Abstract
Macronuclear chromosomes of the ciliated protozoan Oxytricha nova terminate with a single-stranded (T4G4)2 overhang. The (T4G4)2 telomeric overhang is tenaciously bound by a protein heterodimer. We have cloned and sequenced the gene encoding the 41-kDa subunit of this telomere binding protein. The predicted amino acid sequence comprises two distinct regions, a carboxyl-terminal two-thirds that is 23% lysine and bears similarity to histone H1 and an amino-terminal one-third containing a hydrophobic stretch of about 15 amino acids. Two macronuclear versions of the gene differ in nucleotide sequence at several positions, but the derived polypeptides differ only at a single position, Ser-110 or Ala-110. Both versions harbor a small intron. The existence of this intron demonstrates that, despite the elimination of 95% of the micronuclear genome from the developing macronucleus, at least some noncoding DNA is retained during macronuclear development of hypotrichous ciliates.
Full text
PDF


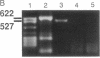
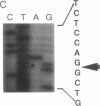
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Allan J., Mitchell T., Harborne N., Bohm L., Crane-Robinson C. Roles of H1 domains in determining higher order chromatin structure and H1 location. J Mol Biol. 1986 Feb 20;187(4):591–601. doi: 10.1016/0022-2836(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Erickson B. W. Optimal sequence alignment using affine gap costs. Bull Math Biol. 1986;48(5-6):603–616. doi: 10.1007/BF02462326. [DOI] [PubMed] [Google Scholar]
- Berman J., Tachibana C. Y., Tye B. K. Identification of a telomere-binding activity from yeast. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3713–3717. doi: 10.1073/pnas.83.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Chiou S. S. Non-nucleosomal packaging of a tandemly repeated DNA sequence at termini of extrachromosomal DNA coding for rRNA in Tetrahymena. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2263–2267. doi: 10.1073/pnas.78.4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Dancis B. M., Holmquist G. P. Telomere replication and fusion in eukaryotes. J Theor Biol. 1979 May 21;78(2):211–224. doi: 10.1016/0022-5193(79)90265-0. [DOI] [PubMed] [Google Scholar]
- Dibb N. J., Newman A. J. Evidence that introns arose at proto-splice sites. EMBO J. 1989 Jul;8(7):2015–2021. doi: 10.1002/j.1460-2075.1989.tb03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Cech T. R. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell. 1984 Sep;38(2):501–510. doi: 10.1016/0092-8674(84)90505-1. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Zakian V. A. Telomere proteins: specific recognition and protection of the natural termini of Oxytricha macronuclear DNA. Cell. 1986 Oct 24;47(2):195–205. doi: 10.1016/0092-8674(86)90442-3. [DOI] [PubMed] [Google Scholar]
- Greslin A. F., Loukin S. H., Oka Y., Prescott D. M. An analysis of the macronuclear actin genes of Oxytricha. DNA. 1988 Oct;7(8):529–536. doi: 10.1089/dna.1.1988.7.529. [DOI] [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Herrick G., Hunter D., Williams K., Kotter K. Alternative processing during development of a macronuclear chromosome family in Oxytricha fallax. Genes Dev. 1987 Dec;1(10):1047–1058. doi: 10.1101/gad.1.10.1047. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983 Apr;20(4):483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Hunter D. J., Williams K., Cartinhour S., Herrick G. Precise excision of telomere-bearing transposons during Oxytricha fallax macronuclear development. Genes Dev. 1989 Dec;3(12B):2101–2112. doi: 10.1101/gad.3.12b.2101. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Comparison of the late H1 histone genes of the sea urchins Lytechinus pictus and Strongelocentrotus purpuratus. Nucleic Acids Res. 1986 Oct 24;14(20):8121–8133. doi: 10.1093/nar/14.20.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Gruissem W., Prescott D. M. Higher order DNA structure in macronuclear chromatin of the hypotrichous ciliate Oxytricha nova. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2495–2499. doi: 10.1073/pnas.79.8.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W. Codon usage in Tetrahymena and other ciliates. J Protozool. 1989 Jan-Feb;36(1):29–34. doi: 10.1111/j.1550-7408.1989.tb02679.x. [DOI] [PubMed] [Google Scholar]
- McClintock B. The Fusion of Broken Ends of Chromosomes Following Nuclear Fusion. Proc Natl Acad Sci U S A. 1942 Nov;28(11):458–463. doi: 10.1073/pnas.28.11.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H., Andreasen P. H., Dreisig H., Kristiansen K., Engberg J. An intron in a ribosomal protein gene from Tetrahymena. EMBO J. 1986 Oct;5(10):2711–2717. doi: 10.1002/j.1460-2075.1986.tb04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Properties of the telomeric DNA-binding protein from Oxytricha nova. Biochemistry. 1989 Jan 24;28(2):769–774. doi: 10.1021/bi00428a053. [DOI] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Telomeric DNA-protein interactions of Oxytricha macronuclear DNA. Genes Dev. 1987 Oct;1(8):783–793. doi: 10.1101/gad.1.8.783. [DOI] [PubMed] [Google Scholar]
- Raghuraman M. K., Cech T. R. Assembly and self-association of oxytricha telomeric nucleoprotein complexes. Cell. 1989 Nov 17;59(4):719–728. doi: 10.1016/0092-8674(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Raghuraman M. K., Dunn C. J., Hicke B. J., Cech T. R. Oxytricha telomeric nucleoprotein complexes reconstituted with synthetic DNA. Nucleic Acids Res. 1989 Jun 12;17(11):4235–4253. doi: 10.1093/nar/17.11.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Access to a messenger RNA sequence or its protein product is not limited by tissue or species specificity. Science. 1989 Apr 21;244(4902):331–334. doi: 10.1126/science.2565599. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Greslin A. F., Prescott D. M. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. doi: 10.1007/BF00329545. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Richman R., Cook R. G., Gorovsky M. A. An intervening sequence in an unusual histone H1 gene of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8674–8678. doi: 10.1073/pnas.83.22.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]