Abstract
Propofol is an intravenous anesthetic that produces its anesthetic effect, largely via the GABAA receptor in the CNS, and also reduces the N-formyl-methionyl-leucyl-phenylalanine (fMLP)-induced neutrophil respiratory burst. Because fMLP-stimulated neutrophils produce leukotriene (LT)B4, we examined the effect of propofol on LTB4 production in vivo and in vitro. Cecal ligation and puncture surgery was performed in mice, with or without exposure to propofol. Propofol attenuated the production of 5-lipoxygenase (5-LOX)-related arachidonic acid (AA) derivatives in the peritoneal fluid. Also, in the in vitro experiments on fMLP-stimulated neutrophils and 5-LOX-transfected human embryonic kidney cells, propofol attenuated the production of 5-LOX-related AA derivatives. Based on these results, we hypothesized that propofol would directly affect 5-LOX function. Using meta-azi-propofol (AziPm), we photolabeled stable 5-LOX protein, which had been used to solve the X-ray crystallographic structure of 5-LOX, and examined the binding site(s) of propofol on 5-LOX. Two propofol binding pockets were identified near the active site of 5-LOX. Alanine scanning mutagenesis was performed for the residues of 5-LOX in the vicinity of propofol, and we evaluated the functional role of these pockets in LTB4 production. We demonstrated that these pockets were functionally important for 5-LOX activity. These two pockets can be used to explore a novel 5-LOX inhibitor in the future.—Okuno, T., Koutsogiannaki, S., Ohba, M., Chamberlain, M., Bu, W., Lin, F.-Y., Eckenhoff, R. G., Yokomizo T., Yuki, K. Intravenous anesthetic propofol binds to 5-lipoxygenase and attenuates leukotriene B4 production.
Keywords: anesthesia, lipid mediator, inhibitor
Leukotriene (LT)B4 is one of the major neutrophil and macrophage chemoattractants. LTB4 is categorized as a secondary chemoattractant and acts as a signal relay molecule for primary chemoattractants such as formyl peptides during neutrophil chemotaxis (1). LTB4 produces its effect mainly through a GPCR called LTB4 receptor (BLT)-1, which is highly expressed in neutrophils and macrophages (2, 3). LTB4 is a breakdown product of arachidonic acid (AA). AA is converted to LTB4 via 5-lipoxygenase (5-LOX) and LTA4 hydrolase (LTA4-H) (Supplemental Fig. 1). The expression of 5-LOX is largely limited to neutrophils and macrophages, whereas LTA4-H is ubiquitously expressed (4, 5). Thus, the production of LTB4 is confined to neutrophils and macrophages.
5-LOX consists of the C2-like domain (residues 1–112) and the catalytic domain (residues 126–673) (6). The catalytic domain contains a catalytic iron. The α2 helix in the catalytic domain is located at the edge of the active site and is proposed to work as a mobile lid to control substrate access to the active site (7). The involvement of 5-LOX is implicated in various disease states (8), and the development of 5-LOX inhibitors has been a target of interest. However, zileuton, the only 5-LOX inhibitor available for clinical use, has an effect nonspecific to 5-LOX (9). The X-ray crystallographic structure of 5-LOX was not available for a long time, which had hindered in silico based quest for 5-LOX-specific inhibitors.
Anesthetic drugs have been administered in various scenarios primarily to affect the CNS. A growing body of literature suggests that anesthetics also affect immune cells. Propofol is a commonly used intravenous anesthetic that works mainly via the GABAA receptor in the CNS (10) and is widely used in various clinical settings. It is used as part anesthesia induction and maintenance drugs in operating rooms and is also administered as a sedative in the intensive care unit, commonly infused continuously. It may be administered to critically ill patients for a prolonged duration, and understanding the impact of propofol on our immune system is important. Studies have shown that propofol attenuates the N-formyl-methionyl-leucyl-phenylalanine (fMLP)–induced neutrophil respiratory burst (11–14). Neutrophils produce LTB4 with fMLP stimulation (1), and LTB4 can enhance the NADPH oxidase system (15). In addition, neutrophils do not express GABAA receptor (16). Taking these factors together, we hypothesized that propofol attenuates LTB4 production via its interaction with an unidentified target.
In this study, we determined the effect of propofol on LTB4 both in vivo and in vitro. We found that propofol reduced the LTB4 level via its interaction with 5-LOX and then examined propofol binding sites on 5-LOX using meta-azi-propofol (AziPm), a photoactive propofol analog (17). Using rigid docking simulation, we identified propofol binding pockets on 5-LOX and examined the role of these pockets in 5-LOX function.
MATERIALS AND METHODS
Mice
Mice on a C57BL/6 background were purchased from the The Jackson Laboratory (Bar Harbor, ME, USA) and housed in specific pathogen-free conditions, with 12-h light and dark cycles. Male mice at 8–10 wk of age were used for the experiments.
Cecal ligation and puncture surgery
All experimental procedures complied with institutional and federal guidelines regarding the use of animals in research. Polymicrobial abdominal sepsis was induced by cecal ligation and puncture (CLP) surgery (18, 19). Mice (8–10 wk old) were anesthetized with the intraperitoneal injection of ketamine 60 mg/kg and xylazine 5 mg/kg. After exteriorization, the cecum was ligated 1.0 cm from its tip and subjected to a single, through-and-through puncture with an 18-gauge needle. A small amount of fecal material was expelled with gentle pressure to maintain the patency of the puncture sites. The cecum was reinserted into the abdominal cavity, and warmed saline was administered subcutaneously at 0.1 ml/g. Buprenorphine was given subcutaneously to alleviate postoperative surgical pain. Some groups of mice were given propofol (Fresenius Kabi USA, Lake Zurich, IL, USA) intravenously over 6 h (10 mg/kg/h). This dosage regimen is clinically relevant for anesthesia practice.
Peritoneal leukocyte count
At 6, 12, and 24 h after CLP surgery, mice were euthanized, and peritoneal lavage was performed by injecting cold sterile PBS into the abdomen and aspirating the peritoneal fluid. The number of peritoneal leukocytes was counted with Turk Blood-Diluting Fluid (Ricca Chemical, Arlington, TX, USA). In addition, the peritoneal leukocyte differential was determined by Giemsa stain with a Heme 3 kit (Thermo Fisher Scientific, Waltham, MA, USA). For lipid mediator analysis, collected fluid was centrifuged immediately at 200 g for 5 min, and supernatant was collected and stored at −80°C until use.
Measurement of various lipid mediators
The reversed-phase mass spectrometry (MS)–based quantitation technique for eicosanoids was used (20, 21). The samples were diluted with 2 ml of methanol and 7 ml of water containing 0.1% formic acid, with a mixture of deuterium-labeled eicosanoids as an internal standard, and then loaded on an Oasis HLB cartridge (Waters, Milford, MA, USA). The column was washed with 1 ml of water, 1 ml of 15% methanol, and 1 ml of petroleum ether and then eluted with 0.2 ml of methanol containing 0.1% formic acid. Eicosanoids were quantified by reverse-phase HPLC-electrospray ionization-tandem mass spectrometry (MS/MS). A Chiralpak AD-RH column (Crawford Scientific, Strathaven, Scotland) was used for chiral separation (22).
Stimulation of mouse neutrophils with fMLP
Mouse neutrophils were purified from bone marrow (23). Neutrophils (1 × 105) were suspended in PBS and 1 mM Ca2+/Mg2+, coated on 12-well plates, and stimulated with 1 µM fMLP (EMD Millipore; Billerica, MA, USA) for 30 min at 37°C, with or without propofol (Sigma-Aldrich; St. Louis, MO, USA) at various concentrations. Supernatant was collected for lipid mediator analysis. Samples were stored at −80°C until use.
Production of 5-LOX-related AA derivatives from human embryonic kidney cells transfected with 5-LOX
The pcDNA3.1_5-LOX wild-type (WT) plasmid was kindly provided by Dr. Dieter Steinhilber (University of Frankfurt, Frankfurt, Germany). Human embryonic kidney (HEK) cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640/10% fetal bovine serum at 37°C in 5% CO2. The plasmid was transfected into HEK cells with Lipofectamine 2000 (Thermo Fisher Scientific) per the company’s protocol. Stimulation experiments were performed as has been described (24). In one experimental condition, transfected HEK cells were harvested and homogenized by sonication (2 × 15 s) on ice. Samples (1 × 106 cells) were stimulated in the presence of 2 mM CaCl2 and 3 µM AA (Cayman Chemical, Ann Arbor, MI, USA), with a total volume of 1 ml at 37°C for 10 min. Propofol was coincubated in some of the samples. The 5-LOX inhibitor zileuton (Sigma-Aldrich) was used as a control. The reaction was stopped with the same volume of methanol, and the supernatant was stored at −80°C until measurement. In another experiment, transfected HEK cells were suspended in PGC buffer (PBS, 0.1% glucose, 1 mM CaCl2) and stimulated with 2.5 µM A23189 (Sigma-Aldrich) and 3 µM AA in the presence or absence of 50 µM propofol at 37°C for 10 min. The reaction was stopped with methanol, and samples were stored at −80°C until use. 5-LOX-related AA derivatives were quantified by reverse-phase HPLC-electrospray ionization-MS/MS method.
Photolabeling of Azi-Pm with stable 5-LOX protein
Stable 5-LOX expression plasmid was kindly provided by Dr. Marcia Newcomer (Louisiana State University, Louisiana, LA, USA). The expression of stable 5-LOX was performed (25). Photolabeling of stable 5-LOX protein using aziPm (17) was also performed as previously described (26). AziPm is a propofol photoaffinity probe, that contains the photoactivatable group in a meta position relative to the hydroxyl group of propofol and possesses pharmacologic characteristics similar to those of propofol. AziPm (final concentration, 10 µM) was equilibrated with stable 5-LOX protein (1 mg/ml) in a reaction volume of 300 µl for 10 min and then exposed to 350-nm light under a Rayonet RPR-3500 Lamp (Southern New England Ultraviolet Co., Branford, CT, USA). The protein was separated on an SDS-polyacrylamide gel and stained with Coomassie G-250. The protein gel band was excised for liquid chromatography (LC)-MS/MS. After trypsin digestion, samples were injected into a nano-LC column with online electrospray into a LTQ linear ion trap (Thermo Fisher Scientific). Raw data were acquired with XCalibur (Thermo Fisher Scientific), and Sequest (Scripps Research Institute, La Jolla, CA, USA) was used to search b and y ions against the sequence of 5-LOX for an AziPm mass modification (216.076 atomic mass units) on any amino acid of every peptide. To evaluate AziPm binding specificity, we coincubated 5-LOX protein with 400 µM propofol to test for labeling protection (competition). The protein and AziPm solution, with or without propofol, were equilibrated and exposed to UV 350-nm light as above.
Clustering analysis
We performed the clustering analysis of lipid mediators using Multiple Experiment Viewer (MeV) software (The Institute for Genomic Research, Rockville, MD, USA; http://mev.tm4.org).
Rigid docking simulation of 5-LOX
Using the reported structure of stable 5-LOX protein (Protein Data Bank ID number: 3O8Y; http://www.rcsb.org/pdb/), we performed a rigid docking simulation of propofol on 5-LOX with the assumption that propofol binding sites would be localized in the vicinity of adducted residues in the photolabeling experiments. The docking program Glide (Schrödinger, Cambridge, MA, USA) was used, and the calculations were performed on an SBGrid workstation (https://sbgrid.org/). Docking grids (12 × 12 × 12 Å) were centered to the adducted residues, and the XP docking protocol with default settings was applied without any additional positional restraints. Docked configuration with the highest affinity based on the Glide score was chosen.
Alanine scanning mutagenesis and transient transfection
Alanine scanning mutagenesis was performed with the pcDNA3.1_5-LOX WT plasmid as a template, using the Quikchange XL kit (Agilent Technologies, Santa Clara, CA, USA), and DNA sequences were confirmed. Plasmids were transfected into HEK cells as above, and transfected cells were subject to Western blot analysis. Their lysates were stimulated with 2 mM CaCl2 and 3 µM AA at 37°C for 10 min as previously described. LTB4 level in the supernatant was determined.
Western blot analysis
Lysed samples (10 µg) of transfected HEK cells were separated on an SDS-polyacrylamide gel and blotted onto nitrocellulose membrane. The membranes were probed with rabbit anti 5-LOX antibody (1:1000) or anti-β actin antibody (1:1000). As a secondary antibody, anti-rabbit IgG-horseradish peroxidase conjugate was used. All the antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The immunoreactive bands from each blot were detected by enhanced chemiluminescence for visualization (Thermo Fisher Scientific).
Statistical analysis
Data were analyzed as indicated in the corresponding figure legends. Statistical analyses were performed with Prism 5 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was defined as P < 0.05.
RESULTS
Propofol exposure reduced peritoneal LTB4 production in mice after CLP surgery
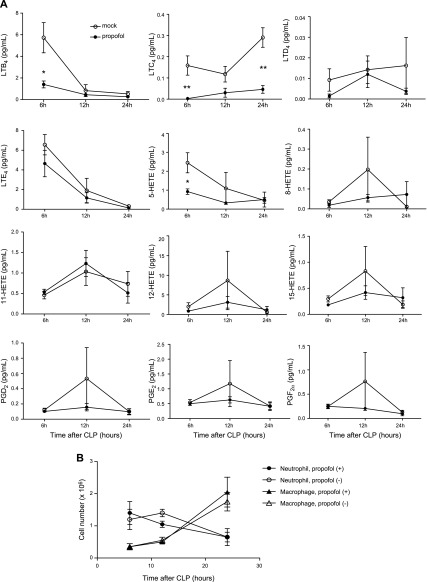
First, we examined the level of LTB4 and other lipid mediators in the peritoneal lavage fluid obtained from mice that underwent CLP surgery. A group of mice received propofol. The LTB4 level at 6 h after CLP surgery was significantly lower in mice that received propofol (Fig. 1A and Table 1). In addition, LTC4 and 5-hydroxyeicosatetraenoic acid (HETE) levels were significantly lower at 6 h after CLP surgery in mice that received propofol. However, 8-, 11-, 12-, and 15-HETE and prostaglandin (PG)D2, -E2, and -F2α levels were not affected by propofol administration at 6 h after CLP surgery. Because LTB4 is mainly produced by neutrophils and macrophages, we measured the number of peritoneal neutrophils and macrophages at various time points after CLP surgery. There was no significant difference in the number of peritoneal neutrophils and macrophages between propofol exposed mice and nonexposed mice, suggesting that propofol attenuates LTB4 production by neutrophils and macrophages (Fig. 1B).
Figure 1.
The level of peritoneal lipid mediators and leukocyte recruitment in mice after CLP surgery and propofol exposure. A) Mice underwent CLP surgery, with or without propofol exposure. Peritoneal lavage was performed at various time points, and lipid mediator levels in the fluid were measured. Data are means ± sd of 4 replicates. Statistical analysis was performed with 2-way ANOVA with Bonferroni post hoc analysis. *P < 0.05, **P < 0.01 of propofol exposure group vs. nonexposure at the same time point after the surgery. B) Mice underwent CLP surgery, with or without propofol exposure. The number of neutrophils and macrophages in the peritoneal cavity was examined at various time points after the surgery. Data are means ± sd of 4 replicates. Statistical analysis was performed with 2-way ANOVA with Bonferroni post hoc analysis. No statistically significant results were observed.
TABLE 1.
Lipid mediator levels in mice undergoing sham surgery
| Lipid mediator | Level (pg/ml) |
|---|---|
| LTB4 | 0.823 ± 0.437 |
| LTC4 | 3.08 ± 2.23 |
| LTD4 | 0.0835 ± 0.0951 |
| LTE4 | 2.67 ± 3.14 |
| 5-HETE | 1.96 ± 0.55 |
| 8-HETE | 0.710 ± 0.475 |
| 11-HETE | 1.17 ± 0.54 |
| 12-HETE | 45.9 ± 21.9 |
| 15-HETE | 5.25 ± 3.74 |
| PGD2 | 5.46 ± 4.25 |
| PGE2 | 11.0 ± 9.91 |
| PGF2α | 0.883 ± 0.819 |
Propofol attenuated LTB4 production in fMLP-stimulated neutrophils
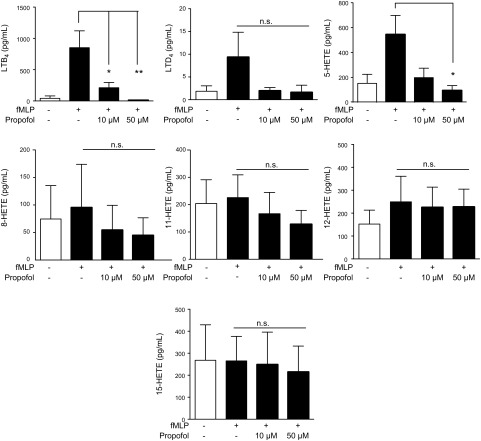
We determined the effect of propofol on LTB4 production from fMLP-stimulated neutrophils in vitro. The plasma concentration of propofol with 2–11 µg/ml (17–62 µM) has been reported for maintenance of anesthesia (27, 28); 2 µg/ml (11.3 µM) in serum level has been reported in sedated patients in intensive care (29). We chose 50 µM propofol for our study. As we observed in vivo, propofol attenuated LTB4, 5-HETE, and LTD4 production from fMLP-stimulated neutrophils (Fig. 2). Thus, we hypothesized that propofol inhibits the production of LTB4, 5-HETE and LTD4 via directly interacting with 5-LOX, a responsible enzyme for LT biosynthesis.
Figure 2.
The production of lipid mediators from fMLP-stimulated mouse neutrophils with propofol. Bone marrow neutrophils were purified from mice and stimulated with fMLP in the presence or absence of propofol for 30 min, and lipid mediators in the supernatant were measured. Data are means ± sd of triplicate experiments. Statistical analysis was performed with 1-way ANOVA with Bonferroni post hoc analysis. *P < 0.05, **P < 0.01, propofol exposure vs. nonexposure. N.s., not significant.
Propofol attenuated the production of 5-LOX-related AA derivatives from 5-LOX transfectants
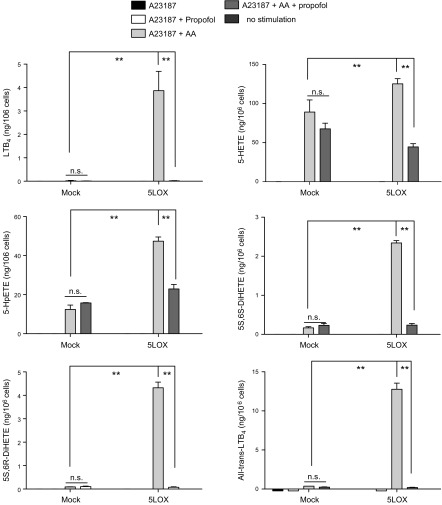
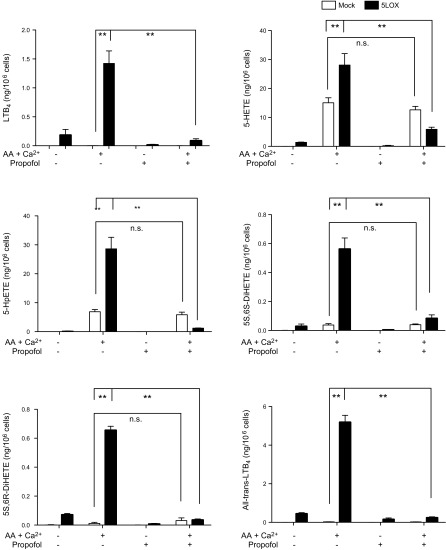
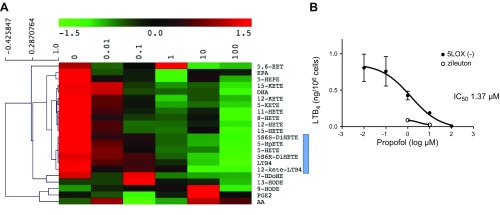
We determined the effect of propofol on the production of 5-LOX-related AA derivatives in HEK cells transfected with 5-LOX WT. First, we stimulated intact 5-LOX transfectants with the calcium ionophore A23187. We found that the production of LTB4, 5-HETE, 5-hydroperoxyeicosatetraenoic acid (HpETE), 5S,6S-dihydroxyl-7E,9E,11Z,14Z-eicosatetraenoic acid (DiHETE), 5S,6R-DiHETE, and all-trans-LTB4 (6-trans-LTB4 and 12-epi-6-trans-LTB4) was induced with AA and A23187 stimulation in 5-LOX transfectants and significantly attenuated by propofol (Fig. 3 and Supplemental Fig. 1B). Similarly, in the experiment using lysates of 5-LOX transfectants, 5-LOX-related AA derivatives were produced with AA and calcium stimulation, and propofol reduced their production (Fig. 4). In addition, the production of 5S-HETE was attenuated by propofol, but the production of 5R-HETE was not attenuated (Supplemental Fig. 2A, B). Because 5R-HETE is nonenzymatically produced, and 5S-HETE is produced both enzymatically via 5-LOX and nonenzymatically (30), propofol would be likely to interact with 5-LOX. Last, we performed cluster analysis of lipid mediators (Fig. 5A). We found that LTB4, 12-keto-LTB4, 5-HETE, 5-HpETE, 5S,6S-DiHETE, and 5S,6R-DiHETE were clustered, all of which are 5-LOX-dependent lipid mediators, further supporting the idea that propofol affects 5-LOX.
Figure 3.
The production of 5-LOX-related AA derivatives from intact 5-LOX-transfected HEK cells with propofol. Intact 5-LOX-transfected HEK cells were stimulated with the calcium ionophore A23187 and AA, and the effect of propofol on the production of 5-LOX-related AA derivatives was examined. Data areas means ± sd of 4 replicates. Statistical analysis was performed with 1-way ANOVA with Bonferroni post hoc analysis. *P < 0.05, **P < 0.01. N.s., not significant.
Figure 4.
The production of 5-LOX-related lipid AA derivatives from lysates of 5-LOX-transfected HEK cells exposed to propofol. Lysates of 5-LOX-transfected HEK cells were stimulated with AA and Ca2+ in the presence of propofol. Data are means ±sd of 4 replicates. Statistical analysis was performed with 1-way ANOVA with Bonferroni post hoc analysis. *P < 0.05, **P < 0.01. N.s., not significant.
Figure 5.
The production of 5-LOX-related lipid AA derivatives from lysates of 5-LOX-transfected HEK cells exposed to propofol at different concentrations. A) Lysates of 5-LOX transfected HEK cells were stimulated with AA and Ca2+ in the presence of propofol at various concentrations, and the supernatant was subjected to lipid mediator analysis. Cluster analysis of lipid mediators was performed with MEV software, with the results shown on a heat map. Lipid mediators clustered with LTB4 are highlighted. B) IC50 of propofol was determined. Data are means ± sd of 4 replicates.
The IC50 of propofol was within a clinically relevant range
We also tested the IC50 of propofol by using 5-LOX lysates. It was 1.37 µM (Fig. 5B), which was within the clinically relevant range. For the comparison, IC50 of zileuton is 0.5–1 µM (31).
AziPm bound to stable 5-LOX protein
The x-ray crystallographic structure of 5-LOX was determined using the 5-LOX protein containing mutation of the destabilizing regions (25). This mutant is called stable 5-LOX and possesses 5-LOX activity analogous to that of WT 5-LOX. Thus, we used stable 5-LOX protein for photolabeling experiment. On the MS analysis of photolabeled stable 5-LOX protein, we detected peptides covering about 80 and 89% of the sequence and found 2 adducted sites (Phe187 and Val431) in the catalytic domain of 5-LOX (Table 2). To determine whether AziPm binding sites were propofol binding sites, we performed a competition assay with propofol. There was no adducted residue in the presence of propofol, confirming that Azi-Pm adducted residues were specific to propofol.
TABLE 2.
Photolabeled residues of 5-LOX
| Sequence coverage (%) | Residues |
|---|---|
| AziPm labeling | |
| 78.5 | F187, V431 |
| 89 | F187, V431 |
| AziPm and propofol competition | |
| 89.1 | No adducted residues |
Propofol docked near the active site of 5-LOX
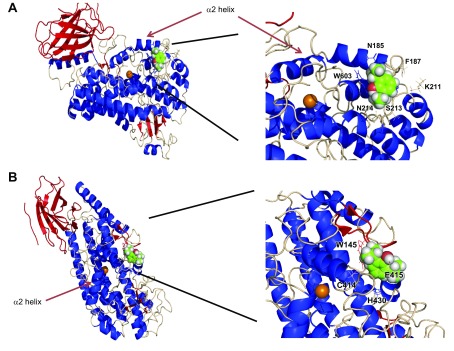
Using the adducted residues as center residues in the grid, we performed rigid docking simulation. Figure 6A shows docked propofol on 5-LOX near Phe187. Propofol was docked to the vicinity of the α2 helix. A heme iron is a catalytic center and the α2 helix acts as a mobile lid, particularly Phe177 and Tyr181 being a cork for the active site. The active site consists of Trp147, Phe177, Tyr181, Asn407, Thr364, Leu420, Phe421, His432, Tyr599, His600, and Ala603 (32). Propofol binding to the region around Phe187 may interfere with substrate entry into the active site (Supplemental Fig. 3A). Val431 is another adducted residue. Figure 6B shows the docked propofol structure on 5-LOX near this residue. This propofol binding site is away from the α2 helix, but is in the vicinity of the active site (Supplemental Fig. 3B). We hypothesized that propofol attenuates 5-LOX activity via its interaction with these 2 sites.
Figure 6.
Rigid docking simulation of propofol on stable 5-LOX with a docking program. Rigid docking was performed, as described in the Materials and Methods section. A) Docking of propofol with the grid center at Phe187. B) Docking of propofol with the grid center at Val431. An orange sphere indicates a heme iron. Both right panels show an enlarged image of the docking sites. Residues with black lines indicate the ones within 4 Å of the docked propofol.
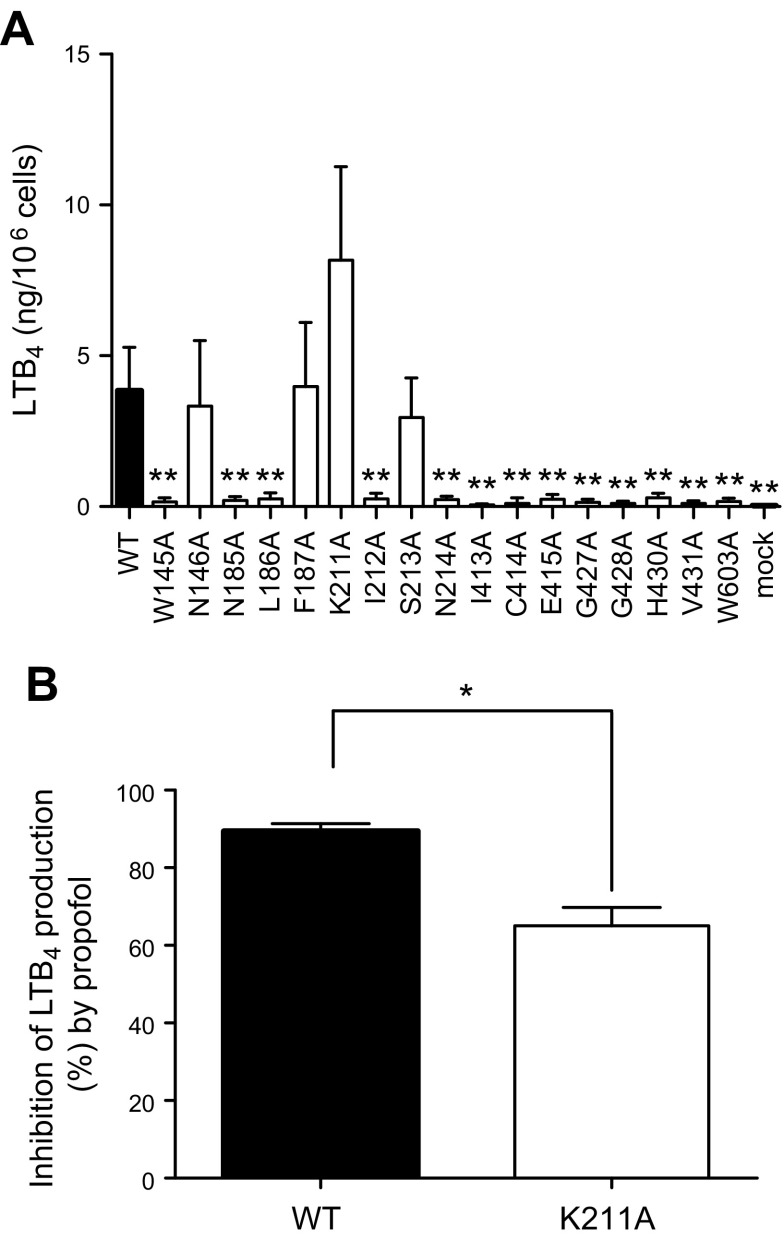
Point mutations of amino acids located at the propofol binding sites attenuated 5-LOX activity
Using docking simulation data, we identified amino acid residues within 4 Å from docked propofol. For propofol docked near Phe187, we identified Asn185, Leu186, Phe187, Lys211, Ile212, Ser213, Asn214, and Trp603 as those. For Val431, we identified Try145, Asn146, Ile413, Cys414, Glu415, Gly427, Gly428, His430, and Val431. We performed alanine scanning mutagenesis of these amino acids and tested 5-LOX activity using lysates of HEK cells transiently transfected with WT 5-LOX or point mutants. We confirmed that the expression levels of WT and all point mutants were similar by Western blot analysis (Supplemental Fig. 4). We noted that LTB4 production was significantly impaired in most of the mutants we tested, suggesting that both of the propofol binding sites were functionally involved in 5-LOX activity (Fig. 7A). Only mutant K211A increased 5-LOX activity, and we tested the effect of propofol on this mutant. Although WT was completely inhibited by propofol (50 µM), K211A was partially inhibited (Fig. 7B). Because propofol bound at 2 sites, our result suggested that both binding sites were functionally necessary for 5-LOX activity.
Figure 7.
LTB4 production in lysates of HEK cells transfected with 5-LOX WT and point mutants. A) Homogenates of HEK cells transiently transfected with WT 5-LOX and mutants were stimulated with Ca2+ and AA. The LTB4 level was measured. Data are means ± sd of 5 replicates. Statistical analysis was performed with 1-way ANOVA with Bonferroni post hoc analysis. *P < 0.05, **P < 0.01. B) The effect of propofol (50 µM) on 5-LOX WT and K211A was examined. Inhibition percentage of LTB4 production by propofol was calculated as [1 − (LTB4 level with propofol by 5-LOX-transfectants − production by mock transfectants)]/[LTB4 level without propofol by 5-LOX transfectants − production by mock transfectants] × 100 (%). Data are means ± sd of 4 replicates. Statistical analysis was performed with the unpaired Student’s t test. *P < 0.05.
DISCUSSION
We showed that commonly used intravenous anesthetic propofol directly bound to 5-LOX at 2 sites and attenuated the production of 5-LOX-related AA derivatives, including LTB4. The docking simulation suggested that propofol binding sites were quite close to the active site of 5-LOX. Both propofol binding sites were functionally necessary for 5-LOX activity, based on our mutagenesis experiment.
5-LOX is implicated in various disease states, including asthma, atherosclerosis, and osteoporosis, and in cancers such as prostate cancer and neuroblastoma (8, 33). Diverse roles of 5-LOX have raised interest in the development of 5-LOX inhibitors. 5-LOX inhibitors are largely classified into 3 types: redox-active inhibitors, iron ligand inhibitors with weak redox-active properties, and nonredox inhibitors (9, 33). In general, redox-active inhibitors have poor selectivity for 5-LOX. In addition, they often produce methemoglobin by interacting with other redox systems. Thus, this class has not been the mainstay in the development of 5-LOX inhibitors. 5-LOX contains nonheme iron in its active site. Chelating iron by hydroxamic acid and N-hydroxy urea derivatives was attempted to make iron ligand inhibitors. Zileuton, the only 5-LOX inhibitor that has been introduced to the clinical market so far, belongs to this class. However, it has a very short half-life and exhibits hepatotoxicity. In addition, this drug has a weak redox property, which may be responsible for its 5-LOX inhibition, and also implies the lack of its specificity. Therefore, the development of inhibitors specific to 5-LOX is warranted. Although the x-ray crystallographic structure of 5-LOX has been reported (25), there has been no reported 5-LOX structure cocrystallized with its inhibitor so far. Because propofol inhibited 5-LOX activity (IC50, 1.37 µM), its binding sites were identified on the 5-LOX structure, and the importance of the propofol binding sites on 5-LOX activity was shown, our data are unique.
Propofol bound to 2 sites near the active site of 5-LOX consisting of Trp147, Phe177, Tyr181, Asn407, Thr364, Leu420, Phe421, His432, Tyr599, His600, and Ala603 (32). One of the propofol binding sites was located near the α2 helix, a gatekeeper for this active site (Fig. 4A). Another binding site was away from the α2 helix and abutted this active site (Fig. 4B). Both of propofol binding sites were functionally significant, as suggested by our mutagenesis data. This finding was further supported by our data on the effect of propofol on the 5-LOX mutant. Therefore, these sites may serve as pockets to explore 5-LOX inhibitors in the future. Also, it is worth noting that propofol did not dock inside the active site. Two different structures of 15-LOX were previously reported and showed that the size of its active site differed between them (34). Thus, it is possible that the active site of 5-LOX may also take different conformations (35). In this study, propofol binding sites were located outside this active site. These results further led us to conclude that a structure-based search for 5-LOX inhibitors can be done to use these sites. We could not cover 10% of 5-LOX sequence in our MS analysis. It is possible that there may be an additional binding site.
We demonstrated that lesser amounts of LTB4, 5-HETE, 5-HpETE, 5S,6S-DiHETE, 5S,6R-DiHETE, and all-trans-LTB4 were produced in the presence of propofol. The effect of propofol on LTB4 was reported by Inada et al. (36–38), and our result is compatible with theirs. 5S,6S-DiHETE, 5S,6R-DiHETE, and all-trans-LTB4 are products of LTA4, which is produced via 5-LOX. Propofol-dependent attenuation was more obvious in LTB4 than in 5-HETE and 5-HpETE (Figs. 4 and 5). Chiral analysis of 5-HETE showed that 5S-HETE was produced less in the presence of propofol, but the production of 5R-HETE was not affected (Supplemental Fig. 2). Because 5R-HETE is nonenzymatically produced and 5S-HETE is produced both enzymatically via 5-LOX and nonenzymatically (30), this result further supported that propofol inhibits 5-LOX. However, it is possible that propofol inhibits LTA4-H, in addition to 5-LOX. The effect of propofol on LTA4-H would be interesting to study in the near future.
In summary, we report that propofol inhibited 5-LOX and its binding sites were located just outside of the active site and necessary for 5-LOX function.
ACKNOWLEDGMENTS
This study was supported, in part, by U.S. National Institutes of Health, Institute of General Medical Sciences Grants GM101345 and GM118277 (to K.Y.) and P01GM055876 (to R.G.E); Children’s Hospital Medical Center (CHMC) Anesthesia Foundation (to K.Y.); Grant-in-Aid S1311011 from the Foundation of Strategic Research Projects in Private Universities from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (to T.Y.); MEXT/Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) 15H05901, 15H05904, and 15H04708 (to T.Y.) and 16K08596 and 15KK0320) (to T.O.); and the Takeda Science Foundation (to T.Y. and T.O.). The authors declare no conflicts of interest.
Glossary
- 5-LOX
5-lipoxygenase
- AA
arachidonic acid
- AziPm
meta-azi-propofol
- DHA
docosahexaenoic acid
- DiHETE
dihydroxyl-7E,9E,11Z,14Z-eicosatetraenoic acid
- EET
epoxyeicosatrienoic acid
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- HdoHE
hydroxydocosahexaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HpETE
hydroperoxyeicosatetraenoic acid
- KETE
oxo-eicosatetraenoic acid
- LC
liquid chromatography
- LT
leukotriene
- LTA4-H
LTA4 hydrolase
- MS
mass spectrometry
- PG
prostaglandin
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. Okuno, S. Koutsogiannaki, T. Yokomizo, and K. Yuki designed the research; T. Okuno, S. Koutsogiannaki, M. Ohba, W. Bu, T. Yokomizo, and K. Yuki analyzed the data; T. Okuno, S. Koutsogiannaki, M. Ohba, M. Chamberlain, W. Bu, FY. Lin, and K. Yuki performed the research; and T. Okuno, S. Koutsogiannaki, W. Bu, R. G. Eckenhoff, T. Yokomizo, and K. Yuki wrote the paper.
REFERENCES
- 1.Afonso P. V., Janka-Junttila M., Lee Y. J., McCann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T., Parent C. A. (2012) LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. (1997) A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387, 620–624 [DOI] [PubMed] [Google Scholar]
- 3.Okuno T., Yokomizo T., Hori T., Miyano M., Shimizu T. (2005) Leukotriene B4 receptor and the function of its helix 8. J. Biol. Chem. 280, 32049–32052 [DOI] [PubMed] [Google Scholar]
- 4.Yokomizo T., Izumi T., Shimizu T. (2001) Leukotriene B4: metabolism and signal transduction. Arch. Biochem. Biophys. 385, 231–241 [DOI] [PubMed] [Google Scholar]
- 5.Ohishi N., Minami M., Kobayashi J., Seyama Y., Hata J., Yotsumoto H., Takaku F., Shimizu T. (1990) Immunological quantitation and immunohistochemical localization of leukotriene A4 hydrolase in guinea pig tissues. J. Biol. Chem. 265, 7520–7525 [PubMed] [Google Scholar]
- 6.Rådmark O., Werz O., Steinhilber D., Samuelsson B. (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 1851, 331–339 [DOI] [PubMed] [Google Scholar]
- 7.Mitra S., Bartlett S. G., Newcomer M. E. (2015) Identification of the substrate access portal of 5-lipoxygenase. Biochemistry 54, 6333–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhilber D., Hofmann B. (2014) Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin. Pharmacol. Toxicol. 114, 70–77 [DOI] [PubMed] [Google Scholar]
- 9.McMillan R. M., Walker E. R. (1992) Designing therapeutically effective 5-lipoxygenase inhibitors. Trends Pharmacol. Sci. 13, 323–330 [DOI] [PubMed] [Google Scholar]
- 10.Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K. E., Ledermann B., Antkowiak B., Rudolph U. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 17, 250–252 [DOI] [PubMed] [Google Scholar]
- 11.Murphy P. G., Ogilvy A. J., Whiteley S. M. (1996) The effect of propofol on the neutrophil respiratory burst. Eur. J. Anaesthesiol. 13, 471–473 [DOI] [PubMed] [Google Scholar]
- 12.Heine J., Jaeger K., Osthaus A., Weingaertner N., Münte S., Piepenbrock S., Leuwer M. (2000) Anaesthesia with propofol decreases FMLP-induced neutrophil respiratory burst but not phagocytosis compared with isoflurane. Br. J. Anaesth. 85, 424–430 [DOI] [PubMed] [Google Scholar]
- 13.Fröhlich D., Rothe G., Schwall B., Schmitz G., Hobbhahn J., Taeger K. (1996) Thiopentone and propofol, but not methohexitone nor midazolam, inhibit neutrophil oxidative responses to the bacterial peptide FMLP. Eur. J. Anaesthesiol. 13, 582–588 [DOI] [PubMed] [Google Scholar]
- 14.Mikawa K., Akamatsu H., Nishina K., Shiga M., Maekawa N., Obara H., Niwa Y. (1998) Propofol inhibits human neutrophil functions. Anesth. Analg. 87, 695–700 [DOI] [PubMed] [Google Scholar]
- 15.Perkins R. S., Lindsay M. A., Barnes P. J., Giembycz M. A. (1995) Early signalling events implicated in leukotriene B4-induced activation of the NADPH oxidase in eosinophils: role of Ca2+, protein kinase C and phospholipases C and D. Biochem. J. 310, 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuki K., Eckenhoff R. G. (2016) Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth. Analg. 123, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall M. A., Xi J., Lor C., Dai S., Pearce R., Dailey W. P., Eckenhoff R. G. (2010) m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J. Med. Chem. 53, 5667–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y., Zou L., Zhang M., Li Y., Chen C., Chao W. (2011) MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology 115, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kita Y., Takahashi T., Uozumi N., Shimizu T. (2005) A multiplex quantitation method for eicosanoids and platelet-activating factor using column-switching reversed-phase liquid chromatography-tandem mass spectrometry. Anal. Biochem. 342, 134–143 [DOI] [PubMed] [Google Scholar]
- 21.Matsunobu T., Okuno T., Yokoyama C., Yokomizo T. (2013) Thromboxane A synthase-independent production of 12-hydroxyheptadecatrienoic acid, a BLT2 ligand. J. Lipid Res. 54, 2979–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. (2011) Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 121, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbo C., Yuki K., Demers M., Wagner D. D., Shimaoka M. (2013) Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J. Anesth. 27, 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstmeier J., Newcomer M. E., Dennhardt S., Romp E., Fischer J., Werz O., Garscha U. (2016) 5-Lipoxygenase-activating protein rescues activity of 5-lipoxygenase mutations that delay nuclear membrane association and disrupt product formation. FASEB J. 30, 1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert N. C., Bartlett S. G., Waight M. T., Neau D. B., Boeglin W. E., Brash A. R., Newcomer M. E. (2011) The structure of human 5-lipoxygenase. Science 331, 217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuki K., Bu W., Xi J., Shimaoka M., Eckenhoff R. (2013) Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1. Anesth. Analg. 117, 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short T. G., Aun C. S., Tan P., Wong J., Tam Y. H., Oh T. E. (1994) A prospective evaluation of pharmacokinetic model controlled infusion of propofol in paediatric patients. Br. J. Anaesth. 72, 302–306 [DOI] [PubMed] [Google Scholar]
- 28.Gepts E., Camu F., Cockshott I. D., Douglas E. J. (1987) Disposition of propofol administered as constant rate intravenous infusions in humans. Anesth. Analg. 66, 1256–1263 [PubMed] [Google Scholar]
- 29.Albanese J., Martin C., Lacarelle B., Saux P., Durand A., Gouin F. (1990) Pharmacokinetics of long-term propofol infusion used for sedation in ICU patients. Anesthesiology 73, 214–217 [DOI] [PubMed] [Google Scholar]
- 30.Serhan C. N. (1995) Leukocyte transmigration, chemotaxis, and oxygenated derivatives of arachidonic acid: when is chirality important? Am. J. Respir. Cell Mol. Biol. 12, 251–253 [DOI] [PubMed] [Google Scholar]
- 31.Carter G. W., Young P. R., Albert D. H., Bouska J., Dyer R., Bell R. L., Summers J. B., Brooks D. W. (1991) 5-lipoxygenase inhibitory activity of zileuton. J. Pharmacol. Exp. Ther. 256, 929–937 [PubMed] [Google Scholar]
- 32.Landa P., Kutil Z., Temml V., Malik J., Kokoska L., Widowitz U., Pribylova M., Dvorakova M., Marsik P., Schuster D., Bauer R., Vanek T. (2013) Inhibition of in vitro leukotriene B4 biosynthesis in human neutrophil granulocytes and docking studies of natural quinones. Nat. Prod. Commun. 8, 105–108 [PubMed] [Google Scholar]
- 33.Werz O., Steinhilber D. (2005) Development of 5-lipoxygenase inhibitors: lessons from cellular enzyme regulation. Biochem. Pharmacol. 70, 327–333 [DOI] [PubMed] [Google Scholar]
- 34.Choi J., Chon J. K., Kim S., Shin W. (2008) Conformational flexibility in mammalian 15S-lipoxygenase: reinterpretation of the crystallographic data. Proteins 70, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 35.Wu Y., He C., Gao Y., He S., Liu Y., Lai L. (2012) Dynamic modeling of human 5-lipoxygenase-inhibitor interactions helps to discover novel inhibitors. J. Med. Chem. 55, 2597–2605 [DOI] [PubMed] [Google Scholar]
- 36.Inada T., Hirota K., Shingu K. (2015) Intravenous anesthetic propofol suppresses prostaglandin E2 and cysteinyl leukotriene production and reduces edema formation in arachidonic acid-induced ear inflammation. J. Immunotoxicol. 12, 261–265 [DOI] [PubMed] [Google Scholar]
- 37.Inada T., Kubo K., Ueshima H., Shingu K. (2011) Intravenous anesthetic propofol suppresses prostaglandin E2 production in murine dendritic cells. J. Immunotoxicol. 8, 359–366 [DOI] [PubMed] [Google Scholar]
- 38.Inada T., Ueshima H., Shingu K. (2013) Intravenous anesthetic propofol suppresses leukotriene production in murine dendritic cells. J. Immunotoxicol. 10, 262–269 [DOI] [PubMed] [Google Scholar]