Abstract
We previously showed that repetitive cyclic loading of the mouse knee joint causes changes that recapitulate the features of osteoarthritis (OA) in humans. By applying a single loading session, we characterized the temporal progression of the structural and compositional changes in subchondral bone and articular cartilage.
We applied loading during a single 5-minute session to the left tibia of adult (26-week-old) C57Bl/6 male mice at a peak load of 9.0N for 1200 cycles. Knee joints were collected at times 0, 1, and 2 weeks after loading. The changes in articular cartilage and subchondral bone were analyzed by histology, immunohistochemistry (caspase-3 and cathepsin K), and microcomputed tomography.
At time 0, no change was evident in chondrocyte viability or cartilage or subchondral bone integrity. However, cartilage pathology demonstrated by localized thinning and proteoglycan loss occurred at 1 and 2 weeks after the single session of loading. Transient cancellous bone loss was evident at 1 week, associated with increased osteoclast number. Bone loss was reversed to control levels at 2 weeks. We observed formation of fibrous and cartilaginous tissues at the joint margins at 1 and 2 weeks.
Our findings demonstrate that a single session of noninvasive loading leads to the development of OA-like morphological and cellular alterations in articular cartilage and subchondral bone. The loss in subchondral trabecular bone mass and thickness returns to control levels at 2 weeks, whereas the cartilage thinning and proteoglycan loss persist.
Keywords: Osteoarthritis, mouse, mechanical loading, bone resorption, chondrocytes
Introduction
The mechanical loading environment in joint tissues plays a crucial role in the development of osteoarthritis (OA). Population cohorts with histories of traumatic joint injuries such as ligament tears have a higher incidence of OA that has been attributed to the resultant alteration in joint mechanics (1). Similarly, excessive physical activity associated with occupational characteristics or athletic activity is associated with the increased incidence of OA (2). Several in vivo animal models demonstrate that the creation of an adverse mechanical loading environment is strongly associated with the development of OA. For example, strenuous running in mice and dogs produces articular cartilage degeneration and peri-articular bone changes (3, 4), while less strenuous running prevents the onset of OA (5, 6). In addition, in vitro studies show that a single event of injurious impact loading of cartilage explants decreases cell viability and increases the production of catabolic mediators such as matrix metalloproteinases (MMPs) and aggrecanases (7–9). These findings suggest, therefore, that a single event of traumatic loading can produce sustained adverse effects on chondrocyte viability and function.
In noninvasive mouse models that apply controlled mechanical loading to joints in vivo, both cyclic and single injurious loading protocols promote OA pathology similar to the degenerative changes in humans joints, including cartilage loss, alterations in subchondral bone, and osteophyte formation (10–15). The traumatic effects of loading are most evident upon loading-induced anterior cruciate ligament (ACL) rupture and direct alteration of joint stability (11, 14, 15). However, in cyclic loading experiments that do not induce macroscopic damage to joint structures (10, 12), it is unclear whether the cartilage and bone changes are related to direct physical damage from repetitive traumatic joint tissue injury or if a single episode of loading is sufficient to initiate cell-mediated processes that can lead to subsequent pathologic alterations in joint tissues.
The present studies were undertaken to address this question by assessing the changes in cartilage and peri-articular bone following a single 5-minute session of in vivo mechanical loading rather than the daily loading protocol employed in our previous studies (12). We characterized the temporal progression of the structural and compositional changes in subchondral bone and articular cartilage after the single loading session. We hypothesized that a single loading session results in more rapid response in the subchondral bone than the articular cartilage and does not result in initial traumatic damage to the tissues.
Methods
Mechanical Loading Conditions
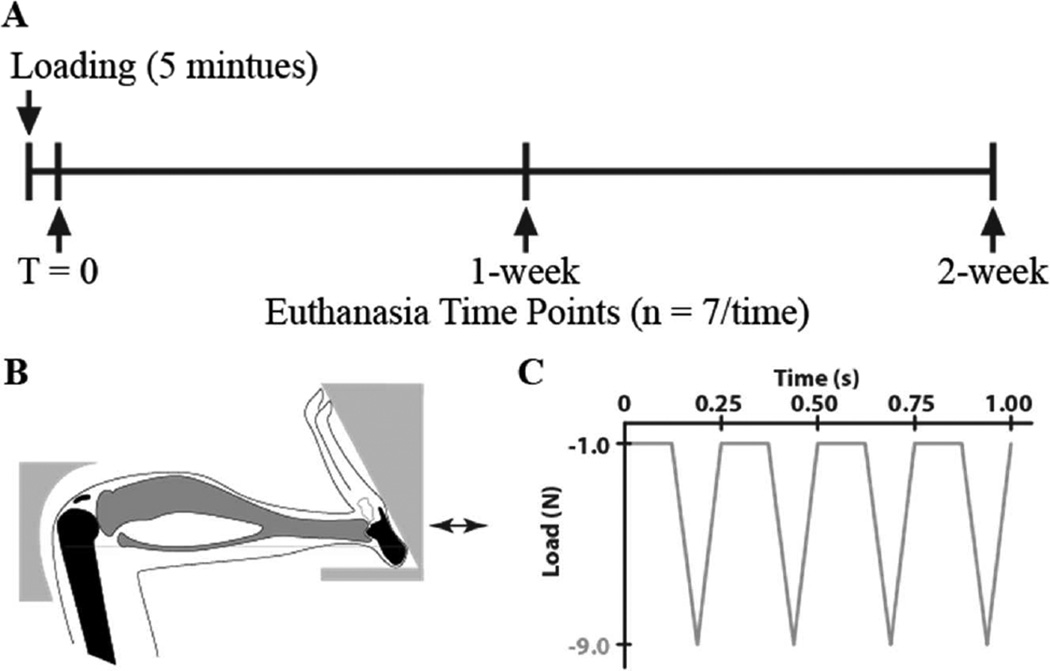
We applied a single session of controlled in vivo compressive loading to the left tibiae of 26-week-old C57Bl/6 male mice (n = 21, Jackson Laboratories, Bar Harbor, ME) at a peak load level of 9.0N and frequency of 4Hz for 1200 cycles (5 minutes) under general anesthesia (2% Isoflurane, 1.0 L/min, Webster). The applied loading was based on protocols demonstrated previously to induce progressive articular cartilage degeneration (12) and to have an anabolic effect on the tibial metaphysis in growing and adult mice (16–18). The left limb was loaded; the right limb served as the non-loaded control. After each loading session, mice resumed normal cage activity. To assess the immediate changes in response to a single session of mechanical loading, analyses were performed at T = 0 (approximately 1 hour after the single loading session) and at 1 and 2 weeks (n = 7/group). All mice were randomly distributed across three different experimental groups. The intact knee joints were collected and fixed in 4% PFA overnight (Fig. 1). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Cornell University.
Figure 1.
(A) Overview of the loading and euthanasia time points, (B) schematic of mouse tibial loading configuration, and (C) in vivo loading waveform used in the study for 1200 cycles.
Articular Cartilage and Bone Tissue Assessment
After tissue fixation in 4% PFA overnight, intact joints in PBS were scanned by microcomputed tomography (microCT) at 10μm resolution (μCT35, Scanco, Switzerland) with an X-ray tube potential of 55 kVp to assess bone morphological changes. Knee joints were then decalcified in formic acid/sodium citrate for one week, dehydrated in an ethanol gradient, and embedded in paraffin. Serial coronal sections of 6 μm thickness were obtained using a rotary microtome (Leica RM2255, Germany). Safranin O/Fast green/hematoxylin staining was performed on sections at 90 μm intervals to assess cartilage morphology. Cartilage degeneration was assessed in the tibial plateau using a modified murine cartilage histological scoring system (19). The presence of osteophytes was identified by the changes in peri-articular bone and articular morphologies in sections used for histological scoring. Subsequently, osteophytes were scored according to their size and maturity (20). The presence of synovial inflammation at the joint margins was examined by hematoxylin and eosin staining.
Localized thickness measurements for articular cartilage and subchondral cortical bone were performed (Osteomeasure histomorphometry system, OsteoMetrics, USA) on Safranin O/Fast green-stained slides previously used for histological scoring. The tibial plateau was first divided into medial and lateral halves, and then further subdivided into anterior, middle, and posterior regions, resulting in six tibial plateau regions for evaluation. A single representative slide from each region was used to measure cartilage and subchondral cortical bone thickness. Five linear projections from the cartilage surface to the boundary between the cartilage and subchondral bone were used to measure cartilage thickness (Ca.Th, μm). The projections were extended into the subchondral bone to circumscribe the region for measuring the subchondral bone thickness (Sub.Pl, μm) (12). Slides from sections adjacent to those used for localized thickness measurements were stained with picrosirius red to identify superficial zone collagen alterations. Damaged superficial zone surface area was quantified under the polarized microscope and normalized by the total linear cartilage surface area (% Loss, mm/mm).
Cancellous bone morphology was assessed in two volumes of interest (VOI) of the microCT scans: the tibial metaphysis and epiphysis. The metaphyseal VOI was located distal to the growth plate extending 10% of the total bone length, excluding primary spongiosa and cortical bone. The epiphyseal VOI was the cancellous tissue proximal to the growth plate. The global threshold was set at 3900 HU and 3200 HU to segment mineralized tissue from epiphyseal and metaphyseal regions, respectively. Trabecular bone from both VOIs was selected by manually contouring inside the cortical bone. For each region, cancellous bone volume fraction (BV/TV, mm3/mm3), trabecular thickness (Tb.Th, μm), and separation (Tb.Sp, μm) were measured.
Cellular Responses
Immunohistochemistry was performed to assess cellular changes in cartilage and subchondral bone following the single session of loading. The effects of loading on chondrocyte phenotype were assessed by examination of cell morphology and expression of the apoptosis-associated protein, caspase-3 (Abcam, Cambridge, MA). Osteoclasts associated with localized regions of bone resorption were identified using an antibody to cathepsin K (Abcam, Cambridge, MA). To assess the effects of the loading on synovial pathology, tissue sections were examined for the presence of synovial lining hyperplasia and for the presence of inflammatory cell infiltration. Immunohistochemistry was applied to three sections, one section each from the anterior, middle, and posterior aspects of the tibial plateau. Sections were dewaxed, rehydrated, and incubated with 1% pepsin at 37°C for antigen retrieval for detection of caspase-3. No antigen retrieval was performed for cathepsin K. Sections were subsequently incubated with 1.5% goat serum for 30 minutes at room temperature and immunostained overnight at 4°C with the respective primary antibody or IgG as a negative control. Secondary antibody incubation and color development were done with avidin/biotin complex (Vector Labs, Burlingame, CA). Chondrocytes that stained positively for caspase-3 were counted under 40× magnification on both medial and lateral halves of tibial cartilage, normalized to total tibial cartilage area, and averaged for all three sections (Chon.N/CA, #/mm2). Osteoclasts that positively stained for cathepsin K with at least two nuclei counterstained with hematoxylin were counted within epiphyseal cancellous bone, normalized to the epiphyseal bone cancellous surface area, and averaged over the three sections (OC.N/BS, #/mm).
Statistics
Statistical analyses were performed using repeated measures two-way ANOVA with interactions (JMP Pro 10.0, SAS Institute Inc), with Loading as the intra-group variable, and Time as an inter-group variable. Post-hoc comparisons of means were performed with Bonferroni correction when interaction effects were significant. P-values of < 0.05 indicated significance.
Results
Articular Cartilage Matrix Changes
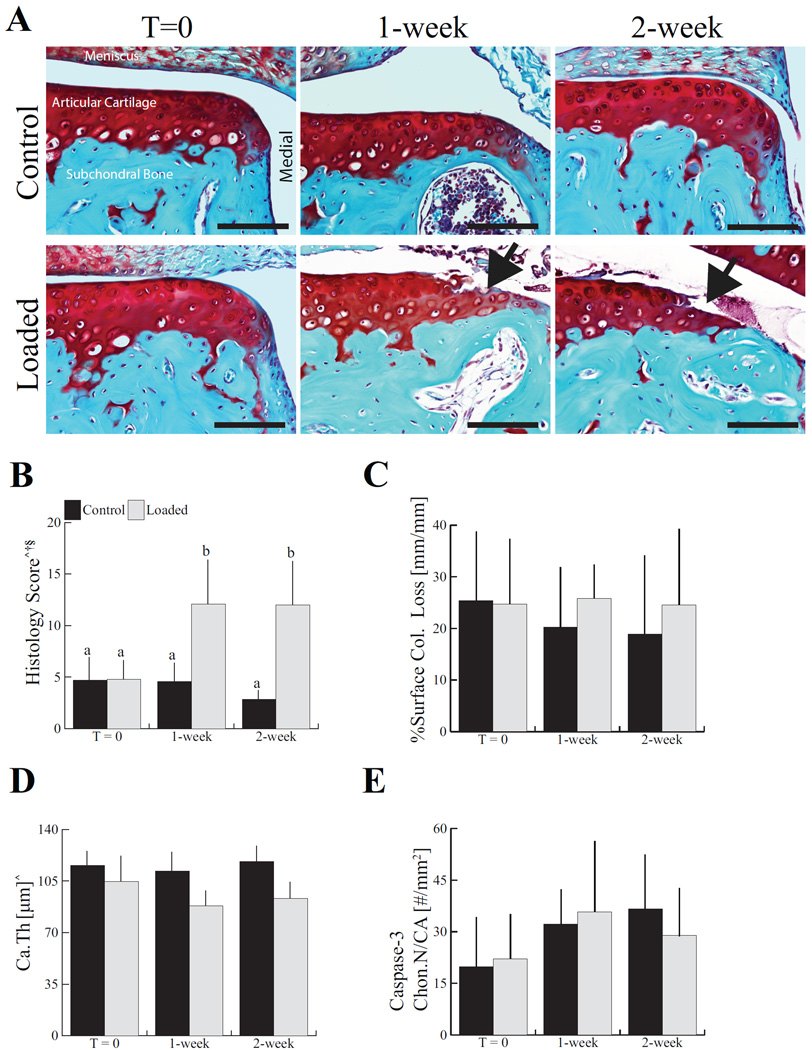
Articular cartilage damage was first evident in the loaded limbs after 1 and 2 weeks (Fig. 2A). The histological scores of loaded limbs increased 2.6-fold after 1 week and 4.2-fold after 2 weeks compared to control limbs. The severity of the damage in the loaded limbs was similar at 1 and 2 weeks (Fig. 2B), with damage mainly localized to the medial peripheral edge of the articular surface. Loading did not induce collagen loss from the joint surface at any time point as assessed by the intensity of picrosirius red staining, although localized fibrillation and fragmentation of the superficial zone cartilage was evident (Fig. 2C).
Figure 2.
Progressive cartilage degeneration occurred following a single loading session in adult mice without creating immediate physical damage (A and B). Superficial zone collagen loss was not altered by the single loading session (C). Loading also thinned articular cartilage (Ca.Th) at the lateral posterior quadrant (D). Chondrocyte apoptosis, measured by immunohistochemical staining for caspase-3 positive chondrocytes in articular cartilage was not altered by the single loading session (E). Control images represent a non-loaded contralateral limb at each time point. Scale bar = 100 μm. Data presented as mean ± SD of 7/group. ^Loading, †time, §loading*time, p < 0.05 by repeated measures two-way ANOVA. Groups with different letters are significantly different by post-hoc comparisons of means with Bonferroni correction.
Articular cartilage thinning in both lateral and medial compartments occurred in the posterior aspect of the tibial plateau of loaded joints (Fig. 2D, Table 1). While articular cartilage located at the lateral-middle aspect of tibial plateau thinned following a single session of mechanical loading, no change was observed in the medial-middle area. Lateral-posterior articular cartilage was 17% thinner and the medial-posterior area was thinner by 13%in loaded limbs compared to control limbs. In contrast to the thinning of the articular cartilage at the posterior aspect of tibial plateau, a single session of loading appeared to increase the cartilage thickness at the medial-anterior aspect by 8.4% with no change in the lateral-anterior area. Control limbs did not undergo cartilage degeneration at any time point in the posterior aspect of tibial plateau, as indicated by similar histological scores and cartilage thicknesses.
Table 1.
Spatial distribution of localized articular cartilage and subchondral 420 cortical bone thickness. Data presented as mean ± SD of 7/group.
| T=0 | 1-week | 2-week | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Loaded | Control | Loaded | Control | Loaded | |||
|
Articular Cartilage Thickness |
Medial | Anterior^ | 100 ± 15 | 118 ± 11 | 117 ± 8.0 | 122 ± 13 | 119 ± 11 | 125 ± 11 |
| Middle | 122 ± 9.4 | 123 ± 15 | 120 ± 10 | 111 ± 9.9 | 123 ± 6.4 | 113 ± 12 | ||
| Posterior^ | 97 ± 14 | 94 ± 11 | 101 ± 9.4 | 84 ± 7.9 | 103 ± 15 | 85 ± 10 | ||
| Lateral | Anterior | 100 ± 12 | 107 ± 3.0 | 99 ± 5.8 | 107 ± 9.7 | 97 ± 16 | 103 ± 11 | |
| Middle^†§ | 118 ± 13 | 116 ± 6.6 | 112 ± 7.8 | 101 ± 5.7 | 122 ± 13 | 96 ± 5.2 | ||
| Posterior^ | 116 ± 9.8 | 105 ± 17 | 112 ± 13 | 88 ± 10 | 118 ± 11 | 93 ± 11 | ||
|
Subchondral Cortical Bone Thickness |
Medial | Anterior | 89 ± 26 | 99 ± 26 | 89 ± 28 | 85 ± 55 | 99 ± 29 | 60 ± 30 |
| Middle§ | 107 ± 32 | 129 ± 33 | 95 ± 25 | 95 ± 32 | 118 ± 17 | 69 ± 31 | ||
| Posterior^ | 63 ± 17 | 55 ± 20 | 67 ± 25 | 47 ± 10 | 76 ± 28 | 51 ± 8.8 | ||
| Lateral | Anterior† | 86 ± 24 | 86 ± 16 | 67 ± 27 | 92 ± 29 | 77 ± 28 | 59 ± 27 | |
| Middle^ | 62 ± 12 | 71 ± 30 | 80 ± 26 | 50 ± 13 | 74 ± 20 | 45 ± 20 | ||
| Posterior | 44 ± 9.4 | 50 ± 6.3 | 54 ± 16 | 48 ± 5.8 | 54 ± 14 | 53 ± 16 | ||
loading,
time,
loading*time, p<0.05 by repeated measures two-way ANOVA.
We next examined the effects of loading on caspase-3, a marker of chondrocyte apoptosis, by immunohistochemistry. The number of chondrocytes immunostained for caspase-3 in the articular cartilage was not altered at any time point after a single loading session (Fig. 2E).
Epiphyseal and Metaphyseal Bone Adaptation
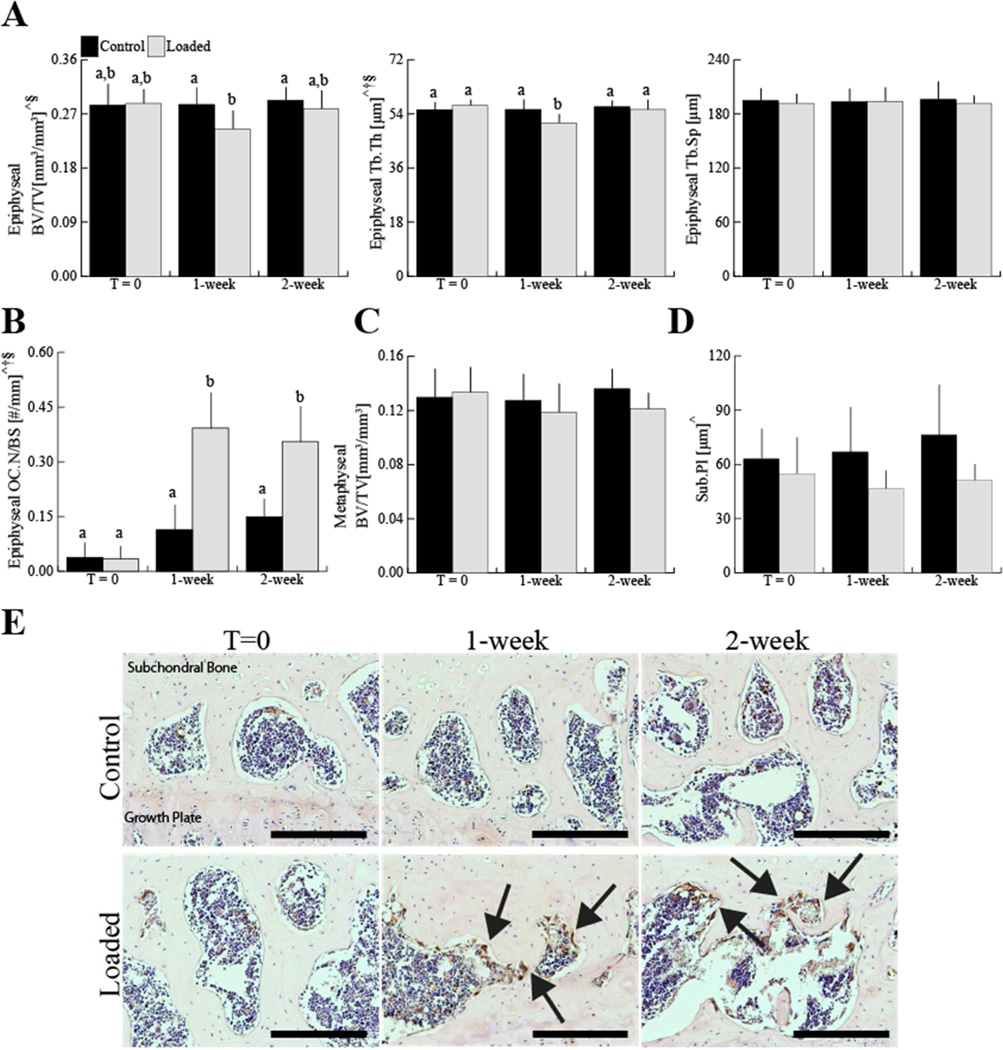
Epiphyseal bone architecture was altered following a single loading session. No change was observed at any time point in control limbs. In the loaded limbs, epiphyseal cancellous bone mass decreased by 12% after 1 week, but returned to control levels by 2 weeks (Fig. 3A). The decreased epiphyseal bone mass at 1 week was attributable to the 8% decrease in trabecular thickness without an increase in trabecular separation (Fig. 3A). The decrease was associated with increased numbers in osteoclasts on the trabecular bone surface. Osteoclast numbers in the epiphysis of loaded limbs, which were identified by cathepsin K expression, increased by 2.4-fold at 1 week and by 1.4-fold at 2 weeks after the single loading session (Fig. 3B, E). Metaphyseal bone mass was unchanged in the loaded and control limbs (Fig. 3C). The subchondral cortical bone thickness decreased by 26% in the medial-posterior area and by 24% decreases in the lateral-middle area after mechanical loading (Fig. 3D, Table 1). At 2 weeks after the single session of mechanical loading, subchondral cortical plate thickness at the medial-middle aspect of tibial plateau decreased by 42%.
Figure 3.
In the epiphysis loading decreased epiphyseal cancellous mass (BV/TV) in adult mice at 1 week. This change was due to decreased trabecular thickness (Tb.Th) at 1 week, but not to increased trabecular separation (Tb.Sp) (A). Osteoclast number (OC.N/BS), which was identified by immunohistochemical staining for Cathepsin K positive osteoclasts, increased in the epiphysis with loading (B, E). Metaphyseal bone mass (BV/TV) did not change with loading (C). Medial posterior subchondral cortical bone thickness (Sub.Pl) decreased with loading (D). Scale bar = 200 μm. Data presented as mean ± SD of 7/group.
^loading, †time, §loading*time, p<0.05 by repeated measures two-way ANOVA. Groups with different letters are significantly different by post-hoc comparisons of means with Bonferroni correction.
Osteophyte Formation
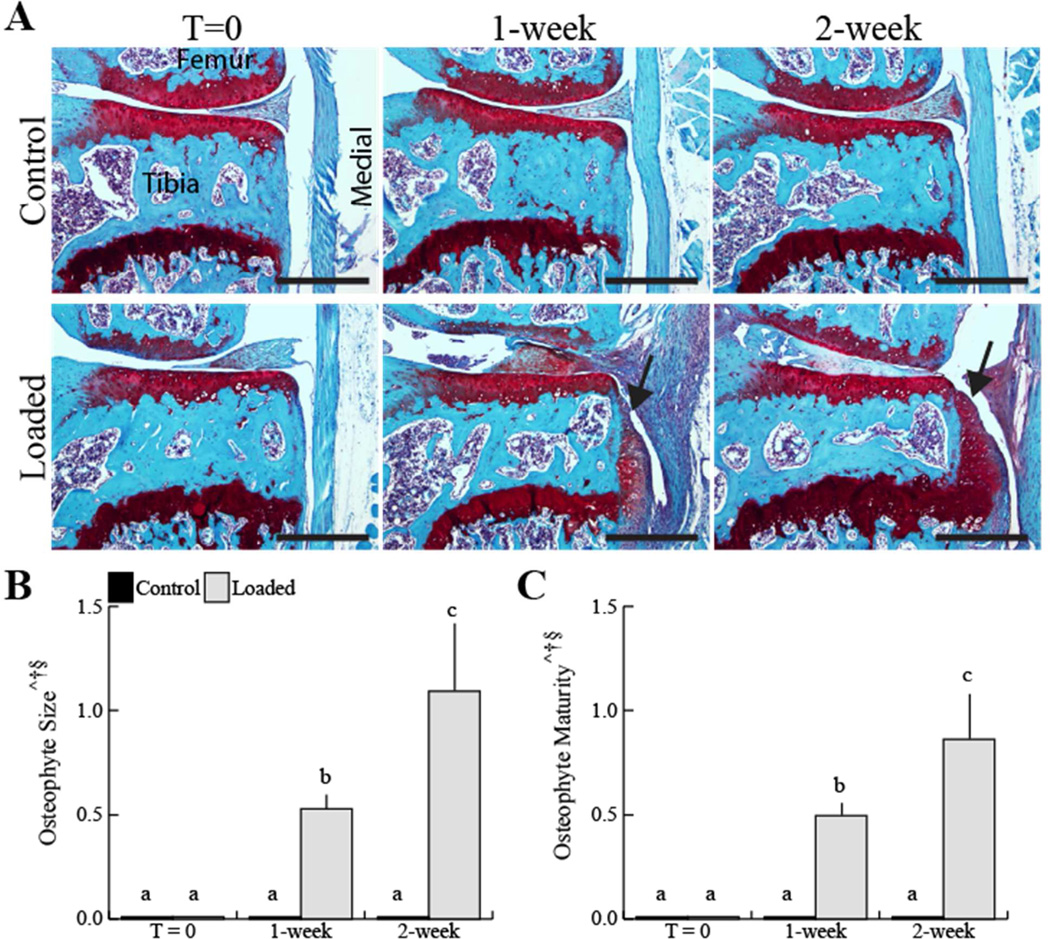
Osteophytes developed at the joint margins following a single loading session in all loaded limbs. Fibrous tissue was evident at the joint margins at 1 week. By 2 weeks, cartilaginous tissue at the joint margins was evident, indicative of the early endochondral phase of osteophyte formation (Fig 4A). Osteophyte size increased by 2.1-fold in the loaded limbs from 1 to 2 weeks (Fig 4B). In addition, osteophyte maturity, indicated by resident hypertrophic chondrocytes and vascular invasion, increased by 1.7 fold in the loaded limbs from 1 to 2 weeks (Fig 4C). Osteophytes were absent in the T=0 loaded limbs and all control limbs.
Figure 4.
A single 5 minute loading session induced osteophyte formation (black arrowheads) 1 and 2 weeks later, but not at time T=0 (A). A single loading session increased osteophyte size and maturity at 1- and 2-week time points (B, C). Osteophytes did not develop in any control or T=0 loaded limb. Scale bar 400 μm. Data presented as mean ± SD of 7/group.
^loading, †time, §loading*time, p<0.05 by repeated measures two-way ANOVA. Groups with different letters are significantly different by post-hoc comparisons of means with Bonferroni correction.
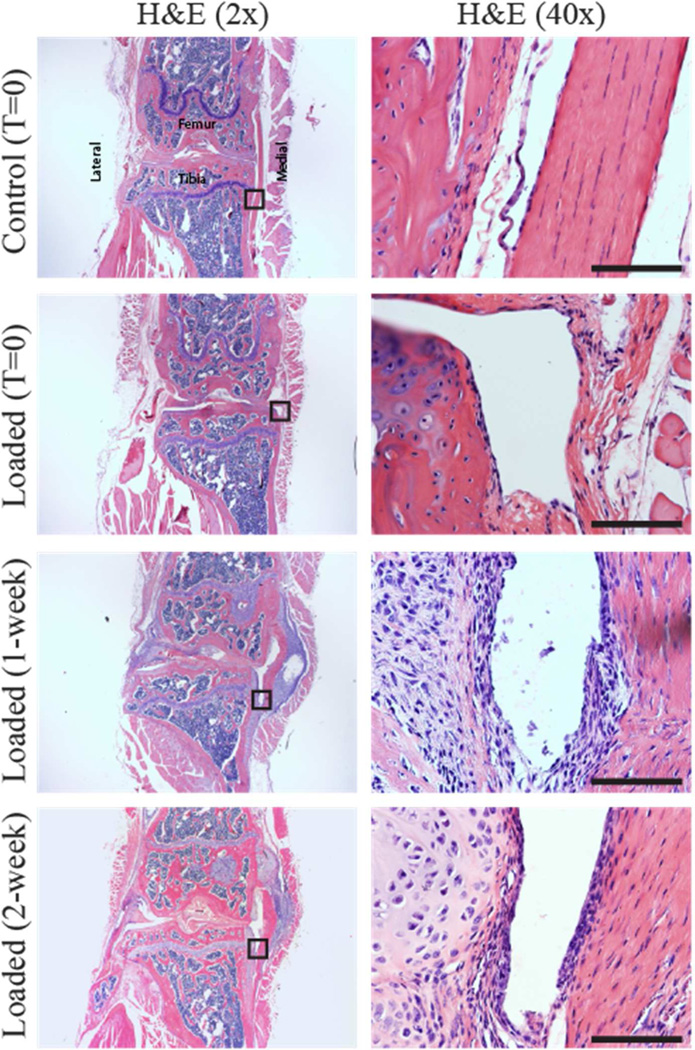
Synovial Inflammation
Enhancement of synovial lining or hyperplasia was not evident in the knee joint of any animal at any time point (Fig 5). No lymphocyte or plasma cell infiltration was present in the synovium. Significant proliferation of cells and columnar cell morphology were observed in the collateral ligaments of loaded limbs.
Figure 5.
A single 5-minute loading session did not induce synovial inflammation indicated by the absence of synovial hyperplasia and lymphocyte infiltration in the synovium at 1 and 2 weeks after the single loading session. Locations of the high magnification images on the right are indicated by the black boxes in the left-hand panels. Scale bar 100 μm.
Discussion
Our results demonstrate that a single episode of mechanical loading can initiate a cell-mediated process leading to cartilage degeneration and bone adaptive changes that evolve over a one- to two-week interval. In addition, subchondral bone demonstrated robust capacity to return to the normal level within two weeks in contrast to articular cartilage. Controlled mechanical loading applied to the mouse knee in other models showed similar changes in the articular cartilage matrix, including loss of proteoglycans and surface fibrillation (10, 11, 14, 15). However, the alterations in the peri-articular bone differed, likely related to differences in the loading protocol parameters used in the other studies. For example, the studies by Poulet et al. included an extended rest period of 10 seconds between loading cycles, and they noted limited osteophyte development at the joint margins 2 weeks after a single loading session (10), whereas in our study all mice developed osteophytes with a shorter rest insertion period of 0.25 second between loading cycles. When higher peak loads (12N) were applied (11, 14), heterotopic ossification and ACL injury were observed, responses that were not observed in our animals using lower loads. The study by Wu et al. demonstrated that single cyclic compressive loading at 9N peak load ruptured the ACL (15), an effect that was not observed in our studies. Analyses for possible damage to knee ligaments or the joint capsule revealed intact ligaments in our model. Subluxation of the knee was not evident. However, we cannot exclude the possibility that the single loading episode resulted in subsequent alterations in joint mechanics. The differential effects with respect to ligament rupture compared to previous studies could be related to the knee holder design. Our device allows normal flexion of the knee, and we speculate that the orientation of the loading (vertical vs. horizontal as in our study) of the loading apparatus in the Wu study may have contributed to the ligament injury. In addition, the mice in our studies were 26 weeks of age compared to 8 weeks of age in the Wu studies, and this age difference may have affected the material properties of the ligament.
In our previous study, mice loaded at a peak load of 4.5N demonstrated mild cartilage fibrillation without the formation of osteophytes in the knee despite daily loading for 6 weeks (12). In contrast, mouse knees loaded daily at 9N for 6 weeks exhibited more extensive and severe cartilage degeneration and developed mineralized osteophytes at joint margins (12). Similarly, the surgically-induced mouse models of OA, such as the destabilized medial meniscus (DMM) and ACL transection models, show more advanced degenerative cartilage changes at 8 weeks post-surgery compared to 4 weeks (21, 22). Our longest evaluation time was limited to two weeks, since our primary objective was to assess rapid morphological and cellular changes in the subchondral bone and articular cartilage after a single loading session. Further studies are needed to establish whether the pathologic cartilage changes that we observed after 2 weeks will progress at later time points or whether a component of the pathology is reversible. In addition, investigating the contributions of loading rate, peak load, frequency, and total number of cycles to changes in the mouse joint will provide a better understanding of the role of the mechanical environment in promoting the cartilage and bone changes and osteophyte formation.
Similar to tissue changes following daily mechanical loading (12), at 1 or 2 weeks after a single session of loading there were alterations in the articular cartilage, including decreased cartilage thickness. In addition, osteophytes had formed on the medial joint margins. In contrast to thinning of articular cartilage at the posterior aspect of tibial plateau observed with repetitive loading, a single session of loading resulted in increased cartilage thickness at the medial-anterior aspect with no change in the lateral-anterior area. The differential patterns observed in the single and repetitive loading sessions suggest that additional mechanical and biological cell-mediated processes are initiated by repetitive episodes versus a single episode of joint loading. Further studies with longer follow-up will be required to determine whether the alterations in the articular cartilage induced by the single loading session are reversible and whether chondrocytes can restore the cartilage matrix to a physiological state after an initial injury.
A single session of loading also produced alterations in the subchondral bone that evolved over the first week. The decreased epiphyseal cancellous mass at 1 week was primarily attributable to thinning of trabeculae rather than increased trabecular separation. These changes were associated with increased osteoclast number. Kennedy et al. (23) examined the effects of cyclic loading on rat ulnae and observed up-regulation of osteoclastic resorption in regions of microdamage. This effect was accompanied by the appearance of osteocyte apoptosis at these sites and up-regulation of osteoclast-inducing factors in adjacent viable osteocytes. We did not directly examine the subchondral bone for evidence of microdamage or effects on osteocyte viability after the acute loading session.
The decrease in the epiphyseal bone at week 1 was no longer detectible at week 2. This contrasts with the effects of repeated daily loading that resulted in progressive decreases in epiphyseal trabecular bone mass. The decrease in epiphyseal trabecular bone mass accompanied corticalization of the epiphysis, which may have contributed to the increased subchondral cortical bone thickness. In contrast, a single session of loading did not produce thickening of the subchondral bone plate. Bone tissues exhibit a unique capacity to adapt to microdamage and to alterations in mechanical loading via the activities of osteoclasts and osteoblasts. The restoration of bone architecture after two weeks indicates that the effects of the acute loading are reversible, and reflect the unique capacity of bone to remodel its extracellular matrix once the loading environment is returned to a physiological loading state. In contrast the cartilage changes persist within the time frame of the study. The different pattern of responses in the cartilage versus the bone reflect the differential capacity of these tissues to remodel their extracellular matrices and likely is a factor that contributes to the pathogenesis of the joint pathology in OA (24).
The single episode of loading did not acutely affect the structure or architecture of the articular cartilage and the subchondral and peri-articular bone and, importantly, did not acutely affect chondrocyte viability. Therefore, the sequential changes in the tissues after the loading session must be related to the induction of alterations in the activities of the resident chondrocytes and bone cells. In addition to effects on articular chondrocytes, bone cells and cells at the joint margins that give rise to osteophytes, the loading episode may also have affected cells in the menisci or synovium. The release of soluble mediators from these tissue may also have indirectly contributed to the de-regulated functional activities of the cartilage and bone cells (25). We did not detect significant signs of inflammation in the synovial tissue or alterations in the histological features of the menisci, but this finding does not exclude their potential roles in contributing to the de-regulated function of the chondrocytes and bone cells.
In conclusion, our results demonstrated that a single loading session that does not adversely affect chondrocyte viability or induce morphological and compositional alterations in cartilage or peri-articular bone tissues is sufficient to induce a cell-mediated program that leads to the development of progressive OA pathologic changes. Notably, the cartilage pathology progressed over the two-week period, whereas the epiphyseal subchondral changes were transient. We speculate that different response profiles of the bone and cartilage may be related in part to the differential capacity of the resident cells in each tissue to remodel and repair the tissue damage. Future studies are needed to define the threshold for induction of bone and cartilage pathology and to define the specific loading parameters that are involved in the initiation of these pathological changes. Importantly, this model provides a system to assess the effects of pharmacological interventions such as anti-resorptive drugs or agents that modulate the catabolic activity of chondrocytes to gain insights into the pathophysiological mechanisms involved in the bone and cartilage changes and for identifying potential therapeutic interventions to prevent the adverse effects of mechanical loading on joint tissues.
Acknowledgments
Lyudmila Lukashova of the HSS microCT core facility and the Cornell CARE staff provided experimental assistance.
Role of the funding source
This work was supported by NIH grants R01-AG028664 (MCHM), and RC4-AR060546 and R01-AG022021 (MBG), R21-AR064034 (MCHM and MBG), NSF GRFP (FCK), and the Clark and Kirby Foundations.
Footnotes
Contributions
Conception and design: FCK, SRG, TMW, MBG, MCHM
Acquisition, analysis, and interpretation of the data: FCK, CD, DAP, AWH, OOA, SRG, TMW, MBG, MCHM
Drafting and critical revision of the article for important intellectual content: FCK, CD, DAP, AWH, OOA, SRG, TMW, MBG, MCHM
Final approval of the article: FCK, CD, DAP, AWH, OOA, SRG, TMW, MBG, MCHM
Conflict of interests
The authors have no conflict of interest related to this work.
References
- 1.Felson D, Lawrence R, Dieppe P, Hirsch R, Helmick C, Jordan J, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Kaila-Kangas L, Arokoski J, Impivaara O, Viikari-Juntura E, Leino-Arjas P, Luukkonen R, et al. Associations of hip osteoarthritis with history of recurrent exposure to manual handling of loads over 20 kg and work participation: a population-based study of men and women. Occup Environ Med. 2011 Oct;68(10):734–738. doi: 10.1136/oem.2010.061390. [DOI] [PubMed] [Google Scholar]
- 3.Oettmeier R, Arokoski J, Roth AJ, Helminen HJ, Tammi M, Abendrothj K. Quantitative Study of Articular Cartilage and Subchondral Bone Remodeling in the Knee Joint of Dogs After Strenuous Running Training. J Bone Miner Res. 1992;7:S419–S424. doi: 10.1002/jbmr.5650071410. [DOI] [PubMed] [Google Scholar]
- 4.Lapvetelainen T, Nevalainen T, Parkkinen JJ, Arokoski J, Kiraly K, Hyttinen M, et al. Lifelong Moderate Running Training Increases the Incidence and Severity of Osteoarthritis in the Knee Joint of C57BL Mice. Anat Rec. 1995;242:159–165. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- 5.Lapveteläinen T, Hyttinen M, Lindblom J, Långsjö TK, Sironen R, Li SW, et al. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage. 2001 Feb;9(2):152–160. doi: 10.1053/joca.2000.0370. [DOI] [PubMed] [Google Scholar]
- 6.Otterness IG, Eskra JD, Bliven ML, Shay AK, Pelletter J, Milici AJ. Exercise Protects Against Articular Cartilage Degeneraiton In The Hamster. Arthritis Rheum. 1998;41(11):2068–2076. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Morel V, Berutto C, Quinn TM. Effects of damage in the articular surface on the cartilage response to injurious compression in vitro. J Biomech. 2006 Jan;39(5):924–930. doi: 10.1016/j.jbiomech.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Natoli RM, Athanasiou Ka. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J Biomech Eng. 2008 Aug;130(4):041012. doi: 10.1115/1.2939368. [DOI] [PubMed] [Google Scholar]
- 9.Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, Defrate LE, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2011 Apr;29(4):501–510. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulet B, Hamilton RW, Shefelbine S, Pitsillides Aa. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011 Jan;63(1):137–147. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
- 11.Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2012 Jul;20(7):773–782. doi: 10.1016/j.joca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013 Jun;65(6):1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulet B, Westerhof TaT, Hamilton RW, Shefelbine SJ, Pitsillides aa. Spontaneous osteoarthritis in Str/ort mice is unlikely due to greater vulnerability to mechanical trauma. Osteoarthritis Cartilage. 2013 May;21(5):756–763. doi: 10.1016/j.joca.2013.02.652. [DOI] [PubMed] [Google Scholar]
- 14.Onur TS, Wu R, Chu S, Chang W, Kim HT, Dang ABC. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J Orthop Res. 2014 Feb;32(2):318–323. doi: 10.1002/jor.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early response of mouse joint tissue to noninvasive knee injury suggests treatment targets. Arthritis Rheumatol. 2014 May;66(5):1256–1265. doi: 10.1002/art.38375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritton JC, Myers ER, Wright TM, van der Meulen MCH. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005 Jun;36(6):1030–1038. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010 Sep;109(3):685–691. doi: 10.1152/japplphysiol.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone. 2011 Sep;49(3):439–446. doi: 10.1016/j.bone.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. Elsevier Ltd. 2010 Oct;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Little CB, Barai A, Burkhardt D, Smith SM, Fosang aJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009 Dec;60(12):3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasson SS, Blanchet TJ, Morris Ea. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007 Sep;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005 Jul;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012 May;50(5):1115–1122. doi: 10.1016/j.bone.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 25.Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, Hachem KEl, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cells Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]