling are a few components that can contribute to failed resolution, as well as drugs that are resolution toxic in animal disease models (14). In humans, new evidence is now available indicating that pathologic conditions associated with reduced SPMs can contribute to chronicity and magnitude of persistent inflammation. Impaired resolution can contribute to acute cardiovascular diseases such as atherosclerosis (110, 111). RvD1 and the ratio of SPM to LTB4 are reduced in vulnerable plaque in human carotid atherosclerotic plaques that may lead to plaque instability (112). In females, reduced ocular lymph node LXA4 correlates with an increase in the number of T-effector cells (T helpers 1 and 17), a decrease in regulatory T cells, and an increase in dry eye pathogenesis (96). Sex differences in resolution mechanism in inflammation have also been observed in humans, where healthy females, on skin challenge, produced higher levels of D-Rvs, which are associated with accelerated resolution than did males (113). These results suggest that specific SPMs may prevent autoimmunity (96, 113) and link resolution to T-cell responses (99). In obese women, supplementation with n-3 PUFAs increases systemic Rvs and upregulation of Rv receptors (79), suggesting that failed resolution can be rescued in humans. It appears that sex differences in SPMs are age, gender, and organ site dependent. Hence, further human studies are needed to appreciate the organ specificity of SPM function.
NEW MEDIATORS AND PHARMACOLOGIC AGENTS TARGETING RESOLUTION
As there are hundreds of endogenous mediators that are involved in the initiation of acute inflammation, there are probably hundreds of molecules that orchestrate the resolution response. Along with lipid mediators, peptides, proteins, and gases, stimulate resolution. For example, PGE2 and PGD2, in addition to their roles in initiation, have precise roles in activating the production of proresolving mediators and resolution of leukocyte traffic by stimulating expression of human 15-LOX type I (20, 34). Glucocorticoids stimulate efferocytosis by macrophages (13) and regulate expression and function of annexin A1. Annexin A1 increases neutrophil apoptosis, blocks endothelial adhesion and transmigration of the cells, and stimulates the macrophage phagocytosis of neutrophils (114, 115)—all activities in resolution that are stimulated by binding and signaling via the LXA4 receptor ALX. Specific miRNAs mediate the actions of SPMs in resolution (116). In human macrophages, miRNA-181b regulates ALX/formyl-peptide receptor (FPR)-2 receptor-evoked resolution responses (117). Erythropoietin promotes resolution, shortening the resolution interval in vivo in mice (118), and several candidate proresolver proteins are identified (27). Endogenous gases, such as H2S and CO, have a role in resolution. H2S promotes resolution of inflammation (119) via actions on microbiota and mucosal barriers (120). Inhaled low-dose CO protects against acute lung injury, reduces proinflammatory mediator production (121), and shifts to SPM production in baboons (122), shortening the resolution of pneumonia.
Novel hybrid compounds of cyclin-dependent kinase inhibitors and NO that enhance apoptosis of neutrophils and are proresolving, such as NO-releasing R-roscovitine hybrid derivatives, possess proresolution properties (123), giving promise for new therapeutics as proresolving agents. Also, inhibitors of LT hydrolase (LTA4) block LTB4 production and stimulate LXA4 biosynthesis via LTA4 conversion to LXA4 (Fig. 1) and show superior therapeutic action over inhibitors of 5-LOX and 5-LOX-activating protein (FLAP) to promote an LX-mediated resolution (124). Identification of novel members of the resolution mediators can provide unique opportunities to address inflammation and infection in many diseases, particularly lung (125–127), diabetes, renal (128), and neurodegenerative diseases; brain injuries (129–131; and vascular diseases (110, 112, 132, 133) that may be caused by failed resolution mechanisms.
CONCLUSIONS
It is clear that dietary supplements containing the n-3 fatty acids, precursors to the Rvs, PDs and MaRs, are not substitutes for prescription drugs (134). There is considerable variation in the quantities and quality of many of the over-the-counter products, some containing other lipids and auto-oxidation products that may cancel the benefits of increasing the availability of resolution pathway substrates. Nonetheless, increasing n-3 fatty acid supplementation can increase Rvs in peripheral blood (79, 81) and in arthritic joints (76), where they can exert beneficial actions, whereas other organs do not appear to be compartments readily able to increase substrate availability, such as human placenta (77). Hence, the dose relationships between oral n-3 fatty acid supplementation and target organ uptake and conversion to specific Rv and other SPM pathways remain to be rigorously investigated.
We have learned much from surgical injury-initiated tissue regeneration in the primordial planaria. In E. coli infectious exudates there are chemical signals, such as Rvs and SPMs, that are mediators of resolution responses in vivo in preclinical models (Table 2), as well as in human milk (74) or in human macrophages that help to regulate the inflammatory response and directly impact tissue regeneration and resolution in primitive model organisms (31, 135). Given that SPMs are agonists of resolution, it appears that they do not evoke unwanted side effects, such as immunosuppression. Hence, the concept of using agonists to stimulate natural resolution circuits and programs is worthy of rigorous testing in human indications, either standing alone or in combination with other traditional therapies for a given outcome. For example, specific SPMs, together with antibiotics lower the amounts of antibiotics needed to clear infections (50) and possibly reduce the potential for increasing bacterial antibiotic resistance by lowering the amounts of antibiotic exposure, which may also reduce viral burden (136). SPMs may also help to increase the effectiveness of adjuvants and antibody production (100) and may normalize differentially expressed proresolution pathways in humans (137). The available evidence from extensive preclinical animal models, human SPM production in vivo, and the limited results of randomized clinical trials in humans suggest that it is indeed time to consider stimulating resolution in the 21st century as a new therapeutic direction for managing unwanted excessive inflammation and infection. Proresolution pharmacology can enhance the host innate response to expedite microbial clearance, limit collateral tissue damage, and stimulate tissue regeneration by enhancing endogenous resolution mechanisms that are programmed into the resolving exudates of the acute inflammatory response.
ACKNOWLEDGMENTS
The author thanks Mary Halm Small (Brigham and Women's Hospital) for expert assistance in manuscript preparation. Research in the C.N.S. laboratory was supported, in part, by grants from the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences (R01GM38765, P01GM095467) and NIH National Institute of Dental and Craniofacial Research (R01DE025020). The author declares no conflict of interest.
Glossary
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- HDHA
hydroxy-docosahexaenoic acid
- LC-MS-MS
liquid chromatography–tandem mass spectrometry
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- MaR
maresin
- MCTR
maresin conjugate in tissue regeneration
- NPD1
neuroprotectin D1
- NSAID
nonsteroidal anti-inflammatory drug
- PAF
platelet activating factor
- PCTR
protectin conjugate in tissue regeneration
- PD
protectin
- PD1
protectin D1
- PG
prostaglandin
- PMN
polymorphonuclear neutrophil
- PUFA
polyunsaturated fatty acid
- RCTR
resolvin conjugate in tissue regeneration
- Rv
resolvin
- SPM
specialized proresolving mediator
- SRS-A
slow-reacting substance of anaphylaxis
AUTHOR CONTRIBUTIONS
C. N. Serhan conceived of and wrote the paper.
REFERENCES
- 1.Majno G., Joris I. (2004) Cells, Tissues, and Disease: Principles of General Pathology, Oxford University Press, New York [Google Scholar]
- 2.Flower R. J. (1985) Cyclooxygenase inhibitors: an overview. In Prostaglandins, Leukotrienes and Lipoxins: Biochemistry, Mechanism of Action, and Clinical Applications (Bailey J. M., ed.), pp. 583–591, Plenum, New York: [Google Scholar]
- 3.Koopman W. J., Moreland L. W., eds. (2005) Arthritis and Allied Conditions: A Textbook of Rheumatology, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 4.Griffiths R. J. (1999) Prostaglandins and inflammation. In Inflammation: Basic Principles and Clinical Correlates (Gallin J. I., Snyderman R., eds.), pp. 349–360, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 5.Robbins S. L., Cotran R. (1979) Pathologic Basis of Disease, W.B. Saunders Co., Philadelphia [Google Scholar]
- 6.Henson P. M. (1991) Resolution of inflammation: a perspective. Chest 99(Suppl), 2S–6S [PubMed] [Google Scholar]
- 7.Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. (1989) Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R.-L. (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong S., Gronert K., Devchand P. R., Moussignac R.-L., Serhan C. N. (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278, 14677–14687 [DOI] [PubMed] [Google Scholar]
- 11.Serhan C. N. (2011) The resolution of inflammation: the devil in the flask and in the details. FASEB J. 25, 1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilroy D. W., Lawrence T., Perretti M., Rossi A. G. (2004) Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 [DOI] [PubMed] [Google Scholar]
- 14.Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O’Neill L. A. J., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones H. R., Robb C. T., Perretti M., Rossi A. G. (2016) The role of neutrophils in inflammation resolution. Semin. Immunol. 28, 137–145 [DOI] [PubMed] [Google Scholar]
- 16.Perretti M. (2015) The resolution of inflammation: new mechanisms in patho-physiology open opportunities for pharmacology. Semin. Immunol. 27, 145–148 [DOI] [PubMed] [Google Scholar]
- 17.Robb C. T., Regan K. H., Dorward D. A., Rossi A. G. (2016) Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 38, 425–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabas I., Glass C. K. (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullerton J. N., Gilroy D. W. (2016) Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 [DOI] [PubMed] [Google Scholar]
- 20.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spite M., Clària J., Serhan C. N. (2014) Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19, 21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan C. N., Dalli J., Colas R. A., Winkler J. W., Chiang N. (2015) Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 1851, 397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan C. N., Chiang N., Dalli J. (2015) The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin. Immunol. 27, 200–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinarello C. A. (2010) Anti-inflammatory agents: present and future. Cell 140, 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan C. (2012) Fresh approaches to anti-infective therapies. Sci. Transl. Med. 4, 140sr2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winyard P. G., Willoughby D. A., eds (2003) Inflammation Protocols, Humana, Totowa, NJ, USA: [Google Scholar]
- 27.Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., Serhan C. N. (2005) Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 [DOI] [PubMed] [Google Scholar]
- 28.Van Dyke T. E. (2008) The management of inflammation in periodontal disease. J. Periodontol. 79(Suppl), 1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan C. N., Jain A., Marleau S., Clish C., Kantarci A., Behbehani B., Colgan S. P., Stahl G. L., Merched A., Petasis N. A., Chan L., Van Dyke T. E. (2003) Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 171, 6856–6865 [DOI] [PubMed] [Google Scholar]
- 30. Van Dyke, T. E., Serhan, C. N. (2006) A novel approach to resolving inflammation. Sci. Am. Oral and Whole Body Health 2006, 42–45. [Google Scholar]
- 31.Dalli J., Ramon S., Norris P. C., Colas R. A., Serhan C. N. (2015) Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J. 29, 2120–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuelsson B. (1983) Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220, 568–575 [DOI] [PubMed] [Google Scholar]
- 33.Dalli J., Chiang N., Serhan C. N. (2015) Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 35.Norris P. C., Gosselin D., Reichart D., Glass C. K., Dennis E. A. (2014) Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc. Natl. Acad. Sci. USA 111, 12746–12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddox J. F., Serhan C. N. (1996) Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J. Exp. Med. 183, 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones C. N., Dalli J., Dimisko L., Wong E., Serhan C. N., Irimia D. (2012) Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc. Natl. Acad. Sci. USA 109, 20560–20565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godson C., Mitchell S., Harvey K., Petasis N. A., Hogg N., Brady H. R. (2000) Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 [DOI] [PubMed] [Google Scholar]
- 39.Börgeson E., McGillicuddy F. C., Harford K. A., Corrigan N., Higgins D. F., Maderna P., Roche H. M., Godson C. (2012) Lipoxin A4 attenuates adipose inflammation. FASEB J. 26, 4287–4294 [DOI] [PubMed] [Google Scholar]
- 40.Martins V., Valença S. S., Farias-Filho F. A., Molinaro R., Simões R. L., Ferreira T. P. T., e Silva P. M., Hogaboam C. M., Kunkel S. L., Fierro I. M., Canetti C., Benjamim C. F. (2009) ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J. Immunol. 182, 5374–5381 [DOI] [PubMed] [Google Scholar]
- 41.Qiu W., Guo K., Yi L., Gong Y., Huang L., Zhong W. (2014) Resolvin E1 reduces hepatic fibrosis in mice with Schistosoma japonicum infection. Exp. Ther. Med. 7, 1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu X., Zhang X., Yao J., Song J., Nikolic-Paterson D. J., Li J. (2012) Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 228, 506–519 [DOI] [PubMed] [Google Scholar]
- 43.Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. (2009) Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 206, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalli J., Zhu M., Vlasenko N. A., Deng B., Haeggström J. Z., Petasis N. A., Serhan C. N. (2013) The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 27, 2573–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan C. N., Dalli J., Karamnov S., Choi A., Park C. K., Xu Z. Z., Ji R. R., Zhu M., Petasis N. A. (2012) Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26, 1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy B. D., Serhan C. N. (2014) Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 76, 27.21–27.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab J. M., Chiang N., Arita M., Serhan C. N. (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan M. M.-Y., Moore A. R. (2010) Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 184, 6418–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 50.Chiang N., Fredman G., Bäckhed F., Oh S. F., Vickery T., Schmidt B. A., Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haas-Stapleton E. J., Lu Y., Hong S., Arita M., Favoreto S., Nigam S., Serhan C. N., Agabian N. (2007) Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One 2, e1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N. A., Serhan C. N. (2005) Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201, 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. (2011) Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 121, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh S. F., Dona M., Fredman G., Krishnamoorthy S., Irimia D., Serhan C. N. (2012) Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 188, 4527–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isobe Y., Arita M., Matsueda S., Iwamoto R., Fujihara T., Nakanishi H., Taguchi R., Masuda K., Sasaki K., Urabe D., Inoue M., Arai H. (2012) Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 287, 10525–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lands W. E. M. (2005) Fish, Omega-3 and Human Health, AOCS Press, Champaign, IL, USA [Google Scholar]
- 57.Bracco U., Deckelbaum R. J., eds. (1992) Polyunsaturated Fatty Acids in Human Nutrition, Raven Press, New York [Google Scholar]
- 58.Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukherjee P. K., Marcheselli V. L., Serhan C. N., Bazan N. G. (2004) Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 101, 8491–8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell E. L., Serhan C. N., Colgan S. P. (2011) Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J. Immunol. 187, 3475–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rius B., Titos E., Morán-Salvador E., López-Vicario C., García-Alonso V., González-Périz A., Arroyo V., Clària J. (2014) Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 28, 836–848 [DOI] [PubMed] [Google Scholar]
- 62.Serhan C. N., Chiang N. (2013) Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 13, 632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiang N., Dalli J., Colas R. A., Serhan C. N. (2015) Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 212, 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooray S. N., Gobbetti T., Montero-Melendez T., McArthur S., Thompson D., Clark A. J., Flower R. J., Perretti M. (2013) Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 110, 18232–18237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filep J. G. (2013) Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc. Natl. Acad. Sci. USA 110, 18033–18034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.-H., Yang R., Petasis N. A., Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norling L. V., Dalli J., Flower R. J., Serhan C. N., Perretti M. (2012) Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler. Thromb. Vasc. Biol. 32, 1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devchand P. R., Arita M., Hong S., Bannenberg G., Moussignac R.-L., Gronert K., Serhan C. N. (2003) Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 17, 652–659 [DOI] [PubMed] [Google Scholar]
- 69.Chiang N., Shinohara M., Dalli J., Mirakaj V., Kibi M., Choi A. M. K., Serhan C. N. (2013) Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J. Immunol. 190, 6378–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biteman B., Hassan I. R., Walker E., Leedom A. J., Dunn M., Seta F., Laniado-Schwartzman M., Gronert K. (2007) Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 21, 2257–2266 [DOI] [PubMed] [Google Scholar]
- 71.Jin S.-W., Zhang L., Lian Q.-Q., Liu D., Wu P., Yao S.-L., Ye D.-Y. (2007) Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth. Analg. 104, 369–377 [DOI] [PubMed] [Google Scholar]
- 72.Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N. (2014) Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalli J., Colas R. A., Quintana C., Barragan-Bradford D., Hurwitz S., Levy B. D., Choi A. M., Serhan C. N., Baron R. M. (2017) Human sepsis eicosanoid and pro-resolving lipid mediator temporal profiles: correlations with survival and clinical outcomes. Crit. Care Med. 45, 58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnardottir H., Orr S. K., Dalli J., Serhan C. N. (2016) Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 9, 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norling L. V., Headland S. E., Dalli J., Arnardottir H. H., Haworth O., Jones H. R., Irimia D., Serhan C. N., Perretti M. (2016) Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 1, e85922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barden A. E., Moghaddami M., Mas E., Phillips M., Cleland L. G., Mori T. A. (2016) Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids 107, 24–29 [DOI] [PubMed] [Google Scholar]
- 77.Keelan J. A., Mas E., D’Vaz N., Dunstan J. A., Li S., Barden A. E., Mark P. J., Waddell B. J., Prescott S. L., Mori T. A. (2015) Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 149, 171–178 [DOI] [PubMed] [Google Scholar]
- 78.Jones M. L., Mark P. J., Keelan J. A., Barden A., Mas E., Mori T. A., Waddell B. J. (2013) Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J. Lipid Res. 54, 2247–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polus A., Zapala B., Razny U., Gielicz A., Kiec-Wilk B., Malczewska-Malec M., Sanak M., Childs C. E., Calder P. C., Dembinska-Kiec A. (2016) Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim. Biophys. Acta 1861, 1746–1755 [DOI] [PubMed] [Google Scholar]
- 80.Mas E., Barden A., Burke V., Beilin L. J., Watts G. F., Huang R. C., Puddey I. B., Irish A. B., Mori T. A. (2015) A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin. Nutr.35, 331–336 [DOI] [PubMed] [Google Scholar]
- 81.Barden A. E., Mas E., Mori T. A. (2016) n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr. Opin. Lipidol. 27, 26–32 [DOI] [PubMed] [Google Scholar]
- 82.Le Faouder P., Baillif V., Spreadbury I., Motta J. P., Rousset P., Chêne G., Guigné C., Tercé F., Vanner S., Vergnolle N., Bertrand-Michel J., Dubourdeau M., Cenac N. (2013) LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 932, 123–133 [DOI] [PubMed] [Google Scholar]
- 83.Elajami T. K., Colas R. A., Dalli J., Chiang N., Serhan C. N., Welty F. K. (2016) Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 30, 2792–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun Y.-P., Oh S. F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S. P., Petasis N. A., Serhan C. N. (2007) Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 282, 9323–9334 [DOI] [PubMed] [Google Scholar]
- 85.Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., Serhan C. N. (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 181, 8677–8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leslie M. (2015) Inflammation’s stop signals. Science 347, 18–21 [DOI] [PubMed] [Google Scholar]
- 87. (2009) Resolvyx announces positive data from Phase 2 clinical trial of the reesolvin RX-10045 in patients with dry eye syndrome. Business Wire Retrieved January 9, 2017, from http://www.businesswire.com/news/home/20090824005320/en/resolvyx-announces-positive-data-phase-20090824005322-clinical.
- 88.De Paiva C. S., Schwartz C. E., Gjörstrup P., Pflugfelder S. C. (2012) Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea 31, 1299–1303 [DOI] [PubMed] [Google Scholar]
- 89.Gronert K. (2010) Resolution, the grail for healthy ocular inflammation. Exp. Eye Res. 91, 478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li N., He J., Schwartz C. E., Gjorstrup P., Bazan H. E. P. (2010) Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 26, 431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erdinest N., Ovadia H., Kormas R., Solomon A. (2014) Anti-inflammatory effects of resolvin-D1 on human corneal epithelial cells: in vitro study. J. Inflamm. (Lond.) 11, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li D., Hodges R. R., Jiao J., Carozza R. B., Shatos M. A., Chiang N., Serhan C. N., Dartt D. A. (2013) Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 6, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hodges R. R., Li D., Shatos M. A., Bair J. A., Lippestad M., Serhan C. N., Dartt D. A. (2016) Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. doi: 10.1038/mi.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dartt D. A., Hodges R. R., Li D., Shatos M. A., Lashkari K., Serhan C. N. (2011) Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J. Immunol. 186, 4455–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cortina M. S., Bazan H. E. (2011) Docosahexaenoic acid, protectins and dry eye. Curr. Opin. Clin. Nutr. Metab. Care 14, 132–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao Y., Min K., Zhang Y., Su J., Greenwood M., Gronert K. (2015) Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J. Immunol. 195, 3086–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S. B., Hu K. M., Seamon K. J., Mani V., Chen Y., Gronert K. (2012) Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 26, 1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu S. H., Chen X. Q., Liu B., Wu H. J., Dong L. (2013) Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br. J. Dermatol. 168, 172–178 [DOI] [PubMed] [Google Scholar]
- 99.Chiurchiù V., Leuti A., Dalli J., Jacobsson A., Battistini L., Maccarrone M., Serhan C. N. (2016) Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 8, 353ra111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramon S., Gao F., Serhan C. N., Phipps R. P. (2012) Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189, 1036–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Russell C. D., Schwarze J. (2014) The role of pro-resolution lipid mediators in infectious disease. Immunology 141, 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dalli J., Chiang N., Serhan C. N. (2014) Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. USA 111, E4753–E4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aursnes M., Tungen J. E., Colas R. A., Vlasakov I., Dalli J., Serhan C. N., Hansen T. V. (2015) Synthesis of the 16S,17S-epoxyprotectin intermediate in the biosynthesis of protectins by human macrophages. J. Nat. Prod. 78, 2924–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dalli J., Vlasakov I., Riley I. R., Rodriguez A. R., Spur B. W., Petasis N. A., Chiang N., Serhan C. N. (2016) Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 113, 12232–12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dalli J., Sanger J. M., Rodriguez A. R., Chiang N., Spur B. W., Serhan C. N. (2016) Identification and actions of a novel third maresin conjugate in tissue regeneration: MCTR3. PLoS One 11, e0149319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. (1987) Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237, 1171–1176 [DOI] [PubMed] [Google Scholar]
- 107.Primdahl K. G., Aursnes M., Walker M. E., Colas R. A., Serhan C. N., Dalli J., Hansen T. V., Vik A. (2016) Synthesis of 13(R)-hydroxy-7Z,10Z,13R,14E,16Z,19Z docosapentaenoic acid (13R-HDPA) and its biosynthetic conversion to the 13-series resolvins. J. Nat. Prod. 79, 2693–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramon S., Dalli J., Sanger J. M., Winkler J. W., Aursnes M., Tungen J. E., Hansen T. V., Serhan C. N. (2016) The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am. J. Pathol. 186, 962–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brezinski D. A., Nesto R. W., Serhan C. N. (1992) Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation 86, 56–63 [DOI] [PubMed] [Google Scholar]
- 110.Ho K. J., Spite M., Owens C. D., Lancero H., Kroemer A. H., Pande R., Creager M. A., Serhan C. N., Conte M. S. (2010) Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 177, 2116–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Libby P., Tabas I., Fredman G., Fisher E. A. (2014) Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 114, 1867–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fredman G., Hellmann J., Proto J. D., Kuriakose G., Colas R. A., Dorweiler B., Connolly E. S., Solomon R., Jones D. M., Heyer E. J., Spite M., Tabas I. (2016) An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rathod K. S., Kapil V., Velmurugan S., Khambata R. S., Siddique U., Khan S., Van Eijl S., Gee L. C., Bansal J., Pitrola K., Shaw C., D’Acquisto F., Colas R. A., Marelli-Berg F., Dalli J., Ahluwalia A. (2017) Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Invest. 127, 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perretti M., D’Acquisto F. (2009) Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 [DOI] [PubMed] [Google Scholar]
- 115.Perretti M., Leroy X., Bland E. J., Montero-Melendez T. (2015) Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 36, 737–755 [DOI] [PubMed] [Google Scholar]
- 116.Recchiuti A., Serhan C. N. (2012) Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front. Immunol. 3, 298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pierdomenico A. M., Recchiuti A., Simiele F., Codagnone M., Mari V. C., Davì G., Romano M. (2015) MicroRNA-181b regulates ALX/FPR2 receptor expression and proresolution signaling in human macrophages. J. Biol. Chem. 290, 3592–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luo B., Wang J., Liu Z., Shen Z., Shi R., Liu Y. Q., Liu Y., Jiang M., Wu Y., Zhang Z. (2016) Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat. Commun. 7, 12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flannigan K. L., Agbor T. A., Motta J. P., Ferraz J. G., Wang R., Buret A. G., Wallace J. L. (2015) Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 29, 1591–1602 [DOI] [PubMed] [Google Scholar]
- 120.Motta J. P., Flannigan K. L., Agbor T. A., Beatty J. K., Blackler R. W., Workentine M. L., Da Silva G. J., Wang R., Buret A. G., Wallace J. L. (2015) Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm. Bowel Dis. 21, 1006–1017 [DOI] [PubMed] [Google Scholar]
- 121.Shinohara M., Kibi M., Riley I. R., Chiang N., Dalli J., Kraft B. D., Piantadosi C. A., Choi A. M., Serhan C. N. (2014) Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L746–L757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dalli J., Kraft B. D., Colas R. A., Shinohara M., Fredenburgh L. E., Hess D. R., Chiang N., Welty-Wolf K., Choi A. M., Piantadosi C. A., Serhan C. N. (2015) The regulation of proresolving lipid mediator profiles in baboon pneumonia by inhaled carbon monoxide. Am. J. Respir. Cell Mol. Biol. 53, 314–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Montanaro G., Bertinaria M., Rolando B., Fruttero R., Lucas C. D., Dorward D. A., Rossi A. G., Megson I. L., Gasco A. (2013) Novel R-roscovitine NO-donor hybrid compounds as potential pro-resolution of inflammation agents. Bioorg. Med. Chem. 21, 2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rao N. L., Dunford P. J., Xue X., Jiang X., Lundeen K. A., Coles F., Riley J. P., Williams K. N., Grice C. A., Edwards J. P., Karlsson L., Fourie A. M. (2007) Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor in comparison with the 5-lipoxygenase inhibitor zileuton. J. Pharmacol. Exp. Ther. 321, 1154–1160 [DOI] [PubMed] [Google Scholar]
- 125.Basil M. C., Levy B. D. (2016) Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 16, 51–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.El Kebir D., Gjorstrup P., Filep J. G. (2012) Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA 109, 14983–14988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Felton J. M., Lucas C. D., Rossi A. G., Dransfield I. (2014) Eosinophils in the lung: modulating apoptosis and efferocytosis in airway inflammation. Front. Immunol. 5, 302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Börgeson E., Godson C. (2012) Resolution of inflammation: therapeutic potential of pro-resolving lipids in type 2 diabetes mellitus and associated renal complications. Front. Immunol. 3, 318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu M., Wang X., Schultzberg M., Hjorth E. (2015) Differential regulation of resolution in inflammation induced by amyloid-β42 and lipopolysaccharides in human microglia. J. Alzheimers Dis. 43, 1237–1250 [DOI] [PubMed] [Google Scholar]
- 130.Harrison J. L., Rowe R. K., Ellis T. W., Yee N. S., O’Hara B. F., Adelson P. D., Lifshitz J. (2015) Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav. Immun. 47, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu G., Fiala M., Mizwicki M. T., Sayre J., Magpantay L., Siani A., Mahanian M., Chattopadhyay M., La Cava A., Wiedau-Pazos M. (2012) Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am. J. Neurodegener. Dis. 1, 60–74 [PMC free article] [PubMed] [Google Scholar]
- 132.Hasturk H., Abdallah R., Kantarci A., Nguyen D., Giordano N., Hamilton J., Van Dyke T. E. (2015) Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler. Thromb. Vasc. Biol. 35, 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kang J. W., Lee S. M. (2016) Resolvin D1 protects the liver from ischemia/reperfusion injury by enhancing M2 macrophage polarization and efferocytosis. Biochim. Biophys. Acta 1861(9 Pt A), 1025–1035 [DOI] [PubMed] [Google Scholar]
- 134.Fialkow J. (2016) Omega-3 fatty acid formulations in cardiovascular disease: dietary supplements are not substitutes for prescription products. Am. J. Cardiovasc. Drugs 16, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dalli J., Serhan C. N. (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Imai Y. (2015) Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim. Biophys. Acta 1851, 496–502 [DOI] [PubMed] [Google Scholar]
- 137.Morris T., Stables M., Colville-Nash P., Newson J., Bellingan G., de Souza P. M., Gilroy D. W. (2010) Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. USA 107, 8842–8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Herrera B. S., Hasturk H., Kantarci A., Freire M. O., Nguyen O., Kansal S., Van Dyke T. E. (2015) Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect. Immun. 83, 792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saito T., Hasegawa-Moriyama M., Kurimoto T., Yamada T., Inada E., Kanmura Y. (2015) Resolution of inflammation by resolvin D1 is essential for peroxisome proliferator-activated receptor-γ-mediated analgesia during postincisional pain development in type 2 diabetes. Anesthesiology 123, 1420–1434 [DOI] [PubMed] [Google Scholar]
- 140.Shevalye H., Yorek M. S., Coppey L. J., Holmes A., Harper M. M., Kardon R. H., Yorek M. A. (2015) Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J. Neurophysiol. 114, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang Y., Zhang M. J., Hellmann J., Kosuri M., Bhatnagar A., Spite M. (2013) Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 62, 618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hellmann J., Tang Y., Kosuri M., Bhatnagar A., Spite M. (2011) Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 25, 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hong S., Tian H., Lu Y., Laborde J. M., Muhale F. A., Wang Q., Alapure B. V., Serhan C. N., Bazan N. G. (2014) Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol. Cell Physiol. 307, C1058–C1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White P. J., St-Pierre P., Charbonneau A., Mitchell P. L., St-Amand E., Marcotte B., Marette A. (2014) Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat. Med. 20, 664–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Salic K., Morrison M. C., Verschuren L., Wielinga P. Y., Wu L., Kleemann R., Gjorstrup P., Kooistra T. (2016) Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 250, 158–165 [DOI] [PubMed] [Google Scholar]
- 146.Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22, 3595–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Viola J. R., Lemnitzer P., Jansen Y., Csaba G., Winter C., Neideck C., Silvestre-Roig C., Dittmar G., Döring Y., Drechsler M., Weber C., Zimmer R., Cenac N., Soehnlein O. (2016) Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 119, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 148.Hiram R., Rizcallah E., Sirois C., Sirois M., Morin C., Fortin S., Rousseau E. (2014) Resolvin D1 reverses reactivity and Ca2+ sensitivity induced by ET-1, TNF-α, and IL-6 in the human pulmonary artery. Am. J. Physiol. Heart Circ. Physiol. 307, H1547–H1558 [DOI] [PubMed] [Google Scholar]
- 149.Clària J., Dalli J., Yacoubian S., Gao F., Serhan C. N. (2012) Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 189, 2597–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Titos E., Rius B., González-Périz A., López-Vicario C., Morán-Salvador E., Martínez-Clemente M., Arroyo V., Clària J. (2011) Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187, 5408–5418 [DOI] [PubMed] [Google Scholar]
- 151.González-Périz A., Horrillo R., Ferré N., Gronert K., Dong B., Morán-Salvador E., Titos E., Martínez-Clemente M., López-Parra M., Arroyo V., Clària J. (2009) Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 23, 1946–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neuhofer A., Zeyda M., Mascher D., Itariu B. K., Murano I., Leitner L., Hochbrugger E. E., Fraisl P., Cinti S., Serhan C. N., Stulnig T. M. (2013) Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 62, 1945–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sasaki A., Fukuda H., Shiida N., Tanaka N., Furugen A., Ogura J., Shuto S., Mano N., Yamaguchi H. (2015) Determination of ω-6 and ω-3 PUFA metabolites in human urine samples using UPLC/MS/MS (published correction in J. Immunol. 407, 2939–2940). Anal. Bioanal. Chem. 407, 1625–1639 [DOI] [PubMed] [Google Scholar]
- 154.Mas E., Croft K. D., Zahra P., Barden A., Mori T. A. (2012) Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 58, 1476–1484 [DOI] [PubMed] [Google Scholar]
- 155.Bouatra S., Aziat F., Mandal R., Guo A. C., Wilson M. R., Knox C., Bjorndahl T. C., Krishnamurthy R., Saleem F., Liu P., Dame Z. T., Poelzer J., Huynh J., Yallou F. S., Psychogios N., Dong E., Bogumil R., Roehring C., Wishart D. S. (2013) The human urine metabolome. PLoS One 8, e73076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Markworth J. F., Vella L., Lingard B. S., Tull D. L., Rupasinghe T. W., Sinclair A. J., Maddipati K. R., Cameron-Smith D. (2013) Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R1281–R1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weiss G. A., Troxler H., Klinke G., Rogler D., Braegger C., Hersberger M. (2013) High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 12, 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang J., Eiserich J. P., Cross C. E., Morrissey B. M., Hammock B. D. (2012) Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic. Biol. Med. 53, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang X., Zhu M., Hjorth E., Cortés-Toro V., Eyjolfsdottir H., Graff C., Nennesmo I., Palmblad J., Eriksdotter M., Sambamurti K., Fitzgerald J. M., Serhan C. N., Granholm A. C., Schultzberg M. (2015) Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 11, 40–50.e1–e.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frediani J. K., Jones D. P., Tukvadze N., Uppal K., Sanikidze E., Kipiani M., Tran V. T., Hebbar G., Walker D. I., Kempker R. R., Kurani S. S., Colas R. A., Dalli J., Tangpricha V., Serhan C. N., Blumberg H. M., Ziegler T. R. (2014) Plasma metabolomics in human pulmonary tuberculosis disease: a pilot study. PLoS One 9, e108854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arnardottir H. H., Dalli J., Norling L. V., Colas R. A., Perretti M., Serhan C. N. (2016) Resolvin D3 is dysregulated in arthritis and reduces arthritic inflammation. J. Immunol. 197, 2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mozurkewich E. L., Greenwood M., Clinton C., Berman D., Romero V., Djuric Z., Qualls C., Gronert K. (2016) Pathway markers for pro-resolving lipid mediators in maternal and umbilical cord blood: a secondary analysis of the Mothers, Omega-3, and Mental Health Study. Front. Pharmacol. 7, 274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Winkler J. W., Orr S. K., Dalli J., Cheng C. Y., Sanger J. M., Chiang N., Petasis N. A., Serhan C. N. (2016) Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 6, 18972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tungen J. E., Aursnes M., Vik A., Ramon S., Colas R. A., Dalli J., Serhan C. N., Hansen T. V. (2014) Synthesis and anti-inflammatory and pro-resolving activities of 22-OH-PD1, a monohydroxylated metabolite of protectin D1. J. Nat. Prod. 77, 2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bazan N. G., Eady T. N., Khoutorova L., Atkins K. D., Hong S., Lu Y., Zhang C., Jun B., Obenaus A., Fredman G., Zhu M., Winkler J. W., Petasis N. A., Serhan C. N., Belayev L. (2012) Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp. Neurol. 236, 122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Serhan C. N., Fredman G., Yang R., Karamnov S., Belayev L. S., Bazan N. G., Zhu M., Winkler J. W., Petasis N. A. (2011) Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 18, 976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tabas I. (2016) Heart disease: death-defying plaque cells. Nature 536, 32–33 [DOI] [PMC free article] [PubMed] [Google Scholar]