Abstract
Lysophosphatidic acid (LPA) has been recognized recently as an endothelium-dependent vasodilator, but several lines of evidence indicate that it may also stimulate vascular smooth muscle cells (VSMCs), thereby contributing to vasoregulation and remodeling. In the present study, mRNA expression of all 6 LPA receptor genes was detected in murine aortic VSMCs, with the highest levels of LPA1, LPA2, LPA4, and LPA6. In endothelium-denuded thoracic aorta (TA) and abdominal aorta (AA) segments, 1-oleoyl-LPA and the LPA1–3 agonist VPC31143 induced dose-dependent vasoconstriction. VPC31143-induced AA contraction was sensitive to pertussis toxin (PTX), the LPA1&3 antagonist Ki16425, and genetic deletion of LPA1 but not that of LPA2 or inhibition of LPA3, by diacylglycerol pyrophosphate. Surprisingly, vasoconstriction was also diminished in vessels lacking cyclooxygenase-1 [COX1 knockout (KO)] or the thromboxane prostanoid (TP) receptor (TP KO). VPC31143 increased thromboxane A2 (TXA2) release from TA of wild-type, TP-KO, and LPA2-KO mice but not from LPA1-KO or COX1-KO mice, and PTX blocked this effect. Our findings indicate that LPA causes vasoconstriction in VSMCs, mediated by LPA1-, Gi-, and COX1-dependent autocrine/paracrine TXA2 release and consequent TP activation. We propose that this new-found interaction between the LPA/LPA1 and TXA2/TP pathways plays significant roles in vasoregulation, hemostasis, thrombosis, and vascular remodeling.—Dancs, P. T., Ruisanchez, E., Balogh, A., Panta, C. R., Miklós, Z., Nüsing, R. M., Aoki, J., Chun, J., Offermanns, S., Tigyi, G., Benyó, Z. LPA1 receptor-mediated thromboxane A2 release is responsible for lysophosphatidic acid-induced vascular smooth muscle contraction.
Keywords: LPA, vasoconstriction, endothelial dysfunction, platelet activation, TP receptor
Lysophosphatidic acid (LPA) is a multifunctional lipid mediator/second messenger with numerous biological actions in mammalian physiology and pathology. It has diverse roles in embryonic development and in the regulation of the immune, reproductive, nervous, and cardiovascular systems as well as tumor biology and fibrosis (1–4). LPA exerts most of its actions via 6 different subtypes of G-protein–coupled receptors (GPCRs). The first GPCR cluster specific to LPA (LPA1/2/3) belongs to the endothelial differentiation gene family (5), which includes the plasma membrane receptors of the other lipid mediator, sphingosine-1-phosphate (6). Subsequently, 3 additional nonendothelial differentiation gene LPA receptors, designated LPA4/5/6, that share similarities with the P2Y (purinergic receptor) gene cluster have been discovered (7). In addition, intracellular LPA activates the peroxisome proliferator–activating receptor γ (PPARγ) (8). LPA is primarily generated by autotaxin (ATX), an enzyme with lysophospholipase D activity, which cleaves the headgroup of lysophospholipids to generate LPA (9). Subsequent studies revealed possible topological and structural connections between ATX and LPA GPCRs (10, 11).
The first biological effect of LPA described in vivo was the regulation of blood pressure (12, 13). Due to these observations, LPA rapidly emerged as a potential mediator in the cardiovascular system. Early studies conducted by Tokumura et al. (13) in rats and guinea pigs revealed a vasoconstrictive and hypertensive response after exposure to different molecular species of LPA. At the same time, the same molecular species elicited a hypotensive response in rabbits and cats. However, the hypotensive effect in cats later proved to be the result of decreased cardiac output induced by pulmonary vasoconstriction produced by platelet aggregation (14). LPA applied to the adventitial surface of pial arteries of newborn piglets in a closed cranial window model caused a dose-dependent vasoconstrictive response (15). Although at that time LPA receptors had not yet been identified, the sensitivity of the pial artery constriction to pertussis toxin indicated the involvement of Gi proteins in mediation of the effect. LPA has also been shown recently to induce endothelium- and shear stress–dependent vasoconstriction when applied to mouse aortic strips (16). In addition, LPA has been implicated in vascular pathologies, such as the development and complications of atherosclerosis (17–20).
We have demonstrated recently that LPA potently elicited vasoldilation in isolated aortae mediated by LPA1, PLC, and activation of eNOS (21). In the absence of endothelium or eNOS, however, LPA caused vasoconstriction, indicating that it also exerts a direct vasoactive action on vascular smooth muscle cells (VSMCs). The objective of the present study was to characterize the vasoconstrictive effect and the underlying signal transduction mechanisms activated by LPA in murine aortic rings denuded of endothelium. Our results using pharmacological tools and gene-knockout (KO) animal models indicate that LPA1 activation in a cyclooxygenase-1 (COX1)-dependent and pertussis toxin–sensitive manner elicits thromboxane A2 (TXA2) production, which in turn causes thromboxane prostanoid (TP) receptor-mediated vasoconstriction.
MATERIALS AND METHODS
All procedures were carried out according to the guidelines of the Hungarian Law of Animal Protection (28/1998) and were approved by the National Scientific Ethical Committee on Animal Experimentation (PEI/001/2706-13/2014).
Animals
C57BL/6 mice were obtained from Charles River Laboratories (Isaszeg, Hungary) and are referred to as wild-type (WT) mice in the text and figures. All transgenic mouse lines were backcrossed to the C57BL/6 genetic background. Mice deficient in LPA1 or LPA2 receptors (LPA1- and LPA2-KO mice, respectively) were generated as previously described (22–25). The LPA1- and LPA2-KO strains have been maintained with constant backcrossing to C57BL/6 since 2008. COX1-KO mice were from Dr. Ingvar Bjarnason (Guy’s, King’s College, and St. Thomas' School of Medical Education, London, United Kingdom) and were backcrossed to C57BL/6 more than 10 times. TP receptor–deficient (TP-KO) mice were kindly provided by Dr. Shuh Narumiya (Kyoto University, Kyoto, Japan) and were backcrossed to C57BL/6 more than 10 times. In experiments performed with LPA1-, LPA2-. and COX1-KO mice, WT animals from the same strain served as controls (Ctrls) and are referred to as LPA1-, LPA2-. and COX1-Ctrl mice, respectively. Because the TP mice had been maintained in our animal facility with KO × KO matings, WT C57BL/6 mice served as Ctrls (TP-Ctrl mice). Pertussis toxin (PTX) was administered intraperitoneally in some of the animals for 5 d prior to the experiments at a dosage of 50 μg/kg body weight to inhibit Gi proteins (26, 27).
Preparation of vessels
Adult male animals were perfused transcardially with 10 ml heparinized (10 IU/ml) Krebs solution under deep ether anesthesia as previously described (28). The aorta was removed and cleaned of fat and connective tissue under a dissection microscope (M3Z; Wild Heerbrugg AG, Gais, Switzerland) and immersed in a Krebs solution of the following composition (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 2.5 CaCl2·2 H2O, 1.2 MgSO4·7 H2O, 20 NaHCO3, 0.03 EDTA, and 10 glucose at room temperature and pH 7.4. Abdominal aortae (AAs) were cut into ∼3-mm-long segments and mounted on stainless steel vessel holders (200 µm in diameter) in a myograph (610 M multiwire myograph system; Danish Myo Technology A/S, Aarhus, Denmark). The endothelium of the segments was removed intentionally by gently rotating the segments on the holder pins. Absence of the endothelium was confirmed by the lack of acetylcholine-induced vasorelaxation of the denuded vessels. Thoracic aortae (TAs) were also cut into segments and subjected, with the endothelium preserved, to TXB2 ELISA as described below in detail.
Myography
Chambers of the myographs were filled with 6 ml gassed (95% O2–5% CO2) Krebs solution. The vessels were allowed a 30-min resting period, during which the bath solution was warmed to 37°C and the passive tension was adjusted to 10 mN, which was determined to be optimal in a previous study (28). Subsequently, the tissues were exposed to 124 mM K+ Krebs solution (made by isoosmolar replacement of Na+ by K+) for 1 min, followed by several washes with normal Krebs solution. A contraction evoked by 10 μM phenylephrine followed by administration of 0.1 μM acetylcholine served as a test of the reactivity of the smooth muscle and the endothelium, respectively. After repeated washing, during which the vascular tension returned to the resting level, the segments were exposed to 124 mM K+ Krebs solution for 3 min to elicit a reference maximal contraction. After a 30-min washout, increasing concentrations of phenylephrine (0.1 nM to 10 μM) and acetylcholine (1 nM to 10 μM) were administered to determine the reactivity of the vessel and to verify the proper denudation of the endothelium. After a 30-min resting period, the vessels at the resting tone were exposed to different concentrations of either LPA, the LPA1–3 agonist VPC31143 (29), or the LPA3 agonist T13 (30). In some experiments, the LPA1&3 receptor antagonist Ki16425 (31) or the selective LPA3 antagonist diacylglycerol pyrophosphate (DGPP) (32) was applied to the bath chambers at a concentration of 10 μM, 30 min prior to the administration of VPC31143. Vasoconstriction is expressed as percentage of the reference contraction induced by 124 mM K+.
Quantification of vascular TXA2 release
TAs were cut into 5 segments and allowed a 2-h resting period. In some of the experiments, 3 μg/ml PTX was applied for 2 h to inhibit Gi (33). Thereafter, the vessels were incubated in 200 μl Krebs solution at 37°C for 2 min to obtain a baseline level of TXA2 release. After the incubation, the supernatant was replaced with 200 μl of Krebs solution containing 10 μM VPC31143 and incubated for 2 min. Supernatants of the resting and the VPC31143-stimulated vessels were snap-frozen and stored at −80°C until the measurement of thromboxane levels. Concentrations of TXB2, a nonenzymatically produced stable metabolite of TXA2, were determined using a TXB2 enzyme immunoassay kit (501020; Cayman Chemical Co., Ann Arbor, MI, USA). TXB2 production was calculated as pg/min. Vessels with a baseline production of TXB2 >20 pg/min were considered preactivated and were excluded from the experiment.
Expression analysis of LPA receptors in the vascular smooth muscle
Endothelium-denuded TAs and AAs were isolated, and the adventitia of the vessels was carefully removed under a dissection microscope. The vessels were fast-frozen and stored at −80°C until PCR analysis. RNA was isolated from vascular smooth muscle with an RNeasy Micro kit (74004; Qiagen, Valencia, CA, USA), and RNA concentration and quality were assessed with Nanodrop (Thermo Fischer Scientific, Waltham, MA, USA). Up to 500 ng total RNA was converted to cDNA using a SuperScript VILO cDNA Synthesis Kit (11754-050; Thermo Fisher Scientific).
Assessment of mRNA expression was performed by quantitative real-time PCR using cDNA corresponding to 20 ng RNA template. PCR reactions were carried out in triplicate with 300 nmol of each primer in a final volume of 25 μl of 2× Maxima SYBR Green/ROX quantitative PCR master mix (K0223; Thermo Fischer Scientific). Amplification was performed after an initial step of 10 min at 95°C for 40 cycles at 94°C/15 s and 60°C/60 s with a StepOnePlus real-time PCR system (Thermo Fisher Scientific). Relative gene expression of each mRNA to glyceraldehyde 3-phosphate dehydrogenase was determined using the dCt method. The primer sequences are listed in Table 1.
TABLE 1.
Primers used for real-time quantitative PCR
| Primer, 5′–3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| GAPDH | CTGCACCACCAACTGCTTAG | GGGCCATCCACAGTCTTCT |
| LPA1 | CACCATGATGAGCCTTCTGA | GCAGCACACATCCAGCAATA |
| LPA2 | CCAGCCTGCTTGTCTTCCTA | GTGTCCAGCACACCACAAAT |
| LPA3 | AGGGCTCCCATGAAGCTAAT | TGCACGTTACACTGCTTGC |
| LPA4 | ACAGTGCCTCCCTGTTTGTC | AAATCAGAGAGGGCCAGGTT |
| LPA5 | TCATCATCTTCCTGCTGTGC | ATCGCGGTCCTGAATACTGT |
| LPA6 | TCGCTCATGAGGACACAGAC | CAAAGCAGCAGTTGGAAACA |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Reagents
LPA (18:1) and VPC31143 were purchased from Avanti Polar Lipids (Alabaster, AL, USA) and dissolved in saline immediately before administration. DGPP was purchased from Avanti Polar Lipids and dissolved in methanol. Ki16425 was purchased from Cayman Chemical Co. and dissolved in DMSO to make a 100-fold concentrated stock solution. In these experiments vehicle treatment served as Ctrl. PTX was purchased from List Biologic Laboratories, Inc. (Campbell, CA, USA) and dissolved in glycerol. T13 was synthesized as previously described (30) and was dissolved in PBS containing 0.1% fatty acid free bovine serum albumin. All other drugs and chemicals used in the present study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Data analysis
An MP100 system and AcqKnowledge 3.72 software from Biopac Systems, Inc. (Goleta, CA, USA) were used to record and analyze changes in the vascular tone. All data are presented as means ± se, and n indicates either the number of vessels tested in myography experiments or the number of animals tested in the case of TXB2 enzyme immunoassay or quantitative PCR. The number in parentheses after n indicates the number of animals the vessel segments were obtained from in the myography experiments. Statistical analysis was performed using GraphPad Prism software (v.6.07; GraphPad Software Inc., La Jolla, CA, USA). Student’s unpaired t test was applied when comparing 2 variables. All other comparisons between the different experimental groups were made by ANOVA followed by Tukey’s or Bonferroni’s post hoc test. A value of P < 0.05 was considered to be statistically significant.
RESULTS
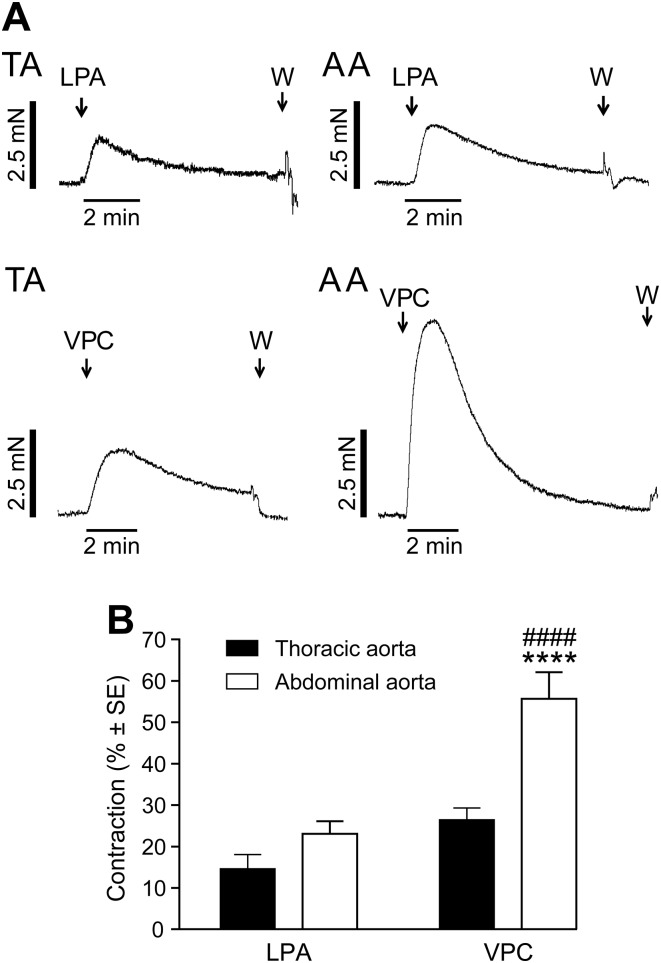
We have previously shown that LPA administration to murine aortic rings with intact endothelium caused relaxation, which disappeared when the endothelium was removed or when eNOS was genetically deleted (21). In our present experiments, LPA elicited a marked vasoconstriction when applied to endothelium-denuded vessels at resting tension (Fig. 1A). In intact vessels, VPC31143 reproduced the vasorelaxant effect of LPA (21). In endothelium-denuded vessels, VPC31143, like LPA, elicited vasoconstriction in segments of the TA and AA (Fig. 1A). Rings exposed to VPC31143 from the AA showed 2-fold higher contraction compared with those from the TA (Fig. 1B). VPC31143 stimulation in the AA evoked higher contraction compared with equimolar LPA (Supplemental Fig. 1), which was similar to that seen in intact vessels for the vasorelaxation response (21). Thus, VPC31143 showed a higher efficacy over the natural ligand LPA in inducing constriction or relaxation, which is consistent with findings obtained in LPA1-expressing HEK cells (29).
Figure 1.
Effects of LPA and VPC31143 on the tone of endothelium-denuded murine TA or AA segments. A) Representative recordings of vessels prepared from WT mice. “W” denotes wash-out with Krebs solution. B) AA segments showed 2-fold higher contraction compared with TA segments when stimulated with VPC31143. VPC31143 stimulation elicited more than twice as forceful contractions in the AA compared with LPA. ****P < 0.0001 vs. VPC effect in the TA; ####P < 0.0001 vs. LPA effect in the AA; 2-way ANOVA with Tukey’s post hoc test (n = 18–32 vessels obtained from 14–32 animals).
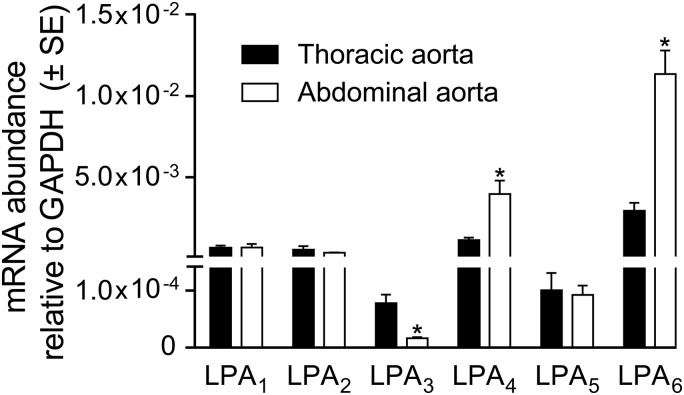
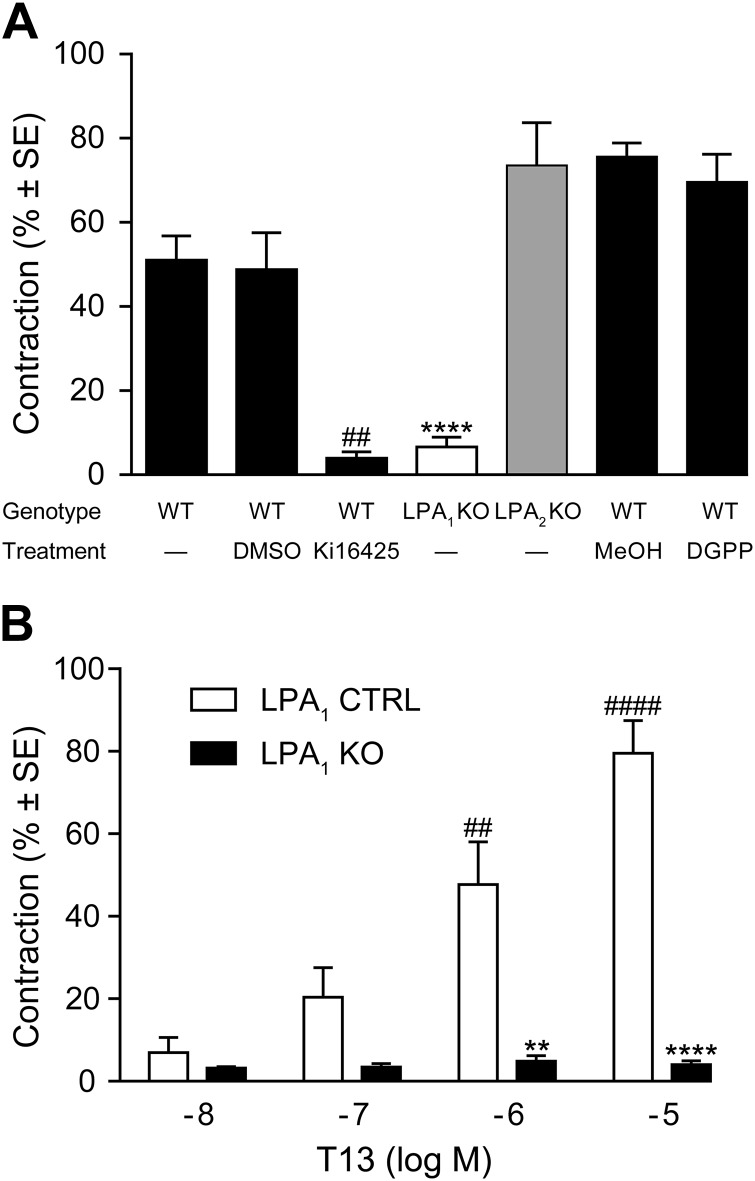
Murine aortic endothelial cells predominantly express LPA1 and LPA4 transcripts. Here we determined the gene expression profile in isolated murine TAs and AAs. LPA1, LPA2, LPA4, and LPA6 transcripts were abundantly detectable in both types of segments, with a slightly higher abundance of LPA4 and LPA6 in the AA specimens (Fig. 2). LPA3 transcripts were the least abundant; however, they showed higher expression in the TA. To identify the LPA receptor mediating the vasoconstriction response, we applied pharmacological and genetic approaches (Fig. 3). The LPA1&3-selective antagonist Ki16425 abolished contraction evoked by VPC31143 compared with vehicle-treated Ctrls isolated from WT mice (Fig. 3A). Similar to its action in rings isolated from WT mice, VPC31143 elicited vasoconstriction in rings prepared from LPA2-KO animals. Furthermore, the LPA3-selective antagonist DGPP failed to alter VPC31143-induced contractions in the AA of WT animals. In contrast, rings prepared from LPA1-KO mice failed to contract in response to the maximally effective concentration (10 µM) of VPC31143, indicating that the vasoconstriction is mediated by LPA1. To evaluate the potential involvement of LPA3 in mediating vasoconstriction, the vasoactive effects of T13 were determined, which had been shown to induce selective and maximal LPA3 activation at 10 nM but to also stimulate other LPA receptors at higher concentrations (30). This compound failed to induce contraction of endothelium-denuded AA segments when 10 nM was applied, whereas dose-dependent vasoconstriction developed in response to higher concentrations (Fig. 3B). However, this vasoconstrictor effect was absent in vessels prepared from LPA1-KO mice, indicating that LPA1 is the sole mediator of the vasoactive effect.
Figure 2.
Expression profile of LPA receptors in freshly isolated murine TA and AA VSMCs, determined by quantitative PCR. Murine aortic VSMCs predominantly express LPA1, LPA2, LPA4, and LPA6. LPA4 and LPA6 showed higher abundance in the AA, whereas LPA3, the least abundant subtype, showed higher expression in the TA (n = 3–9). *P < 0.05 vs. TA (unpaired Student’s t test).
Figure 3.
VPC31143-evoked contraction is mediated by LPA1 receptors. A) The LPA1&3 antagonist Ki16425 and the lack of LPA1 receptors, but not that of LPA2 receptors nor the selective LPA3 antagonist DGPP, abolish VPC31143-elicited vasoconstriction. Vessels of C57BL/6, LPA1-Ctrl, and LPA2-Ctrl mice showed identical responses and are therefore pooled and referred to as WT in the figure. Both Ki16425 and DGPP were applied at 10 μM for 30 min before the administration of VPC31143. Vehicle-treated Ctrl vessels were exposed to 1% DMSO or methanol (MeOH). ****P < 0.0001 vs. WT; ##P < 0.01 vs. WT with DMSO treatment; 1-way ANOVA with Tukey’s post hoc test (n = 5–23 vessels obtained from 4–13 animals). B) T13 at 10 nM concentration, where it selectively activates LPA3, failed to elicit vasoconstriction in Ctrl vessels. However, the administration of higher concentrations of T13, which are known to stimulate LPA1, resulted in dose-dependent contraction that was completely missing in LPA1-KO vessels. **P < 0.01, ****P < 0.0001 vs. LPA1 Ctrl; ##P < 0.01, ####P < 0.0001 vs. 10 nM LPA1 Ctrl (n = 16–22 vessels obtained from 7–12 animals).
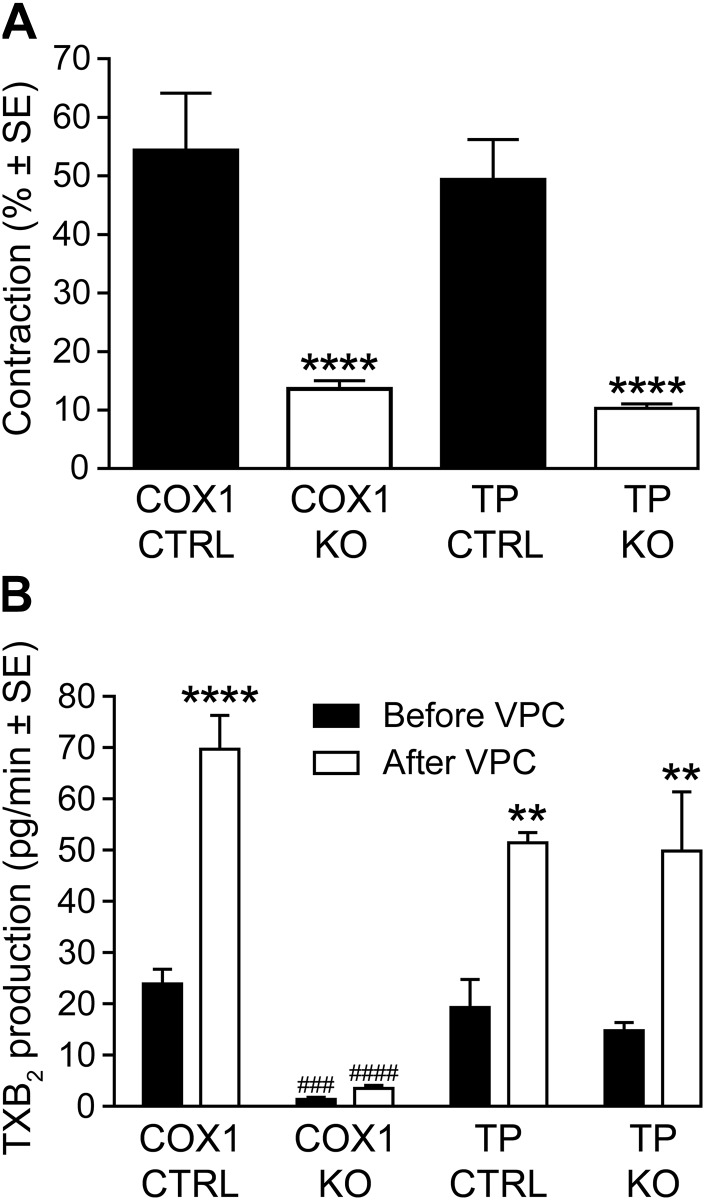
Because LPA was reported to induce COX1-mediated effects (34) and LPA-induced contractions of the longitudinal smooth muscle layer of the ileum were shown to be sensitive to indomethacin (35), we hypothesized that TXA2, a potent activator of VSMC contraction (36), might be involved in LPA1-mediated vasoconstriction. To test this hypothesis, we isolated aortic rings from WT, COX1-KO, and TP-KO mice and exposed them to maximally effective concentrations of VPC31143. Vessels from COX1- and TP-KO mice showed markedly reduced contractions in response to VPC31143 (Fig. 4A), indicating that COX1-derived TXA2 could be involved in the downstream activation of TP, which in turn causes contraction.
Figure 4.
Involvement of constrictor prostanoids in VPC31143-induced contraction. A) Reduced vasoconstrictor effects of 10 μM VPC31143 in AA segments prepared from COX1-KO and TP-KO mice as compared with WT animals. ****P < 0.0001 vs. the corresponding Ctrl; 1-way ANOVA with Tukey’s post hoc test (n = 8–23 vessels obtained from 5–16 animals). B) VPC31143 treatment markedly increased TXA2 release in COX1-Ctrl TA segments, whereas COX1-KO vessels showed diminished basal rate of TXA2 production that did not increase in response to VPC31143. In contrast, basal and VPC31143-stimulated TXA2 release from vessels of TP-KO mice showed no difference compared with TP-Ctrl vessels. **P < 0.01, ****P < 0.0001 vs. before VPC; ###P < 0.001, ####P < 0.0001 vs. corresponding COX1 Ctrl; 2-way ANOVA with Bonferroni’s post hoc test (n = 3–7).
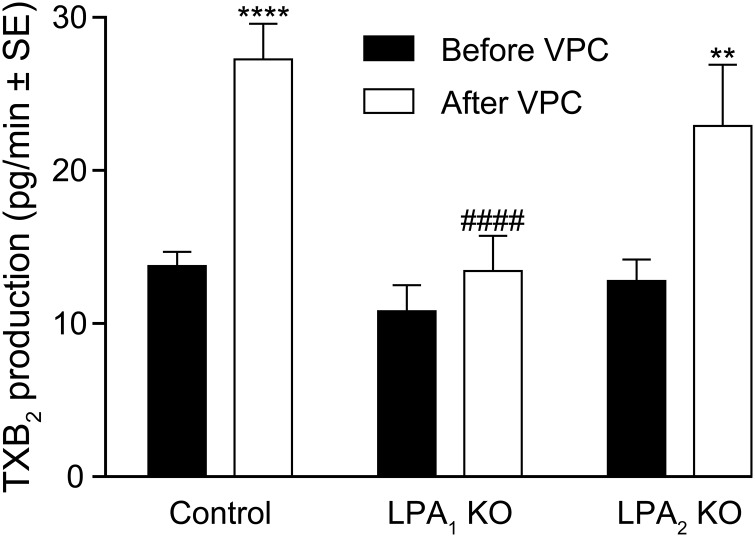
To gain further insight into this mechanism, we determined the levels of TXB2 in supernatants collected after exposure of the vessels to VPC31143 for 2 min (Figures 4B and 5). After VPC31143 treatment, a 2-fold increase of TXB2 production was detected in the supernatant collected from aortae of WT mice, which was absent in the vessels of LPA1-KO mice but remained unaltered in LPA2-KO vessels (Fig. 5). Furthermore, in the supernatants of vessels taken from COX1-KO mice, we found a diminished basal rate and a lack of VPC31143-activated elevation of TXB2 production, whereas deletion of TP receptors failed to alter the resting or stimulated release of TXB2 (Fig. 4B). These results are in agreement with our hypothesis that LPA1 elicits vasoconstriction via COX1-mediated production of TXA2, leading to the activation of the TP GPCRs in VSMCs.
Figure 5.
LPA1 mediates VPC31143-induced vascular TXA2 release. A lack of LPA1 but not LPA2 abolishes the VPC31143-elicited elevation in TXA2 production of thoracic aortic segments. LPA1- and LPA2-Ctrl mice showed identical responses and were therefore pooled and referred to as control. **P < 0.01, ****P < 0.0001 vs. before VPC; ####P < 0.0001 vs. control after VPC; 2-way ANOVA with Bonferroni’s post hoc test (n = 6–16).
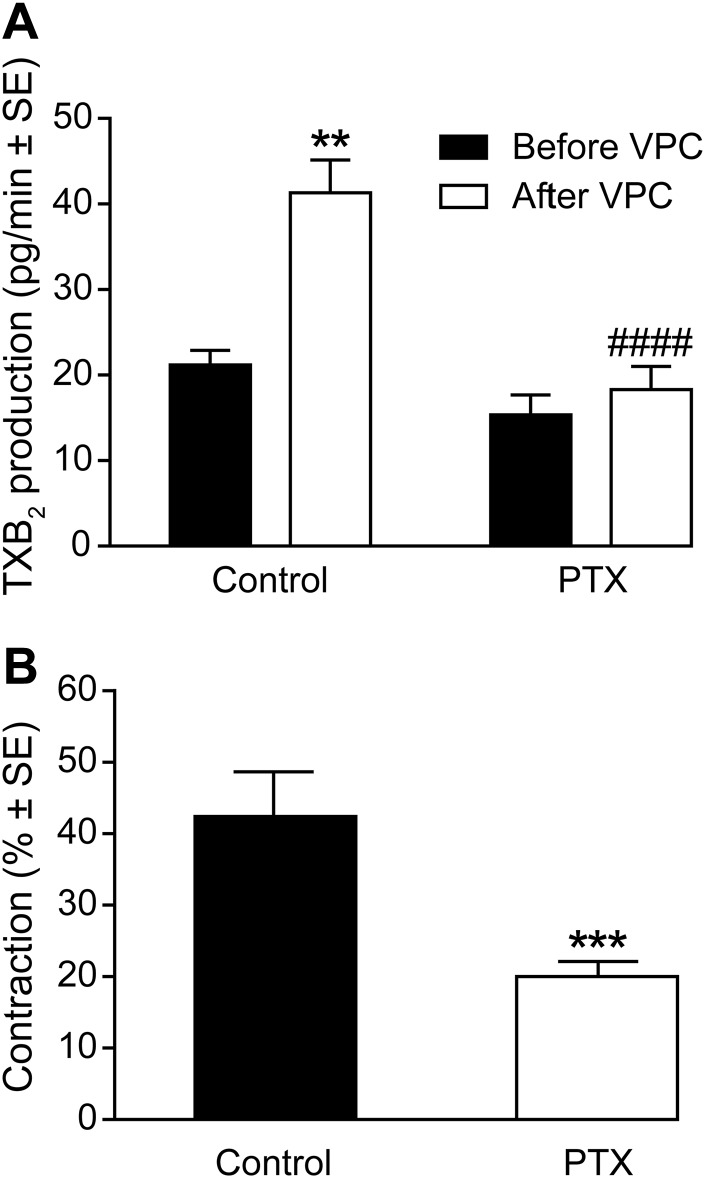
LPA- and VPC31143-activated vasorelaxation involves the activation of the canonical PLC–inositol trisphosphate–Ca2+ signal transduction pathway in the endothelium (21). Here we hypothesized that the LPA1-mediated autocrine/paracrine release of TXA2 and the consequent vasoconstriction may be mediated by Gi, a well-documented regulator of phospholipase A2 activity and TXA2 production (37–39). In support of this hypothesis, preincubation of aortic segments with PTX abolished the enhancement of TXB2 production upon VPC31143-stimulation (Fig. 6A). Furthermore, aortic segments isolated from PTX-pretreated mice showed reduced contractile responses after administration of VPC31143 (Fig. 6B).
Figure 6.
LPA-induced TXA2 production and constriction is mediated by Gi protein. A) Effect of PTX pretreatment on VPC31143-stimulated TXA2 production in WT vessels. **P < 0.01 vs. before VPC; ####P < 0.0001 vs. Ctrl after VPC; 2-way ANOVA with Bonferroni’s post hoc test (n = 5–8). B) PTX treatment inhibited VPC31143-elicited contraction in WT vessels. ***P < 0.001 vs. Ctrl; unpaired Student’s t test (n = 14–19 vessels obtained from 6–10 animals).
DISCUSSION
The diversity of the cardiovascular effects of LPA has long been known, yet no mechanistic explanation has been provided for the marked differences between the experimental findings. The results we present here indicate for the first time that LPA induces vasoconstriction in vessels with damaged endothelium, in contrast to its recently reported vasodilator effect in intact vessels. Interestingly, both the constrictive and dilatory effects are mediated by LPA1. The role of LPA1 is supported by the absence of vasoconstriction in LPA1 KO mice, the constrictive effect of the LPA1–3 agonist VPC31143 (29), and inhibition of constriction by the LPA1&3 antagonist Ki16425 but not by the LPA3 antagonist DGPP and the lack of LPA2 receptors. In addition, T13 failed to induce contraction at a concentration that selectively stimulates LPA3, whereas its contractile effect at higher concentrations was abolished by the absence of LPA1. Quantitative PCR results obtained from freshly isolated thoracic and abdominal aortic VSMCs showed a rank order of expression of LPA receptor subtype transcripts as 6 > 4 > 1 ≥ 2 > 5 > 3. This rank order of receptor expression also argues in favor of LPA1 rather than LPA3 being responsible for the VPC31143 and Ki16425 effects.
In search of the mediator of the vasoconstrictor response, we hypothesized that the constrictor prostanoid TXA2 could be responsible for this effect. This hypothesis is supported by our finding that LPA increased TXA2 production in isolated vessels that required COX1 and LPA1 but not LPA2. Moreover, the vasoconstrictor response was diminished in COX1- and TP-KO vessels. Our results support a mechanism whereby TXA2 is generated by LPA1-mediated COX1 activation. In addition, we found that PTX treatment of the vessels abolished production of TXA2 and diminished the vasoconstrictor response, indicating that this LPA1 receptor–mediated effect is coupled to the Gi protein in VSMCs.
LPA has also been reported to induce endothelium-dependent vasoactive effects mediated by TP receptors. Ohata et al. (40, 41) demonstrated that under conditions of increased shear stress, LPA stimulates Ca2+ influx in murine and bovine aortic endothelial cells via mechanosensitive cation channels. LPA in the presence of shear stress increased phenylephrine-induced contraction and reduced acetylcholine-induced relaxation in rat mesenteric arteries in an endothelium-dependent manner. These effects of LPA were sensitive to the COX inhibitor indomethacin and the TP receptor antagonist SQ29548 (16, 42). In addition, LPA induced endothelial shear stress-dependent Ca2+ elevation in VSMCs and contraction of the mouse aorta, which could be blocked by inhibitors of COX, TXA2 synthase, and the TP receptor (16).
In spite of the similar paracrine signaling, the mechanism of this vascular reaction is completely different from that described in the present study because the former develops only in the presence of shear stress and depends on the presence of intact endothelium, whereas our experiments have been performed in the absence of endothelium and shear stress. Nevertheless, these 2 lines of findings together indicate that under pathophysiological conditions involving increased shear stress or endothelial damage, LPA stimulates TXA2 release from the endothelium or VSMCs, leading to vasoconstriction. Reduction in vascular diameter in turn might further increase shear stress and mechanical stimulation or injury of the endothelium, leading to a vicious circle.
Regarding a potential interaction between LPA and prostanoids in the regulation of smooth muscle contraction, the available literary data are sparse and controversial. In an earlier study, Tokumura et al. (35) reported that the constrictor action of LPA in the guinea pig ileum was sensitive to indomethacin, whereas in a subsequent report by the same group, COX inhibition failed to influence LPA-induced contractions of the rat colon (43). Interestingly, in cultured ileal smooth muscle cells, LPA has also been reported to enhance the intracellular Ca2+ response to mechanical stimulation, an effect similar to that observed by Ohata et al. (44, 45) in endothelial cells, although in these studies the potential involvement of TXA2 or TP receptors has not been investigated. Moreover, in the uterus, LPA was shown to regulate COX2 expression (46). However, in contrast to our findings, this effect is mediated by LPA3 and results in the release of prostaglandin E2 and I2. The present observations provide direct evidence for an LPA-stimulated TXA2 release from the smooth muscle and a consequent change of the smooth muscle tone.
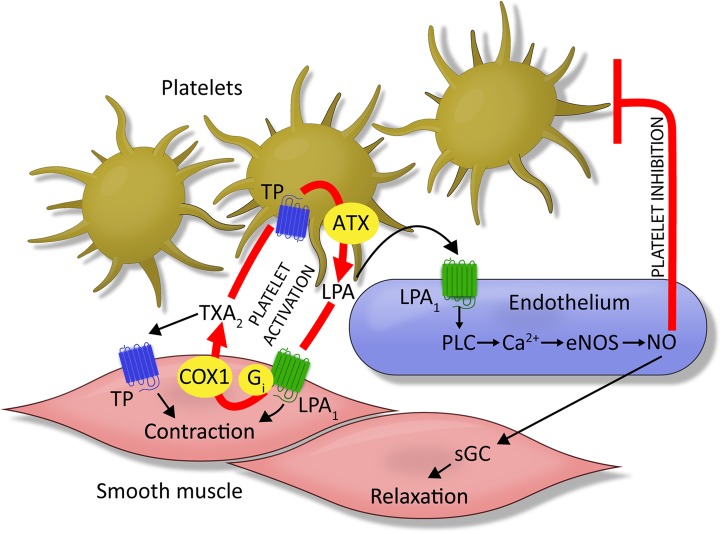
Our present and previous findings (21) together support the conclusion that LPA, acting on LPA1 receptors, represents a Janus-faced mediator in the regulation of vascular tone. To understand the physiological importance of this paradoxical behavior, the mechanism of intravascular LPA production should be considered. LPA production in blood is linked to platelet activation. At the site of vascular injury, platelets can come into direct contact with VSMCs, and LPA produced by ATX can activate the LPA1–COX1–TXA2 axis (Fig. 7). TXA2, in turn, could lead to vasoconstriction and augmented platelet aggregation, preventing hemorrhage through the damaged vessel wall. A putative positive feedback loop between the release of LPA and TXA2 production ensures efficient hemostasis, but it may initiate a vicious circle by promoting thrombus formation. Plausibly, when a growing thrombus reaches the intact vascular wall, localized LPA generation can induce NO release via the endothelial LPA1 receptor–PLC–eNOS pathway (Fig. 7) (21). In such a case, endothelial NO, as the negative feedback regulator of the vascular LPA1 system, can oppose TXA2-mediated vasoconstriction and inhibit platelet activation.
Figure 7.
Integrated hypothesis of LPA1-mediated vasoactive effects in intact vessels vs. after endothelial damage. Under physiological conditions, LPA stimulates endothelial NO production in an LPA1/PLC-dependent manner (21), resulting in vasorelaxation and inhibition of platelets. In the absence of the endothelium, however, platelet activation initiates LPA production, which in turn acts on LPA1 in VSMCs and induces TXA2 production. TXA2 elicits contraction via the activation of TP in VSMCs but also promotes further platelet activation acting on TP in platelets. TP-mediated activation of platelets results in additional LPA production. This mechanism represents a potential positive feedback loop in which platelet activation promotes contraction and further platelet activation via a vicious circle involving LPA/LPA1 and TXA2/TP receptors, resulting in a pathophysiological vasoconstriction.
The opposite effects of LPA on the vascular tone mediated by LPA1 in VSMCs and the endothelium may not be equally potent in every species. For instance, it was reported that intravenous injection of LPA induces hypertension in rats and guinea pigs, whereas hypotension is induced in cats and rabbits (13). In mice, LPA induces only weak and transient hypertension (47), which may indicate a balance between vasoconstrictor and vasodilator effects. Interestingly, the pressor effect of LPA is enhanced significantly in spontaneously hypertensive rats as compared with Wistar-Kyoto rats (48). This latter report indicates that the balance between the vasoconstrictor and vasodilator properties of LPA might become disturbed under pathologic conditions. Our present and previous observations indicate that dysfunction or damage of the endothelium can tilt the vasodilator effect of LPA toward vasoconstriction and potentially even to vasospasm. Accumulation of LPA in atherosclerotic plaques has been reported (18–20, 49). Release of LPA from plaque-associated sources coupled with disruption of normal endothelial functions might lead to sustained vasoconstriction. The vasoconstrictor properties of LPA can become dominant after hemorrhage, where it can directly stimulate VSMCs, bypassing the physical and functional barrier provided by the endothelium. Experiments conducted in the late 1990s showed that LPA applied to the subarachnoid space attenuated vasodilator responses and elicited vasospasm (15, 50). Furthermore, in an experimental subarachnoid hemorrhage model 4 d after intrathecal injection of autologous blood, an LPA-like bioactive mediator was detected in the cerebrospinal fluid (15). These results are consistent with the actions of LPA in posthemorrhagic hydrocephalus, which has been reported to be coupled to LPA1 (51). The present results might offer a potential mechanism of LPA-induced vasoconstriction/vasospasm after subarachnoid hemorrhage. Platelet-derived LPA in the cerebrospinal fluid comes into direct contact with LPA1 on the abluminal surface of VSMCs, where it can selectively trigger TXA2-mediated contraction, the very mechanism we described in the present study. Interestingly, with the same time course (i.e., 3–4 d after subarachnoid hemorrhage), when the LPA-like biologic activity was detected in the cerebrospinal fluid (15), pharmacological inhibition of TXA2 synthesis attenuated the development of cerebral vasospasm (52, 53). Nevertheless, the role of a potentially vicious circle between LPA and TXA2 production requires further investigation in models of subarachnoid hemorrhage.
In summary, the present study demonstrates that mouse aortic VSMCs express all known LPA receptor transcripts, with the highest abundance of LPA1, LPA2, LPA4, and LPA6. The direct effect of LPA on VSMCs is contraction, which is in contrast to its endothelium-dependent vasorelaxant action, even though both are mediated by LPA1 receptors. The signaling pathways include LPA-induced COX1-mediated autocrine/paracrine TXA2 release in VSMCs and consequent activation of TP receptors, which is responsible for the development of vasoconstriction. We propose that LPA acts as a Janus-faced mediator in the regulation of vascular tone and that its overall effect is determined by the functional integrity of the endothelium. The interaction between the LPA/LPA1 and TXA2/TP pathways described in the present study might play a significant role not only in vasoregulation but also in hemostasis, thrombosis, and vascular remodeling.
ACKNOWLEDGMENTS
This work was supported by the Hungarian Scientific Research Fund (K-101775 and K-112964; to Z.B.), the Fulbright Specialist Program (Project ID 6458; to G.T.), the U.S. National Institutes of Health (NIH) National Cancer Institute (Grant CA092160; to G.T.), and the NIH National Institute of Neurological Disorders and Stroke (Grant NS084398; to J.C.). The authors thank Margit Kerék, László Hricisák, Ildikó Murányi, and Ágnes Fülöp (Institute of Clinical Experimental Research, Semmelweis University, Budapest, Hungary) for expert technical assistance and for managing the transgenic mouse colonies and András Kucsa (Semmelweis University) for graphic artwork of Figure 7.
Glossary
- AA
abdominal aorta
- ATX
autotaxin
- COX
cyclooxygenase
- Ctrl
control
- DGPP
diacylglycerol pyrophosphate
- GPCR
G-protein–coupled receptor
- KO
knockout
- LPA
lysophosphatidic acid
- PPARγ
peroxisome proliferator–activated receptor γ
- PTX
pertussis toxin
- TA
thoracic aorta
- TP
thromboxane prostanoid
- TXA2
thromboxane A2
- VSMC
vascular smooth muscle cell
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. T. Dancs, É. Ruisanchez, J. Aoki, G. Tigyi, and Z. Benyó designed research; P. T. Dancs, É. Ruisanchez, A. Balogh, and C. R. Panta performed experiments; P. T. Dancs, Z. Miklós, and É. Ruisanchez analyzed data and prepared figures; P. T. Dancs, G. Tigyi, and Z. Benyó wrote the paper; R. M. Nüsing, J. Aoki, J. Chun, and S. Offermanns corrected the paper; and J. Chun, R. M. Nüsing, and S. Offermanns contributed transgenic mice to the study.
REFERENCES
- 1.Tigyi G. (2010) Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol. 161, 241–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaho V. A., Hla T. (2011) Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 111, 6299–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yung Y. C., Stoddard N. C., Mirendil H., Chun J. (2015) Lysophosphatidic acid signaling in the nervous system. Neuron 85, 669–682 [Erratum] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng X., Yung Y. C., Chen A., Chun J. (2015) Lysophosphatidic acid signalling in development. Development 142, 1390–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht J. H., Weiner J. A., Post S. R., Chun J. (1996) Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 135, 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kihara Y., Mizuno H., Chun J. (2015) Lysophospholipid receptors in drug discovery. Exp. Cell Res. 333, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagida K., Kurikawa Y., Shimizu T., Ishii S. (2013) Current progress in non-Edg family LPA receptor research. Biochim. Biophys. Acta 1831, 33–41 [DOI] [PubMed] [Google Scholar]
- 8.McIntyre T. M., Pontsler A. V., Silva A. R., St Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., Prestwich G. D. (2003) Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA 100, 131–136 [Erratum] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., Fukuzawa K. (2002) Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 277, 39436–39442 [DOI] [PubMed] [Google Scholar]
- 10.Moolenaar W. H., Perrakis A. (2011) Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12, 674–679 [DOI] [PubMed] [Google Scholar]
- 11.Chrencik J. E., Roth C. B., Terakado M., Kurata H., Omi R., Kihara Y., Warshaviak D., Nakade S., Asmar-Rovira G., Mileni M., Mizuno H., Griffith M. T., Rodgers C., Han G. W., Velasquez J., Chun J., Stevens R. C., Hanson M. A. (2015) Crystal structure of antagonist bound human lysophosphatidic acid receptor 1. Cell 161, 1633–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen S., Smeby R. R., Bumpus F. M. (1968) Antihypertensive effect of an isolated phospholipid. Am. J. Physiol. 214, 337–341 [DOI] [PubMed] [Google Scholar]
- 13.Tokumura A., Fukuzawa K., Tsukatani H. (1978) Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids 13, 572–574 [DOI] [PubMed] [Google Scholar]
- 14.Schumacher K. A., Classen H. G., Späth M. (1979) Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: identity with substances in aged serum (DAS). Thromb. Haemost. 42, 631–640 [PubMed] [Google Scholar]
- 15.Tigyi G., Hong L., Yakubu M., Parfenova H., Shibata M., Leffler C. W. (1995) Lysophosphatidic acid alters cerebrovascular reactivity in piglets. Am. J. Physiol. 268, H2048–H2055 [DOI] [PubMed] [Google Scholar]
- 16.Niioka T., Ohata H., Momose K., Honda K. (2013) Lysophosphatidic acid induces shear stress-dependent contraction in mouse aortic strip in situ. J. Cardiovasc. Pharmacol. 62, 530–538 [DOI] [PubMed] [Google Scholar]
- 17.Zhang C., Baker D. L., Yasuda S., Makarova N., Balazs L., Johnson L. R., Marathe G. K., McIntyre T. M., Xu Y., Prestwich G. D., Byun H. S., Bittman R., Tigyi G. (2004) Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 199, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siess W., Zangl K. J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., Aepfelbacher M. (1999) Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 96, 6931–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schober A., Siess W. (2012) Lysophosphatidic acid in atherosclerotic diseases. Br. J. Pharmacol. 167, 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth S. S., Mueller P., Yang F., Brandon J. A., Morris A. J. (2014) Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 34, 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruisanchez É., Dancs P., Kerék M., Németh T., Faragó B., Balogh A., Patil R., Jennings B. L., Liliom K., Malik K. U., Smrcka A. V., Tigyi G., Benyó Z. (2014) Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J. 28, 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contos J. J., Fukushima N., Weiner J. A., Kaushal D., Chun J. (2000) Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA 97, 13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contos J. J. A., Ishii I., Fukushima N., Kingsbury M. A., Ye X., Kawamura S., Brown J. H., Chun J. (2002) Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 22, 6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsbury M. A., Rehen S. K., Contos J. J., Higgins C. M., Chun J. (2003) Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci. 6, 1292–1299 [DOI] [PubMed] [Google Scholar]
- 25.Yang A. H., Ishii I., Chun J. (2002) In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta 1582, 197–203 [DOI] [PubMed] [Google Scholar]
- 26.Mallem Y., Holopherne D., Reculeau O., Le Coz O., Desfontis J. C., Gogny M. (2005) Beta-adrenoceptor-mediated vascular relaxation in spontaneously hypertensive rats. Auton. Neurosci. 118, 61–67 [DOI] [PubMed] [Google Scholar]
- 27.Van Meijeren C. E., Vleeming W., van de Kuil T., Manni J., Kegler D., Hendriksen C. F. M., de Wildt D. J. (2004) In vivo pertussis toxin treatment reduces contraction of rat resistance arteries but not that of mouse trachea. Eur. J. Pharmacol. 488, 127–135 [DOI] [PubMed] [Google Scholar]
- 28.Horváth B., Orsy P., Benyó Z. (2005) Endothelial NOS-mediated relaxations of isolated thoracic aorta of the C57BL/6J mouse: a methodological study. J. Cardiovasc. Pharmacol. 45, 225–231 [DOI] [PubMed] [Google Scholar]
- 29.Heise C. E., Santos W. L., Schreihofer A. M., Heasley B. H., Mukhin Y. V., Macdonald T. L., Lynch K. R. (2001) Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol. Pharmacol. 60, 1173–1180 [DOI] [PubMed] [Google Scholar]
- 30.Tamaruya Y., Suzuki M., Kamura G., Kanai M., Hama K., Shimizu K., Aoki J., Arai H., Shibasaki M. (2004) Identifying specific conformations by using a carbohydrate scaffold: discovery of subtype-selective LPA-receptor agonists and an antagonist. Angew. Chem. Int. Ed. Engl. 43, 2834–2837 [DOI] [PubMed] [Google Scholar]
- 31.Ohta H., Sato K., Murata N., Damirin A., Malchinkhuu E., Kon J., Kimura T., Tobo M., Yamazaki Y., Watanabe T., Yagi M., Sato M., Suzuki R., Murooka H., Sakai T., Nishitoba T., Im D. S., Nochi H., Tamoto K., Tomura H., Okajima F. (2003) Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 64, 994–1005 [DOI] [PubMed] [Google Scholar]
- 32.Fischer D. J., Nusser N., Virag T., Yokoyama K., Wang Da, Baker D. L., Bautista D., Parrill A. L., Tigyi G. (2001) Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol. Pharmacol. 60, 776–784 [PubMed] [Google Scholar]
- 33.Petitcolin M. A., Vandeputte C., Spitzbarth-Régrigny E., Bueb J. L., Capdeville-Atkinson C., Tschirhart E. (2001) Lack of involvement of pertussis toxin-sensitive G-proteins in norepinephrine-induced vasoconstriction of rat aorta smooth muscle. Biochem. Pharmacol. 61, 485–491 [DOI] [PubMed] [Google Scholar]
- 34.Li H., Wang D., Zhang H., Kirmani K., Zhao Z., Steinmetz R., Xu Y. (2009) Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol. Cancer Ther. 8, 1692–1701 [DOI] [PubMed] [Google Scholar]
- 35.Tokumura A., Fukuzawa K., Tsukatani H. (1982) Contractile actions of lysophosphatidic acids with a chemically-defined fatty acyl group on longitudinal muscle from guinea-pig ileum. J. Pharm. Pharmacol. 34, 514–516 [DOI] [PubMed] [Google Scholar]
- 36.Sellers M. M., Stallone J. N. (2008) Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 294, H1978–H1986 [DOI] [PubMed] [Google Scholar]
- 37.Nebigil C., Malik K. U. (1993) Alpha adrenergic receptor subtypes involved in prostaglandin synthesis are coupled to Ca++ channels through a pertussis toxin-sensitive guanine nucleotide-binding protein. J. Pharmacol. Exp. Ther. 266, 1113–1124 [PubMed] [Google Scholar]
- 38.LaBelle E. F., Polyak E. (1998) Norepinephrine stimulates arachidonic acid release from vascular smooth muscle via activation of cPLA2. Am. J. Physiol. 274, C1129–C1137 [DOI] [PubMed] [Google Scholar]
- 39.Mong S., Wu H. L., Clark M. A., Gleason J. G., Crooke S. T. (1986) Leukotriene D4 receptor-mediated synthesis and release of arachidonic acid metabolites in guinea pig lung: induction of thromboxane and prostacyclin biosynthesis by leukotriene D4. J. Pharmacol. Exp. Ther. 239, 63–70 [PubMed] [Google Scholar]
- 40.Ohata H., Ikeuchi T., Kamada A., Yamamoto M., Momose K. (2001) Lysophosphatidic acid positively regulates the fluid flow-induced local Ca(2+) influx in bovine aortic endothelial cells. Circ. Res. 88, 925–932 [DOI] [PubMed] [Google Scholar]
- 41.Ohata H., Yamada H., Momose K. (2011) Lysophosphatidic acid induces shear stress-dependent Ca2+ influx in mouse aortic endothelial cells in situ. Exp. Physiol. 96, 468–475 [DOI] [PubMed] [Google Scholar]
- 42.Shibata K., Miyazaki T., Ohata H., Honda K. (2011) Shear stress-dependent effects of lysophosphatidic acid on agonist-induced vasomotor responses in rat mesenteric artery. J. Cardiovasc. Pharmacol. 57, 604–610 [DOI] [PubMed] [Google Scholar]
- 43.Tokumura A., Yube N., Fujimoto H., Tsukatani H. (1991) Lysophosphatidic acids induce contraction of rat isolated colon by two different mechanisms. J. Pharm. Pharmacol. 43, 774–778 [DOI] [PubMed] [Google Scholar]
- 44.Ohata H., Seito N., Aizawa H., Nobe K., Momose K. (1995) Sensitizing effect of lysophosphatidic acid on mechanoreceptor-linked response in cytosolic free Ca2+ concentration in cultured smooth muscle cells. Biochem. Biophys. Res. Commun. 208, 19–25 [DOI] [PubMed] [Google Scholar]
- 45.Ohata H., Aizawa H., Momose K. (1996) Mechanisms of mechanical stress-induced Ca(2+)-mobilization sensitized by lysophosphatidic acid in cultured smooth muscle cells. Life Sci. 58, 2217–2223 [DOI] [PubMed] [Google Scholar]
- 46.Ye X., Hama K., Contos J. J., Anliker B., Inoue A., Skinner M. K., Suzuki H., Amano T., Kennedy G., Arai H., Aoki J., Chun J. (2005) LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panchatcharam M., Miriyala S., Yang F., Rojas M., End C., Vallant C., Dong A., Lynch K., Chun J., Morris A. J., Smyth S. S. (2008) Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ. Res. 103, 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokumura A., Yotsumoto T., Masuda Y., Tanaka S. (1995) Vasopressor effect of lysophosphatidic acid on spontaneously hypertensive rats and Wistar Kyoto rats. Res. Commun. Mol. Pathol. Pharmacol. 90, 96–102 [PubMed] [Google Scholar]
- 49.Bot M., Bot I., Lopez-Vales R., van de Lest C. H., Saulnier-Blache J. S., Helms J. B., David S., van Berkel T. J., Biessen E. A. (2010) Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am. J. Pathol. 176, 3073–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakubu M. A., Liliom K., Tigyi G., Leffler C. W. (1997) Role of lysophosphatidic acid in endothelin-1- and hematoma-induced alteration of cerebral microcirculation. Am. J. Physiol. (Comp. & Integrative Physiol.) 42, R703–R709 [DOI] [PubMed] [Google Scholar]
- 51.Yung Y. C., Mutoh T., Lin M. E., Noguchi K., Rivera R. R., Choi J. W., Kingsbury M. A., Chun J. (2011) Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med. 3, 99ra87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki T., Wakai S., Asano T., Takakura K., Sano K. (1982) Prevention of cerebral vasospasm after SAH with a thromboxane synthetase inhibitor, OKY-1581. J. Neurosurg. 57, 74–82 [DOI] [PubMed] [Google Scholar]
- 53.Chan R. C., Durity F. A., Thompson G. B., Nugent R. A., Kendall M. (1984) The role of the prostacyclin-thromboxane system in cerebral vasospasm following induced subarachnoid hemorrhage in the rabbit. J. Neurosurg. 61, 1120–1128 [DOI] [PubMed] [Google Scholar]