Abstract
Nonalcoholic fatty liver disease (NAFLD) is widespread in adults and children. Early exposure to maternal obesity or Western-style diet (WD) increases steatosis and oxidative stress in fetal liver and is associated with lifetime disease risk in the offspring. Pyrroloquinoline quinone (PQQ) is a natural antioxidant found in soil, enriched in human breast milk, and essential for development in mammals. We investigated whether a supplemental dose of PQQ, provided prenatally in a mouse model of diet-induced obesity during pregnancy, could protect obese offspring from progression of NAFLD. PQQ treatment given pre- and postnatally in WD-fed offspring had no effect on weight gain but increased metabolic flexibility while reducing body fat and liver lipids, compared with untreated obese offspring. Indices of NAFLD, including hepatic ceramide levels, oxidative stress, and expression of proinflammatory genes (Nos2, Nlrp3, Il6, and Ptgs2), were decreased in WD PQQ-fed mice, concomitant with increased expression of fatty acid oxidation genes and decreased Pparg expression. Notably, these changes persisted even after PQQ withdrawal at weaning. Our results suggest that supplementation with PQQ, particularly during pregnancy and lactation, protects offspring from WD-induced developmental programming of hepatic lipotoxicity and may help slow the advancing epidemic of NAFLD in the next generation.—Jonscher, K. R., Stewart, M. S., Alfonso-Garcia, A., DeFelice, B. C., Wang, X. X., Luo, Y., Levi, M., Heerwagen, M. J. R., Janssen, R. C., de la Houssaye, B. A., Wiitala, E., Florey, G., Jonscher, R. L., Potma, E. O., Fiehn, O. Friedman, J. E. Early PQQ supplementation has persistent long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice.
Keywords: antioxidant, PGC-1α, ceramide, lipidomics, CARS
Nonalcoholic fatty liver disease (NAFLD) describes a broad spectrum of chronic liver abnormalities, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH, the more severe form of the disease), characterized by different degrees of inflammation and fibrosis. NAFLD is the most common liver disease in the world and affects 20–30% of all adults in the United States and well over 60% of adults with obesity, increasing the risk of cardiovascular events, type 2 diabetes and hepatocellular carcinoma (1). There is compelling evidence from nonhuman primate and rodent models linking a maternal obesogenic environment and development of NAFLD in offspring (2, 3). Indeed, exposure to a high-fat diet (HFD) or Western-style diet (WD) in utero, compared with only postnatal high-energy diet feeding, rapidly worsens the liver phenotype to fibrosis in rodents (2, 4, 5).
Periconception obesity in both parents affects reproductive health (reviewed in ref. 6), potentially by affecting embryonic mitochondrial metabolism, which may underlie the progression of liver damage observed in offspring born to obese mothers (7–9). How oxidative metabolism, including that of offspring exposed to a maternal HFD, may be restored and whether restoration will effectively blunt development of NAFLD, remain open questions. Therapeutic approaches targeting mitochondrial oxidative function, delivered both during gestation and lactation, may prevent metabolic complications in offspring in later life. Sen and Simmons (10) demonstrated that high doses of vitamins A, E, and C and selenium provided during pregnancy and lactation to WD-fed rats, effectively decreased adiposity and improved glucose tolerance in offspring switched to a lower fat control diet after birth; however, effects on liver were not tested. Likewise, we recently established that supplementing the diet of obese nonhuman primates fed an HFD with the antioxidant resveratrol prevented development of fatty liver in the fetus (11); however, it altered fetal pancreatic morphology, suggesting the need for further studies.
Pyrroloquinoline quinone (PQQ) is a ubiquitous natural bacterial cofactor found in soil, plants, and interstellar dust and is essential for reproductive health and normal development in mammals (12, 13). PQQ is a powerful antioxidant. In the reduced form, its aroxyl radical-scavenging activity is 7.4-fold higher than that of vitamin C, the most active water-soluble antioxidant (14). PQQ stimulates mitochondrial biogenesis in vitro by activating peroxisome proliferator-activated receptor (PPAR)-γ coactivator (PGC)-1α (15), an important regulator of metabolism and mitochondrial oxidative defense. PQQ supplementation increased the concentration of lysozyme in plasma (a component of the innate immune system) in broiler chicks (16), mitigated streptozotocin-induced oxidative damage and diabetes in mice (17), and protected mice from thioacetamide-induced liver fibrosis (18), whereas diets devoid of PQQ resulted in elevated plasma lipids in rats (19), suggesting PQQ targets the lesions of NAFLD. The major source of this important antioxidant in mammals is dietary (20), and PQQ is highly enriched in human breast milk (21), making it an intriguing dietary therapeutic for preventing excess maternal obesity-induced oxidative stress or ameliorating mitochondrial dysfunction and inflammation caused by exposure to an overabundance of toxic lipids during development.
The goal of this study was to test the hypothesis that PQQ supplementation, provided prenatally at conception and through lactation in obese pregnancy, improves offspring metabolic outcomes, enhances oxidative defense, and prevents NAFLD in the livers of obese offspring exposed to HFD both prenatally and after weaning. Furthermore, using coherent anti-Stokes Raman spectroscopy (CARS) imaging and lipidomics profiling, we sought to determine whether levels of bioactive lipids, frequently linked to hepatic apoptosis and metabolic dysfunction, are reduced in livers of obese mice given supplemental PQQ.
MATERIALS AND METHODS
Animals and diets
All experiments were reviewed, approved, and monitored by the University of Colorado Institutional Animal Care and Use Committee in accordance with the Guide for Care and Use of Laboratory Animals (National Research Council, Washington DC, USA). C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and used for 2 different studies: a maternal–fetal study (study 1) and a developmental programming study in offspring (study 2), as outlined in Supplemental Fig. S1. For study 1, 8-wk-old females were randomly assigned to either a low-fat diet (LFD; 3.85 kcal/g, 10% kcal from fat) (D12450B; Research Diets, New Brunswick, NJ, USA) or a high-fat diet (HFD; 4.73 kcal/g, 45% kcal from fat) (D12451; Research Diets) matched for micronutrients. The diets were fed to the mice ad libitum for 8 wk before mating and throughout gestation. At ∼16 wk of age, females were mated with standard chow-fed males, and PQQ supplementation was initiated. PQQ (obtained as a gift from Dr. Robert Rucker, University of California, Davis) was dissolved in water at a concentration of 1.25 mg/l (3.8 μM) providing ∼7.5 μg/d based on standard C57BL/6J mouse water consumption (22). This daily dose was derived from human studies (23) and adjusted for increased caloric expenditure in mice vs. humans. Successful pregnancy was confirmed by the presence of a vaginal plug. Mothers were individually housed during gestation, and mice and food were weighed weekly.
Blood and tissues of mothers and pups were harvested in very late gestation [embryonic d (E)18.5] (24). The animals were unfed for 4 h before glucose analysis and then were euthanized. Maternal blood and tissues were quickly collected with simultaneous dissection and isolation of fetal–placental units. Each fetus and placenta was weighed separately, and each fetus was subsequently euthanized by decapitation and exsanguination. Fetal blood was pooled between littermates for subsequent analysis. Fetal livers were dissected, snap frozen, and stored at −80°C for later analysis; stabilized in RNALater (Qiagen, Valencia, CA, USA); or embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) and used for cryosections. Livers from 1 fetus per litter were selected for analysis; therefore n represents the number of litters in study 1. Maternal weight was determined as gross weight before harvest minus aggregate weight of fetal–placental units. Placentas were measured with a micrometer, lengthwise and across the placental diameter after dissection (24). The placental surface area was calculated as length × width.
For study 2, sibling offspring of breeders (fed a standard chow diet) were randomly stratified into breeding groups and fed standard chow [CH; 22% kcal from fat, 23% protein, 55% carbohydrate, 3.3 kcal/g; (CH) 2019, Teklad; Envigo, Indianapolis, IN, USA] or WD [TD.88137, Envigo; 42% kcal from fat, 15% protein, 43% carbohydrate (34% sucrose by weight), 0.2% cholesterol, and 4.5 kcal/g], with or without PQQ supplementation. In the maternal–fetal studies, dams were fed an HFD to focus on the effects of a high lipid load on fetal hepatic metabolism. We changed from HFD to WD for the postnatal studies, because WD accelerates steatosis and mild fibrosis (25) and at 20 wk of age, the WD replicates a phenotype similar to human NASH (26). In previous studies, we found that long-term feeding of mice with an LFD adversely affected their health and led to an increased rate of early death from undetermined causes in the vivarium. The standard chow diet represented a more physiologically relevant diet that reflected a healthier level of fat consumption; therefore, we decided to use that diet, even though it was not matched for micronutrients with WD. Second-generation offspring were either continued on the maternal diet or were switched after weaning to WD without PQQ (WD PQQ/WD). Last, we tested whether a subset of WD-fed offspring supplemented with PQQ after weaning at a higher concentration of 12.1 μM, providing ∼24 μg/d per mouse, provided increased protection from weight gain (WD PQQ 3X). Tissues from offspring were either harvested at the time of weaning [postnatal d (PND) 21] or at 20 wk of age. Offspring were unfed for 4 h and then were euthanized via isoflurane anesthetic followed by cervical dislocation and cardiac exsanguination. Livers were dissected, weighed, snap frozen in liquid nitrogen, and stored at −80°C until they were used or were embedded in OCT compound and used for cryosections. The n in study 2 represents the number of animals studied. The study mice were derived from repeated multiple litters, representing 2 or more breeding pairs. The data presented herein include males (except for E18.5 mice), because they have been shown to respond more robustly to diet-induced obesity than females (24, 27).
Indirect calorimetry
Energy balance (energy intake, resting and nonresting energy expenditure, and fuel utilization) was determined via indirect calorimetry at PND 21 or 20 wk. The mice were individually placed in an 8-chamber system (Oxymax Comprehensive Lab Animal Monitoring System; Columbus Instruments, Columbus, OH, USA), equipped with control of temperature, humidity, and light/dark cycle. The mice were acclimated to the system for 2–3 d before data acquisition and then monitored for 3–5 d, during which time food and water intake were measured, as well as oxygen consumption (Vo2) and carbon dioxide production (Vco2). The respiratory quotient (RQ) was calculated as the ratio of CO2 production to O2 consumption (Vco2/Vo2) (28–30). Activity was measured and thermic energy from food (TEF; kilocalories above resting energy expenditure that are necessary to metabolize or store foods consumed) was inferred from total energy expenditure and activity (31). After monitoring, the mice were returned to group housing for up to 48 h and then euthanized.
Body composition
Body composition was determined in adult male mice by quantitative magnetic resonance imaging (qMRI; Echo MRI Whole Body Composition Analyzer; Echo Medical Systems, Houston, TX, USA). Body composition was measured either before acclimation to the calorimeter or 24–48 h before death.
Analysis of metabolic markers
Maternal and fetal glucose concentrations were determined at death with a OneTouch Ultra Meter (Lifescan, Milpitas, CA, USA); unfed offspring glucose levels were quantified using blood from the tail vein 1–2 wk before euthanasia. Blood was collected via cardiac puncture for the mother/offspring or by decapitation of the fetus after euthanasia. Fetal blood was pooled from each litter and treated as a single sample for analysis. After incubation at either room temperature for 15 min (fetuses) or on ice for 30 min (offspring), blood samples were centrifuged for 10 min at 4000 g. Insulin concentration was determined in serum using an Ultrasensitive Insulin ELISA (Crystal Chem, Downers Grove, IL, USA), according to the manufacturer’s instructions. Maternal homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting blood glucose (in mg/dl) × fasting insulin (in microU/ml) divided by 405. Serum triglycerides (TGs) were determined with the Infinity Triglycerides Liquid Stable Reagent (Thermo Fisher Scientific, Waltham, MA, USA) with an internal glycerol standard and quantified with a spectrophotometer reading at 540 nm. Liver TGs were extracted from ∼50 mg of frozen tissue (3) and quantified as for serum. Final concentrations were normalized to starting tissue weight at homogenization.
CARS imaging
CARS images of 12-μm-thick liver cryosections from selected adult mice were obtained by combining 2 laser beams: a Stokes beam fixed at 1064 nm and a pump beam tuned at 817 nm. The CARS microscope has been described (32). We also analyzed E18.5 and PND 21 liver sections using an Olympus FV1000 FCS/RICS microscope (Olympus, Tokyo, Japan) in the Advanced Light Microscopy Core at the University of Colorado Anschutz Medical Campus.
Lipidomics
Sample
An additional WD-fed cohort of male mice was studied, with or without constant exposure to PQQ (n = 6–7 mice/group, mean age 5.7 ± 0.2 wk). These mice were slightly older than PND 21 because they were subjected to glucose tolerance testing, qMRI, and metabolic chamber analysis for 5–7 d after weaning. Allowing for 2–3 d between tests for stress reduction and accounting for availability in the metabolic chamber, which accommodates only 8 cages, led to a delay in tissue harvesting. Liver tissue samples were harvested and kept at −80°C before analysis. Lipids were extracted from 5 mg of tissue (33), albeit slightly modified. In brief, tissues were homogenized in ice-cold methanol containing deuterated and odd-chain lipid standards. Subsequently, ice-cold methyl tert-butyl ether was added to each sample, followed by vortexing and addition of water. After centrifugation, the upper organic phase was removed, dried, and resuspended in 9:1 methanol:toluene. A portion of each resuspended sample was removed and diluted 20-fold for triacylglycerol analysis.
Liquid chromatography and mass spectrometry
Chromatographic separation was performed with a 1290 Infinity Binary LC (Agilent Technologies, Santa Clara, CA, USA) equipped with a charged surface hybrid (CSH) C18 column (Acquity CSH C18 column; 1.7 µm 2.1 × 100 mm; Waters, Milford, MA, USA). Mobile phase A consisted of 60:40 v/v acetonitrile:water and mobile phase B consisted of 90:10 v/v isopropanol:acetonitrile. Ammonium formate (10 mM) and formic acid (0.1% v/v) were added equally to both mobile phases as positive-ion mode modifiers, and ammonium acetate (10 mM) was used for negative-ion mode analysis. Gradient program for both positive- and negative-ionization mode was as follows: 15% B (initial), gradient to 30% B at 2.0 min, gradient to 48% B at 2.5 min, gradient to 82% B at 11.0 min, gradient to 99% B at 11.5 min, hold at 99% B until 12.0 min, gradient to initial conditions at 12.1 min, and hold until 15 min. Flow rate was 0.6 ml/min. Sample (1.67 μl) was injected in positive mode 2 times: once at standard resuspension concentration and a second time 20-fold diluted for triacylglycerol analysis. Five microliters of sample were injected in negative mode. All mass spectrometry data were collected on a 6530 Accurate Mass quadrupole time-of-flight (QTOF; Agilent Technologies) system, using both positive and negative mode electrospray ionization. Pools of each sample group (WD PQQ and WD) were created and injected to collect tandem MS data for annotation purposes.
Data processing
Samples were annotated via in-house accurate-mass/retention-time libraries previously generated from tandem mass spectrometry (MS/MS) data collected by using the specific liquid chromatography (LC)/MS method implemented and via MS/MS data collected from treatment pools and analyzed using LipidBlast and NIST MS Search 2.2 (34). MassHunter Workstation Software Quantitative Analysis B.07.00 (Agilent Technologies) was used for targeted peak picking and integration.
Total RNA extraction and real-time quantitative PCR
RNA isolation from snap-frozen liver tissue from fetuses and offspring was performed with the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Real-time quantitative PCR (qPCR) was performed on an iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) or an ABI Prism Step One (Thermo Fisher Scientific) (24, 35). Gene expression of each target sequence was normalized to expression of the reference genes Ppia and Sdha in fetal livers and 18S rRNA in 20 wk livers. Forward and reverse sequences of primers are summarized in Table 1. Equal efficiency of the reverse transcription of RNA was confirmed through quantification of expression of the reference genes, which did not differ between groups (for diet, PQQ, and interaction, all P > 0.1).
TABLE 1.
Forward and reverse sequences of primers used to probe expression of selected genes
| Primer, 5′–3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| 18S | TCCGATAACGAACGAGAC | CTAAGGGCATCACAGACC |
| Acadm | GGCGATGAAGGTTGAACTC | TACTCTGTGTTGAATCCATAGC |
| Cpt1aa | AGAAGAAGTTCATCCGATTCAAG | CCACCACCACGATAAGCC |
| Cpt1ab | GTCCCTCCAGCTGGCTTATC | CATGCGGCCAGTGGTGTCTA |
| Il1b | AGTTGACGGACCCCAAAAGA | GGACAGCCCAGGTCAAAGG |
| Il6 | TCCATCCAGTTGCCTTCTTG | TTTCTCATTTCCACGATTTCCC |
| Il10 | GGTTGCCAAGCCTTATCG | TCTTCACCTGCTCCACTG |
| Nfkb1 | TGTAGATAGGCAAGGTCAGAATG | AACACTGGAAGCACGGATG |
| Nlrp3 | GCCTTGAAGAAGAGTGGATGG | GCGTGTAGCGACTGTTGAG |
| Nos2 | AGAGAGATCCGATTTAGAGTCTTGGT | TGACCCGTGAAGCCATGAC |
| Pparaa | TCGCTATCCAGGCAGAAGG | AACAACAATAACCACAGA |
| Pparab | TGCCCTGAACATCGAGTGTCGAAT | TCGTACACCAGCTTCAGCCGAATA |
| Pparg1 | TGTTGACCCAGAGCATGGTG | TGCGAGTGGTCTTCCATCAC |
| Pparg2 | CTGACGGGGTCTCGGTTG | AACCATGGTAATTTCAGTAAAGGGT |
| Ppargc1aa | GTCAGAGTGGATTGGAGTTG | AAGTCATTCACATCAAGTTCAG |
| Ppargc1ab | ACCCACAGGATCAGAACAAACCCT | TTGGTGTGAGGAGGGTCATCGTTT |
| Ppia | GTCTCCTTCGAGCTGTTTGC | TAAAGTCACCACCCTGGCAC |
| Ptgs2 | GGTCTGGTGCCTGGTCTG | CTCTCCTATGAGTATGAGTCTGC |
| Sdha | TGCAGGCCTGGAGATAAAGTTCCT | AACACTGCAGCATGGTTCTGCATC |
| Sod1 | CTTCTCGTCTTGCTCTCTC | TCCTGACAACACAACTGG |
| Sod2 | ATGTAGGTGTCTTCAGCCACA | AATTCCCAGCAAACACAGAGT |
| Tlr4 | TTCAAGACCAAGCCTTTCAG | GTCACATCACATAGTCCTTCC |
| Tnf | GGTTCTGTCCCTTTCACTCAC | TGCCTCTTCTGCCAGTTCC |
Primer used for analysis of mRNA from 20-wk-old mouse livers.
Primer used for analysis of mRNA from fetal livers.
SOD activity assay
Mitochondrial-enriched supernatants were isolated from 30 mg frozen liver samples in buffer containing 210 mM mannitol, 70 mM sucrose, 5 mM HEPES, and 1 mM EGTA (pH 7.4) at 5% w/v. After homogenization, the samples were centrifuged 5 min at 600 g. Superoxide dismutase (SOD) activity was measured in a 10 μg sample (protein content measured by bicinchoninic acid assay) with a kit from Cayman Chemical (Ann Arbor, MI, USA), according to the manufacturer’s directions.
Western blot analysis
Frozen livers (∼50 mg) were homogenized with tissue protein extraction reagent buffer (Thermo Fisher Scientific) containing protease and phosphatase inhibitors, and equivalent amounts of total extracted proteins (determined by Bradford assay) were separated on 4–20% SDS-polyacrylamide gel and then transferred onto a PVDF membrane. Anti-MnSOD antibody (Cell Signaling Technology, Danvers, MA, USA) was used to probe the blot, and a UVP BioSpectrum Imaging System (Upland, CA, USA) was used to acquire the chemiluminescent signal. β-Actin (Cell Signaling Technology) was used as the control. Pixel densities in bands were quantified with ImageJ (National Institutes of Health, Bethesda, MD, USA).
Histology
Samples from snap-frozen livers were fixed for 24 h in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin by the Pathology Core at the University of Colorado Anschutz Medical Campus.
Statistics
Data are expressed as means ± sem. The significance of differences between groups was determined by 2-way ANOVA or Mann-Whitney U test with Prism 6.0 Software (GraphPad, La Jolla, CA, USA). A Student’s t test was performed on LC/MS data, to determine statistical significance of peak heights. Differences were deemed significant at P < 0.05. Principle component analysis was performed on LC/MS data by using MetaboAnalyst 3.0 (36).
RESULTS
Treating with PQQ during pregnancy does not affect maternal weight gain but increases placental growth
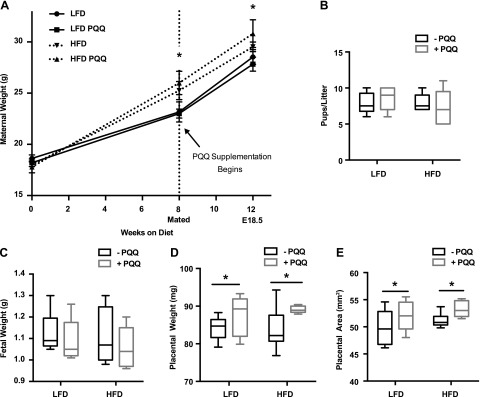
We tested the hypothesis that PQQ supplementation, provided both prenatally (at the time of mating) and after weaning, would improve metabolic outcomes of offspring of obese dams. Female mice were fed an LFD or HFD before mating with LFD-fed males and continued to be fed the same diet throughout gestation and lactation, adding PQQ supplementation at mating. Body weight of HFD-fed mice was higher before mating, and this difference was maintained during pregnancy, with no effect of PQQ supplementation in LFD- or HFD-fed mothers (Fig. 1A). The increased energy density of the HFD (4.73 kcal/g) as compared with the LFD (3.85 kcal/g) led to increased energy intake for the obese group, although food consumption (in grams per day) did not differ between groups (Supplemental Fig. S2). Neither diet nor PQQ treatment affected the number of pups per litter (Fig. 1B), and the average fetal weight was not different between groups (Fig. 1C). However, PQQ treatment led to a significant increase in placental weight (Fig. 1D) and placental surface area (Fig. 1E), regardless of maternal diet.
Figure 1.
PQQ supplementation does not protect against maternal weight gain but increases placental size in pregnant obese mice. A) Maternal weights for matched groups were recorded from the initiation of experimental diet to E18.5. PQQ supplementation began at mating. Weights at 8 and 12 wk after diet initiation were compared. B) Number of pups per litter. C, D) Fetal (C) and matched placental (D) weights were measured. E) Placenta length and width were measured with a micrometer, and placental surface area was calculated. Data are means ± sem (n = 5–7/group). *P < 0.05, with 2-way ANOVA comparing diets in A and PQQ treatment in D and E.
Metabolic phenotypes of male offspring of obese dams are modulated by PQQ throughout development
The effect of PQQ supplementation on diet-induced obesity had not been studied, to our knowledge, prompting us to measure levels of glucose, insulin, and TGs in both lean and obese dams and offspring in our model. Results are summarized in Table 2. Short-term supplementation with PQQ during pregnancy increased maternal glucose levels significantly, in both LFD- (control) and HFD-fed mothers, without changing fasting insulin. We investigated cohorts of offspring at E18.5 (fetuses), PND 21 (weanlings), and 20 wk (adults). In contrast to mothers, glucose levels of E18.5 fetuses of HFD-fed dams exposed to PQQ were not changed; nor was glucose affected by PQQ treatment at PND 21. At 20 wk, glucose was markedly increased by the WD in the group without PQQ. Although glucose and insulin levels were lower in the WD PQQ-fed mice, as compared to levels in the WD-fed cohort, the effect on HOMA-IR reduction did not reach significance (assessed by t test). Fetuses from HFD-fed dams and offspring fed the WD had significantly increased hepatic TGs vs. control-fed offspring, whereas PQQ supplementation significantly reduced TG accumulation in both control and HFD- or WD-fed mice, with the exception of PND 21 WD-fed mice. Twenty-wk-old offspring of WD-fed dams given PQQ only during gestation and lactation showed significant reductions in glucose and hepatic TGs compared to WD-fed offspring without maternal PQQ.
TABLE 2.
Metabolic phenotype of mothers and offspring throughout development
| Phenotype | CTL/CTLa n = 5–11 | HFD/HFD n = 7–9 | CTL PQQ/CTL PQQ n = 5–7 | HFD PQQ/HFD PQQ n = 5–7 |
|---|---|---|---|---|
| Maternal | ||||
| Glucose (mg/dl) | 77.5 ± 5.5 | 90.3 ± 6.3 | 119.8 ± 10.4 | 106.2 ± 13.0# |
| Insulin (ng/ml) | 0.83 ± 0.05 | 0.77 ± 0.03 | 0.86 ± 0.13 | 0.68 ± 0.01 |
| HOMA-IR | 4.57 ± 0.43 | 4.94 ± 0.39 | 7.17 ± 1.25 | 5.13 ± 0.63 |
| Serum TGs (mg/dl) | 23.3 ± 5.8 | 23.7 ± 6.0 | 20.0 ± 10.3 | 21.8 ± 2.3 |
| E18.5 (averaged per litter) | ||||
| Glucose (mg/dl) | 72.4 ± 13.8 | 116.9 ± 21.7 | 85.2 ± 21.6 | 92.7 ± 9.2 |
| Insulin (ng/ml) | 1.27 ± 0.13 | 0.81 ± 0.05* | 1.02 ± 0.08 | 0.78 ± 0.07 |
| HOMA-IR | 6.54 ± 1.41 | 6.73 ± 1.32 | 6.05 ± 1.61 | 5.14 ± 0.69 |
| Hepatic TGs (mg/dl) | 10.9 ± 0.8 | 22.4 ± 2.9* | 7.3 ± 1.5 | 8.5 ± 0.8# |
| PND 21b | ||||
| Glucose (mg/dl) | 193.3 ± 10.7 | 200.6 ± 9.3 | 179.2 ± 18.5 | 197.0 ± 18.5 |
| Hepatic TGs (mg/dl) | 14.9 ± 1.5 | 17.3 ± 1.5* | 11.6 ± 0.9 | 24.3 ± 2.6 |
| 20 wk | ||||
| Glucose (mg/dl) | 154.8 ± 8.7 | 172.9 ± 10.6* | 124.3 ± 10.9 | 167.8 ± 14.4 |
| Insulin (ng/ml)b | 0.87 ± 0.11 | 1.34 ± 0.31 | – | 0.84 ± 0.19 |
| HOMA-IRb | 9.58 ± 1.32 | 16.48 ± 3.94 | – | 10.02 ± 2.42 |
| Hepatic TGs (mg/dl) | 14.4 ± 4.5 | 63.8 ± 5.0* | 9.3 ± 0.7 | 37.0 ± 4.0# |
| 20 wk: WD PQQ/WD supplemented during gestation and lactation only | ||||
| Glucose (mg/dl) | 172.9 ± 10.6 | 125.2 ± 8.8# | ||
| Hepatic TGs (mg/dl) | 63.8 ± 5.0 | 44.8 ± 5.0# | ||
Data are means ± sem.
Control diet: LFD for materna/fetal studies, CH for offspring studies; HFD: high-fat-diet for maternal/fetal studies, WD for offspring studies.
Insulin concentrations were not measured for PND 21 or 20 wk CH-PQQ/CH-PQQ-fed mice. *P < 0.05 vs. CTL for diet effect; #P < 0.05 vs. CTL PQQ for PQQ effect.
Prolonged treatment with PQQ improves body composition but does not protect male offspring of obese dams from WD-induced weight gain
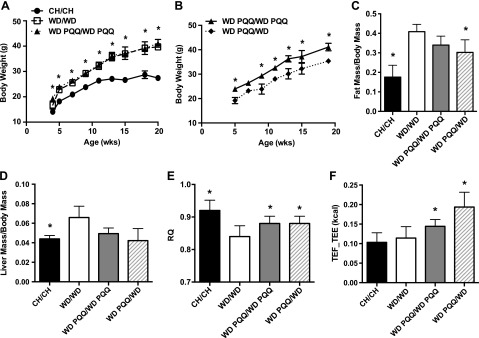
We charted growth through adulthood for offspring continued with WD feeding and for a subset of WD-PQQ–fed offspring with PQQ withdrawn after weaning (WD PQQ/WD). WD-fed mice supplemented with PQQ throughout pregnancy and postnatally were not protected from weight gain (PQQ supplementation similarly did not affect weight gain of CH-fed mice; Supplemental Fig. S3 and Fig. 2A). Mice with PQQ withdrawn postnatally gained weight at the same rate as their WD-PQQ– and WD-fed counterparts; however, they were smaller at weaning and maintained the same weight difference throughout life (Fig. 2B). WD-fed litters were not significantly smaller than WD PQQ-fed litters (4.4 ± 0.3 vs. 5.0 ± 0.4 pups/litter); thus, litter size was unlikely to contribute to this disparity. Increasing the concentration of PQQ after weaning did not alter growth trajectories (Supplemental Fig. S4). PQQ did not affect energy or drink intake (Supplemental Fig. S5). Body composition was measured by ECHO-MRI. The ratio of fat mass to body mass of CH-fed mice (0.176 ± 0.02) was similar to that obtained with PQQ supplementation (CH PQQ/CH PQQ 0.128 ± 0.03; P = 0.3); however, PQQ treatment reduced WD-induced increases in fat mass (Fig. 2C) and liver hypertrophy (Fig. 2D) at 20 wk, even when PQQ was provided only during gestation and lactation.
Figure 2.
Growth and energy metabolism characteristics of male offspring continued on the maternal diet or with PQQ removed after weaning. Mice were weighed weekly after weaning. A) Growth curves for mice continued to 20 wk of age on the maternal diet. B) Effect of removing PQQ after weaning. PQQ was removed from offspring of WD PQQ-fed dams after weaning, and they were fed the WD for an additional 17 wk. C, D) At death, the percentage of fat mass (C) was measured, and the ratio of liver mass to body mass (D) was determined. E, F) Before death, mice were placed in a metabolic chamber and the RQ (E) and TEF (F) consumption and storage on total energy expenditure (TEF_TEE) were calculated. Data are means ± sem (n = 3–10/group). *P < 0.05 vs. CH mice in A vs. drink-switched mice (WD PQQ/WD) in B and vs. WD-fed mice in C–F.
“Metabolic flexibility” defines the ability to switch between lipid and glucose oxidation, depending on substrate availability, and is measured by a change in the RQ. Impaired metabolic flexibility may result in poor glycemic control, reduced energy expenditure, and weight gain. To elucidate whether PQQ reduces liver TGs and fat mass through a change in energy metabolism or metabolic flexibility, we measured CO2 production and O2 consumption in a metabolic chamber at 20 wk and calculated the RQ. RQs were affected by both diet and PQQ, with the WD reducing RQ and PQQ increasing RQ in WD-fed animals (Fig. 2E). The increase in RQ suggests that PQQ treatment improves the ability of WD-fed mice to switch from lipid to glucose oxidation during a meal, thus increasing their metabolic flexibility (37), even when they received PQQ supplementation only during gestation and lactation. Energy expenditure for mice in the metabolic chamber was calculated and, notably, the TEF was significantly increased for WD-fed mice treated with PQQ (Fig. 2F). Taken together, these results suggest that although PQQ does not protect from overall weight gain, it may increase metabolic flexibility in a high fat/high carbohydrate environment.
PQQ treatment increases expression of regulators of lipid catabolism in WD-fed offspring
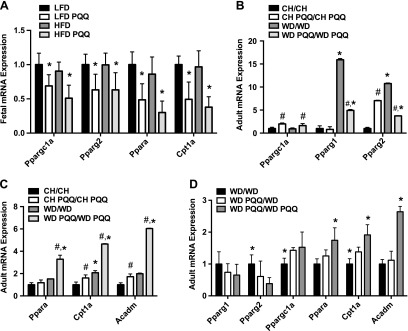
Given the striking reduction of hepatic TGs and potential changes in fuel metabolism associated with PQQ supplementation, we next investigated the effect of PQQ on expression of genes that regulate lipid metabolism. PGC-1α mediates regulation of both PPARα and -γ (reviewed in refs. 38, 39). We therefore determined the effects of WD and PQQ on those gene sets in fetal and male adult offspring. At E18.5, maternal feeding of the HFD did not significantly alter fetal gene expression; however, maternal supplementation with PQQ modestly decreased expression of Ppargc1a, Pparg2, Ppara, and Cpt1a, regardless of diet (Fig. 3A). In adults maintained on the maternal diet, PQQ treatment significantly increased Ppargc1a expression (Fig. 3B). Pparg1 mediates lipogenesis, whereas Pparg2 in the liver is protective and prevents lipotoxicity (40). Expression levels of both isoforms were significantly increased by both maternal and postnatal exposure to the WD, although Pparg1 expression was more highly up-regulated than Pparg2. PQQ supplementation significantly decreased expression levels of both isoforms in the WD PQQ/WD PQQ-fed group compared with levels in the WD/WD-fed group (Fig. 3B). PQQ treatment did not affect Pparg1 expression in CH-fed mice. PQQ significantly altered Pparg2, increasing expression in the CH-fed group and decreasing it in obese adult offspring (Fig. 3B). Unlike E18.5, Ppara and Cpt1a mRNA expression in adult offspring were significantly increased in WD PQQ/WD PQQ-fed animals (Fig. 3C). We also investigated expression of Acadm, another gene involved in fatty acid oxidation and found similar significant up-regulation in WD PQQ/WD PQQ-fed offspring (Fig. 3C). We again investigated persistent effects in our cohort with PQQ withdrawn at weaning (Fig. 3D) and found that mRNA expression levels of the WD PQQ/WD-fed group followed the same trends as WD-fed offspring maintained with continuous PQQ treatment. Taken together, these data suggest that PQQ up-regulates catabolic and oxidative genes in the livers of animals exposed to excess lipids during early life, and this altered response persists into adulthood.
Figure 3.
Regulation of hepatic metabolism is shifted toward catabolic mechanisms by PQQ treatment. A–C) Real-time quantitative PCR was used to measure mRNA expression of key metabolic transcription factors in fetal livers (A) and livers (B) from male adult (20 wk) offspring, and expression of fatty acid oxidation genes was measured in adult males (C). Gene expression was normalized to LFD-fed results and Ppia and Sdha expression levels (fetal) and CH-fed results and 18S rRNA expression levels (adult). Data are means ± sem (n = 3–9/group). *P < 0.05, effect of diet, for WD-fed compared with CH-fed animals; #P < 0.05, main effect of supplementation with PQQ. D) Persistent effects were assessed in offspring of WD-fed mothers with either no PQQ (WD/WD), the WD-fed cohort with PQQ withdrawn at weaning (WD PQQ/WD), or those maintained on the maternal diet (WD PQQ/WD PQQ) (n = 5–6/group). Gene expression was normalized to WD-fed results and 18S rRNA expression levels. *P < 0.05 compared with WD PQQ/WD-fed mice by Mann-Whitney U test.
Hepatic lipid composition and lipid droplet size is affected by PQQ treatment
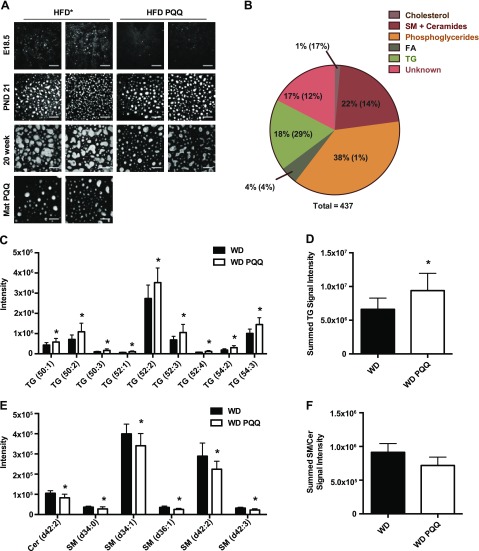
The reduction of total hepatic TGs after PQQ treatment in E18.5 and 20-wk-old obese offspring was consistent with gene expression patterns; however, the slight increase in TGs in PND 21 obese offspring on PQQ was unexpected; therefore, we used CARS microscopy to further investigate lipid droplet accumulation in liver cryosections from all offspring groups. Few lipid droplets were observed in livers from chow-fed mice (data not shown). Displayed in Fig. 4A are representative CARS images of livers from HFD-exposed fetuses and WD-fed offspring (2 mice/group). At E18.5, lipid droplet accumulation was lower in mice exposed to HFD PQQ compared with HFD-exposed fetuses, in agreement with the total TG measurements in Table 2 for these groups. By PND 21, offspring had accumulated much more fat, likely through suckling from the dams. Although variability was observed between mice, liver sections from offspring treated with PQQ appeared to have larger lipid droplets, on average, than offspring without PQQ. Fat accretion continued into adulthood; however, by 20 wk, droplets were much larger in the WD-fed mice with no PQQ treatment as compared to those with long-term consumption of WD with PQQ, and fewer and smaller droplets were observed in the WD PQQ/WD images as compared with those of the WD-fed mouse livers, which again is consistent with the total TG measurements above. Similar patterns were observed in hematoxylin and eosin -stained images (Supplemental Fig. S6).
Figure 4.
PQQ treatment alters hepatic lipid profiles around the time of weaning, increasing protective TGs and decreasing SM and ceramide concentrations. A) CARS microscopy was used to visualize lipid droplets in 12-μm-thick cryosections from mouse livers at E18.5, PND 21 (males), and 20 wk (males). Representative images from 2 mice are shown for offspring of dams treated with or without PQQ. Mat PQQ, 20-wk-old WD PQQ/WD-fed mice that received PQQ via maternal supplementation only. Magnification, ×60; field of view, 350 μm. HFD*, E18.5 fetuses were from HFD-fed mothers, PND 21 and 20-wk-old offspring were WD fed. B) Untargeted lipidomics analysis was performed on 6–7 mice/group (WD- and WD PQQ-fed) from 6-wk-old males. When multiple instances of a compound were present, unique ions were selected based on highest mean abundance and were used for subsequent analysis. Lipids were organized into general classes, with percentage of total identified shown for each class. Student’s t test was performed to determine significance. The percentage of compounds with significant changes are shown in parentheses for each class. P < 0.05. C, D) A subset of the most abundant significantly changed TGs (C) and the total PQQ-induced change in TGs (D). E, F) A subset of the most abundant significantly changed SMs and ceramides (E) and the total PQQ-induced change in SMs and ceramide concentrations from the lipidomics analysis (F) are plotted. Data are means ± sem. *P < 0.05, by Mann-Whitney U test.
Given that TGs and lipid droplet sizes were increased in WD PQQ-fed PND 21 mice, we were curious as to whether PQQ treatment results in altered lipid composition in the liver. We performed untargeted MS-based lipidomics in liver tissue from the additional WD-fed cohort of ∼6-wk-old mice, with or without constant exposure to PQQ. Differences in lipidomics data were examined by principle component analysis (Supplemental Fig. S7), highlighting the multivariate separation between treatment classes (WD vs. WD PQQ). Summarized in Fig. 4B is the relative distribution of classes of unique compounds identified in both positive and negative ion mode. The percentage of compounds within each class that were significantly different between groups is indicated within each section of the chart in parentheses. Most of the identified ions were phosphoglycerides (38%); however, abundance of only 1% of the >150 phosphoglycerides identified changed significantly between groups. Lipid classes showing the most significant changes were the TGs and the sphingomyelins (SMs)/ceramides, representing 18 and 22% of the compounds detected, respectively. Of the lipid classes, TGs were the most affected by maternal PQQ treatment, with 29% of the unique TG moieties changing significantly. The next most altered class of lipids was the combined SMs/ceramides, with 14% of unique compounds changing significantly. A subset of the significantly changed TGs is plotted in Fig. 4C, and, consistent with the total TG data for WD-fed PND 21 offspring, peak abundance was increased for age-matched offspring of WD PQQ-treated dams as compared with untreated counterparts (summed TG intensity; Fig. 4D). Notably, TG (54:3), identified as triolein, a bioactive lipid associated with protection from oxidative stress (41), was increased in WD PQQ-treated mice. In contrast, concentrations of SMs and ceramides, associated with lesions of NAFLD such as insulin resistance, oxidative stress, and inflammation (42–45), were decreased with PQQ treatment in mice fed the WD (Fig. 4E; summed intensity in Fig. 4F). Thus, PQQ supplementation of WD-fed mothers during pregnancy and lactation appeared to shift the liver lipid composition of offspring around the time of weaning to increase lipid mediators of oxidative defense and decrease proinflammatory mediators. Whether this protective lipid profile persists in adults remains to be determined.
Oxidative defense response of obese offspring is differentially affected by PQQ throughout development
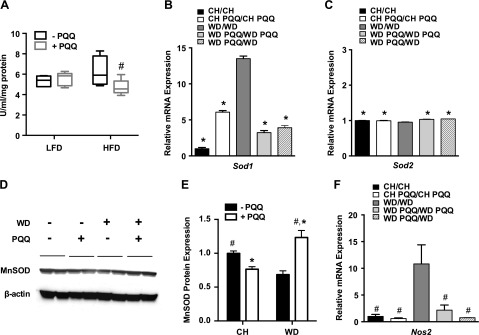
The significant increase of triolein, a marker for protective response to oxidative stress (41), in mice supplemented with PQQ prompted us to investigate the activity and expression of the oxidative defense enzyme SOD. The activity of mitochondrial manganese-dependent (Mn)SOD in fetal livers was significantly increased in HFD-exposed E18.5 fetuses, whereas maternal supplementation with PQQ significantly decreased SOD activity for HFD- but not LFD-exposed fetuses (Fig. 5A). We examined mRNA expression levels of Sod1 (cytoplasmic) and Sod2 (mitochondrial) in adult mouse livers and found that, after birth, expression of Sod1 was markedly increased in WD/WD-fed mice, as compared with CH/CH-fed animals and was reduced in PQQ-supplemented mice. Notably, Sod1 expression was significantly decreased in WD-fed animals with PQQ withdrawn at weaning (WD PQQ/WD), demonstrated a persistent effect of PQQ on Sod1 (Fig. 5B). Expression of Sod2 was unchanged by diet or PQQ treatment (Fig. 5C). We further investigated mitochondrial MnSOD protein expression by Western blot analysis. A representative blot shown in Fig. 5D and quantified in Fig. 5E, shows that expression of the mitochondrial gene product, MnSOD, was significantly decreased in adult male offspring fed a WD diet and increased by PQQ treatment in the WD-fed offspring, suggesting changes in MnSOD protein abundance, which may not be reflected by mRNA expression levels, are affected by both diet and PQQ.
Figure 5.
Response of obese offspring to oxidative stress in early life and in later life is altered by PQQ treatment. Offspring of dams fed a LFD or HFD were assessed for response to oxidative stress by measuring activity or expression of SOD and Nos2. A) MnSOD activity was measured in E18.5 fetuses (n = 5/group). B, C) Expression levels of cytoplasmic Sod1 (B) and mitochondrial Sod2 (C) were measured by qPCR for male adult offspring continued on the maternal diet or drink switched after weaning (n = 3–9/group). *P < 0.05, compared with WD/WD determined by unpaired Student’s t test with Welch’s correction. D) Protein expression was determined by Western blot, and a representative blot from n = 2/group is shown. E) Image analysis was performed using ImageJ, and the densitometry was measured. After background subtraction, integrated pixel densities were normalized to control values (CH/CH) (n = 4 mice/group). F) mRNA expression of Nos2 was measured for the same groups as in B and C. *P < 0.05; #P < 0.1 compared with WD/WD feeding, determined by unpaired Student’s t test with Welch’s correction. Data are means ± sem.
Inducible NOS-2 is up-regulated in the liver in response to injury, oxidative stress, and inflammation (46, 47), which are lesions of NAFLD in addition to TG accretion. In 20-wk-old offspring, we found that the WD resulted in significant up-regulation of Nos2 expression as compared to the CH diet, whereas PQQ treatment selectively diminished hepatic Nos2 expression in WD-fed offspring (Fig. 5F). Furthermore, Nos2 expression in WD-fed mice with PQQ withdrawn at weaning (WD PQQ/WD) was even lower than that of the WD PQQ/WD PQQ-fed group. Taken together, these data demonstrate that subpharmacological supplementation with PQQ can robustly reverse lipid-induced increases in expression of enzymes associated with oxidative stress and inflammation, which manifest even before birth. Supplementation with this potent antioxidant is effective in adulthood, even when provided only during gestation and lactation.
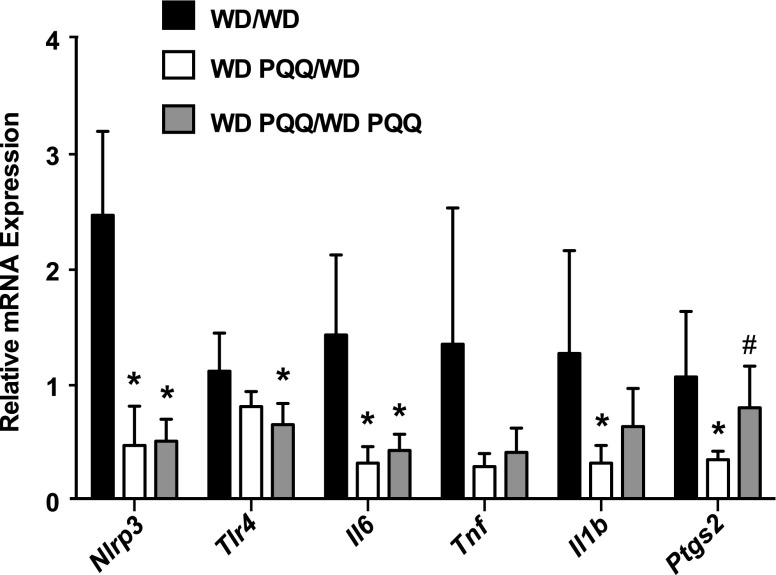
PQQ supplementation reduces expression of proinflammatory genes in adult WD-fed offspring
The marked reversal of Nos2 mRNA expression in PQQ-treated WD-fed offspring suggested that the action of PQQ is targeted to inflammatory pathways. Therefore, we used qPCR to probe mRNA expression levels of proinflammatory genes in livers of adult male offspring maintained with the maternal diet. Results are summarized in Table 3, where we observed a general pattern of increased inflammatory gene expression associated with consumption of WD. Supplementing with PQQ inhibited WD-induced up-regulation of these proinflammatory mediators. Expression of the p50 subunit of NFκB (Nfkb1) and IL10 did not change significantly with diet or PQQ. TLR4, TNF, and IL1β gene expression did not show significant effects of PQQ, although significance was reached in some cases when compared pair-wise by t test. A significant effect of both diet (increase) and PQQ (decrease) was observed for the inflammasome pathway genes NLRP3 and IL6, and cyclooxygenase (COX)-2 (Ptgs2) gene expression was markedly and specifically modulated by PQQ. Figure 6 shows, again, that PQQ supplementation given only during gestation and lactation persistently protected WD-fed offspring from up-regulation of inflammatory programs into adulthood.
TABLE 3.
mRNA expression of proinflammatory genes in 20-wk-old male mice
| Gene | CH n = 4–6 | WD n = 4–6 | CH PQQ n = 4–5 | WD PQQ n = 5–7 |
|---|---|---|---|---|
| Nlrp3 | 1.00 ± 0.11 | 4.67 ± 0.73* | 1.52 ± 0.14 | 2.12 ± 0.25# |
| Tlr4 | 1.00 ± 0.06 | 2.51 ± 0.33* | 1.38 ± 0.27 | 1.66 ± 0.26 |
| Nfkb1 | 1.00 ± 0.13 | 1.38 ± 0.14 | 1.19 ± 0.14 | 1.29 ± 0.13 |
| Tnf | 1.00 ± 0.17 | 10.51 ± 3.8* | 1.55 ± 0.65 | 3.15 ± 0.67 |
| Il10 | 1.00 ± 0.31 | 1.98 ± 0.69 | 1.58 ± 0.65 | 1.55 ± 0.10 |
| Il6 | 1.00 ± 0.22 | 4.10 ± 0.90* | 0.54 ± 0.15 | 1.21 ± 0.18# |
| Il1b | 1.00 ± 0.22 | 3.65 ± 1.05* | 0.81 ± 0.31 | 0.93 ± 0.07 |
| Ptgs2 | 1.00 ± 0.09 | 1.09 ± 0.04 | 0.71 ± 0.09 | 0.76 ± 0.06# |
Data are means ± sem normalized to CH. *P < 0.05 vs. CH for diet effect; #P < 0.05 vs. CH PQQ for PQQ effect.
Figure 6.
PQQ supplementation during gestation and lactation only leads to mRNA expression profiles of proinflammatory genes in adults similar to those of WD-fed offspring receiving PQQ throughout their lifespan. mRNA expression of proinflammatory genes was measured in livers of adult male offspring. Mothers were fed a WD, with or without PQQ, and and either offspring continued on the maternal diet to 20 wk (WD/WD- or WD PQQ/WD PQQ-fed), or PQQ was withdrawn at weaning (WD PQQ/WD). Data are means ± sem (n = 5–6/group). Mann-Whitney U test was used in pair-wise comparison of groups. *P < 0.05 compared with WD/WD-fed mice; #P < 0.05 compared with WD PQQ/WD-fed mice.
DISCUSSION
Collectively, multiple lines of evidence from human and animal studies have converged to suggest that pregnancy and the postnatal period are uniquely crucial windows of opportunity for prevention of metabolic disease in this and subsequent generations (2, 48–50). However, nontoxic, nonsynthetic compounds that can be safely used in pregnancy to target specific pathways for disease risk are few and far between. In the current study, supplementation with PQQ, a naturally occurring antioxidant found in foods and enriched in human breast milk, given at subpharmacological levels throughout pregnancy and lactation, protected obese mouse offspring from hepatic lipotoxicity, increased metabolic flexibility, and suppressed liver inflammation without affecting growth or weight gain.
PQQ, given at the time of conception, had no effect on maternal weight gain but increased maternal glucose. PQQ at the molecular level enhances the production of pyruvate from lactate (51), possibly changing the cellular redox state and favoring increased gluconeogenesis (52) in the mothers. However, there was no effect of PQQ on fetal glucose, litter size, or fetal weights, suggesting that although statistically significant, maternal glucose had no effect on fetal growth, despite increasing placental weight. The lower fetal insulin levels in the HFD-fed group may have been caused by impaired insulin secretion or by the immaturity of the islets at E18.5 (53). With no significant changes observed in insulin levels in adulthood, we suspect that this is a transient effect; however, follow-up studies are necessary to pursue this notion further. Notably, PQQ given to mothers fed both LFD and HFD led to significantly reduced levels of fetal hepatic TGs compared to unexposed mice at E18.5. More studies are needed to identify whether the mechanisms underlying this change reflect effects on the mother or the placenta or represent a direct effect of PQQ on fetal development in utero.
PQQ reduced expression of both Ppara and Pparg2 in the livers of E18.5 mice compared to HFD-fed controls, an effect that persisted for Pparg2 but not Ppara in adult mice fed WD and exposed to PQQ. PQQ activates PGC-1α, a transcriptional coactivator implicated in regulation of reactive oxygen species–detoxifying genes, such as SOD, as well as many other metabolic mediators, including PPARα and -γ (as reviewed in ref. 36). In adult but not fetal livers, Ppara expression was significantly increased by PQQ supplementation, but only for obese offspring; diet did not have a significant effect. This result is consistent with findings reported by Bauerly et al. (19), where PQQ supplementation did not elevate PPARα expression in chow-fed rat livers or in Hepa 1–6 cells. We also observed similar up-regulation in expression of the fatty acid oxidation genes Cpt1a and Acadm at 20 wk, suggesting that PQQ increases fatty acid oxidation under conditions of overnutrition to reduce hepatic TGs. Notably, we observed a significant increase in expression of Pparg isoforms with WD feeding that was normalized by PQQ treatment in adult obese offspring, consistent with Sen and Simmons (10), who observed a similar trend for total Pparg expression in adipose tissue from offspring of obese dams treated with antioxidants. PPAR-γ2 is ectopically induced in liver in response to overnutrition and may prevent lipotoxicity by facilitating TG accumulation and increasing the lipid-buffering capacity of the liver (40); PQQ may activate similar pathways.
In adult mice, we measured significant decreases in WD-induced hepatic TGs and expression of proinflammatory cytokines associated with PQQ supplementation, particularly for NLRP-3, a major component of the inflammasome; NOS-2; and IL-6. We also observed PQQ-specific up-regulation of COX-2 gene expression (Ptgs2). The significant effect of PQQ on IL-6, as compared with that of TNF and IL-1β, is similar to that observed in a thioacetamide model of induced fibrosis, where PQQ normalized expression of IL6 more effectively than other genes (18). These results also complement observations by Tchaparian et al. (54) and suggest that PQQ activates IL-6 to affect Jak-Stat signaling. IL-6 production was also significantly diminished by PQQ treatment in synoviocytes, via attenuating phosphorylation of p38 and JNK (55). In addition, an in vivo model of osteoarthritis has demonstrated an effect of PQQ in decelerating inflammatory responses via inhibition of NO production by suppressing IκBα degradation (56), suggesting PQQ acts systemically to modulate IL-6 and NO levels through different pathways. Notably, the ceramide content in livers of WD-fed weanling mice was lowered by PQQ, suggesting that the disease-causing effects of excess lipids on inflammation and oxidative stress had already been mitigated in early life. Taken together, these data show that PQQ targets multiple proinflammatory pathways in the liver to protect against WD-induced liver lipotoxicity, although there was no change in WD-induced weight gain in the offspring of WD-fed mothers.
As reviewed by Akagawa et al. (14), in numerous in vitro and in vivo models, PQQ acts as an antioxidant by scavenging O2− and protects mitochondria from oxidative stress-induced damage. Whether PQQ acts preconception to reduce oxidative stress in oocytes or sperm cells or improves placental function by reducing oxidative stress, indirectly protecting the fetus, is beyond the scope of the present study but is important to determine. The progressive development of NAFLD involves excessive TG accumulation in hepatocytes and results in cellular dysfunction caused by oxidative stress, lipotoxicity, or bacterial endotoxin activation of liver resident macrophages (Kupffer cells), inducing secretion of proinflammatory cytokines that activate neighboring stellate cells (57). Inflammatory cells, particularly the Kupffer cells, are recruited to the liver in response to liver injury or after exposure to danger signals. PQQ reduced macrophage infiltration in thioacetamide-induced injury (18) and inhibited osteoclast formation (58), suggesting that PQQ targets macrophage activity. Oxidative stress, associated with maternal obesity (59, 60), may connect the liver to intrauterine programming sequelae in the macrophage, particularly via epigenetic mechanisms (61–64). Whether PQQ simply limits macrophage infiltration or actually blocks the impact of maternal nutrition on developmental programming in the macrophage to reduce inflammation in the adult offspring is a critical area for future study.
We tested the effect of early life PQQ supplementation on metabolic flexibility in adult mice. We measured an increase in RQ and TEF for PQQ-supplemented mice that may be interpreted as improvement in metabolic flexibility (37). Insulin resistance and altered glucose disposal rate, as well as dysfunction in fatty acid oxidation in mitochondria, impair metabolic flexibility in patients with type 2 diabetes (65). Decreased RQ has also been associated with impaired insulin-mediated suppression of lipolysis in adipose tissue (66); however, the action of PQQ in adipose tissue has not yet been established. The RQ for animals in which PQQ was removed after weaning was increased similarly to that for the continually supplemented mice, suggesting that PQQ supplementation during gestation and lactation permanently alters fuel utilization in the offspring. The further increase in TEF for the WD-fed animals with PQQ withdrawn at weaning may account for their decreased weight, although the reason underlying the increase remains unclear. Nevertheless, PQQ supplementation during this early-life period conferred persistent effects into adulthood, reducing the accumulation of TGs, suppressing induction of proinflammatory pathways, and increasing fatty acid oxidation. PQQ in early life protected against diet-induced lipotoxicity and diminished NAFLD, suggesting that it might be useful in pregnancy and lactation for conferring future health benefits for offspring. Whether these effects are related to persistent epigenetic changes in genes that regulate liver health or adipose tissue remains to be determined.
CONCLUSIONS
We have demonstrated for the first time, to our knowledge, that subpharmacological supplementation of PQQ, when provided prenatally in obese pregnant mice, protects against disease pathways involved in NAFLD and NASH, decreasing hepatic PPARγ expression in early life. Continued treatment after weaning showed similar trends and results in reduced hepatic TG accumulation, inhibition of proinflammatory programs, and increased oxidative defense. Protection against weight gain was not achieved; however, PQQ altered fuel utilization and increased metabolic flexibility in obese animals and significantly reduced adiposity, resulting in an overall healthier metabolic state in offspring exposed to the WD. Our novel observation of a reduction in ceramides in the postnatal livers of mice and normalization of PPARγ by PQQ in adults suggests that prolonged treatment with PQQ protects against hepatic lipotoxicity, as suggested in other studies (18). In vivo tests in preclinical models of diabetes and NASH and in vitro tests have reported no genotoxic potential of PQQ (67) and recent short-term human studies have shown PQQ-related improvements in cognitive function (68), dry skin (69), and lipid metabolism (70). Preclinical and human studies have also demonstrated that supplemental PQQ improves oxidative stress (23, 71), suggesting that dietary supplementation with PQQ is an emerging therapeutic target worthy of further study in the battle to reduce the risks of NAFLD in infants exposed to maternal overnutrition.
ACKNOWLEDGMENTS
The authors thank Dr. Robert Rucker (University of California, Davis) for invaluable mentorship of K.R.J. and helpful discussions; Greg Glazner and Radu Moldovan [Advanced Light Microscopy Core Facility, U.S. National Institutes of Health (NIH)/National Center for Advancing Translational Science Colorado County Technical Services, University of Colorado Anschutz Medical Campus; Grant UL1 TR001082] for help with the CARS analysis; Matt Jackman and the Nutrition Obesity Research Center Metabolic Phenotyping Core [NIH P30 National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK48520 and NIH P50 and National Institute of Child Health and Human Development Grant HD073063, University of Colorado Anschutz Medical Campus] for aid with processing data from the metabolic chamber. Funding was received through NIH NIDDK Grant R01 DK104351 (to O.F., which provided funds for B.C.D.); NIH NIDDK Grant U24 DK097154, and the QTOF mass spectrometer was funded by NIH National Institute of General Medical Sciences Grant S10-RR031630 (to O.F.); NIH NIGMS Grant P41-RR01192 (Laser Microbeam and Medical Program) (to A.A.G. and E.O.P.); NIH NIDDK Grant 5R01DK098336 and Virtual, Augmented and Mixed Reality Grant 1I01BX001954 (to X.X.W., Y.L., and M.L.); and NIH NIDDK Grant K25DK098615 and an unrestricted grant from the Mitsubishi Gas and Chemical Company (to K.R.J.) The authors declare no conflicts of interest.
Glossary
- CARS
coherent anti-Stokes Raman spectroscopy
- CH
cholesterol-enriched standard chow
- COX
cyclooxygenase
- E18.5
embryonic d 18.5
- HFD
high-fat diet
- HOMA-IR
homeostasis model assessment of insulin resistance
- LC
liquid chromatography
- LFD
low-fat diet
- MnSOD
manganese-dependent SOD
- MS
mass spectrometry
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PGC
peroxisome proliferator-activated receptor γ coactivator
- PND
postnatal day
- PPAR
peroxisome proliferator-activated receptor
- PQQ
pyrroloquinoline quinone
- PWR
placental weight ratio
- QTOF
quadrupole time-of-flight
- RQ
respiratory quotient
- qMRI
quantitative magnetic resonance imaging
- qPCR
quantitative PCR
- SM
sphingomyelin
- SOD
superoxide dismutase
- TEF
thermic energy from food
- TG
triglyceride
- WD
Western-style diet
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. R. Jonscher and J. E. Friedman designed the overall research; O. Fiehn designed the lipidomics data acquisitions; K. R. Jonscher, M. S. Stewart, B. C. DeFelice, M. J. R. Heerwagen, R. C. Janssen, B. A. de la Houssaye, E. Wiitala, G. Florey, and R. L. Jonscher performed research; X. X. Wang, Y. Luo, and M. Levi contributed reagents and analytical tools; A. Alfonso-Garcia, and E. O. Potma conducted CARS experiments and image analysis; K. R. Jonscher, B. C. DeFelice and R. C. Janssen analyzed data and O. Fiehn advised on the lipidomics data analysis; K. R. Jonscher and J. E. Friedman wrote the paper; and R. C. Janssen assisted with editing the manuscript.
REFERENCES
- 1.Armstrong M. J., Adams L. A., Canbay A., Syn W. K. (2014) Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 59, 1174–1197 [DOI] [PubMed] [Google Scholar]
- 2.Bruce K. D., Cagampang F. R., Argenton M., Zhang J., Ethirajan P. L., Burdge G. C., Bateman A. C., Clough G. F., Poston L., Hanson M. A., McConnell J. M., Byrne C. D. (2009) Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50, 1796–1808 [DOI] [PubMed] [Google Scholar]
- 3.McCurdy C. E., Bishop J. M., Williams S. M., Grayson B. E., Smith M. S., Friedman J. E., Grove K. L. (2009) Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 119, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouralidarane A., Soeda J., Visconti-Pugmire C., Samuelsson A. M., Pombo J., Maragkoudaki X., Butt A., Saraswati R., Novelli M., Fusai G., Poston L., Taylor P. D., Oben J. A. (2013) Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 58, 128–138 [DOI] [PubMed] [Google Scholar]
- 5.Pruis M. G., Lendvai A., Bloks V. W., Zwier M. V., Baller J. F., de Bruin A., Groen A. K., Plösch T. (2014) Maternal western diet primes non-alcoholic fatty liver disease in adult mouse offspring. Acta Physiol. (Oxf.) 210, 215–227 [DOI] [PubMed] [Google Scholar]
- 6.Lane M., Zander-Fox D. L., Robker R. L., McPherson N. O. (2015) Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends Endocrinol. Metab. 26, 84–90 [DOI] [PubMed] [Google Scholar]
- 7.Wu L. L., Dunning K. R., Yang X., Russell D. L., Lane M., Norman R. J., Robker R. L. (2010) High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 151, 5438–5445 [DOI] [PubMed] [Google Scholar]
- 8.Igosheva N., Abramov A. Y., Poston L., Eckert J. J., Fleming T. P., Duchen M. R., McConnell J. (2010) Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 5, e10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesolowski S. R., Kasmi K. C., Jonscher K. R., Friedman J. E. (2016) Developmental origins of NAFLD: a womb with a clue. [E-pub ahead of print] Nat. Rev. Gastroenterol. Hepatol., doi: 10.1038/nrgastro.2016.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen S., Simmons R. A. (2010) Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes 59, 3058–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts V. H., Pound L. D., Thorn S. R., Gillingham M. B., Thornburg K. L., Friedman J. E., Frias A. E., Grove K. L. (2014) Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 28, 2466–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killgore J., Smidt C., Duich L., Romero-Chapman N., Tinker D., Reiser K., Melko M., Hyde D., Rucker R. B. (1989) Nutritional importance of pyrroloquinoline quinone. Science 245, 850–852 [DOI] [PubMed] [Google Scholar]
- 13.Steinberg F., Stites T. E., Anderson P., Storms D., Chan I., Eghbali S., Rucker R. (2003) Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp. Biol. Med. (Maywood) 228, 160–166 [DOI] [PubMed] [Google Scholar]
- 14.Akagawa M., Nakano M., Ikemoto K. (2015) Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci. Biotechnol. Biochem. 80, 13–22 [DOI] [PubMed] [Google Scholar]
- 15.Chowanadisai W., Bauerly K. A., Tchaparian E., Wong A., Cortopassi G. A., Rucker R. B. (2010) Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J. Biol. Chem. 285, 142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel K. G., Zhang H. J., Wang J., Wu S. G., Yue H. Y., Sun L. L., Qi G. H. (2015) Effects of dietary pyrroloquinoline quinone disodium on growth performance, carcass yield and antioxidant status of broiler chicks. Animal 9, 409–416 [DOI] [PubMed] [Google Scholar]
- 17.Kumar N., Kar A. (2015) Pyrroloquinoline quinone (PQQ) has potential to ameliorate streptozotocin-induced diabetes mellitus and oxidative stress in mice: a histopathological and biochemical study. Chem. Biol. Interact. 240, 278–290 [DOI] [PubMed] [Google Scholar]
- 18.Jia D., Duan F., Peng P., Sun L., Ruan Y., Gu J. (2015) Pyrroloquinoline-quinone suppresses liver fibrogenesis in mice. PLoS One 10, e0121939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauerly K., Harris C., Chowanadisai W., Graham J., Havel P. J., Tchaparian E., Satre M., Karliner J. S., Rucker R. B. (2011) Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS One 6, e21779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rucker R., Chowanadisai W., Nakano M. (2009) Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. 14, 268–277 [PubMed] [Google Scholar]
- 21.Mitchell A. E., Jones A. D., Mercer R. S., Rucker R. B. (1999) Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal. Biochem. 269, 317–325 [DOI] [PubMed] [Google Scholar]
- 22.Bachmanov A. A., Reed D. R., Beauchamp G. K., Tordoff M. G. (2002) Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 32, 435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris C. B., Chowanadisai W., Mishchuk D. O., Satre M. A., Slupsky C. M., Rucker R. B. (2013) Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J. Nutr. Biochem. 24, 2076–2084 [DOI] [PubMed] [Google Scholar]
- 24.Heerwagen M. J., Stewart M. S., de la Houssaye B. A., Janssen R. C., Friedman J. E. (2013) Transgenic increase in N-3/n-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One 8, e67791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lytle K. A., Jump D. B. (2016) Is Western diet-induced nonalcoholic steatohepatitis in Ldlr−/− mice reversible? PLoS One 11, e0146942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeek J., Jacobs A., Spincemaille P., Cassiman D. (2016) Development of a representative mouse model with nonalcoholic steatohepatitis. Curr. Protoc. Mouse Biol. 6, 201–210 [DOI] [PubMed] [Google Scholar]
- 27.Samuelsson A. M., Matthews P. A., Argenton M., Christie M. R., McConnell J. M., Jansen E. H., Piersma A. H., Ozanne S. E., Twinn D. F., Remacle C., Rowlerson A., Poston L., Taylor P. D. (2008) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–392 [DOI] [PubMed] [Google Scholar]
- 28.MacLean P. S., Higgins J. A., Johnson G. C., Fleming-Elder B. K., Peters J. C., Hill J. O. (2004) Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R288–R297 [DOI] [PubMed] [Google Scholar]
- 29.Jackman M. R., Steig A., Higgins J. A., Johnson G. C., Fleming-Elder B. K., Bessesen D. H., MacLean P. S. (2008) Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1117–R1129 [DOI] [PubMed] [Google Scholar]
- 30.Giles E. D., Jackman M. R., Johnson G. C., Schedin P. J., Houser J. L., MacLean P. S. (2010) Effect of the estrous cycle and surgical ovariectomy on energy balance, fuel utilization, and physical activity in lean and obese female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1634–R1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steig A. J., Jackman M. R., Giles E. D., Higgins J. A., Johnson G. C., Mahan C., Melanson E. L., Wyatt H. R., Eckel R. H., Hill J. O., MacLean P. S. (2011) Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R656–R667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim R. S., Kratzer A., Barry N. P., Miyazaki-Anzai S., Miyazaki M., Mantulin W. W., Levi M., Potma E. O., Tromberg B. J. (2010) Multimodal CARS microscopy determination of the impact of diet on macrophage infiltration and lipid accumulation on plaque formation in ApoE-deficient mice. J. Lipid Res. 51, 1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matyash V., Liebisch G., Kurzchalia T. V., Shevchenko A., Schwudke D. (2008) Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kind T., Liu K. H., Lee D. Y., DeFelice B., Meissen J. K., Fiehn O. (2013) LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 10, 755–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang T., Wang Z., Proctor G., Moskowitz S., Liebman S. E., Rogers T., Lucia M. S., Li J., Levi M. (2005) Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J. Biol. Chem. 280, 32317–32325 [DOI] [PubMed] [Google Scholar]
- 36.Xia J., Sinelnikov I. V., Han B., Wishart D. S. (2015) MetaboAnalyst 3.0: making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GDS group (2016) Metabolic flexibility and oxidative capacity independently associate with insulin sensitivity in individuals with newly diagnosed type 2 diabetes. Diabetologia 59, 2203–2207 [DOI] [PubMed] [Google Scholar]
- 38.Puigserver P., Spiegelman B. M. (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24, 78–90 [DOI] [PubMed] [Google Scholar]
- 39.Sugden M. C., Caton P. W., Holness M. J. (2010) PPAR control: it’s SIRTainly as easy as PGC. J. Endocrinol. 204, 93–104 [DOI] [PubMed] [Google Scholar]
- 40.Medina-Gomez G., Gray S. L., Yetukuri L., Shimomura K., Virtue S., Campbell M., Curtis R. K., Jimenez-Linan M., Blount M., Yeo G. S., Lopez M., Seppänen-Laakso T., Ashcroft F. M., Oresic M., Vidal-Puig A. (2007) PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 3, e64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo T., Deng Z. Y., Li X. P., Rao H., Fan Y. W. (2014) Triolein and trilinolein ameliorate oxidized low-density lipoprotein-induced oxidative stress in endothelial cells. Lipids 49, 495–504 [DOI] [PubMed] [Google Scholar]
- 42.Pagadala M., Kasumov T., McCullough A. J., Zein N. N., Kirwan J. P. (2012) Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 23, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavez J. A., Siddique M. M., Wang S. T., Ching J., Shayman J. A., Summers S. A. (2014) Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem. 289, 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bikman B. T. (2012) A role for sphingolipids in the pathophysiology of obesity-induced inflammation. Cell. Mol. Life Sci. 69, 2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maceyka M., Spiegel S. (2014) Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwakiri Y. (2015) Nitric oxide in liver fibrosis: the role of inducible nitric oxide synthase. Clin. Mol. Hepatol. 21, 319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassanin A., Malek H. A., Saleh D. (2014) Heparin modulation on hepatic nitric oxide synthase in experimental steatohepatitis. Exp. Ther. Med. 8, 1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brumbaugh D. E., Tearse P., Cree-Green M., Fenton L. Z., Brown M., Scherzinger A., Reynolds R., Alston M., Hoffman C., Pan Z., Friedman J. E., Barbour L. A. (2013) Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J. Pediatr. 162, 930–936, e931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oben J. A., Mouralidarane A., Samuelsson A. M., Matthews P. J., Morgan M. L., McKee C., Soeda J., Fernandez-Twinn D. S., Martin-Gronert M. S., Ozanne S. E., Sigala B., Novelli M., Poston L., Taylor P. D. (2010) Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol. 52, 913–920 [DOI] [PubMed] [Google Scholar]
- 50.Saben J. L., Boudoures A. L., Asghar Z., Thompson A., Drury A., Zhang W., Chi M., Cusumano A., Scheaffer S., Moley K. H. (2016) Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 16, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akagawa M., Minematsu K., Shibata T., Kondo T., Ishii T., Uchida K. (2016) Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci. Rep. 6, 26723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hausler N., Browning J., Merritt M., Storey C., Milde A., Jeffrey F. M., Sherry A. D., Malloy C. R., Burgess S. C. (2006) Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem. J. 394, 465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herrera P. L., Huarte J., Sanvito F., Meda P., Orci L., Vassalli J. D. (1991) Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development 113, 1257–1265 [DOI] [PubMed] [Google Scholar]
- 54.Tchaparian E., Marshal L., Cutler G., Bauerly K., Chowanadisai W., Satre M., Harris C., Rucker R. B. (2010) Identification of transcriptional networks responding to pyrroloquinoline quinone dietary supplementation and their influence on thioredoxin expression, and the JAK/STAT and MAPK pathways. Biochem. J. 429, 515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z., Sun C., Tao R., Xu X., Xu L., Cheng H., Wang Y., Zhang D. (2016) Pyrroloquinoline quinone decelerates rheumatoid arthritis progression by inhibiting inflammatory responses and joint destruction via modulating NF-κB and MAPK pathways. Inflammation 39, 248–256 [DOI] [PubMed] [Google Scholar]
- 56.Tao R., Wang S., Xia X., Wang Y., Cao Y., Huang Y., Xu X., Liu Z., Liu P., Tang X., Liu C., Shen G., Zhang D. (2015) Pyrroloquinoline quinone slows down the progression of osteoarthritis by inhibiting nitric oxide production and metalloproteinase synthesis. Inflammation 38, 1546–1555 [DOI] [PubMed] [Google Scholar]
- 57.Liu W., Baker R.D., Bhatia T., Zhu L. Baker S.S. (2016) Pathogenesis of nonalcoholic steatohepatitis. Cell. Mol. Life Sci. 73, 1969–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odkhuu E., Koide N., Haque A., Tsolmongyn B., Naiki Y., Hashimoto S., Komatsu T., Yoshida T., Yokochi T. (2012) Inhibition of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast formation by pyrroloquinoline quinine (PQQ). Immunol. Lett. 142, 34–40 [DOI] [PubMed] [Google Scholar]
- 59.Perrone S., Santacroce A., Picardi A., Buonocore G. (2016) Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J. Clin. Pediatr. 5, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson L. P., Al-Hasan Y. (2012) Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 582748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hitchler M. J., Domann F. E. (2007) An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 43, 1023–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cerda S., Weitzman S. A. (1997) Influence of oxygen radical injury on DNA methylation. Mutat. Res. 386, 141–152 [DOI] [PubMed] [Google Scholar]
- 63.Rees W. D., Hay S. M., Brown D. S., Antipatis C., Palmer R. M. (2000) Maternal protein deficiency causes hypermethylation of DNA in the livers of rat fetuses. J. Nutr. 130, 1821–1826 [DOI] [PubMed] [Google Scholar]
- 64.Lillycrop K. A., Phillips E. S., Torrens C., Hanson M. A., Jackson A. A., Burdge G. C. (2008) Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br. J. Nutr. 100, 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van de Weijer T., Sparks L. M., Phielix E., Meex R. C., van Herpen N. A., Hesselink M. K., Schrauwen P., Schrauwen-Hinderling V. B. (2013) Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS One 8, e51648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shulman G. I. (2014) Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 1131–1141 [DOI] [PubMed] [Google Scholar]
- 67.Nakano M., Suzuki H., Imamura T., Lau A., Lynch B. (2013) Genotoxicity of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™). Regul. Toxicol. Pharmacol. 67, 189–197 [DOI] [PubMed] [Google Scholar]
- 68.Itoh Y., Hine K., Miura H., Uetake T., Nakano M., Takemura N., Sakatani K. (2016) Effect of the antioxidant supplement pyrroloquinoline quinone disodium salt (BioPQQ™) on cognitive functions. Adv. Exp. Med. Biol. 876, 319–325 [DOI] [PubMed] [Google Scholar]
- 69.Nakano M., Kamimura A., Watanabe F., Kamiya T., Watanabe D., Yamamoto E., Fukagawa M., Hasumi K., Suzuki E. (2015) Effects of orally administered pyrroloquinoline quinone disodium salt on dry skin conditions in mice and healthy female subjects. J. Nutr. Sci. Vitaminol. (Tokyo) 61, 241–246 [DOI] [PubMed] [Google Scholar]
- 70.Nakano M., Kawasaki Y., Suzuki N., Takara T. (2015) Effects of pyrroloquinoline quinone disodium salt intake on the serum cholesterol levels of healthy Japanese adults. J. Nutr. Sci. Vitaminol. (Tokyo) 61, 233–240 [DOI] [PubMed] [Google Scholar]
- 71.Zhu B. Q., Simonis U., Cecchini G., Zhou H. Z., Li L., Teerlink J. R., Karliner J. S. (2006) Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 11, 119–128 [DOI] [PubMed] [Google Scholar]