Abstract
Cardiotrophin (CT)-1 is a regulator of glucose and lipid homeostasis. In the present study, we analyzed whether CT-1 also acts to peripherally regulate metabolic rhythms and adipose tissue core clock genes in mice. Moreover, the circadian pattern of plasma CT-1 levels was evaluated in normal-weight and overweight subjects. The circadian rhythmicity of oxygen consumption rate (Vo2) was disrupted in aged obese CT-1-deficient (CT-1−/−) mice (12 mo). Although circadian rhythms of Vo2 were conserved in young lean CT-1−/− mice (2 mo), CT-1 deficiency caused a phase shift of the acrophase. Most of the clock genes studied (Clock, Bmal1, and Per2) displayed a circadian rhythm in adipose tissue of both wild-type (WT) and CT-1−/− mice. However, the pattern was altered in CT-1−/− mice toward a lower percentage of the rhythm or lower amplitude, especially for Bmal1 and Clock. Moreover, CT-1 mRNA levels in adipose tissue showed significant circadian fluctuations in young WT mice. In humans, CT-1 plasma profile exhibited a 24-h circadian rhythm in normal-weight but not in overweight subjects. The 24-h pattern of CT-1 was characterized by a pronounced increase during the night (from 02:00 to 08:00). These observations suggest a potential role for CT-1 in the regulation of metabolic circadian rhythms.—López-Yoldi, M., Stanhope, K. L., Garaulet, M., Chen, X. G., Marcos-Gómez, B., Carrasco-Benso, M. P., Santa Maria, E. M., Escoté, X., Lee, V., Nunez, M. V., Medici, V., Martínez-Ansó, E., Sáinz, N., Huerta, A. E., Laiglesia, L. M., Prieto, J., Martínez, J. A., Bustos, M., Havel, P. J., Moreno-Aliaga, M. J. Role of cardiotrophin-1 in the regulation of metabolic circadian rhythms and adipose core clock genes in mice and characterization of 24-h circulating CT-1 profiles in normal-weight and overweight/obese subjects.
Keywords: adipose tissue, obesity, Bmal1, Per2, Cry1
Obesity [defined as a body mass index (BMI) >30 kg/m2] is a growing public health problem that has reached epidemic proportions in many developed and developing countries (1). Among the different causes of obesity, the modern human lifestyle, including dietary components and timing and altered patterns of physical activity, can contribute to desynchrony between internal rhythms and external cues in humans. This disruption has been proposed to be an important contributor to the onset of metabolic syndrome (MetS), obesity and type 2 diabetes (2–4). In this context, it has been described that different phenomena, such as short sleep duration (5), shift work (6), and jet lag (7), among others, could be involved in the development of obesity and type 2 diabetes. In addition, other factors, such as altered patterns of physical activity (8) and the time of feeding (9), can contribute to the desynchrony of circadian rhythms.
Recent studies have suggested important interactions between internal circadian rhythms (i.e., clock) and the regulation of energy metabolism. Indeed, disruption of circadian rhythms (chronodisruption; CD) may result in desynchrony, which impairs metabolic homeostasis and may contribute to the development of obesity and MetS (10, 11). In mammals, a circadian molecular complex is integrated by a central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus and peripheral oscillators are present in most tissues and cells, including adipose tissue (12). Peripheral clocks are controlled by the central clock to ensure a temporal synchronization of physiologic events (13, 14). The positive limb of the molecular core clock machinery is composed and directed by the CLOCK and BMAL1 proteins, which heterodimerize to regulate the expression of the negative elements and circadian genes. On the other hand, the negative limb comprises PER and CRY, which form heterodimers and repress their own transcription by negatively regulating the transcriptional activity of the CLOCK:BMAL1 heterodimer (15). The importance of the clock gene network in the control of metabolism and energy balance has become clear, given that mice in which some of these genes are genetically inactivated have been reported to develop obesity and related metabolic disorders (16, 17). In this context, a previous study demonstrated that clock mutant mice exhibit disrupted diurnal feeding rhythm, being hyperphagic and obese, and exhibit elements of metabolic syndrome (18).
Cardiotrophin (CT)-1 belongs to the IL-6 family of cytokines (19). A study by our group has revealed that CT-1 is a key regulator of energy homeostasis and of glucose and lipid metabolism (20). CT-1 null (CT-1−/−) mice develop adult-onset obesity, insulin resistance, and hypercholesterolemia, despite reduced energy intake (20). In addition, chronic administration of recombinant (r)CT-1 to mice reduces body weight and improves insulin resistance in ob/ob and in high-fat–fed obese mice by reducing food intake and enhancing energy expenditure. However, some studies have described that circulating levels of CT-1 are higher in obese subjects (21) with metabolic syndrome (19). Taking into account the beneficial effects of CT-1 on energy homeostasis, as well as on glucose and lipid metabolism, the observed elevation of CT-1 in obesity may represent a compensatory mechanism to attenuate obesity-related metabolic dysfunctions.
Adipose tissue has been established as a peripheral oscillator capable of modulating central core clock genes (22). However, the regulation of the peripheral clock and the clock-controlled genes present in the adipose depots remains unclear (23). In this context, it has been proposed that several molecules secreted by adipose tissue such as leptin and adiponectin, which are important metabolic mediators, could be peripheral regulators of the circadian clocks in the brain and peripheral organs/tissues (24, 25). Some of these adipokines are controlled by clock genes, and their expression in adipose tissue exhibit diurnal variation (26–28). Circulating concentrations of leptin, adiponectin, and other hormones involved in metabolic regulation, such as ghrelin and IL-6, exhibit 24-h variations that have circadian and meal-related patterns (24, 29, 30). There is no information available regarding the potential involvement of CT-1 in the regulation of core clock genes in adipose tissue, and the potential for diurnal variations of circulating CT-1 in humans has not been investigated.
Therefore, the purpose of the present study was to analyze whether CT-1 deficiency would regulate circadian rhythmicity and the expression of core circadian clock genes in white adipose tissue in mice. Moreover, we tested the potential circadian rhythm of CT-1 mRNA levels in adipose tissue. Finally, we sought to evaluate whether there is a circadian rhythm of CT-1 concentrations in plasma of normal-weight and overweight/obese men and women.
MATERIALS AND METHODS
Animal studies
Recombinant protein CT-1
rCT-1 was obtained as described in Beraza et al. (31), and contained <0.04 ng LPS per 1 μg protein, as determined by the Limulus amoebocyte lysate assay (Cambrex, East Rutherford, NJ, USA).
Animal models
CT-1−/− mice
CT-1−/− mice were generated as described by Oppenheim et al. (32). We analyzed mice backcrossed into a C57BL/6J background for 11 generations (provided by Diane Pennica; Genentech, South San Francisco, CA, USA, and Bettina Holtmann, University of Würzburg, Germany). C57BL/6J mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained under specific pathogen-free conditions and controlled temperature and humidity with a 12-h light (08:00–20:00), 12-h dark (20:00–08:00) cycle. Body weight and food intake were measured in wild-type (WT) and CT-1−/− mice from 2 to 12 mo old. At the end of the different experimental periods (2 and 12 mo), WT and CT-1−/− animals were euthanized in parallel between 8 and 11 am after 16 h of food withdrawal. To better characterize the role of CT-1 deficiency on the regulation of peripheral clocks, we obtained epididymal fat samples from WT and CT-1−/− mice (2 mo old) at the following times (08:00, 14:00, 20:00, and 02:00). On the other hand, to test the effects of rCT-1 treatment, CT-1−/− animals were divided into 3 subgroups: one that received rCT-1 IV (0.2 mg/kg/d) for 6 d, another given saline instead of rCT-1, and a pair-fed (PF) group given saline. In this case, animals were euthanized between 8 and 11 am after remaining unfed for 16 h. Epididymal adipose depots were excised and kept at −80°C for subsequent analysis. All experimental procedures were approved by the University of Navarra Ethics Committee.
Prolonged administration of rCT-1 to ob/ob mice
Eight-week-old ob/ob mice were obtained from the Janvier Laboratory (Le Genest St. Isle, France). C57BL/6J mice were obtained from Harlan Laboratories (Barcelona, Spain) and were placed on a high-fat diet (HFD; 60% kcal from fat, 20% from carbohydrates, and 20% from protein; Research Diets, New Brunswick, NJ, USA) ad libitum. Both WT and ob/ob mice were maintained under specific pathogen-free conditions and controlled temperature and humidity with a 12-h light (08:00–20:00), 12-h dark (20:00–08:00) cycle. Ob/ob mice were divided into 3 groups, one that received rCT-1 intravenously (0.2 mg/kg/d) for 10 d and fed ad libitum, another group given saline instead of rCT-1 and fed ad libitum, and a PF group given saline and receiving the same amount of food as ingested by the rCT-1 treated animals. This group is necessary to distinguish what rCT-1 actions are likely to be independent of the effects of rCT-1 on food intake. Food intake and body weight were measured daily. All groups of animals were euthanized in parallel between 8 and 11 am after remaining unfed overnight. Epididymal fat depots were excised, weighed, and kept at −80°C for subsequent analysis (20). All experimental procedures were approved by the University of Navarra Ethics Committee.
Body composition analysis
Whole-animal body composition was measured in live conscious animals with Quantum molecular resonance technology (EchoMRI-100-700; Echo Medical Systems, Houston, TX, USA).
Biochemistry
All serum measurements were performed in samples collected from mice left unfed for 16 h, unless otherwise indicated, by using a Cobas Mira Autoanalyzer (Roche Diagnostic, Basel, Switzerland) (20). Insulin was analyzed with a murine-specific ELISA kit from EMD-Millipore (Billerica, MA, USA) and Crystal Chem, Inc. (Downers Grove, IL, USA).
Whole-body oxygen consumption
As previously reported, 24-h whole-body oxygen consumption (Vo2) was measured in WT and CT-1−/− mice (2 and 12 mo old) using the Oxylet System (Panlab, Spain) (20).
Real-time PCR
Total RNA was extracted from epididymal fat depots by using Trizol reagent (Thermo Fisher Scientific, Carlsbad, CA, USA), according to the manufacturer’s instructions. RNA concentrations and quality were measured by Nanodrop Spectrophotometer 1000 (Thermo Fisher Scientific, Waltham, MA, USA). RNA was then incubated with an RNAse-free kit DNase (Thermo Fisher Scientific, Austin, TX, USA) for 30 min at 37°C. RNA (2 μg) was reverse transcribed into complementary DNA with Moloney murine leukemia virus reverse transcriptase (Thermo Fisher Scientific). mRNA levels were determined by using predesigned TaqMan Assays-on-Demand (Clock: Mm00455959_m1; Bmal: Mm00500226_m1; Per2: Mm00478113_m1; Cry1: Mm00514392_m1; Ctf1: Mm00432772_m1). Taqman Universal Master Mix (Thermo Fisher Scientific) was provided by the same supplier. The reaction conditions were followed as described by the manufacturer’s instructions. Amplification and detection of specific products were performed on the Prism 7900HT system (Thermo Fisher Scientific). mRNA levels were normalized by the housekeeping gene cyclophilin (Mm02342430_g1), obtained from Thermo Fisher Scientific. All samples were analyzed in duplicate. The relative expression level of each gene was calculated as 2−ΔΔCt.
Human study
Subjects
Participants in this study were a subgroup from a U.S. National Institutes of Health-funded investigation (NCT01103921; http://www.clinicaltrials.gov/), in which 187 participants assigned to 8 experimental groups were studied. This paper reports only the results of baseline samples collected before the dietary sugar intervention.
Participants were recruited through an internet listing (Craigslist.com) and local postings of flyers and were interviewed on telephone and in person. A medical history, complete blood count, and serum biochemistry panel were obtained to assess eligibility. Inclusion criteria encompassed men and women (age 18–40 yr and BMI 18–35 kg/m2), with a self-report of stable body weight during the prior 6 mo. Exclusion criteria included diabetes (fasting glucose >125 mg/dl), evidence of renal or hepatic disease, fasting plasma triacylglycerol (TAG) >400 mg/dl, hypertension (>140/90 mmHg), hemoglobin <8.5 g/dl, and surgery for weight loss. Individuals who smoked, habitually ingested more than 2 alcoholic beverages per day, exercised more than 3.5 h/wk at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight loss medications were also excluded.
Methods
The first phase of this study consisted of a 3.5-d inpatient period during which subjects resided at the University of California, Davis Clinical and Translational Science Center’s Clinical Research Center, consumed a standardized baseline diet, and participated in experimental procedures.
Inpatient diets
During d 2 and 3 of the inpatient period, subjects consumed energy-balanced meals consisting of conventional foods. Daily energy requirements were calculated by the Mifflin equation. With this equation, the resting energy expenditure (REE) is calculated by using the following formula: REE (males) = 10 × weight (kg) + 6.25 × height (cm) − 5 × age (yr) + 5; REE (females) = 10 × weight (kg) + 6.25 × height (cm) − 5 × age (yr) − 161 (33), with adjustment of 1.3 for activity on the days of the 24-h serial blood collections, and adjustment of 1.5 for the other days. The diet provided 55% energy requirements mainly as low-fiber complex carbohydrate (i.e., white bread, white rice, regular pasta), 30% from fat, 15% from protein, and 22 g fiber/2000 kcal. The timing of inpatient meals and the energy distribution were: breakfast at 09:00 (25%); lunch at 13:00 (35%); and dinner at 18:00 (40%).
Procedures
We performed 24-h serial blood collections (34) on the third day of the inpatient period. Three fasting blood samples were collected at 08:00, 08:30, and 09:00. Twenty-nine postprandial blood samples were collected at 30–60 min intervals until 08:00 the following morning. Fasting plasma concentrations of TAG and total and low- and high-density lipoprotein-cholesterol (LDL-C and HDL-C) were determined with a Polychem Chemistry Analyzer (PolyMedCo, Inc., Cortland, NY, USA) with reagents from MedTest DX (Cortlandt Manor, NY, USA). Glucose concentrations were measured with an automated glucose analyzer (YSI, Inc. Yellow Springs, OH, USA) and insulin by radioimmunoassay (EMD-Millipore). Free fatty acids (FFAs) were assayed with an enzymatic colorimetric assay (Wako Chemicals, Richmond, VA, USA) adapted to a microtiter plate.
Ethics
The study was conducted in accordance with experimental protocol that was approved by the University of California, Davis Institutional Review Board, and the participants provided written informed consent.
Determination of serum CT-1 plasma concentrations
Plasma CT-1 concentrations were measured by ELISA, as described previously (35). In brief, human plasma samples were added to 96-well plates precoated overnight with a rat monoclonal anti-CT-1 antibody (R&D Systems, Minneapolis, MN, USA), washed with PBS, and blocked in 1% bovine serum albumin (BSA)/PBS. Then, samples were incubated overnight at 4°C. Following incubation, plates were washed 3 times with PBS Tween-0.05% (PBST) and incubated with a rabbit anti-human CT-1 antibody (R&D Systems) for 4 h at room temperature. After incubation, unbound antibodies were washed off with PBST, and plates were incubated with a peroxidase conjugated rabbit specific antibody (Pierce, Rockford, IL, USA) added for detection of the bound anti-CT-1. Finally, plates were washed, and labeled antibody binding was determined using 3,3,5,5-tetramethylbenzidine substrate. Absorbance was read at 450 nm and CT-1 plasma concentrations determined by comparison with a standard curve of serially diluted recombinant CT-1 in dilution buffer.
Statistical analysis
Data are presented as means ± sem. A repeated-measures ANOVA was performed to analyze significant time-dependent changes in CT-1 plasma levels during 24 h, as well as during the light period (from 08:00 to 20:00) and the dark period (from 20:00 to 08:00). Comparisons between the values for different variables were analyzed by Student’s t test or Mann-Whitney U test once the normality had been screened using Kolmogorov-Smirnoff and Shapiro-Wilk tests. Statistical analyses were performed and graphs created with Prism 6 software (Graph-Pad Software, Inc., La Jolla, CA, USA) and Stata Statistical Software (Release 12; StataCorp LP College Station, TX, USA). Overall, a value of P < 0.05 was indicated significant.
To investigate the presence of circadian rhythm for Vo2 in WT and CT-1−/− in mice and 24-h circulating CT-1 concentrations in humans, a least-squares periodic regression (36) was used to fit a sinusoidal function to the data (t = 24 h). Cosinor analysis was used to characterize the rhythm, calculating its mesor (mean value of Vo2 rhythm fitted to a cosine function), amplitude [difference between the maximum (or minimum) value of the cosine function and mesor], acrophase (timing of the maximum value of the cosine fitted curve relative to local 00:00 h). These parameters are represented graphically in a polar plot (hourly) to visualize the characteristics of the rhythm by a vector (cosinor) whose length corresponds to the amplitude and the direction to the acrophase. All the data from all individuals were used simultaneously to directly estimate the population parameters (1-step method) using a model that includes a sinusoid and a constant for each individual, which corresponds to the individual mesor, eliminating the effect of interindividual variation in the estimated global rhythm. A JTK_Cycle analysis (37) was also performed to better characterize the Vo2 rhythmicity in mice and the CT-1 rhythmicity in human plasma.
RESULTS
CT-1−/− mice exhibit altered metabolic circadian rhythm and expression of core clock genes in adipose tissue
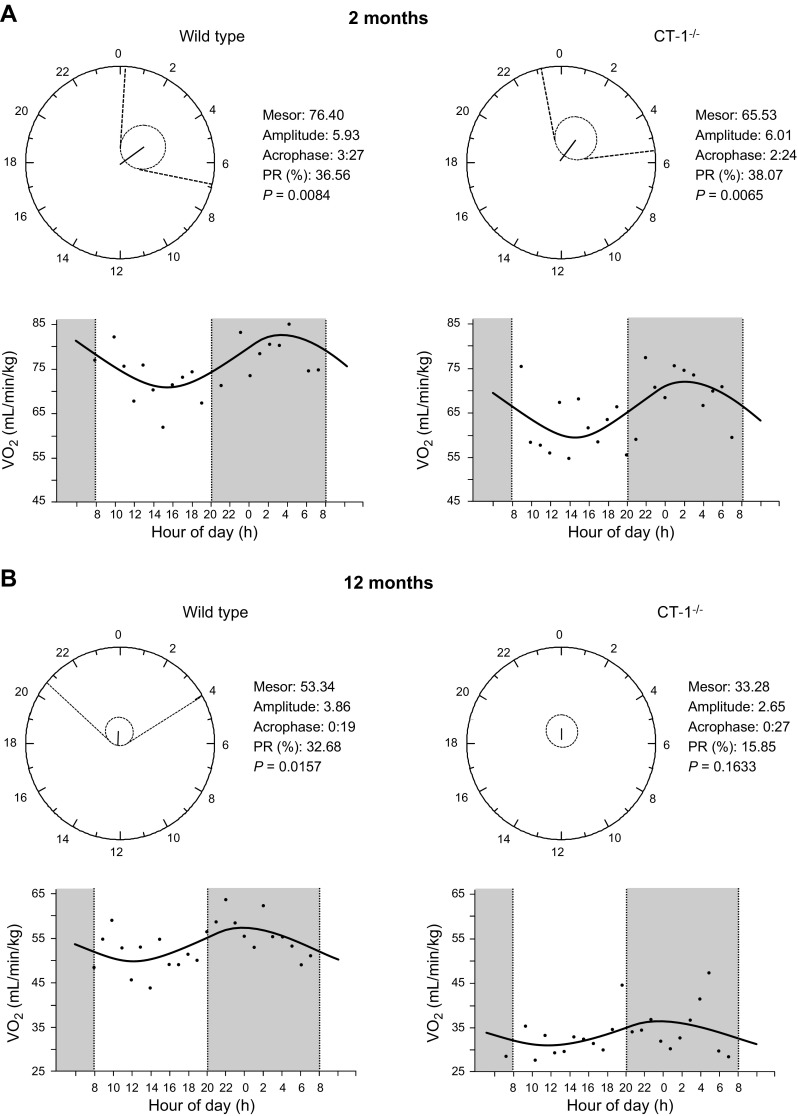
As reported (20), CT-1−/− and WT mice had similar body weights at 2 mo of age; however, CT-1−/− mice developed adult onset obesity (Supplemental Table 1). CT-1−/− mice exhibited decreased energy expenditure and oxygen consumption at 2 mo of age, which preceded and accompanied the development of obesity (20). To determine whether CT-1 deficiency could also result in disturbances in circadian patterns, we analyzed the daily rhythm of 24-h Vo2 (38) in WT and CT-1−/− mice at 2 and 12 mo of age. Parameters imputed from each group were obtained by cosinor analysis, and defined the characteristics of the rhythms as mesor, amplitude, acrophase, and percent rhythm are reported in Fig. 1. Although both young WT and CT-1−/− mice (2 mo old) exhibited robust circadian rhythms for Vo2 values (P < 0.05), it is important to note that CT-1 deficiency caused a phase shift characterized by an advance of ∼1 h of acrophase (Fig. 1A). Aging per se (comparisons between WT mice at 2 and 12 mo of age) lead to CDs characterized by an advance of the acrophase together with a reduction of the amplitude of the rhythm (Fig. 1A , B, left panels). Whereas the 24-h circadian rhythm for Vo2 was maintained in 12-mo-old WT mice (Fig. 1B), the circadian rhythm for Vo2 was completely absent in CT-1−/− obese mice, suggesting that CT-1 deficiency not only promotes obesity, but also appears to aggravate the CD associated with aging in mice. Similar outcomes were obtained when the rhythmicity of Vo2 was analyzed by JTK_Cycle (Supplemental Table 2).
Figure 1.
Cosinor analysis of the 24-h circadian rhythm for Vo2 in WT and CT-1−/− mice at 2 and 12 mo of age. Top: polar (clock-like) representation of the estimates of the parameters of the rhythm for Vo2 in the form of a 24-h clock in WT and CT-1−/− mice at 2 (A) and 12 (B) mo of age. Bottom: x–y plot showing the best-fitting waveform profile of the rhythm.
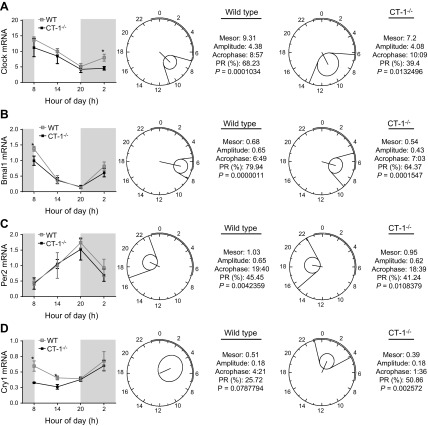
To test whether CT-1 deficiency also modulates mRNA expression of core clock genes in peripheral organs, such as adipose tissue, we analyzed 24-h gene expression (08:00, 14:00, 20:00 and 02:00) of Clock, Bmal1, Per2, and Cry1 in epididymal fat and compared between 2-mo-old WT and CT-1−/− mice fed ad libitum (Fig. 2). The expressions of the positive elements Clock and Bmal1 mRNA levels were moderately lower in CT-1−/− animals, significantly different (P < 0.05) from the Clock levels in WT mice at 02:00 (Fig. 2A) and Bmal1 levels at 08:00 (Fig. 2B, left panel). Cosinor analysis revealed that Clock and Bmal1 mRNA levels exhibited circadian rhythmicity in both WT and CT-1−/− mice. However, the pattern was altered in CT-1−/− mice toward a lower percentage of the rhythm and lower amplitude (Fig 2A, B). Per2 expression also exhibited a circadian rhythm in WT and CT-1−/− mice, with no significant differences between both. This rhythm characterized by a continuous rise during the light period, reached the acrophase at 20:00. As expected Per2 rhythms (a negative element of the clock) were in antiphase with Clock and Bmal1 (positive elements). No significant changes were observed between WT and CT-1−/− mice (Fig. 2C). The gene expression of the other negative element Cry1 was significantly lower (P < 0.05) in CT-1-knockout mice compared with WT during the morning (at 08:00 and 14:00) (Fig. 2D). Cosinor analyses revealed that the circadian rhythmicity of Cry1 was observed only in CT-1−/−, but not in WT mice (Fig. 2D). The similarity in body composition between the WT and CT-1−/− at 2 mo of age suggests that the alteration in the circadian patterns is caused by CT-1 deficiency and it is not a consequence of increased adiposity.
Figure 2.
Daily mRNA expression profiles and cosinor analysis of the circadian rhythms for core clock genes. Left: around the clock gene expression levels of Clock (A), Bmal1 (B), Per2 (C), and Cry1 (D) in epididymal fat of 2-mo-old WT and CT-1−/− mice. Data are means ± sem (n = 4–6). Polar (clock-like) representation of the estimates of the parameters of the rhythm for clock genes in the form of a 24-h clock in WT (middle panels) and CT-1−/− (right panels) mice at 2 mo of age. *P < 0.05 vs. WT control group.
In 12-mo-old obese CT-1−/− mice, the expression of Clock, Bmal1, Per2, and Cry1 in adipose tissue at morning times were also markedly different from those in WT mice (Supplemental Fig. 1). However, it is difficult to discern between the effects derived from CT-1 deficiency and those secondary to the obesity and insulin resistance observed in old CT-1−/−mice and from induced by aging.
On the other hand, our data suggest that the exogenous administration of rCT-1 (0.2 mg/kg/d) could also regulate the expression of some peripheral clocks in adipose tissue of 2-mo-old CT-1−/− mice (Supplemental Fig. 2) and in ob/ob mice (Supplemental Fig. 3), further supporting the involvement of this cytokine in the regulation of adipose tissue peripheral clocks. However, gene expression was measured at a single time point, and it is hard to establish whether the changes observed were a direct effect of the cytokine or secondary to a potential phase shift of the rhythm of these core clock genes induced by rCT-1 administration.
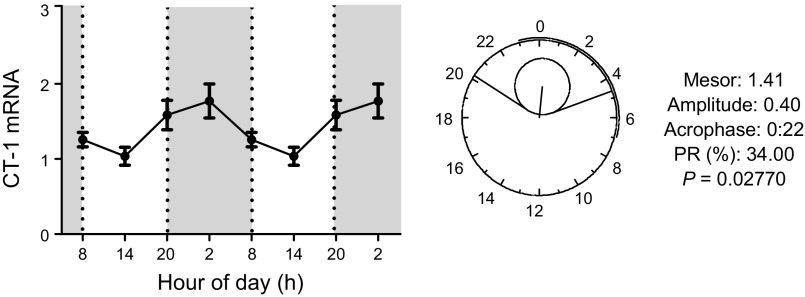
Finally, because CT-1 deficiency leads to impairments in adipose tissue peripheral clock genes, we asked whether it has a daily mRNA expression rhythm. The data revealed that adipose tissue CT-1 mRNA levels exhibited significant fluctuations throughout the 24-h period and a significant circadian rhythm (Fig. 3).
Figure 3.
Adipose tissue daily mRNA expression profile and cosinor analysis of CT-1. Around the clock gene expression levels of CT-1 in epididymal fat of 2-mo-old WT mice. Data are means ± sem (n = 4–6). Polar (clock-like) representation of the estimates of the parameters of the rhythm for CT-1 mRNA in the form of a 24-h clock in WT mice at 2 mo of age.
All these observations suggest an involvement of CT-1 in the regulation of the expression of clock genes in adipose tissue in mice.
CT-1 plasma 24-h profiles in normal-weight and overweight subjects
Table 1 shows the characteristics of the subjects included in the study. The overweight (group including overweight and obese individuals) exhibited hyperinsulinemia (P < 0.05) and higher levels of cholesterol (P < 0.01) than normal-weight subjects, because of increased levels of LDL-C (P < 0.001). CT-1 levels were moderately higher in overweight/obese than in normal-weight subjects but the difference was not statistically significant.
TABLE 1.
Baseline anthropometric and biochemical parameters of normal-weight vs. overweight subjects
| Variable | All subjects n = 84 | Normal weight n = 37 | Overweight n = 47 | P |
|---|---|---|---|---|
| Anthropometric parameter |
||||
| Age (yr) |
26.80 ± 0.67 |
24.85 ± 0.82 |
28.65 ± 0.99 |
<0.01 |
| Weight (kg) |
74.70 ± 1.45 |
65.05 ± 1.53 |
82.29 ± 1.59 |
<0.0001 |
| BMI (kg/m2) |
25.36 ± 0.40 |
22.15 ± 0.30 |
27.88 ± 0.40 |
<0.0001 |
| Waist (cm) |
77.94 ± 1.08 |
70.28 ± 1.00 |
83.96 ± 1.17 |
<0.0001 |
| Waist/hip ratio |
0.79 ± 0.01 |
0.76 0.01 |
0.81 ± 0.01 |
0.002 |
| Metabolic parameter |
||||
| Glucose (mg/dl) |
90.39 ± 0.72 |
89.19 ± 0.97 |
91.38 ± 1.03 |
0.127 |
| Insulin (mU/L) |
13.6 ± 1.02 |
11.31 ± 0.67 |
15.37 ± 1.67 |
0.046 |
| HOMA |
2.91 ± 0.24 |
2.39 ± 0.15 |
3.30 ± 0.39 |
0.057 |
| TAG (mg/dl) |
102.9 ± 4.82 |
88.52 ± 5.12 |
114.2 ± 7.25 |
0.007 |
| FFA (mM) |
0.39 ± 0.01 |
0.37 ± 0.02 |
0.40 ± 0.02 |
0.236 |
| Cholesterol (mg/dl) |
156.7 ± 3.31 |
145 ± 4.62 |
165.9 ± 4.25 |
0.001 |
| HDL-C (mg/dl) |
45.21 ± 1.39 |
46.77 ± 1.78 |
43.99 ± 2.06 |
0.325 |
| LDL-C (mg/dl) |
91.35 ± 2.73 |
80.99 ± 3.58 |
99.51 ± 3.58 |
0.0005 |
| CT-1 baseline (ng/ml) | 10.61 ± 0.96 | 9.67 ± 1.19 | 11.34 ± 1.44 | 0.390 |
Data are means ± sem. Underlined values indicate a significant difference between normal-weight and overweight individuals. HOMA: homeostasis model assessment.
When the correlations between plasma CT-1 concentrations and anthropometric and biochemical parameters were analyzed, Spearman’s correlation analysis revealed that CT-1 levels correlated positively with weight (r = 0.349; P = 0.034), waist circumference (r = 0.372; P = 0.023), and waist/hip ratio (r = 0.398; P = 0.015), but negatively with LDL-C (r = −0.341; P = 0.039) in normal-weight subjects. These associations were not observed in the overweight group (Table 2).
TABLE 2.
Correlation analysis between plasma CT-1 and anthropometric and biochemical variables in normal-weight and overweight subjects at baseline
| All subjects n = 84 |
Normal weight n = 37 |
Overweight n = 47 |
||||
|---|---|---|---|---|---|---|
| Variable | ρ | P | ρ | P | ρ | P |
| Weight | 0.134 | 0.225 | 0.349 | 0.034 | −0.003 | 0.982 |
| BMI | 0.129 | 0.244 | 0.112 | 0.509 | 0.101 | 0.500 |
| Waist | 0.149 | 0.175 | 0.372 | 0.023 | −0.048 | 0.750 |
| Waist/hip ratio | 0.172 | 0.118 | 0.398 | 0.015 | 0.018 | 0.905 |
| Glucose | 0.088 | 0.434 | 0.157 | 0.362 | −0.003 | 0.985 |
| Insulin | 0.019 | 0.889 | 0.043 | 0.840 | 0.004 | 0.983 |
| HOMA | 0.081 | 0.561 | 0.196 | 0.371 | 0.063 | 0.738 |
| TAG | 0.021 | 0.849 | 0.253 | 0.130 | −0.131 | 0.378 |
| FFA | −0.011 | 0.924 | −0.058 | 0.732 | −0.009 | 0.952 |
| Cholesterol | −0.083 | 0.454 | −0.298 | 0.073 | −0.102 | 0.497 |
| HDL-C | −0.078 | 0.480 | −0.058 | 0.732 | −0.078 | 0.601 |
| LDL-C | −0.093 | 0.398 | −0.341 | 0.039 | −0.034 | 0.823 |
All: glucose (n = 82), insulin (n = 55), HOMA (n = 54); normal-weight: glucose (n = 36), insulin (n = 24), HOMA (n = 23); overweight: glucose (n = 46), insulin (n = 31), HOMA (n = 31). Underlined values indicate a statistically significant difference.
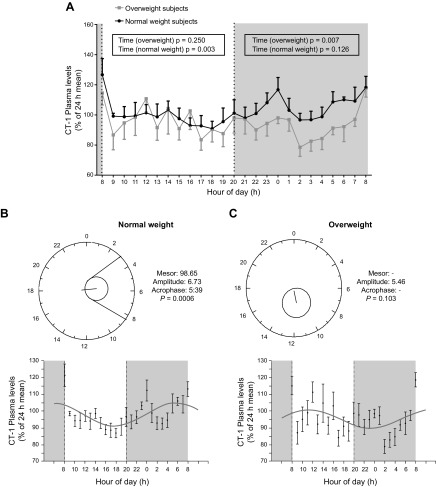
We next focused on analyzing the possible circadian pattern of circulating CT-1 concentrations in plasma samples from human subjects. For that purpose, 24-h profiles for plasma CT-1 were determined in 11 selected subjects (6 normal weight and 5 overweight/obese) matched for similar CT-1 plasma levels at baseline (characteristics of these 11 subjects are summarized in Supplemental Table 4). Figure 4A shows the 24-h plasma CT-1 profile expressed as a percentage of 24-h mean plasma CT-1 concentrations in both the normal-weight and overweight groups. Relevant differences in CT-1 patterns during light and dark periods were observed between both groups. Thus, Fig. 4A shows that during the light period, CT-1 plasma levels showed significant (P = 0.003) daytime changes characterized by the marked fall from 08:00 to 09:00 in normal-weight individuals, whereas no significant daytime changes were observed in the overweight group (P = 0.250). Conversely, CT-1 excursions during the dark period (from 20:00 to 08:00) exhibited significant changes (P = 0.007) only in the overweight subjects without significant modifications in the normal-weight individuals. It is important to note that at 02:00, CT-1 levels were significantly lower in overweight/obese subjects (P < 0.05) than in normal-weight subjects (Fig. 4A).
Figure 4.
Circadian CT-1 plasma profile in humans. A) Plasma 24-h CT-1 profile in normal-weight (n = 6) and overweight (n = 5) groups. Plasma CT-1 levels are expressed as a percentage relative to 24-h mean. Data are means ± sem. *P < 0.05, vs. normal weight. B) Cosinor analysis of the circadian rhythm for plasma CT-1 in normal-weight and overweight subjects. Top: polar (clock-like) representation of the estimates of the parameters of the rhythm for plasma CT-1 in the form of a 24-h clock in normal-weight and overweight subjects. Bottom: x–y plots showing the best fitting waveform profile of the rhythm in normal-weight and overweight subjects.
A cosinor analysis was performed to evaluate the circadian rhythm of CT-1. The main parameters defining the circadian rhythm as mesor, amplitude, relative amplitude, acrophase, and percent rhythm are shown in Fig. 4B and Supplemental Table 5. The results indicate that the normal-weight group had a significant 24-h circadian rhythm of CT-1 (Fig. 4B), whereas the rhythm was reduced or not significant in the overweight group. These data were additionally confirmed by the JTK_Cycle analysis (Supplemental Table 6).
DISCUSSION
The current study provides evidence of a role of CT-1 in the regulation of circadian rhythms and core clock genes in adipose tissue. A novel observation from this study is that 24-h circadian rhythms of Vo2 were altered in CT-1−/− mice compared with WT mice at 12 mo of age. It is possible that this circadian alteration contributes to the mature-onset obesity and metabolic disturbances observed in these animals (20). Although it cannot be concluded whether the CD observed in old CT-1−/− mice is a cause or a consequence of obesity, the fact that young lean CT-1−/− mice (2 mo) exhibited an early-onset phase shift of acrophase for Vo2 rhythm suggests that CD both precedes and accompanies development of obesity in the adult state in these animals.
The lack of CT-1 also dramatically alters the expression pattern of core clock genes in adipose tissue. Several observations suggest that disruption of adipose tissue clock genes could precede the development of obesity in CT-1−/− mice. That the body fat is similar in both WT and CT-1−/− mice suggests that the alterations of these clock genes are specific to CT-1 deficiency and not secondary to the increased adiposity observed in adult mice. Moreover, the comparison of the daily rhythms of core clock gene expression in adipose tissue of young WT and CT-1−/− mice suggests that there is truly an alteration of the core clock gene expression as a consequence of CT-1 deficiency, which is linked to a reduced percentage of the rhythm, amplitude or both, and especially for the positive elements Clock and Bmal1. Although, some moderate changes in the acrophase were observed for Clock and Per2 in CT-1−/− mice, CT-1 deficiency does not seem to cause a phase shift in the expression of these core clock genes in adipose tissue. It is important to note that CT−/− mice exhibit decreased energy expenditure and oxygen consumption at 2 mo of age, which precedes and accompanies the development of obesity (20). Hence, it can be proposed that alterations in energy expenditure and oxygen consumption observed in young CT-1−/− mice could be, at least in part, secondary to the disruption of the clock machinery observed in the adipose tissue of these animals. It has also been reported that ClockΔ19 mutant mice, which express a loss-of-function mutation in the Clock gene, exhibit features of the metabolic syndrome (18). Moreover, Cry1/2 deficiency results in an increased vulnerability to obesity induced by an HFD, despite reduced food intake, and this may be related to more efficient lipid storage in adipose tissue (17). Adult CT-1−/− mice also exhibit obesity and increased expression of lipogenic genes, despite reduced food intake compared to WT mice (20). In addition, it has been reported that Bmal1−/− male and female mice exhibit increased adiposity on a standard chow diet, but consumption of an HFD does not exaggerate these differences (39). However, other studies have reported conflicting outcomes regarding the role of Bmal1 and Cry in obesity susceptibility (40, 41).
It has been reported that an impairment of peripheral circadian clocks in adipose tissue and liver precedes the metabolic abnormalities in ob/ob mice (42). These abnormalities were attenuated by leptin treatment, but not by caloric restriction. On the other hand, we have previously described that treatment of ob/ob mice with CT-1 promoted body weight loss and fat mass reduction, accompanied by a dramatic remodeling in adipose tissue gene profile (20). Our current data also suggest that exogenous CT-1 has a potential role as a regulator of adipose tissue clock gene machinery. However, further studies are needed to better characterize the relevance of these observations and, if it exists, a true alteration in the expression of clock genes mediated by rCT-1 treatment, or it is secondary to a phase shift of the rhythm. In this context, a previous study suggested that cardiotrophin-like cytokine is likely to be an output signal from the SCN, which is involved in daily rhythms of behavior (43).
Circulating concentrations of many hormones (notably glucocorticoids and growth hormone) exhibit circadian and ultradian patterns. As an example for a circulating cytokine, there are circadian variations of circulating IL-6 concentrations, with lower levels during daytime and higher levels during the night (44–46). In the present study, circulating CT-1 concentrations also exhibited diurnal variations and a 24-h circadian rhythm. The most prominent changes of plasma CT-1 concentrations over 24 h include the increase of plasma levels during the night, reaching a maximum peak at 08:00, followed by a marked decrease that occurs in the morning between 08:00 and 09:00.
It has been proposed that the role of the circadian system in the regulation of appetite during sleep/wake cycles is to serve as a mechanism to facilitate the typical overnight fast (47). A circadian system may be involved in reduced hunger in the morning, despite a prolonged overnight fast. Taking into account the previously mentioned properties of CT-1 that reduce food intake, it can be hypothesized that the nocturnal increase in CT-1 during the late night hours contributes to the regulation of patterns of hunger and appetite in humans. The observation that CT-1 levels increase during the late night and early morning hours and then decline fairly soon after awakening suggest a close relationship with the sleep–wake cycles.
The nocturnal increase in CT-1 has a similar pattern to the increase of FFAs when lipolysis in adipose tissue is increased during the night (48), suggesting that the changes in plasma CT-1 levels may also be related to metabolic homeostasis during the prolonged fasting that occurs during the late night and early morning hours. In a previous study, our group reported that CT-1 is a nutritionally regulated gene that increases markedly in several tissues when mice are subjected to 48-h food withdrawal and decreases promptly upon refeeding (20). However, it is important to note that plasma CT-1 levels do not appear to be acutely regulated by meals, given that CT-1 levels do not change significantly after breakfast, lunch, or dinner.
An interesting observation of the current study is that the circadian rhythm of CT-1 observed in normal-weight subjects was not significant in overweight/obese individuals. Obesity has been associated with a loss of circadian patterns, a pathology that has been labeled “chronodisruption” (49). CD suggests that rhythms can become desynchronized and that this may have adverse effects on health (50–52). CD can be assessed in physiologic studies as a reduction in rhythm amplitude, sometimes as a total loss of rhythm, by a delayed or advanced phase between different peripheral clocks and the SCN, or a phase inversion of circadian rhythms, as observed in night workers (50, 52). The current results for plasma CT-1 concentrations, suggesting a dampening of CT-1 rhythms in overweight/obese subjects, parallel previous results reported for the adipocyte hormones leptin, adiponectin, and resistin. These hormones exhibit clear circadian rhythms in 24-h plasma concentrations, and these rhythms are attenuated or even absent in obese individuals (53, 54). Several factors could contribute to this, including differential metabolic responses to overnight fasting between normal-weight and obese subjects (55). Although our previous studies have clearly demonstrated that the lack of CT-1 causes adult onset obesity in mice, the relationship between CT-1 circulating levels and obesity in humans is still unclear. Thus, although some studies have reported that obese individuals exhibit increased levels of circulating CT-1 (19, 21, 35), no significant changes in CT-1 serum levels in overweight/obese subjects were observed in the current or another study (56), suggesting that other factors associated with obesity, and not the increased adiposity directly, could determine the levels of this cytokine. Therefore, it would be interesting to compare the 24-h diurnal rhythm of CT-1 between healthy normal-weight and overweight/obese individuals (matched by insulin and HOMA levels) and lean and obese subjects with metabolic syndrome or type 2 diabetes.
In summary, the results presented herein suggest a role for CT-1 in the regulation of metabolic circadian rhythms and core clock genes in adipose tissue. In addition, the current results also indicate that circulating CT-1 concentrations exhibit a 24-h circadian pattern in normal-weight subjects and that this rhythm is impaired in overweight/obese subjects. Further research is needed to better define the processes involved in the circadian/diurnal regulation of CT-1 secretion and the role of CT-1 in metabolic physiology and the pathophysiology of obesity.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Grant 2007/10 from the Navarra Department of Health; the Mutua Madrileña Foundation; Fondo de Investigación Sanitaria (FIS) Grants PI10/01516 and PI13/01851; CIBERobn; Línea Especial ‘‘Nutrición, Obesidad y Salud” and Universidad de Navarra Centre for Nutrition Research; by agreement between International Agricultural Machinery Fair (FIMSA) and the “Temporary Business Association (UTE) project CIMA” Spanish Government of Science and Innovation Grant SAF2014-52480-R; and European Regional Development Fund ERDF grant (to M.G.). M.L.-Y. was supported by a fellowship from Asociación de Amigos University of Navarra. The human study was supported by U.S. National Institutes of Health (NIH)/National Heart, Lung and Blood Institute Grants 1R01 HL09133 and 1R01 HL107256 (to P.J.H.); National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research, National Institute of General Medical Sciences Grant UL1 RR024146. K.L.S. was supported by Building Interdisciplinary Research Careers in Women’s Health Award K12 HD051958, funded by the NIH National Institute of Child Health and Human Development, Office of Research on Women’s Health, Office of Dietary Supplements, and the NIH National Institute of Aging. The authors declare no conflicts of interest.
Glossary
- BMI
body mass index
- CD
chronodisruption
- CT-1
cardiotrophin-1
- FFA
free fatty acid
- HDL-C
high-density lipoprotein-cholesterol
- HFD
high-fat diet
- HOMA
homeostasis model assessment
- LDL-C
low-density lipoprotein-cholesterol
- MetS
metabolic syndrome
- PBST
PBS Tween
- PF
pair fed
- rCT-1
recombinant protein CT-1
- REE
resting energy expenditure
- SCN
suprachiasmatic nucleus
- TAG
triacylglycerol
- Vo2
oxygen consumption
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M López-Yoldi performed the research, analyzed the data, and wrote the paper; K. L. Stanhope and P. J. Havel designed the research for the human study, analyzed the data, and reviewed the manuscript; M. Garaulet and M. P. Carrasco-Benso analyzed the circadian rhythm data and contributed to writing the manuscript; X. G. Chen, V. Medici, V. Lee, and M. V. Nunez performed research in the human study; B. Marcos-Gómez, E. M. Santa Maria, N. Sáinz, and L. M. Laiglesia performed the research in the animal models; X. Escoté performed the research and analyzed the data; E. Martínez-Ansó contributed new reagents or analytic tools; A. E. Huerta analyzed the data; M. Bustos designed and performed the research and reviewed the manuscript; J. Prieto and J. A. Martínez designed the research and reviewed the manuscript; and M. J. Moreno-Aliaga designed the research, analyzed the data, and wrote the paper.
REFERENCES
- 1.Reichman T. W., Therapondos G., Serrano M. S., Seal J., Evers-Meltzer R., Bohorquez H., Cohen A., Carmody I., Ahmed E., Bruce D., Loss G. E. (2015) “Weighing the risk”: obesity and outcomes following liver transplantation. World J. Hepatol. 7, 1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garaulet M., Madrid J. A. (2009) Chronobiology, genetics and metabolic syndrome. Curr. Opin. Lipidol. 20, 127–134 [DOI] [PubMed] [Google Scholar]
- 3.Gimble J. M., Sutton G. M., Bunnell B. A., Ptitsyn A. A., Floyd Z. E. (2011) Prospective influences of circadian clocks in adipose tissue and metabolism. Nat. Rev. Endocrinol. 7, 98–107 [DOI] [PubMed] [Google Scholar]
- 4.Morris C. J., Yang J. N., Scheer F. A. (2012) The impact of the circadian timing system on cardiovascular and metabolic function. Prog. Brain Res. 199, 337–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton O. M., Cain S. W., O’Connor S. P., Porter J. H., Duffy J. F., Wang W., Czeisler C. A., Shea S. A. (2012) Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4, 129ra43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peplonska B., Bukowska A., Sobala W. (2015) Association of rotating night shift work with BMI and abdominal obesity among nurses and midwives. PLoS One 10, e0133761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar Jha P., Challet E., Kalsbeek A. (2015) Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 418, 74–88 [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Sastre P., Gómez-Abellán P., Martinez-Nicolas A., Ordovás J. M., Madrid J. A., Garaulet M. (2014) Evening physical activity alters wrist temperature circadian rhythmicity. Chronobiol. Int. 31, 276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garaulet M., Gómez-Abellán P. (2014) Timing of food intake and obesity: a novel association. Physiol. Behav. 134, 44–50 [DOI] [PubMed] [Google Scholar]
- 10.Antunes L. C., Levandovski R., Dantas G., Caumo W., Hidalgo M. P. (2010) Obesity and shift work: chronobiological aspects. Nutr. Res. Rev. 23, 155–168 [DOI] [PubMed] [Google Scholar]
- 11.Bass J., Takahashi J. S. (2010) Circadian integration of metabolism and energetics. Science 330, 1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel-Mahan K., Sassone-Corsi P. (2013) Metabolism and the circadian clock converge. Physiol. Rev. 93, 107–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reppert S. M., Weaver D. R. (2002) Coordination of circadian timing in mammals. Nature 418, 935–941 [DOI] [PubMed] [Google Scholar]
- 14.Sahar S., Sassone-Corsi P. (2012) Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol. Metab. 23, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garaulet M., Ordovás J. M., Madrid J. A. (2010) The chronobiology, etiology and pathophysiology of obesity. Int. J. Obes. 34, 1667–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paschos G. K., Ibrahim S., Song W. L., Kunieda T., Grant G., Reyes T. M., Bradfield C. A., Vaughan C. H., Eiden M., Masoodi M., Griffin J. L., Wang F., Lawson J. A., Fitzgerald G. A. (2012) Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 18, 1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barclay J. L., Shostak A., Leliavski A., Tsang A. H., Jöhren O., Müller-Fielitz H., Landgraf D., Naujokat N., van der Horst G. T., Oster H. (2013) High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am. J. Physiol. Endocrinol. Metab. 304, E1053–E1063 [DOI] [PubMed] [Google Scholar]
- 18.Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R., Eckel R. H., Takahashi J. S., Bass J. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natal C., Fortuño M. A., Restituto P., Bazán A., Colina I., Díez J., Varo N. (2008) Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 294, E52–E60 [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Aliaga M. J., Pérez-Echarri N., Marcos-Gómez B., Larequi E., Gil-Bea F. J., Viollet B., Gimenez I., Martínez J. A., Prieto J., Bustos M. (2011) Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. 14, 242–253 [DOI] [PubMed] [Google Scholar]
- 21. Malavazos, A. E., Ermetici, F., Morricone, L., Delnevo, A., Coman, C., Ambrosi, B., Corsi, M. M. (2008) Association of increased plasma cardiotrophin-1 with left ventricular mass indexes in normotensive morbid obesity. Hypertension51, e8–e9; author reply e10. [DOI] [PubMed]
- 22.Mohawk J. A., Green C. B., Takahashi J. S. (2012) Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35, 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukumaran S., Xue B., Jusko W. J., Dubois D. C., Almon R. R. (2010) Circadian variations in gene expression in rat abdominal adipose tissue and relationship to physiology. Physiol. Genomics 42A, 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froy O. (2012) Circadian rhythms and obesity in mammals. ISRN Obes. 2012, 437198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashinaga T., Wada N., Otabe S., Yuan X., Kurita Y., Kakino S., Tanaka K., Sato T., Kojima M., Ohki T., Nakayama H., Egashira T., Tajiri Y., Yamada K. (2013) Modulation by adiponectin of circadian clock rhythmicity in model mice for metabolic syndrome. Endocr. J. 60, 483–492 [PubMed] [Google Scholar]
- 26.Ando H., Yanagihara H., Hayashi Y., Obi Y., Tsuruoka S., Takamura T., Kaneko S., Fujimura A. (2005) Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 146, 5631–5636 [DOI] [PubMed] [Google Scholar]
- 27.Bray M. S., Young M. E. (2007) Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes. Rev. 8, 169–181 [DOI] [PubMed] [Google Scholar]
- 28.Garaulet M., Ordovás J. M., Gómez-Abellán P., Martínez J. A., Madrid J. A. (2011) An approximation to the temporal order in endogenous circadian rhythms of genes implicated in human adipose tissue metabolism. J. Cell. Physiol. 226, 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodosi B., Gardi J., Hajdu I., Szentirmai E., Obal F. Jr., Krueger J. M. (2004) Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1071–R1079 [DOI] [PubMed] [Google Scholar]
- 30.Johnston J. D. (2012) Adipose circadian rhythms: translating cellular and animal studies to human physiology. Mol. Cell. Endocrinol. 349, 45–50 [DOI] [PubMed] [Google Scholar]
- 31.Beraza N., Marqués J. M., Martínez-Ansó E., Iñiguez M., Prieto J., Bustos M. (2005) Interplay among cardiotrophin-1, prostaglandins, and vascular endothelial growth factor in rat liver regeneration. Hepatology 41, 460–469 [DOI] [PubMed] [Google Scholar]
- 32.Oppenheim R. W., Wiese S., Prevette D., Armanini M., Wang S., Houenou L. J., Holtmann B., Gotz R., Pennica D., Sendtner M. (2001) Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J. Neurosci. 21, 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mifflin M. D., St Jeor S. T., Hill L. A., Scott B. J., Daugherty S. A., Koh Y. O. (1990) A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 51, 241–247 [DOI] [PubMed] [Google Scholar]
- 34.Stanhope K. L., Bremer A. A., Medici V., Nakajima K., Ito Y., Nakano T., Chen G., Fong T. H., Lee V., Menorca R. I., Keim N. L., Havel P. J. (2011) Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J. Clin. Endocrinol. Metab. 96, E1596–E1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rendo-Urteaga T., García-Calzón S., Martínez-Ansó E., Chueca M., Oyarzabal M., Azcona-Sanjulián M. C., Bustos M., Moreno-Aliaga M. J., Martínez J. A., Marti A. (2013) Decreased cardiotrophin-1 levels are associated with a lower risk of developing the metabolic syndrome in overweight/obese children after a weight loss program. Metabolism 62, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 36.Batschelet E. (1981) Circular Statistics in Biology, Academic Press, London [Google Scholar]
- 37.Hughes M. E., Hogenesch J. B., Kornacker K. (2010) JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythms 25, 372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubal A., Choshniak I., Haim A. (1992) Daily rhythms of metabolic rate and body temperature of two murids from extremely different habitats. Chronobiol. Int. 9, 341–349 [DOI] [PubMed] [Google Scholar]
- 39.Kennaway D. J., Varcoe T. J., Voultsios A., Boden M. J. (2013) Global loss of bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS One 8, e65255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmeryckx B., Himmelreich U., Hoylaerts M. F., Lijnen H. R. (2011) Impact of clock gene Bmal1 deficiency on nutritionally induced obesity in mice. Obesity (Silver Spring) 19, 659–661 [DOI] [PubMed] [Google Scholar]
- 41.Griebel G., Ravinet-Trillou C., Beeské S., Avenet P., Pichat P. (2014) Mice deficient in cryptochrome 1 (cry1 (−/−)) exhibit resistance to obesity induced by a high-fat diet. Front. Endocrinol. (Lausanne) 5, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ando H., Kumazaki M., Motosugi Y., Ushijima K., Maekawa T., Ishikawa E., Fujimura A. (2011) Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 152, 1347–1354 [DOI] [PubMed] [Google Scholar]
- 43.Kraves S., Weitz C. J. (2006) A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat. Neurosci. 9, 212–219 [DOI] [PubMed] [Google Scholar]
- 44.Bauer J., Hohagen F., Ebert T., Timmer J., Ganter U., Krieger S., Lis S., Postler E., Voderholzer U., Berger M. (1994) Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Clin. Investig. 72, 315 [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas A. N., Bixler E. O., Lin H. M., Prolo P., Trakada G., Chrousos G. P. (2005) IL-6 and its circadian secretion in humans. Neuroimmunomodulation 12, 131–140 [DOI] [PubMed] [Google Scholar]
- 46.Agorastos A., Hauger R. L., Barkauskas D. A., Moeller-Bertram T., Clopton P. L., Haji U., Lohr J. B., Geracioti T. D. Jr., Patel P. M., Chrousos G. P., Baker D. G. (2014) Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology 44, 71–82 [DOI] [PubMed] [Google Scholar]
- 47.Scheer F. A., Morris C. J., Shea S. A. (2013) The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 21, 421–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saloranta C., Taskinen M. R., Widen E., Härkönen M., Melander A., Groop L. (1993) Metabolic consequences of sustained suppression of free fatty acids by acipimox in patients with NIDDM. Diabetes 42, 1559–1566 [DOI] [PubMed] [Google Scholar]
- 49.Garaulet M., Madrid J. A. (2010) Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv. Drug Deliv. Rev. 62, 967–978 [DOI] [PubMed] [Google Scholar]
- 50.Colas D., London J., Gharib A., Cespuglio R., Sarda N. (2004) Sleep-wake architecture in mouse models for Down syndrome. Neurobiol. Dis. 16, 291–299 [DOI] [PubMed] [Google Scholar]
- 51.Stewart L. S., Persinger M. A., Cortez M. A., Snead O. C. III (2007) Chronobiometry of behavioral activity in the Ts65Dn model of Down syndrome. Behav. Genet. 37, 388–398 [DOI] [PubMed] [Google Scholar]
- 52.Erren T. C., Reiter R. J. (2009) Defining chronodisruption. J. Pineal Res. 46, 245–247 [DOI] [PubMed] [Google Scholar]
- 53.Mingrone G., Manco M., Granato L., Calvani M., Scarfone A., Mora E. V., Greco A. V., Vidal H., Castagneto M., Ferrannini E. (2005) Leptin pulsatility in formerly obese women. FASEB J. 19, 1380–1382 [DOI] [PubMed] [Google Scholar]
- 54.Yildiz B. O., Suchard M. A., Wong M. L., McCann S. M., Licinio J. (2004) Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. USA 101, 10434–10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H., Xia F., Lam K. S., Wang Y., Bao Y., Zhang J., Gu Y., Zhou P., Lu J., Jia W., Xu A. (2011) Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin. Chem. 57, 691–700 [DOI] [PubMed] [Google Scholar]
- 56. Jung, C., Fritzenwanger, M., Figulla, H. R. (2008) Cardiotrophin-1 in adolescents: impact of obesity and blood pressure. Hypertension52, e6; author reply e7. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.