Abstract
In the peripheral nervous system, Schwann cells (SCs) demonstrate surveillance activity, detecting injury and undergoing trans-differentiation to support repair. SC receptors that detect peripheral nervous system injury remain incompletely understood. We used RT-PCR to profile ionotropic glutamate receptor expression in cultured SCs. We identified subunits required for assembly of N-methyl-d-aspartic acid (NMDA) receptors (NMDA-Rs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, and kainate receptors. Treatment of SCs with 40–100 µM glutamate or with 0.5–1.0 µM NMDA robustly activated Akt and ERK1/2. The response was transient and bimodal; glutamate concentrations that exceeded 250 µM failed to activate cell signaling. Phosphoprotein profiling identified diverse phosphorylated proteins in glutamate-treated SCs in addition to ERK1/2 and Akt, including p70 S6-kinase, glycogen synthase kinase-3, ribosomal S6 kinase, c-Jun, and cAMP response element binding protein. Activation of SC signaling by glutamate was blocked by EGTA and dizocilpine and by silencing expression of the NMDA-R NR1 subunit. Phosphoinositide 3-kinase/PI3K functioned as an essential upstream activator of Akt and ERK1/2 in glutamate-treated SCs. When glutamate or NMDA was injected directly into crush-injured rat sciatic nerves, ERK1/2 phosphorylation was observed in myelinated and nonmyelinating SCs. Glutamate promoted SC migration by a pathway that required PI3K and ERK1/2. These results identified ionotropic glutamate receptors and NMDA-Rs, specifically, as potentially important cell signaling receptors in SCs.—Campana, W. M., Mantuano, E., Azmoon, P., Henry, K., Banki, M. A., Kim, J. H., Pizzo, D. P., Gonias, S. L. Ionotropic glutamate receptors activate cell signaling in response to glutamate in Schwann cells.
Keywords: NMDA receptor, AMPA receptor, kainate receptor, peripheral nerve injury, migration
Ionotropic glutamate receptors (IGRs) are ligand-gated transmembrane channels that selectively transport cations (1). Sixteen separate genes and multiple alternatively spliced mRNAs encode subunits for intact tetrameric IGRs, including N-methyl-d-aspartic acid (NMDA) receptors (NMDA-Rs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and kainate receptors. Although IGRs are best characterized as neuronal receptors, other cell types also express IGRs (1, 2). We and others have reported that Schwann cells (SCs) in culture express NMDA-Rs (3–5), and we also provided evidence that SCs express NMDA-Rs in vivo in injured sciatic nerves (3). Others have shown that SCs express AMPA receptors and kainate receptors (5–7). The function of IGRs in SCs remains incompletely understood. In SCs and oligodendrocytes, different IGRs may control intracellular calcium levels in cell bodies and myelin, with distinct effects on cell physiology (8–10).
In neurons, IGR-mediated calcium influx activates cell signaling factors, including Src family kinases, PI3K, and ERK1/2 (11, 12). Activated Src family kinases and other cell signaling factors are known to modify NMDA-R subunits, regulating channel activity (13). These reactions, which involve IGRs and cell signaling factors that are activated downstream of IGRs, form positive feedback loops that regulate neuronal cell physiology (13–15). NMDA-Rs may regulate cell signaling independently of calcium influx by binding cell signaling and/or adapter proteins (16). NMDA-Rs may also initiate cell signaling after interaction with or cleavage by the extracellular protease, tissue-type plasminogen activator (tPA) (17, 18).
Although we have shown that NMDA-Rs activate cell signaling in SCs in response to tPA (3), the function of IGRs in SCs as glutamate-triggered cell signaling receptors remains unexplored. This is an important question, because many of the cell signaling factors that are reported to be activated by IGRs in neurons have also been reported to control SC gene expression and phenotype in response to peripheral nervous system (PNS) injury (19, 20). In addition to IGRs, many cells express metabotropic glutamate receptors, which are GPCRs that are capable of initiating cell signaling (21). Saitoh et al. (22) reported that in SCs, high concentrations of glutamate (2 mM) augment ERK1/2 activation by neuregulin-1 by binding to metabotropic glutamate receptors. This pathway was dependent on c-Src, which functioned as an essential upstream signaling factor.
Herein, we define SC signaling responses that are generated by IGRs in response to glutamate. ERK1/2, Akt, and factors downstream of ERK1/2 and Akt, including p70 S6-kinase (p70 S6K), glycogen synthase kinase-3 (GSK-3), and ribosomal S6 kinase (RSK), were phosphorylated in SCs treated with 40–100 µM glutamate; however, the cell signaling response was bimodal and entirely absent when glutamate concentration exceeded 250 μM. NMDA-Rs were principally responsible for glutamate-activated cell signaling in SCs. PI3K functioned as an essential upstream activator of IGR signaling in response to glutamate in SCs; c-Src was uninvolved. When glutamate was injected into crush-injured sciatic nerves in rats, ERK1/2 phosphorylation was observed in myelinated and nonmyelinating SCs in vivo. As a result of IGR activation by glutamate, SCs demonstrated increased cell migration. We conclude that IGRs and NMDA-Rs, specifically, function as key regulators of cell signaling in SCs. The robust activity of IGRs in glutamate-activated cell signaling may not have been identified previously because of the novel glutamate concentration dependency of the response.
MATERIALS AND METHODS
Materials
l-Glutamic acid monosodium salt hydrate (glutamate), NMDA, and 1-naphthyl-acetyl spermine trihydrochloride (NAS) were from Sigma-Aldrich (St. Louis, MO, USA). Dizocilpine (MK801), 2-(2-amino-3-methoxy phenyl)-4h-1-benzopyran-4-one (PD98059), and 2-morpholin-4-yl-8-phenyl chromen-4-one (LY294004) were from Millipore (Billerica, MA, USA). Abs used in immunoblotting experiments that detect phospho-ERK1/2, total ERK1/2, phospho-Akt, total Akt, and phospho-p70 S6 kinase (p70 S6K) were from Cell Signaling Technologies (Beverly, MA, USA). Horseradish peroxidase–conjugated secondary Ab also was purchased from Cell Signaling Technologies. For immunofluorescence (IF) microscopy studies, rabbit polyclonal Ab that detects phospho-ERK1/2 was from Cell Signaling Technologies. Ab that targeted glial fibrillary acidic protein (GFAP) was from Dako (Carpentaria, CA, USA). Nerve growth factor-β (NGF-β) and neurotrophin-3 were from R&D Systems (Minneapolis, MN, USA).
Cell culture
Rat SCs (rSCs) were isolated from sciatic nerves, extracted from 1-d-old Sprague-Dawley rats, and purified from fibroblasts by using Thy1.1 Ab and rabbit complement cytolysis (Sigma-Aldrich) (23). Final rSC primary cultures consisted of 98% SCs, as determined by IF microscopy for S100 (23). rSCs were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) that was supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, 21 μg/ml bovine pituitary extract (Lonza Biosciences, Walkersville, MD, USA) and 4 μM forskolin (Millipore). DMEM contains 0.4 mM glycine. Primary cultures of human SCs (hSCs), which were isolated from human spinal nerve, were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and were maintained in the medium recommended by the manufacturer. Experiments were performed with cells that were passaged no more than 7 times. Rat PC12 pheochromocytoma cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM with 10% FBS, 5% heat-inactivated horse serum (Omega Scientific, Tarzana, CA, USA), penicillin (100 U/ml), and streptomycin (1 mg/ml).
NR1 gene silencing
Rat NMDA-R NR1/glutamate N1 (GluN1) subunit gene silencing was performed as previously described (3). In brief, NR1-specific small interfering RNA (siRNA) On-TargetPlus SMARTpool and pooled nontargeting control (NTC) siRNA were purchased from Dharmacon (Lafayette, CO, USA). rSCs (2 × 106) were transfected with NR1-specific siRNA (50 nM) or NTC siRNA (50 nM) by electroporation using the Cell Nucleofector Kit V (Amaxa; Lonza Biosciences). NR1 gene-silencing was >85% at the mRNA level as determined by quantitative RT-PCR and confirmed by immunoblot analysis, as previously described (3). Cell signaling experiments were performed 24–36 h after introducing siRNAs.
RT-PCR analysis of IGR expression by rSCs
Total RNA was collected from rSCs and PC12 cells, which were differentiated by treatment with 50 ng/ml NGF for 7 d, by using the Absolutely RNA Miniprep Kit (Agilent Technologies, Palo Alto, CA, USA). cDNA was generated by using 1 µg of total RNA and the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Control reactions were conducted without reverse transcriptase to assure absence of genomic DNA. DNA oligonucleotide PCR primers were prepared on the basis of published sequences (24–30) to specifically amplify IGR subunit cDNAs, including the following: NR1, (forward) 5′-GCGACTCCCGCAGCAAT-3′ and (reverse) 3′-CCCCTGCCATGTTCTCAAAA-5′; NR2A, (forward) 5′-TCCACTCAAGGAATCTTGTGAGATAT-3′ and (reverse) 3′-ACTTGCCCATGTGTATTTATTTGTTT-5′; NR2B, (forward) 5′-AACCCTCGTGGCCAGCA-3′ and (reverse) 3′-ACTTGCCCATGTGTATTTATTTGTTT-5′; NR2C, (forward) 5′-GGCCCAGCTTTTGACCTTAGT-3′ and (reverse) 3′-CCTGTGACCACCGCAAGAG-5′; NR2D, (forward) 5′-TTTTTAACCTTTCATTCATCAGGACTC-3′ and (reverse) 3′-TGGCGGGCCAAGGTCT-5′; NR3A, (forward) 5′-GAAGAAAAGCAGCCACGTT-CC-3′ and (reverse) 3′-CTAGAGCAATGTCCTCCCAGG-5′; NR3B, (forward) 5′-CCTCTATAGCCTTTCCCGAGG-3′ and (reverse) 3′-CTAGAGCAATGTCCTCCCAGG-5′; GluA1, (forward) 5′-AAATTGCTTATGGGACATTG-3′ and (reverse) 3′-GCAGACTTCATGTATGTCCA-5′; GluA2, (forward) 5′-GACTCTGGCTCCACTAAAGA-3′ and (reverse) 3′-AGTCCTCACAAACACAGAGG-5′; GluA3, (forward) 5′-GTGAACAAGGCATCTTAGAC-3′ and (reverse) 3′-ACTGGTCTTGTCCTTACTC-5′; GluA4, (forward) 5′-ACAGCAGCTATTGAGAACAG-3′ and (reverse) 3′-GCCTTCGTACTTGTCATTTCC-5′; GluK1, (forward) 5′-TGCTAAATAGTTTCTGGTTTGG-3′ and (reverse) 3′-ATTCCTCCAACTATTCTGGTC-5′; GluK2, (forward) 5′-CCCAGGATGATGTGAACGG-3′ and (reverse) 3′-TCACGAACATAGGTTATAGCCA-5′; GluK3, (forward) 5′-GATGACTGTGAACTCCCT-3′ and (reverse) 3′-TTAATCCTTCCCATTGAGCC-5′; GluK4, (forward) 5′-ACGCCTCCAACATCTCTG-3′ and (reverse) 3′-CATTGCCTTCCATGTCCTG-5′ and; GluK5, (forward) 5′-GCCATGAATGCTACCACC-3′ and (reverse) 3′-GCATGACATACGGGTTCTC-5′. cDNA (1.0 µl) was amplified in separate reactions by using each pair of primers and Taqman Gene Expression Master Mix (10 µl total volume; Thermo Fisher Scientific). Each of 40 amplification cycles included incubation at 95°C for 0.5 min, incubation at primer-specific temperatures for 1 min, and then incubation at 72°C for 1 min. PCR products were subjected to 2–3% agarose gel electrophoresis and visualized by GelRed Nucleic Acid Stain (Biotium, Fremont, CA, USA) fluorescence. Bioneer 100 bp DNA ladder (D-1030; Bioneer, Daejeon, South Korea) was used as a size standard.
Phosphoprotein array analysis
rSCs and hSCs were plated at a density of 2 × 106 cells/well and cultured until ∼80% confluent. Cells were then transferred to serum-free medium (SFM) for 1 h and treated with 80 µM glutamate or vehicle for 10 min. Cells were rinsed extensively with PBS and cell extracts were prepared by using the lysis buffer provided by the human phospho-kinase array kit (R&D Systems). Protein content in cell extracts was determined by bicinchoninic acid assay. An equivalent amount of cellular protein (300 µg) was incubated with the 2 nitrocellulose membranes with Abs printed in duplicate. Phosphorylated proteins were detected by using biotinylated detection Abs, as described by the manufacturer.
Immunoblot analysis of cell signaling
rSCs and hSCs were plated at a density of 2 × 105 cells/well in serum-containing medium and cultured until ∼85% confluent. Cultures were then transferred into SFM for 1 h. Some cultures were pretreated for 30 min with PD98059 (50 µM), LY294002 (20 µM), MK801 (1 µM), or NAS (20 µM). Cells were then treated with the indicated concentration of glutamate or vehicle for 10 min or for the indicated time. Cells were rinsed twice with ice-cold PBS. Cell extracts were prepared in RIPA buffer (PBS with 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor mixture, and sodium orthovanadate). Protein concentration in cell extracts was determined by bicinchoninic acid assay. An equivalent amount of cellular protein (40 µg) was subjected to 10% SDS-PAGE and electrotransferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in 20 mM Tris-HCl, 150 mM NaCl, pH 7.4, with Tween 20 and incubated with primary Abs. Membranes were then washed and treated with horseradish peroxidase–conjugated secondary Abs for 1 h. Immunoblots were developed by ECL (PerkinElmer, Waltham, MA, USA). Each experiment was performed at least 3 times.
SC migration
Migration of rSCs was studied by using 6.5 mm Transwell units with 8-µm pores, as previously described (31). The bottom surface of each membrane was coated with 10 µg/ml fibronectin. We selected fibronectin as substratum because after PNS injury, fibronectin expression is induced to provide a provisional matrix for nerve regeneration (32). rSCs (1 × 105 cells) in DMEM-based Sato medium that was supplemented with 1 mg/ml bovine serum albumin (33) were added to the top Transwell chamber. In some cases, cells were pretreated with MK801 (1 µM), LY29004 (20 µM), or PD98059 (50 µM) for 15 min before adding cells to the Transwells. Reagents were added at the same concentrations to the medium in Transwells, together with 80 µM glutamate or vehicle. The bottom chamber contained 10% FBS. Cell migration was allowed to proceed for 4 h at 37°C. The upper surface of each membrane was cleaned with a cotton swab. Membranes were then stained with Diff-Quik (Dade-Behring, Deerfield, IL, USA). The number of cells on the bottom surface of each membrane was counted by using the open source software ImageJ [National Institutes of Health (NIH), Bethesda, MD, USA]. Each condition was studied with 5 internal replicates. Each experiment was performed 3 times.
Activation of ERK1/2 in SCs in vivo
Sprague-Dawley rats (200 g) from Harlan Laboratories (Indianapolis, IN, USA) were housed in pairs with a 12-h light/dark cycle and ad libitum access to food and water. Before surgery, rats were anesthetized with 5% isoflurane (Fluriso; VetOne, Boise, ID, USA). Anesthesia was maintained with 3% isoflurane. By using a sterile field, the left sciatic nerve was exposed at the midthigh level and crushed once for 15 s at the sciatic nerve notch by using flat forceps. The muscle layer then was closed by using 6.0 silk sutures and small surgical staples over the skin. Twenty-four hours later, when SC NMDA-Rs are apparently up-regulated (3), rats were reanesthetized and slowly injected immediately below the crush injury site with 2 μl glutamate (100–400 μM), NMDA (20 μM), or vehicle (PBS). A separate cohort of rats with crush-injured nerves underwent the same procedure without receiving an injection. Ten minutes later, 5 mm of sciatic nerve distal to the injury site was collected, together with uninjured contralateral nerve. Experiments were performed 3 times with duplicate or triplicate internal replicates. All procedures were performed according to protocols approved by the University of California, San Diego, Committee on Animal Research and conform to the NIH guidelines for animal use.
For immunoblot analysis, sciatic nerve tissue was extracted in RIPA buffer. Immunoblotting was performed as described above. For IF microscopy studies, deeply anesthetized rats were subjected to intracardiac perfusion with fresh PBS followed by 4% paraformaldehyde. Tissue was paraffin-embedded and tissue sections (4 µm) were immunostained sequentially with Abs specific for GFAP (1:6000) and phospho-ERK1/2 (1:3600) by using the tyramide signal amplification system (Thermo Fisher Scientific) and a Ventana Discovery Ultra (Ventana Medical Systems, Tucson, AZ, USA). This procedure allows for visualization of 2 different rabbit antibodies. First, antigen retrieval was performed by incubation with Cell-Conditioning Solution 1 (CC1; Ventana Medical Systems) for 40 min at 95°C. Next, GFAP-specific Ab was incubated with tissue sections and detected by using OmniMap donkey anti-rabbit secondary Ab followed by fluorescent-labeling with TSA-Alexa Fluor 594. Ab was fully denatured, inactivated, and removed from the tissue by treatment in CC2 (Ventana Medical Systems) for 20 min at 95°C. Next, Ab targeting phospho-ERK1/2 was applied to the slides and detected with UltraMap donkey anti-rabbit secondary Ab followed by fluorescent-labeling with TSA-Alexa Fluor 488. Fully immunostained slides were rinsed and coverslipped with ProLong gold antifade with DAPI (Thermo Fisher Scientific). Microscopic slides were imaged by using a Zeiss Upright Widefield fluorescence microscope (Zeiss, Jena, Germany). The presented images were captured at ×630 magnification.
Statistics
Studies were analyzed by Student’s t test or by 1-way ANOVA with Tukey’s post hoc analysis, using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
RESULTS
IGR subunit mRNA expression by SCs
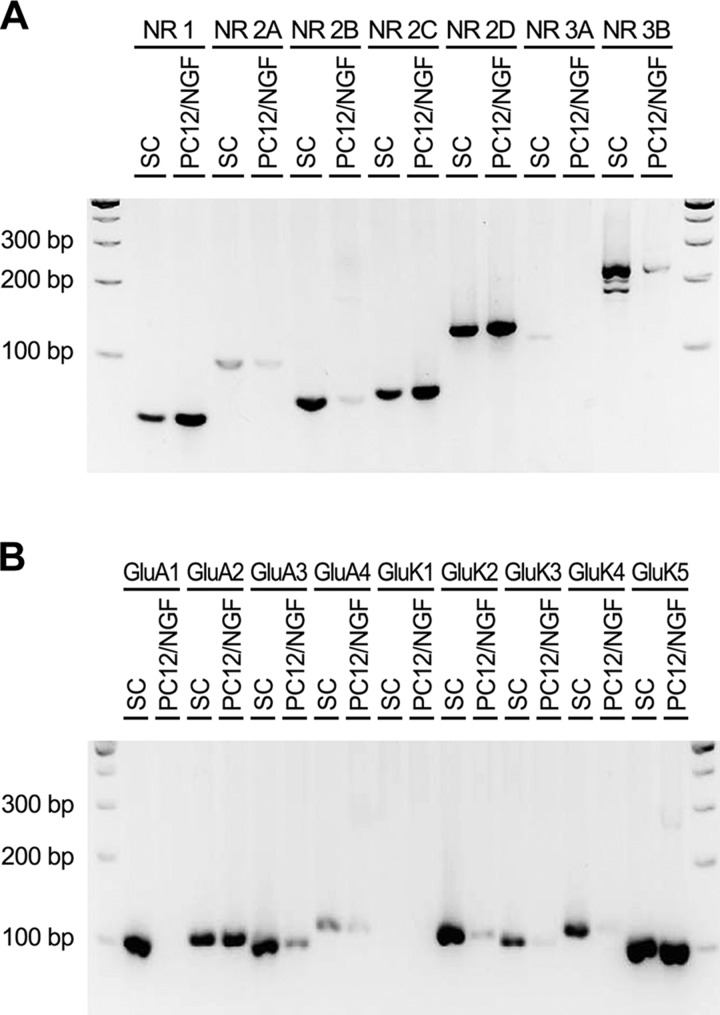
We previously demonstrated that rSCs in culture express NMDA-R NR1/GluN1 and NR2B/GluN2B protein subunits (3). rSCs express mRNAs for NR1 and NR2B in vivo in sciatic nerves (3). In the present study, we performed RT-PCR to comprehensively survey rSCs in primary culture for expression of mRNAs that encode protein subunits that are involved in IGR assembly. Figure 1A shows that cultured rSCs express mRNAs that encode NR1 and NR2B, which confirmed the results of previous immunoblotting experiments (3). rSCs also expressed mRNAs for NR2C/GluN2C, NR2D/GluN2D, and NR3B/GluN3B. Figure 1B shows that rSCs express mRNA for the essential AMPA receptor subunit, GluA2, together with mRNAs that encode GluA1, GluA3, and, perhaps, GluA4. Kainate receptor mRNAs expressed by rSCs included GluK2, GluK3, GluK4, and GluK5. Of interest, kainate receptor mRNAs were expressed at higher levels in SCs compared with NGF-β–differentiated PC12 cells. These results demonstrate that rSCs express genes that are needed for assembly of all 3 major IGRs.
Figure 1.
Rat SCs express mRNA the encodes essential subunits for expression of all 3 major IGRs. Specific oligonucleotides were used to amplify cDNA that was isolated from cultured rSCs and, as a control, NGF-β–differentiated PC12 cells. RT-PCR products were subjected to 2–3% agarose gel electrophoresis, together with 100 bp ladder standards. A) NMDA-R subunits are shown. B) Equivalent cDNA was subjected to RT-PCR to amplify subunits of AMPA and kainate receptors.
Glutamate activates cell signaling in rSCs
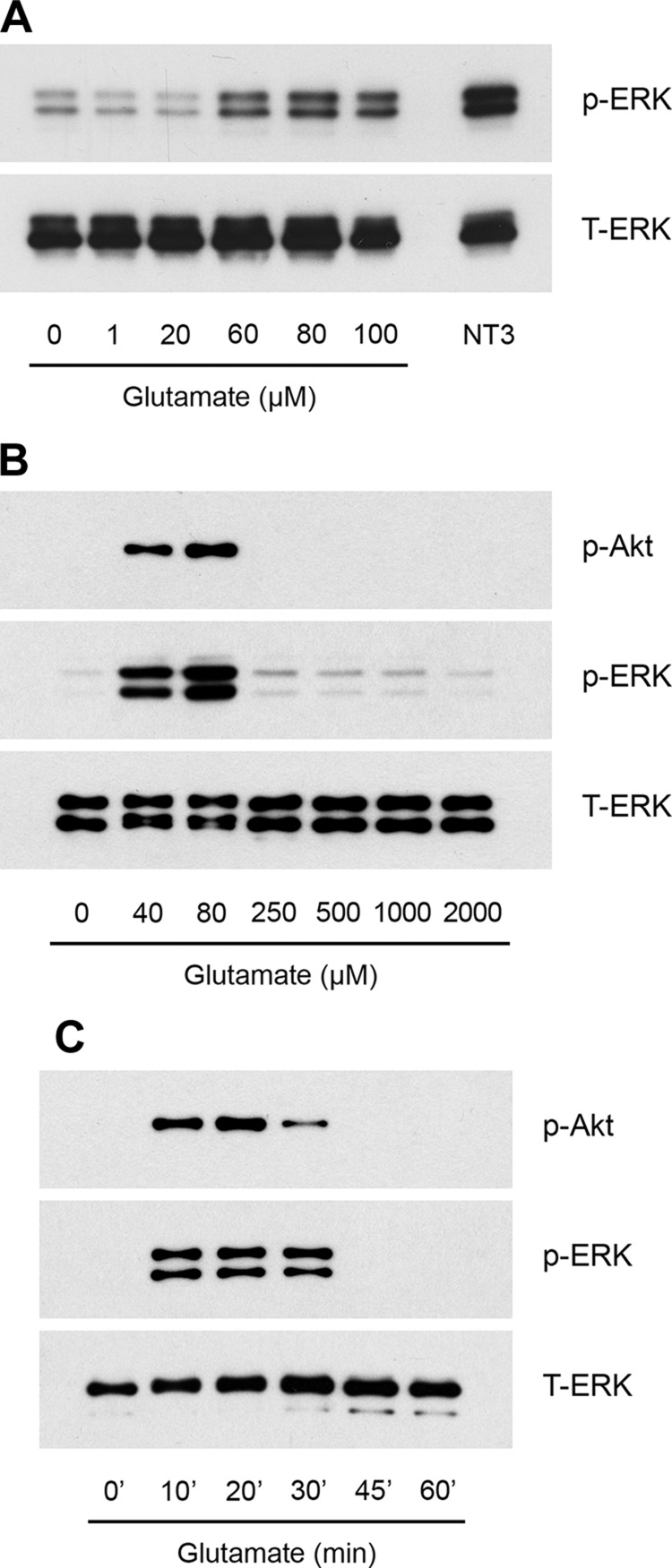
rSCs were transferred to SFM for 30 min and stimulated with increasing concentrations of glutamate for 10 min in medium that contained 0.4 mM glycine. Figure 2A shows that glutamate, at concentrations of 60, 80, and 100 µM, induced robust phosphorylation of ERK1/2. An expanded analysis of the glutamate concentration dependency of ERK1/2 activation showed that the response was bimodal. Glutamate concentrations of ≥250 µM failed to activate ERK1/2 (Fig. 2B). This bimodal response is important because glutamate was previously reported to activate cell signaling in rSCs by its effects on metabotropic glutamate when added at a concentration of 2.0 mM (22). Akt also was activated in glutamate-treated rSCs. Concentration dependency of Akt phosphorylation paralleled that observed with ERK1/2. Robust Akt activation was induced by 40 or 80 µM glutamate. Higher concentrations of glutamate failed to elicit a response.
Figure 2.
Glutamate activates cell signaling in cultured rSCs. A, B) Cells were treated with the indicated concentrations of glutamate in SFM for 10 min. As a control, cells were treated with 10 nM neurotrophin-3 (NT3; A) for 10 min. Immunoblot analysis was performed to detect phospho-ERK1/2, phospho-Akt, and total ERK1/2. C) Primary cultures of rSCs were treated for indicated times with 80 µM glutamate. Phospho-ERK1/2 and phospho-Akt were determined by immunoblot analysis. The presented studies are representative of experiments performed at least in triplicate.
We next performed time-course experiments and demonstrated that activation of ERK1/2 and Akt by 80 µM glutamate in rSCs is transient (Fig. 2C). Maximum phosphorylation of ERK1/2 and Akt was observed at 20 min. Levels of phosphorylated ERK1/2 and Akt returned to baseline by 45 min.
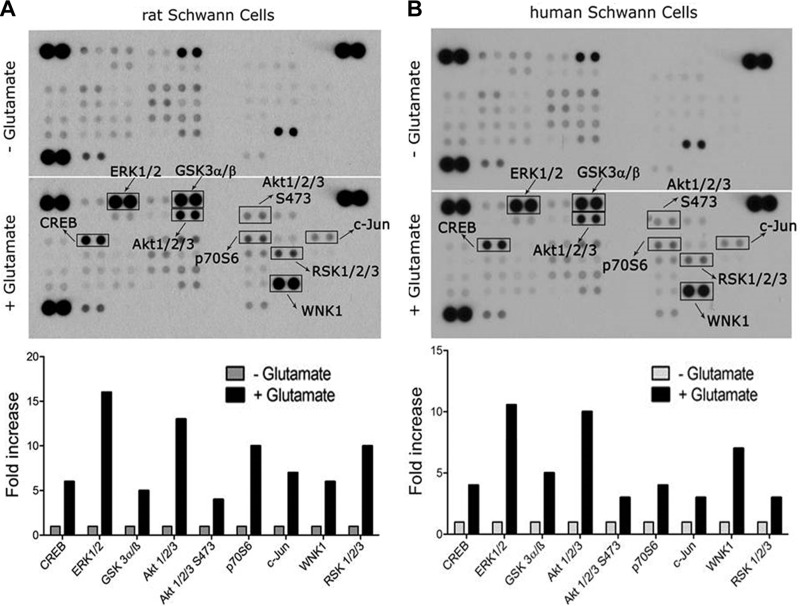
To analyze glutamate-activated cell signaling in rSCs in an unbiased manner, we applied an array approach using the phospho-kinase proteome profiler, which profiles 43 distinct protein phosphorylation events. rSCs were treated with 80 µM glutamate or vehicle for 10 min. ERK1/2 phosphorylation was detected (Fig. 3A), as anticipated on the basis of immunoblotting studies presented in Fig. 2, together with phopho-RSK, which is known to be activated downstream of ERK1/2 (34). Akt and p70 S6K were phosphorylated, which suggested activation of the PI3K/Akt/mTOR pathway (35). GSK-3 also is an Akt substrate (35, 36) that was phosphorylated in glutamate-treated rSCs. GSK-3 phosphorylation is inactivating. In SCs, GSK-3 may control cellular differentiation (37). Phosphorylation of Akt at Ser473 reflects the activity of mTORC2 or other so-called PDK2s in a pathway that complements PDK1-dependent phosphorylation of Akt Thr308 to generate the most fully activated form of Akt (38, 39).
Figure 3.
Comprehensive analysis of protein phosphorylation in glutamate-treated SCs. A) rSCs were treated with 80 µM glutamate or with vehicle for 10 min. Protein phosphorylation was analyzed by array. Bar graph shows increases in protein phosphorylation associated with glutamate treatment compared with cells treated with vehicle. Although the majority of the presented phosphorylation events are activating, some, such as GSK-3, are inactivating. B) hSCs were treated with 80 µM glutamate or with vehicle for 10 min. Protein phosphorylation was determined by array.
The transcription factor, cAMP response element binding protein (CREB), was phosphorylated in glutamate-treated rSCs. CREB is phosphorylated downstream of multiple cell signaling factors, including Akt and ERK1/2 (40). Phospho-CREB may promote SC proliferation (41). c-Jun was phosphorylated in glutamate-treated rat SCs, which is interesting given the central role played by c-Jun in activation of the SC repair program (3, 19). JNK phosphorylation was not observed at the time point examined. WNK1 is a serine-threonine kinase that is poorly characterized in SCs.
When cell signaling is activated by IGRs in neurons, PI3K or c-Src may function upstream of ERK1/2, Akt, and CREB (11, 12). We did not detect phosphorylation of the Src family kinases, c-Src, Lyn, Lck, Fyn, Yes, and Fgr, in glutamate-treated rSCs. All of these Src family kinases were represented in the array. When ERK1/2 is activated downstream of metabotropic glutamate receptors in SCs, reportedly c-Src activation serves as an essential upstream event (22).
To test whether the observed signaling responses are conserved in hSCs, we obtained SCs that were isolated from human spinal nerve and were immunopositive for S100β, GFAP, and CD90. Figure 3B shows that hSCs that were treated with 80 µM glutamate demonstrated a spectrum of phosphorylation events that were equivalent to those observed in glutamate-treated rSCs.
NMDA-Rs are principally responsible for glutamate signaling in rSCs
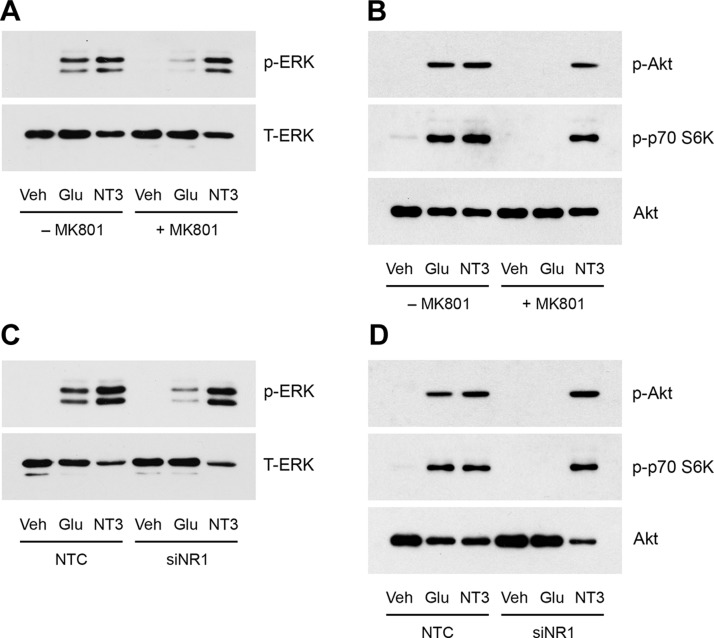
To determine whether NMDA-Rs contribute to the cell signaling response observed in glutamate-treated rSCs, we pretreated rSCs with the selective NMDA-R inhibitor, MK801 (1.0 µM), for 30 min. Figure 4A shows that MK801 almost completely blocked ERK1/2 activation in response to 80 µM glutamate. MK801 did not affect ERK1/2 activation by neurotrophin-3. Figure 4B shows that pretreating rSCs with MK801 blocked Akt phosphorylation by glutamate. Treating rSCs with glutamate activated p70 S6K, as would be predicted by our array studies—this response also was blocked by MK801.
Figure 4.
The role of NMDA-Rs in glutamate-activated cell signaling in SCs. A) rSCs were pretreated with MK801 (1 µM) or vehicle for 30 min, then with 80 µM glutamate or 10 nM neurotrophin-3 (NT3) for 10 min. Phospho-ERK1/2 and total ERK1/2 were determined by immunoblot analysis. B) rSCs were treated with MK801 or vehicle, then with glutamate, as shown in panel A. Phospho-Akt, phospho-p70 S6K, and total Akt were determined by immunoblot analysis. C) rSCs that were transfected with NR1-specific or NTC siRNA were treated with glutamate (80 µM) or NT3 (10 nM) for 10 min. Phospho-ERK1/2 and total ERK1/2 were determined by immunoblot analysis. D) rSCs that were transfected with NR1-specific or NTC siRNA were treated with glutamate (80 µM) or NT3 (10 nM) for 10 min. Phospho-Akt, phospho-p70 S6K, and total Akt were determined by immunoblot analysis. The presented images are representative of studies performed at least in triplicate.
To confirm that NMDA-Rs are largely responsible for glutamate-activated cell signaling in rSCs, we silenced expression of the essential NMDA-R NR1 subunit, as previously described (3). Control cells were transfected with NTC siRNA. Figure 4C shows that ERK1/2 activation in response to 80 µM glutamate was substantially inhibited by NR1 gene silencing. Activation of Akt and p70 S6K also was almost completely blocked by NR1 gene-silencing (Fig. 4D), which suggested that NMDA-Rs are responsible for the entire spectrum of glutamate-triggered cell signaling events.
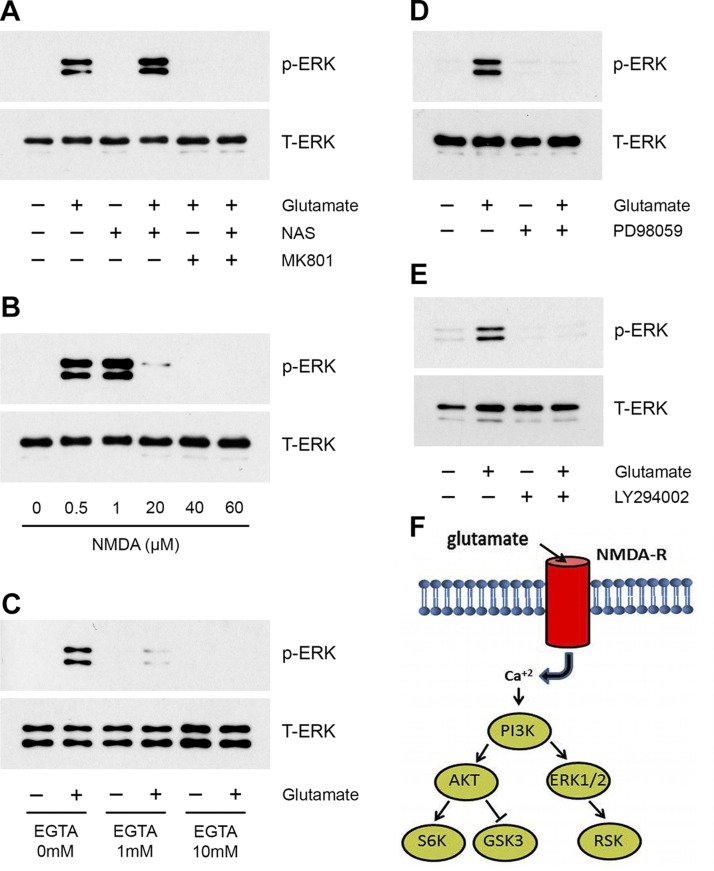
We next treated rSCs with the selective calcium-permeable AMPA receptor inhibitor, NAS (20 µM), for 30 min. NAS failed to inhibit ERK1/2 activation in response to 80 µM glutamate (Fig. 5A). By contrast, MK801, alone or in combination with NAS, was highly effective in blocking ERK1/2 activation.
Figure 5.
IGR activity in glutamate-triggered ERK1/2 activation. A) rSCs were pretreated with MK801 (1 µM), NAS (20 µM), or MK801 + NAS, then with glutamate (80 µM) or vehicle, as indicated, for 10 min. Phospho-ERK1/2 and total ERK1/2 were determined by immunoblot analysis. B) rSCs were treated with increasing concentrations of NMDA for 10 min. Phospho-ERK1/2 and total ERK1/2 were determined. C) rSCs were treated with the indicated concentrations of EGTA and glutamate (80 µM) or vehicle for 10 min. Phospho-ERK1/2 and total ERK1/2 were determined by immunoblot analysis. D) rSCs were pretreated with PD98059 (50 µM) or vehicle for 30 min, then with glutamate (80 µM) as indicated for 10 min. E) rSCs were pretreated with LY294002 (20 µM) or vehicle for 30 min, then with glutamate (80 µM) as indicated for 10 min. Phospho-ERK1/2 and total ERK1/2 were determined by immunoblot analysis. The presented images are representative of studies performed at least in triplicate. F) Model summarizing the pathway by which glutamate activates cell signaling in rSCs in an NMDA-R– and PI3K-dependent manner.
We also treated rSCs with different concentrations of the NMDA-R–selective agonist, NMDA, for 10 min. As shown in Fig. 5B, NMDA activated ERK1/2 similarly to glutamate; however, the concentration dependency of the response was shifted down compared with glutamate. ERK1/2 phosphorylation was observed with 0.5 and 1.0 µM NMDA. The response to NMDA was bimodal; higher concentrations of NMDA failed to activate ERK1/2, which mimicked the bimodal response observed with glutamate.
Next, rSCs were pretreated with the cell-impermeable calcium chelator, EGTA, to test whether calcium transport into SCs is necessary for activation of cell signaling by glutamate. EGTA blocked ERK1/2 activation in response to 80 µM glutamate (Fig. 5C).
PI3K is an essential upstream mediator of ERK1/2 activation in glutamate-treated SCs
Akt, GSK3, and p70 S6K, which are phosphorylated in glutamate-treated rSCs, are well-described substrates for kinases downstream of PI3K (35, 36). Cell signaling factors that function upstream of ERK1/2 are more diverse. We therefore conducted experiments to identify the pathway that couples IGR activation to ERK1/2. MEK inhibitor PD98059 blocked ERK1/2 activation in response to glutamate in rSCs, as anticipated (Fig. 5D). PI3K inhibitor LY294003 also completely blocked ERK1/2 activation by 80 µM glutamate (Fig. 5E). These results suggest that PI3K serves as an upstream activator of both Akt and ERK1/2 when NMDA-Rs are activated in glutamate-treated rSCs. p70 S6K, GSK3, and RSK are then phosphorylated downstream of Akt and ERK1/2, as shown in the model presented in Fig. 5F.
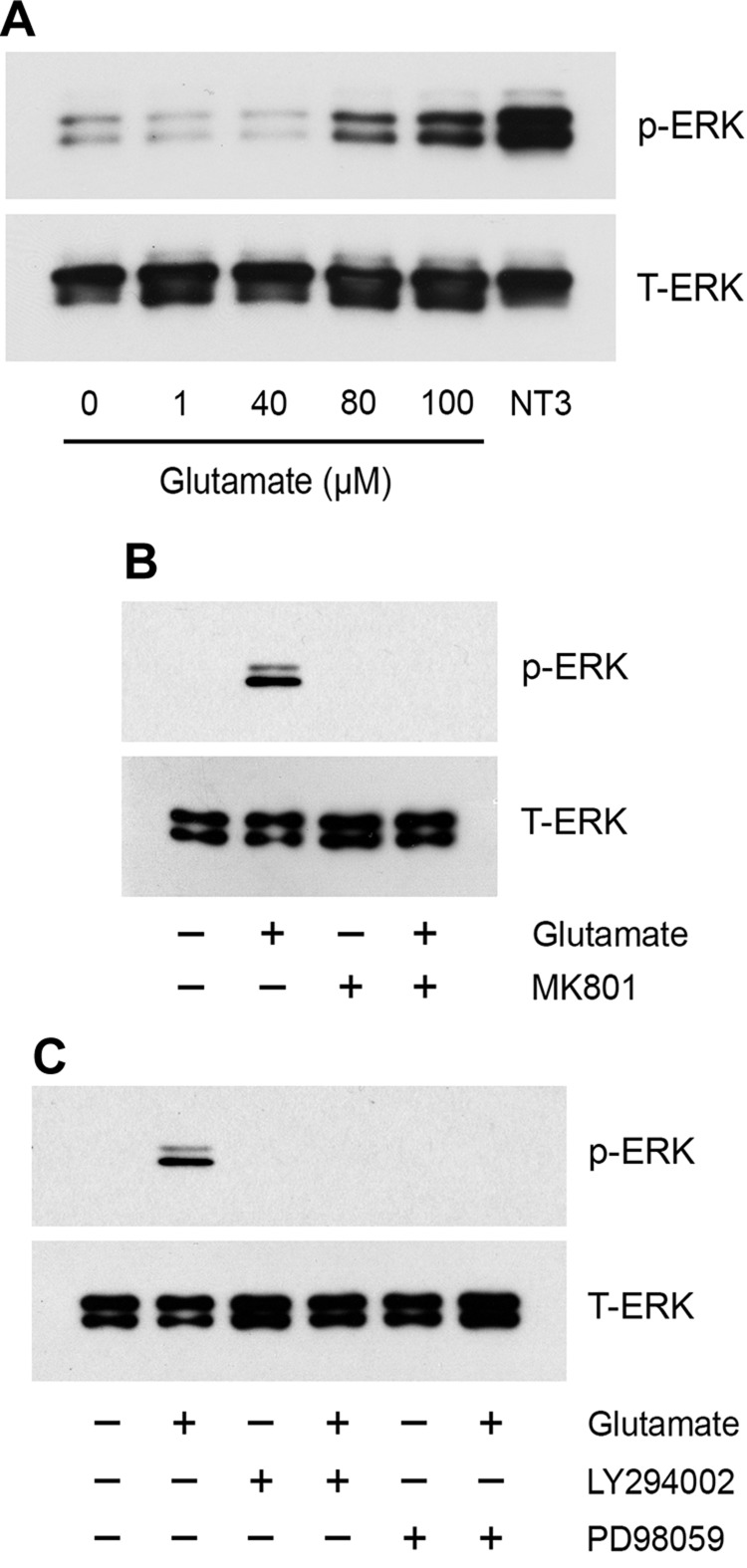
Next, we conducted experiments to examine cell signaling in hSCs. Glutamate (80 and 100 µM) activated ERK1/2 (Fig. 6A). ERK1/2 activation was blocked by MK801 (Fig. 6B), which suggested a role for NMDA-Rs, and by either PD98059 or LY294002 (Fig. 6C). These results and the results of the phosphoprotein array studies demonstrate that the signaling response, elicited by glutamate, is qualitatively equivalent in rSCs and hSCs.
Figure 6.
Glutamate-activated cell signaling in hSCs. A) hSCs were treated with the indicated concentrations of glutamate for 10 min, with neurotrophin-3 (NT3; 10 nM), or with vehicle. B) hSCs were pretreated with MK801 (1 µM) for 30 min, then with glutamate (80 µM) or vehicle for 10 min. C) hSCs were pretreated with PD98059 (50 µM) or LY294002 (20 µM) for 30 min, then with glutamate (80 µM) or vehicle as indicated. Phospho-ERK1/2 and total ERK1/2 were determined by immunoblot analysis. The presented images are representative of studies performed at least in duplicate.
Glutamate activates ERK1/2 in SCs in vivo in injured sciatic nerves
Rat sciatic nerves were subjected to crush injury and studied 24 h later, because we previously demonstrated that NMDA-Rs may be up-regulated in the distal segment under these conditions (3). Glutamate, NMDA, or vehicle (2.0 µl) were injected directly into the injured nerve, as previously described (3, 31). Nerve tissue, immediately distal to the injury site, was harvested 10 min later and analyzed by immunoblotting to determine ERK1/2 phosphorylation.
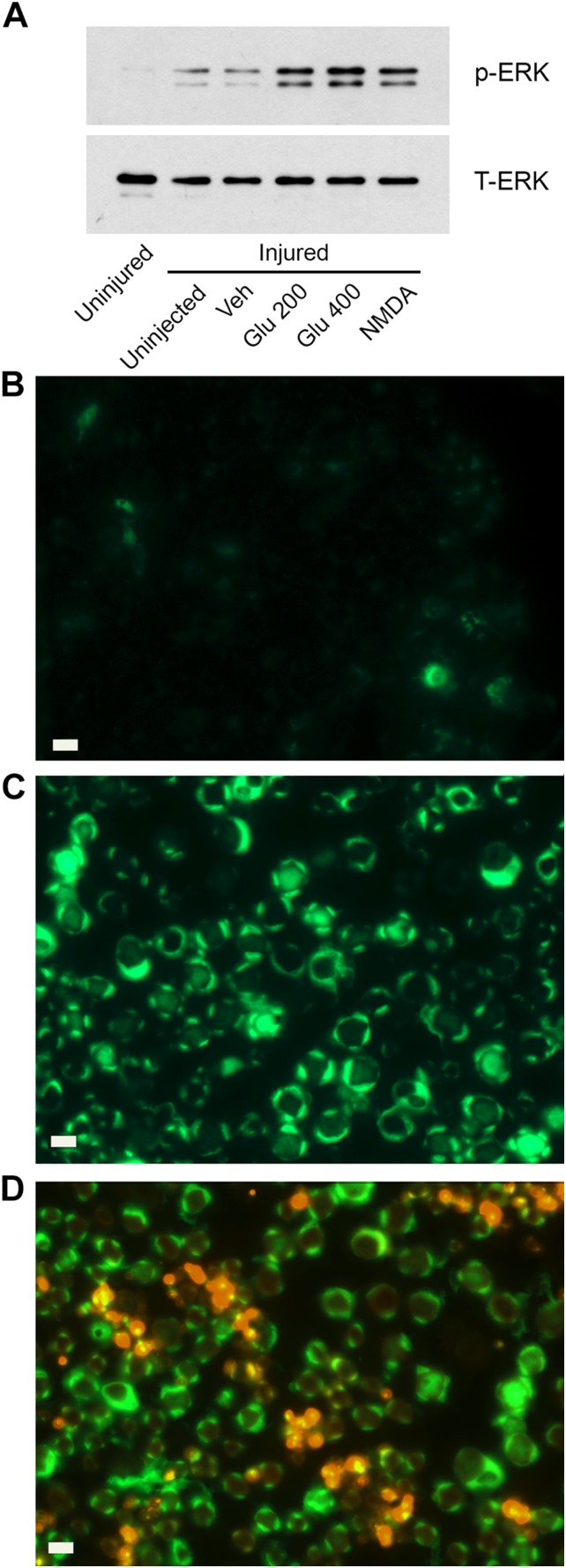
Immunoblots in Fig. 7A show that minimal phospho-ERK1/2 was detected in uninjured contralateral sciatic nerve, as anticipated (3, 31). A modest increase in phospho-ERK1/2 was noted in crush-injured nerve before injection of reagents or after injection of vehicle (PBS). By contrast, phospho-ERK1/2 was substantially increased after injection of glutamate (200 or 400 µM) or NMDA (20 µM). Agonist concentrations were increased in these studies, compared with those used in in vitro studies, to account for dilution of samples in the sciatic nerve.
Figure 7.
Glutamate activates ERK1/2 in SCs in vivo. A) Crush-injured sciatic nerves were injected with 2.0 µl PBS (vehicle), 200 µM glutamate, 400 µM glutamate, or 20 µM NMDA. Nerve tissue was harvested 10 min later. As controls, we also harvested crush-injured nerve that was not injected (uninjected) and contralateral nerve that was not injured (uninjured). Immunoblot analysis was performed to detect phospho-ERK1/2 and total ERK1/2. B–D) Crush-injured sciatic nerve was injected with 100 µM glutamate (C, D) or harvested before (B). IF microscopy was performed to detect phospho-ERK1/2 (green immunofluorescence). Tissue was also immunostained to detect GFAP (red; D). Scale bars, 20 μm.
To determine whether ERK1/2 was phosphorylated in SCs in vivo after injection of glutamate, we analyzed distal segments of crush-injured sciatic nerves by IF microscopy. Photomicrographs of control, uninjected nerve, and glutamate-injected nerve are shown in Fig. 7B, C, respectively. These photomicrographs are presented at equal exposure. All images were prepared avoiding epineurium.
Phospho-ERK1/2 immunoreactivity increased after glutamate injection and was readily identified morphologically in myelinated SC crescents and inner glial loops. To examine nonmyelinating SCs, we performed dual-labeling IF microscopy for GFAP and phospho-ERK1/2. GFAP is a biomarker of nonmyelinating SCs, although late in the course of PNS injury, GFAP may also be up-regulated in myelinated SCs (42). Figure 7D shows colocalization of phospho-ERK1/2 with GFAP (yellow costaining) in glutamate-injected nerves, which suggested activation of ERK1/2 in nonmyelinating SCs. We conclude that both myelinated SCs and nonmyelinating SCs respond to glutamate and activate cell signaling in vivo.
IGRs promote SC migration
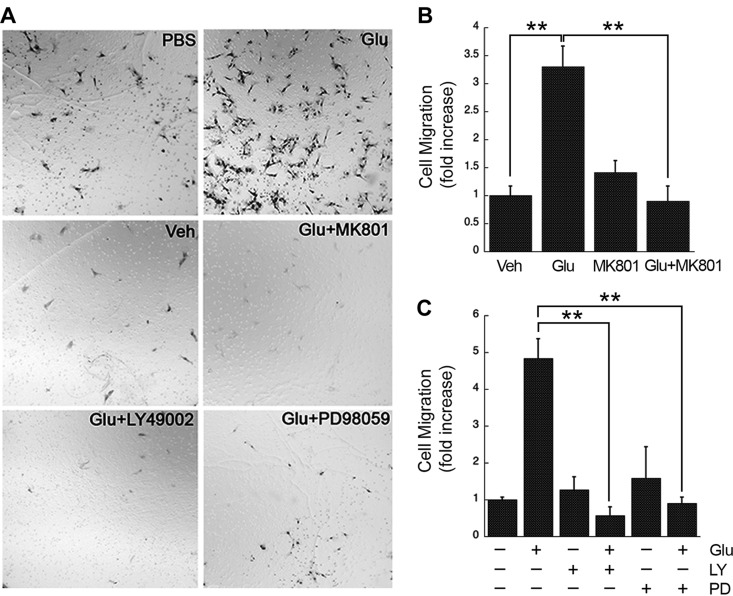
We and others have shown that activation of cell signaling factors, including ERK1/2, Akt, and Rac1, promotes SC migration (3, 31, 43, 44). We therefore studied the effects of glutamate on SC migration, as a representative metric for reporting the effects of activated cell signaling on SC physiology. Glutamate (80 µM) significantly increased migration of rSCs in Transwell units (Fig. 8A, B; P < 0.01). The response to glutamate was blocked by MK801, which indicated an essential role for NMDA-Rs. LY29004 and PD98059 also blocked rSC migration in response to glutamate (Fig. 8C). The activity of PD98059 implicates ERK1/2 activation. The activity of LY29004 implicates PI3K, which may be important because of its role in activating ERK1/2 or because it activates ERK1/2 in combination with other cell signaling factors.
Figure 8.
Glutamate induces SC migration by an NMDA-R– and PI3K-dependent mechanism. A) Images of rSCs that migrated to the underside of Transwell membranes. Agents added to each Transwell, including 80 µM glutamate, MK801 (1 µM), PI3K inhibitor LY294008 (LY; 20 µM), and MEK inhibitor PD98059 (PD; 50 µM), are specified in each panel. Migration was allowed to proceed for 4 h. B) Quantification of cell migration results showing the effects of MK801 on glutamate-stimulated cell migration (means ± sem, n = 3). C) Quantification of cell migration results showing the effects of cell signaling inhibitors on glutamate-stimulated cell migration (means ± sem, n = 3). **P < 0.01
DISCUSSION
In neurons, various IGRs collaborate to control excitatory synaptic transmission; however, many other activities have been described for these receptors (1). NMDA-Rs control differentiation of neuronal progenitors and neurite development in addition to their well-established function in synaptic plasticity (45, 46). In SCs, we showed that NMDA-Rs respond to their protein ligand, tPA, and activate cell signaling (3). We also showed that NMDA-Rs function together with LDL receptor–related protein-1 as coreceptors to more effectively activate SC signaling in response to tPA. When we silenced expression of the obligate NMDA-R subunit, NR1, in cultured SCs, SC survival was compromised and apoptosis was observed in low-serum medium or in the presence of TNF-α (3).
In the current study, we explored the ability of IGRs to activate cell signaling in SCs in response to glutamate. We are interested in SC signaling because of the important surveillance role played by these cells. In the injured PNS, SCs serve as first responders (19, 20), undergoing robust modifications in gene transcription and cell physiology to clear damaged tissue and facilitate PNS repair. The spectrum of extracellular molecules recognized by SCs in the injured nerve milieu remains incompletely understood; however, proteins that are mainly intracellular and released only when neurons are injured and small molecules, such as glutamate, deserve consideration. These extracellular mediators may serve to temporally regulate glial-axon interactions in PNS injury.
Our data indicate that glutamate triggers a diverse cell signaling response in rSCs and hSCs in primary culture. Many of the regulated cell signaling factors, observed by phosphoprotein array, are phosphorylated downstream of PI3K. Although our analysis of mRNA expression suggests that SCs may assemble all 3 major IGRs, cell signaling in response to glutamate in cultured SCs was mainly a function of NMDA-Rs. Treating SCs with MK801 and silencing of the NR1 NMDA-R subunit substantially inhibited activation of cell signaling in response to glutamate. We cannot entirely rule out a contributing role for AMPA or kainate receptors, although a selective AMPA receptor antagonist was ineffective at blocking ERK1/2 activation by glutamate when added to cultures of SCs in the absence of MK801.
We confirmed that cell signaling is activated by glutamate in SCs in vivo by injecting glutamate or NMDA directly into crush-injured sciatic nerves in rats. By using IF microscopy, we obtained evidence that suggested that SCs with different phenotypes are glutamate responsive, including nonmyelinating SCs that form Remak bundles and SCs that myelinate axons before crush injury. Although our experiments in rats do not definitively identify the IGR responsible for activating ERK1/2 in SCs in vivo, the fact that NMDA was active suggests that NMDA-Rs are involved.
Activation of cell signaling by IGRs in response to glutamate differed in a number of ways from the previously reported glutamate response mediated by metabotropic glutamate receptors (22). First and foremost, the concentration of glutamate required to activate IGRs was substantially lower than that used to activate metabotropic glutamate receptor (2.0 mM). When we studied concentrations of glutamate >1 mM, the glutamate response was no longer apparent. ERK1/2 phosphorylation in response to IGR activation was robust even when glutamate was added in isolation. In the previous study on metabotropic glutamate receptors (22), glutamate was shown to mainly augment ERK1/2 activation in response to a stronger stimulant, neuregulin-1. Furthermore, the previously reported activation of metabotropic receptors by glutamate required c-Src as an upstream factor. In the IGR response, Src family kinases were not involved. Instead, PI3K functioned upstream of both Akt and ERK1/2.
Because NMDA-Rs activated ERK1/2, Akt, and cell signaling factors that are known to function downstream of these kinases, NMDA-Rs may be clustered with other SC receptors that also regulate these 2 cell signaling factors simultaneously (3, 43, 47–49). These SC receptors have been reported to regulate cell cycle progression during development, SC differentiation and myelination, SC migration, and transformations in SC phenotype that accompany the response to PNS injury. The importance of NMDA-Rs in SC survival was previously reported (3). We have now shown that activation of NMDA-Rs by glutamate promotes SC migration. The full spectrum of consequences of glutamate-activated IGR signaling in SCs remains to be investigated.
In response to glutamate, activation of Akt and ERK1/2 was transient. Whether a signaling response is sustained or transient can be very important in determining the effects on cell physiology. A classic example involves cultured PC12 cells, which can be induced to proliferate or differentiate depending on the duration of ERK1/2 activation (50). Protein phosphatases play a key role in limiting the duration of kinase activation. For example, phosphatase and tensin homolog opposes PI3K and may thereby eliminate a key upstream stimulus that is required to maintain activation of ERK1/2 and Akt (51).
The first recognized NMDA-R–targeting drug was ketamine, which has been used in anesthesia, sedation, and pain management (52). Namenda is an NMDA-R antagonist, indicated for treatment of Alzheimer’s disease (53). NMDA-R–targeting drugs are in development to treat depression (54–56). It is therefore feasible that numerous patients may receive long-term therapy with drugs that target NMDA-Rs. The studies presented here suggest that antagonizing the NMDA-Rs may have significant effects on cell signaling in SCs and SC physiology. Ultimately, this may dysregulate glial-axon interactions and facilitate development of painful peripheral neuropathies (19, 57). Understanding the off-target effects of NMDA-R–targeting drugs in such cells as SCs, especially in conditions in which PNS injury may be present, is thus an important problem.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R01-HL60551 (to S.L.G.), and by FIRB 2013 (RBFR13RBK9) from the Italian Ministry of Education, Universities, and Research (to E.M.). The authors thank Katie Fichter [Department of Anesthesiology, University of California, San Diego (UCSD) School of Medicine] for technical assistance. IF microscopy was performed using the UCSD School of Medicine Light Microscopy Core Facility, sponsored by the NIH National Institute of Neurological Disorders and Stroke Grant P30-NS047101.
Glossary
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CREB
cAMP response element binding protein
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- Glu
glutamate
- GSK-3
glycogen synthase kinase-3
- hSC
human Schwann cell
- IF
immunofluorescence
- IGR
ionotropic glutamate receptor
- MK801
dizocilpine
- NAS
1-naphthyl-acetyl spermine trihydrochloride
- NGF-β
nerve growth factor-β
- NMDA
N-methyl-d-aspartic acid
- NMDA-R
N-methyl-d-aspartic acid receptor
- NTC
nontargeting control
- p70 S6K
p70 S6-kinase
- PNS
peripheral nervous system
- rSC
rat Schwann cell
- RSK
ribosomal S6 kinase
- SC
Schwann cell
- SFM
serum-free medium
- siRNA
small interfering RNA
- tPA
tissue-type plasminogen activator
AUTHOR CONTRIBUTIONS
W. M. Campana, E. Mantuano, K. Henry, and S. L. Gonias conceived of and designed the experiments; W. M. Campana, E. Mantuano, P. Azmoon, K. Henry, M. A. Banki, J. H. Kim, D. P. Pizzo, and S. L. Gonias performed experiments and/or performed data analysis; W. M. Campana, E. Mantuano, and S. L. Gonias wrote the paper; and all authors agreed on the manuscript before submission.
REFERENCES
- 1.Traynelis S. F., Wollmuth L. P., McBain C. J., Menniti F. S., Vance K. M., Ogden K. K., Hansen K. B., Yuan H., Myers S. J., Dingledine R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill S. S., Pulido O. M. (2001) Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol. Pathol. 29, 208–223 [DOI] [PubMed] [Google Scholar]
- 3.Mantuano E., Lam M. S., Shibayama M., Campana W. M., Gonias S. L. (2015) The NMDA receptor functions independently and as an LRP1 co-receptor to promote Schwann cell survival and migration. J. Cell Sci. 128, 3478–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans P. D., Reale V., Merzon R. M., Villegas J. (1991) N-methyl-D-aspartate (NMDA) and non-NMDA type glutamate receptors are present on squid giant axon Schwann cells. J. Exp. Biol. 157, 593–600 [DOI] [PubMed] [Google Scholar]
- 5.Fink T., Davey D. F., Ansselin A. D. (1999) Glutaminergic and adrenergic receptors expressed on adult guinea pig Schwann cells in vitro. Can. J. Physiol. Pharmacol. 77, 204–210 [PubMed] [Google Scholar]
- 6.Liu G. J., Bennett M. R. (2003) ATP secretion from nerve trunks and Schwann cells mediated by glutamate. Neuroreport 14, 2079–2083 [DOI] [PubMed] [Google Scholar]
- 7.Lieberman E. M., Abbott N. J., Hassan S. (1989) Evidence that glutamate mediates axon-to-Schwann cell signaling in the squid. Glia 2, 94–102 [DOI] [PubMed] [Google Scholar]
- 8.Micu I., Plemel J. R., Lachance C., Proft J., Jansen A. J., Cummins K., van Minnen J., Stys P. K. (2016) The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 276, 41–50 [DOI] [PubMed] [Google Scholar]
- 9.Micu I., Jiang Q., Coderre E., Ridsdale A., Zhang L., Woulfe J., Yin X., Trapp B. D., McRory J. E., Rehak R., Zamponi G. W., Wang W., Stys P. K. (2006) NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 [DOI] [PubMed] [Google Scholar]
- 10.Christensen P. C., Welch N. C., Brideau C., Stys P. K. (2016) Functional ionotropic glutamate receptors on peripheral axons and myelin. Muscle Nerve 54, 451–459 [DOI] [PubMed] [Google Scholar]
- 11.Perkinton M. S., Ip J. K., Wood G. L., Crossthwaite A. J., Williams R. J. (2002) Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurones. J. Neurochem. 80, 239–254 [DOI] [PubMed] [Google Scholar]
- 12.Crossthwaite A. J., Valli H., Williams R. J. (2004) Inhibiting Src family tyrosine kinase activity blocks glutamate signalling to ERK1/2 and Akt/PKB but not JNK in cultured striatal neurones. J. Neurochem. 88, 1127–1139 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y. T., Salter M. W. (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369, 233–235 [DOI] [PubMed] [Google Scholar]
- 14.Salter M. W., Kalia L. V. (2004) Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328 [DOI] [PubMed] [Google Scholar]
- 15.Xu J., Weerapura M., Ali M. K., Jackson M. F., Li H., Lei G., Xue S., Kwan C. L., Manolson M. F., Yang K., Macdonald J. F., Yu X. M. (2008) Control of excitatory synaptic transmission by C-terminal Src kinase. J. Biol. Chem. 283, 17503–17514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weilinger N. L., Lohman A. W., Rakai B. D., Ma E. M., Bialecki J., Maslieieva V., Rilea T., Bandet M. V., Ikuta N. T., Scott L., Colicos M. A., Teskey G. C., Winship I. R., Thompson R. J. (2016) Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 19, 432–442 [DOI] [PubMed] [Google Scholar]
- 17.Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E. T., Vivien D., Buisson A. (2001) The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 7, 59–64 [DOI] [PubMed] [Google Scholar]
- 18.Mantuano E., Lam M. S., Gonias S. L. (2013) LRP1 assembles unique co-receptor systems to initiate cell signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein. J. Biol. Chem. 288, 34009–34018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campana W. M. (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav. Immun. 21, 522–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jessen K. R., Mirsky R. (2016) The repair Schwann cell and its function in regenerating nerves. J. Physiol. 594, 3521–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niswender C. M., Conn P. J. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitoh F., Wakatsuki S., Tokunaga S., Fujieda H., Araki T. (2016) Glutamate signals through mGluR2 to control Schwann cell differentiation and proliferation. Sci. Rep. 6, 29856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campana W. M., Li X., Dragojlovic N., Janes J., Gaultier A., Gonias S. L. (2006) The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J. Neurosci. 26, 11197–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan P. H., Yang L. C., Shih H. C., Lan K. C., Cheng J. T. (2005) Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 12, 59–66 [DOI] [PubMed] [Google Scholar]
- 25.Tan P. H., Chia Y. Y., Chow L. H., Chen J. J., Yang L. C., Hung K. C., Chen H. S., Kuo C. H. (2010) Gene knockdown of the N-methyl-D-aspartate receptor NR1 subunit with subcutaneous small interfering RNA reduces inflammation-induced nociception in rats. Anesthesiology 112, 1482–1493 [DOI] [PubMed] [Google Scholar]
- 26.Allgaier C., Scheibler P., Müller D., Feuerstein T. J., Illes P. (1999) NMDA receptor characterization and subunit expression in rat cultured mesencephalic neurones. Br. J. Pharmacol. 126, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L., Margolis F. L., Shipley M. T., Lidow M. S. (1998) Identification of a long variant of mRNA encoding the NR3 subunit of the NMDA receptor: its regional distribution and developmental expression in the rat brain. FEBS Lett. 441, 392–396 [DOI] [PubMed] [Google Scholar]
- 28.Nishi M., Hinds H., Lu H. P., Kawata M., Hayashi Y. (2001) Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J. Neurosci. 21, RC185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau J. C., Kroes R. A., Moskal J. R., Linsenmeier R. A. (2013) Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol. Vis. 19, 1538–1553 [PMC free article] [PubMed] [Google Scholar]
- 30.Meinhardt M. W., Hansson A. C., Perreau-Lenz S., Bauder-Wenz C., Stählin O., Heilig M., Harper C., Drescher K. U., Spanagel R., Sommer W. H. (2013) Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J. Neurosci. 33, 2794–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantuano E., Inoue G., Li X., Takahashi K., Gaultier A., Gonias S. L., Campana W. M. (2008) The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J. Neurosci. 28, 11571–11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akassoglou K., Strickland S. (2002) Nervous system pathology: the fibrin perspective. Biol. Chem. 383, 37–45 [DOI] [PubMed] [Google Scholar]
- 33.Bottenstein J. E., Sato G. H. (1980) Fibronectin and polylysine requirement for proliferation of neuroblastoma cells in defined medium. Exp. Cell Res. 129, 361–366 [DOI] [PubMed] [Google Scholar]
- 34.Anjum R., Blenis J. (2008) The RSK family of kinases: emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 9, 747–758 [DOI] [PubMed] [Google Scholar]
- 35.Asnaghi L., Bruno P., Priulla M., Nicolin A. (2004) mTOR: a protein kinase switching between life and death. Pharmacol. Res. 50, 545–549 [DOI] [PubMed] [Google Scholar]
- 36.Nicholson K. M., Anderson N. G. (2002) The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 14, 381–395 [DOI] [PubMed] [Google Scholar]
- 37.Ogata T., Iijima S., Hoshikawa S., Miura T., Yamamoto S., Oda H., Nakamura K., Tanaka S. (2004) Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 24, 6724–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman M. E., Apsel B., Uotila A., Loewith R., Knight Z. A., Ruggero D., Shokat K. M. (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayard E., Xue G., Parcellier A., Bozulic L., Hemmings B. A. (2010) Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr. Top. Microbiol. Immunol. 346, 31–56 [DOI] [PubMed] [Google Scholar]
- 40.Mayr B., Montminy M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2, 599–609 [DOI] [PubMed] [Google Scholar]
- 41.Lee M. M., Badache A., DeVries G. H. (1999) Phosphorylation of CREB in axon-induced Schwann cell proliferation. J. Neurosci. Res. 55, 702–712 [DOI] [PubMed] [Google Scholar]
- 42.Triolo D., Dina G., Lorenzetti I., Malaguti M., Morana P., Del Carro U., Comi G., Messing A., Quattrini A., Previtali S. C. (2006) Loss of glial fibrillary acidic protein (GFAP) impairs Schwann cell proliferation and delays nerve regeneration after damage. J. Cell Sci. 119, 3981–3993 [DOI] [PubMed] [Google Scholar]
- 43.Yu H., Zhu L., Li C., Sha D., Pan H., Wang N., Ma S. (2015) ERK1/2 and AKT are vital factors in regulation of the migration of rat Schwann cells. J. Vet. Med. Sci. 77, 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantuano E., Jo M., Gonias S. L., Campana W. M. (2010) Low density lipoprotein receptor-related protein (LRP1) regulates Rac1 and RhoA reciprocally to control Schwann cell adhesion and migration. J. Biol. Chem. 285, 14259–14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nacher J., McEwen B. S. (2006) The role of N-methyl-D-asparate receptors in neurogenesis. Hippocampus 16, 267–270 [DOI] [PubMed] [Google Scholar]
- 46.Rajan I., Cline H. T. (1998) Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J. Neurosci. 18, 7836–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monje P. V., Bartlett Bunge M., Wood P. M. (2006) Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia 53, 649–659 [DOI] [PubMed] [Google Scholar]
- 48.Mantuano E., Mukandala G., Li X., Campana W. M., Gonias S. L. (2008) Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J. Biol. Chem. 283, 19904–19911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamazaki T., Sabit H., Oya T., Ishii Y., Hamashima T., Tokunaga A., Ishizawa S., Jie S., Kurashige Y., Matsushima T., Furuta I., Noguchi M., Sasahara M. (2009) Activation of MAP kinases, Akt and PDGF receptors in injured peripheral nerves. J. Peripher. Nerv. Syst. 14, 165–176 [DOI] [PubMed] [Google Scholar]
- 50.Marshall C. J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 [DOI] [PubMed] [Google Scholar]
- 51.Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 [DOI] [PubMed] [Google Scholar]
- 52.Anis N. A., Berry S. C., Burton N. R., Lodge D. (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br. J. Pharmacol. 79, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson D. M., Keating G. M. (2006) Memantine: a review of its use in Alzheimer’s disease. Drugs 66, 1515–1534 [DOI] [PubMed] [Google Scholar]
- 54.Zarate C. A. Jr., Singh J. B., Carlson P. J., Brutsche N. E., Ameli R., Luckenbaugh D. A., Charney D. S., Manji H. K. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 [DOI] [PubMed] [Google Scholar]
- 55.Ghasemi M., Phillips C., Trillo L., De Miguel Z., Das D., Salehi A. (2014) The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci. Biobehav. Rev. 47, 336–358 [DOI] [PubMed] [Google Scholar]
- 56.Moskal J. R., Burch R., Burgdorf J. S., Kroes R. A., Stanton P. K., Disterhoft J. F., Leander J. D. (2014) GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin. Investig. Drugs 23, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orita S., Henry K., Mantuano E., Yamauchi K., De Corato A., Ishikawa T., Feltri M. L., Wrabetz L., Gaultier A., Pollack M., Ellisman M., Takahashi K., Gonias S. L., Campana W. M. (2013) Schwann cell LRP1 regulates remak bundle ultrastructure and axonal interactions to prevent neuropathic pain. J. Neurosci. 33, 5590–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]