Abstract
Alterations in hippocampal neuronal Ca2+ and Ca2+-dependent systems have been implicated in mediating some of the long-term neuroplasticity changes associated with acquired epilepsy (AE). However, there are no studies in an animal model of AE that directly evaluate alterations in intracellular calcium concentration ([Ca2+]i) and Ca2+ homeostatic mechanisms (Ca2+ dynamics) during the development of AE. In this study, Ca2+ dynamics were evaluated in acutely isolated rat CA1 hippocampal, frontal, and occipital neurons in the pilocarpine model by using [Ca2+]i imaging fluorescence microscopy during the injury (acute), epileptogenesis (latency), and chronic-epilepsy phases of the development of AE. Immediately after status epilepticus (SE), hippocampal neurons, but not frontal and occipital neurons, had significantly elevated [Ca2+]i compared with saline-injected control animals. Hippocampal neuronal [Ca2+]i remained markedly elevated during epileptogenesis and was still elevated indefinitely in the chronic-epilepsy phase but was not elevated in SE animals that did not develop AE. Inhibiting the increase in [Ca2+]i during SE with the NMDA channel inhibitor MK801 was associated in all three phases of AE with inhibition of the changes in Ca2+ dynamics and the development of AE. Ca2+ homeostatic mechanisms in hippocampal neurons also were altered in the brain-injury, epileptogenesis, and chronic-epilepsy phases of AE. These results provide evidence that [Ca2+]i and Ca2+-homeostatic mechanisms are significantly altered during the development of AE and suggest that altered Ca2+ dynamics may play a role in the induction and maintenance of AE and underlie some of the neuroplasticity changes associated with the epileptic phenotype.
Keywords: neuronal plasticity, pilocarpine model, calcium homeostasis, seizure
Epilepsy is one of the most common neurological disorders (1), and ≈40% of epilepsies are acquired, meaning that the epileptic condition is acquired through an injury to the nervous system (2, 3). Epileptogenesis is the process by which an injury such as status epilepticus (SE), stroke, or traumatic brain injury produces long-term plasticity changes in neurons, resulting in spontaneous recurrent seizures [acquired epilepsy (AE)] in previously normal brain tissue (4–6). AE develops in three phases: injury (brain insult), epileptogenesis (latency), and, finally, chronic epilepsy (spontaneous recurrent seizure) (7). The molecular basis for developing AE is still not completely understood. However, there is growing evidence from the SE and glutamate injury-induced models of AE that elevated intracellular calcium concentration ([Ca2+]i) and altered Ca2+-homeostatic mechanisms (Ca2+ dynamics) may play a role in the development of AE (6, 8–13). In addition, altered Ca2+ dynamics have been observed in the hippocampus of chronic epileptic animals as long as 1 year after the induction of seizures in the in vivo pilocarpine model of AE (14). This model of AE shares many of the clinical and pathophysiological characteristics of partial-complex or temporal-lobe epilepsy in humans (14–19). The hippocampus has been shown to be the focus for many of the plasticity, pathophysiological, and epileptogenic alterations in the pilocarpine model of AE (14–19). Thus, if Ca2+ is involved as a second messenger in the inductions and maintenance of AE in the pilocarpine model, it would be expected that Ca2+ dynamics should be altered immediately after SE and in the three phases of the development of AE.
This study was undertaken to determine whether hippocampal neuronal Ca2+ dynamics are altered immediately after SE and in the three phases of the development of AE.
Ca2+ dynamics were evaluated in acutely isolated CA1 hippocampal, frontal, and occipital neurons at several time points during the injury, epileptogenesis, and chronic-epilepsy phases of AE. The effects of NMDA receptor inhibition by (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK801) on both the development of seizures and Ca2+ dynamics were determined. Comparisons of sham (saline-treated), pilocarpine without SE, and pilocarpine with SE but without AE control animals with SE animals with AE indicated that Ca2+ dynamics were significantly altered during the development of AE and that both changes in Ca2+ dynamics and the development of AE could be blocked by inhibition of the NMDA receptor during SE. The results demonstrate that altered Ca2+ dynamics were associated with the development of AE and that inhibition of these changes in Ca2+ dynamics was associated with the inhibition of the development of AE. The results provide direct evidence that Ca2+ dynamics are significantly altered during epileptogenesis and implicate Ca2+ as a major second messenger that may mediate some of the long-term plasticity changes in AE.
Materials and Methods
Pilocarpine Treatment and Induction of Epilepsy. SE and epilepsy were induced in male Sprague–Dawley rats (Harlan–Sprague–Dawley) of 120 days of age by i.p. injection of pilocarpine (14–19). In summary, animals were injected s.c. with 1 mg/kg scopolamine methyl nitrate 30 min before being injected with pilocarpine (350 mg/kg i.p.). The pilocarpine-induced SE was terminated after 1 h by injecting diazepam (4 mg/kg i.p.) at 1, 3, and 5 h after the onset of SE. Sham control animals were injected with equal volumes of normal saline and then diazepam. Pilocarpine-injected animals that did not develop SE or that developed SE but did not develop AE were also used as controls (Fig. 2B). MK801-treated animals were injected with 4 mg/kg MK801 20 min before injection with pilocarpine or saline and diazepam (sham conditions) (19). Animals were killed immediately or 1, 2, 6, 10, or 30 days or 1 year after SE. To determine the time after SE of the development of the first seizure, animals were analyzed by continuous video and electroencephalogram (EEG) monitoring for the appearance of spontaneous seizures, as described in ref. 20. Video and EEG monitoring was performed by using surface electrodes that were implanted bilaterally 3 mm anterior and posterior to the bregma under ketamine/xylazine anesthesia and secured to the scull with dental acrylic (19). It was established that video monitoring alone was able to detect animals that developed AE by comparing EEG and video recordings and monitoring animals for 5 days. No animals in this monitoring protocol manifested electrographic seizures without clinical seizures detected by video monitoring. In some experiments, animals also had a hippocampal depth electrode implanted and were subjected to continuous EEG and video monitoring. These studies documented that the video and surface EEG seizure activity observed during monitoring were associated with seizure discharges from the hippocampus, as described in ref. 21. Limbic seizures produced during pilocarpine SE were identical to those described in ref. 16. Spontaneous recurrent seizures (SRS) that developed after SE in ≈70% of the animals have been well characterized (14–17) and consisted of SRS activity similar to the kindled class 4–5 seizures, as described by Racine (22). To determine the onset of seizure activity (Fig. 2D), some animals were monitored as early as 5 days after SE. Animals at the 6- and 10-day time points that developed SRS manifested only electrographic seizures. Electrographic seizures were defined as epileptiform activity lasting >20 s. Clinical seizures were not observed until after 10 days. The time after SE of the first seizure was identified, and animals were monitored for an additional 5 days to evaluate seizure frequency. All chemicals were purchased from Sigma unless stated otherwise.
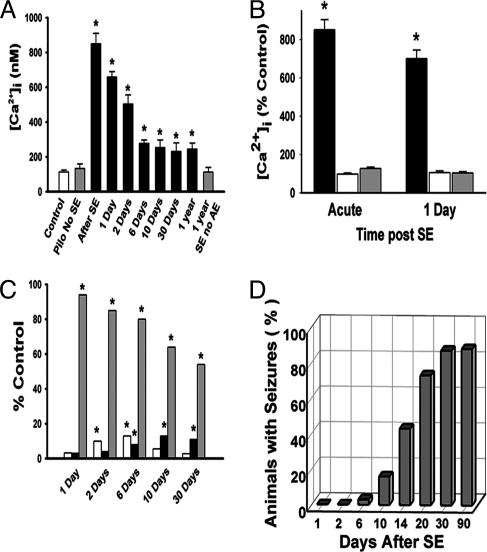
Fig. 2.
Effect of epileptogenesis on hippocampal and frontal and occipital cortex [Ca2+]i, apoptosis and cell death, and development of AE. (A) The data give the mean ± SEM for CA1 hippocampal [Ca2+]i immediately after and 1, 2, 6, 10, and 30 days after SE (black bars). The controls were a saline-injected animal (white bar) and a pilocarpine-injected with no SE animal (gray bar) and were obtained 1 day after SE. Animals that had SE but did not develop AE were studied 1 year after SE (1 year SE with no AE, gray bar). [Ca2+]i in sham control animals for each time point also was studied, and [Ca2+]i values for these conditions were not significantly different from the control and pilocarpine with no SE values shown and thus were omitted for clarity (*, P < 0.01, compared with control). Pilo, pilocarpine. (B) The data show the mean ± SEM of [Ca2+]i expressed as a percentage of control for hippocampal CA1 (black bars), frontal (white bars), and occipital (gray bars) pyramidal neurons immediately and 1 day after SE (*, P < 0.01, compared with controls). Controls were saline-injected animals sampled at the same time and from the three different brain regions (*, P < 0.01, compared with controls). (C) The data present the percentage of neurons positive for apoptosis (white bars), neuronal cell death (black bars), and neurons with elevated [Ca2+]i (gray bars) at different times after SE. The largest SEM was 9%, and thus these SEMs were omitted for clarity. (*, P < 0.01, compared with sham controls.) (D) Percentage of animals that developed seizures (AE) at different times after SE (n = 30).
Isolation of Hippocampal CA1 Pyramidal Neurons and Loading with Ca2+ Indicators. Acute isolation of CA1 hippocampal, frontal, and occipital neurons was performed by established procedures (14, 23). Briefly, brains were rapidly dissected and placed in 4°C chilled, oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (CSF), and hippocampal, frontal, and occipital slices of 450 μm were cut on a 12° agar ramp with a Vibratome. Slices were equilibrated for 10 min in CSF and then treated for 6 min with 8 mg/ml protease XXIII in CSF and rinsed. The hippocampal CA1 and cellular layers 3–5 in the frontal and occipital cortical slices were visualized on a dissecting microscope, and 1-mm2 thick pieces from the cellular regions were excised. These tissue samples were triturated with a series of Pasteur pipettes of decreasing diameter (14). To control for the enzymatic treatment used to disrupt the cells, we also isolated neurons by the identical procedure of trituration without the use of proteolytic enzymes and obtained identical results, except that the yield of neurons was lower. Although acutely isolated neurons were prepared from tissue punches from the pyramidal neuron-enriched stratum pyramidale of the CA1 and layers 3–5 of the frontal and occipital cortex, it was necessary to evaluate possible minimal contamination from nonprincipal neurons or interneurons. Pyramidal neurons were identified morphologically and by the absence of immunoreactivity for specific protein markers for interneurons, including parvalbumin, cholecystokinin, vasoactive intestinal peptide, somatostatin, and neuropeptide Y (24, 25).
Intracellular [Ca2+]i Measurements. Cells were loaded with fura-2 acetoxymethyl ester by using established procedures (14). Briefly, cells were equilibrated at 4°C in medium containing the acetoxymethyl ester (AM) forms of 1 μM fura-2 in the trituration solution. The resulting cell suspension was placed in the center of poly(l-lysine)-coated viewing microscope chambers and allowed to equilibrate with the cells for 1 h in a humidified, oxygenated dark chamber at 37°C. After equilibration, fura-2 was carefully washed off the cells, and the cells were incubated for another 15 min to further allow intracellular esterases to cleave the dyes from the AM forms. The dish was then transferred to the fluorescence imaging system [Olympus (Melville, NY)/PerkinElmer]. The cells attached to the dish substrate were constantly perfused with a gravity-feed perfusion system at a rate of 2–3 ml/min. For physiological studies, glutamate (10 μM) was applied by using a rapid perfusion system in the presence of glycine (10 μM). All morphologically intact and viable pyramidal neurons that were phase-bright were randomly selected by systematically sampling the microscopic field. Large numbers of neurons were evaluated, and the investigator was blinded to experimental conditions. The indicator-loaded cells were transferred to a heated stage (maintained at 37°C) of an Olympus IX-70 inverted microscope coupled to an ultra-high-speed fluorescence imaging system (Olympus/PerkinElmer), as described in ref. 14. Experiments were performed by using a ×20 fluorite water-immersion objective, and images were recorded by a cooled digital charge-coupled device camera (Astrocam, Cambridge, U.K.). The fluorescence excitation source was a 75-W xenon arc lamp (Olympus). Neutral density filters of variable opacities were used to attenuate unwanted excitation. Ratio images were acquired by using alternating excitation wavelengths (340/380 nm) with a filter wheel (Sutter Instruments, Novato, CA) and fura filter cube at 510/540 nm emissions with a dichroic mirror at 400 nm (Olympus). Image acquisition and processing were controlled by a computer connected to the camera and filter wheel by using merlin 2.1 (Olympus/PerkinElmer). To calculate ratio values, image pairs at each wavelength were captured and digitized every 15 s, and the images at each wavelength were averaged over four frames. The background fluorescence was obtained by imaging a field lacking fluorescent indicators. Fluorescence ratio values were converted to absolute Ca2+ concentrations by using calibration curves generated by an in situ procedure after the loading of the isolated neurons with fura-2 and use of standard procedures described in ref. 14. [Ca2+]i data were collected by using merlin 2.1 and were statistically analyzed and plotted by using origin 6.0 (OriginLab, Northampton, MA) or sigmaplot 5 (SPSS, Chicago). The significance of data were tested by Student's paired or unpaired t test wherever appropriate. The distributions of [Ca2+]i for the four phases of AE shown in Fig. 1 were evaluated by logisticregression analysis.
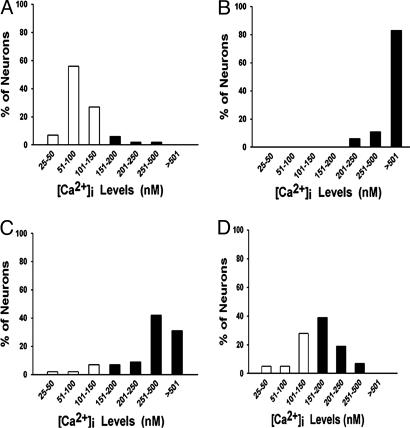
Fig. 1.
Distribution of CA1 neuronal [Ca2+]i. Shown are data for control animals (n = 21) (A) and for animals in the acute (day 1) (B), latency (day 6) (C), and chronic (day 30) (D) phases of AE after SE. The data represent the distribution in neuronal calcium levels as a percentage of neurons for each concentration range of [Ca2+]i. Normal [Ca2+]i (25–150 nM) is represented by white bars, and elevated levels are represented by black bars (151 to >500 nM). [Ca2+]i is represented in concentration ranges (25–50, 51–100, 101–150, 151–200, 201–250, 251–500, and >500 nM) for ease of comparison. Sham control animals sampled at the same time points for the injury, epileptogenesis, and chronic phases gave results identical to those for the control animals shown in A and were omitted for clarity. Statistical analysis using a linear regression comparing the number of neurons with abnormal [Ca2+]i (black bars) for the injury, latency, and chronic phases of AE yielded results that were significantly different statistically from those for the control group (P < 0.01).
Assay for Apoptosis and Cell Death. Before and after glutamate treatments (10 μM), the neurons were analyzed by immunofluorescence microscopy with the spatial module of ultraview 2.21 (Olympus/PerkinElmer) for the presence of apoptosis by using the Vybrant Apoptosis Assay Kit No. 3 (Molecular Probes), which detects the externalization of phosphatidylserine in apoptotic cells as described in ref. 14. The total number of apoptotic cells was routinely determined in each preparation. Dead (necrotic), apoptotic, and viable neurons were determined in the acutely isolated preparations by using specific markers (14). Propidium iodide is impermeant to viable cells and apoptotic cells but stains necrotic cells with red fluorescence. Fluorescent labeled annexin V (green fluorescence) detects apoptotic cells. Thus, acutely isolated neurons were stained with these agents, and the percentages of dead (red), apoptotic (green), and viable (phase-bright) neurons were determined for each sample. Cell loss in the CA1 region of the hippocampus was determined by direct histological evaluation in fixed tissue from animals perfused and killed before and at different times after SE by using procedures established in ref. 17.
Results
[Ca2+]i in Acutely Isolated Hippocampal and Cortical Neurons from Control Animals and Animals in the Acute Injury Phase of Epileptogenesis. The distribution of [Ca2+]i in control animals before SE is shown in Fig. 1 A (n = 21). Ninety-two percent of the neurons had [Ca2+]i in the normal range (25–150 nM), and only 8% had elevated levels (>151 nM). None of the control neurons had [Ca2+]i > 500 nM. Twenty-four hours after SE in the acute phase, 100% of the isolated neurons had abnormally elevated [Ca2+]i, exceeding 500 nM in 85% of the neurons (Fig. 1B; n = 67). The mean ± SEM neuronal [Ca2+]i values for animals killed immediately after SE, sham control animals, and pilocarpine without the development of SE control animals were 850 ± 59, 90 ± 22, and 112 ± 31 nM, respectively (Fig. 2A). Because seizure activity and neuronal plasticity changes are enriched in the hippocampus in this model of AE (7, 15, 16, 31), [Ca2+]i in other brain regions was evaluated after SE and compared with levels in hippocampal neurons. Frontal and occipital neurons did not manifest significant elevation of [Ca2+]i immediately or 1 day after SE in comparison with no-SE controls, whereas hippocampal neurons showed a marked increase in [Ca2+]i (Fig. 2B), demonstrating that the effect of SE on hippocampal neuronal [Ca2+]i was not occurring diffusely throughout the brain (Fig. 2B). Other hippocampal CA1 nonpyramidal neurons (bipolar neurons and interneurons) also manifested elevations in [Ca2+]i after SE (data not shown), indicating that SE had a more widespread effect on [Ca2+]i in hippocampal neurons.
[Ca2+]i in Hippocampal Neurons Acutely Isolated from Animals in the Epileptogenesis and Chronic-Epilepsy Phases of AE. The distribution of [Ca2+]i in CA1 neurons during epileptogenesis (latency phase) demonstrated that [Ca2+]i still remained elevated during epileptogenesis (Fig. 1C). The percentage of neurons manifesting elevated levels at 6 days after SE was 81% (Fig. 1C; n = 38). At 10 days after SE, 62% of the neurons still manifested elevated [Ca2+]i. The mean ± SEM [Ca2+]i for the 2- (n = 38), 6- (n = 48), and 10-day (n = 21) time points in the latency phase after SE are shown in Fig. 2B and still remained elevated. The distribution of [Ca2+]i in CA1 neurons in the chronic-epilepsy phase at 30 days after SE is shown in Fig. 1D (n = 23). The percentage of neurons manifesting elevated [Ca2+]i during the chronic phase of epileptogenesis (30 days) was 57%. There were no neurons with [Ca2+]i > 501 nM in the chronic phase of epileptogenesis. The mean ± SEM [Ca2+]i values for the chronic phase of epileptogenesis at 30 days (n = 23) and 1 year (n = 35) after SE were 305 ± 27 and 325 ± 35 nM, respectively, and these values were significantly elevated compared with controls (Fig. 2 A). In contrast, the SE with no AE animals at 1 year after SE did not have significantly elevated [Ca2+]i (112 ± 27 nM) in comparison with the animals with AE (Fig. 2 A; n = 28). This result strongly implicates changes in [Ca2+]i in the development of AE by controlling for the effect of SE alone without AE.
Elevated [Ca2+]i During the Injury, Epileptogenesis, and Chronic Phases of AE Were Not the Result of Apoptosis or Cell Death. The percentage of acutely isolated CA1 neurons positive for apoptosis and cell death were evaluated at the 1-, 2-, 6-, 10-, and 30-day time points after SE and compared with the percentage of CA1 neurons with abnormally elevated [Ca2+]i (Fig. 2C). These data demonstrate that only a small fraction (<10%) of the CA1 neurons that manifested abnormally elevated [Ca2+]i developed apoptosis and necrosis. Apoptosis was maximal at 6 days post-SE, returned to control levels by 10 days, and was the same at 30 days, indicating that after the acute phase of injury (1–6 days), CA1 hippocampal neurons were not undergoing further necrosis or apoptosis in comparison with control neurons. Thus, the small amount of cell death after SE under these conditions could not account for the elevation of [Ca2+]i in the large percentage of CA1 neurons observed and did not continue past 6 days after the acute-injury phase. Thus, the vast majority of neurons (>90%) survived the SE injury and still manifested markedly increased [Ca2+]i long after SE. This issue is important, because dead neurons do not seize. The increase in [Ca2+]i was not sufficient to kill these CA1 neurons but may have triggered and sustained Ca2+ second-messenger-mediated long-term plasticity changes associated with the induction and maintenance of epilepsy.
Seizure Initiation Occurs as [Ca2+]i Decreases at the End of Epileptogenesis. The development of seizures at 1, 2, 6, 10, 14, 20, 30, and 90 days after SE was evaluated (see Materials and Methods). Fig. 2D demonstrates that none of the animals had seizures until the sixth day after SE. Electrographic seizures were observed in 3% of the animals at 6 days and 16% of the animals at 10 days; clinical seizures really began to develop at 14 days (43%) and reached full development by 30 days (87%). Not all animals that had SE developed AE, and in this experiment 87% of the animals developed AE. Comparing the decrease in mean [Ca2+]i for CA1 neurons shown in Fig. 2 A with the development of seizures shown in Fig. 2D, it is apparent that the seizures began to develop after the very high [Ca2+]i observed during the latency or epileptogenesis phase of AE, demonstrating that the massive but not lethal rise in [Ca2+]i immediately and for several days after SE is ideally temporally placed for a second messenger to be able to initiate the plasticity changes associated with epileptogenesis.
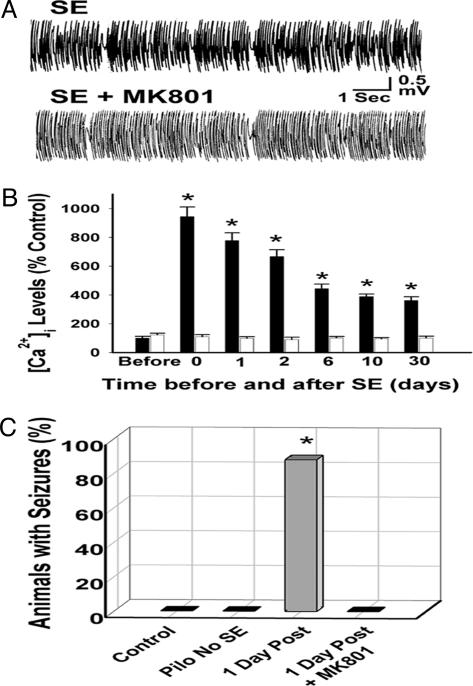
Inhibition of Elevated [Ca2+]i and the Development of Epileptogenesis by MK801. MK801 did not decrease the intensity or duration of SE (Fig. 3A). The effects of MK801 administered during SE on the development of both increased [Ca2+]i in CA1 hippocampal neurons (Fig. 3B) and seizures (Fig. 3C) were evaluated in the pilocarpine model. The increases in CA1 hippocampal neuronal [Ca2+]i during all three phases of the development of AE produced by SE were completely blocked by treatment with MK801 (Fig. 3B). MK801 also completely prevented the development of seizures in the pilocarpine model (Fig. 3C). Thus, treating with MK801 during SE without decreasing the severity of SE completely blocked the increases in CA1 hippocampal neuronal [Ca2+]i after SE and also inhibited the development of spontaneous recurrent seizures.
Fig. 3.
Effect of MK801 on severity of SE, CA1 hippocampal neuronal [Ca2+]i, and development of seizures. (A) Representative EEG tracings from SE and SE plus MK801 animals recorded during SE. MK801 did not decrease the severity of SE. (B) The mean ± SEM [Ca2+]i expressed as a percentage of control values for animals treated with (white bars) or without (black bars) MK801 during SE and evaluated before, immediately after, and 1, 2, 6, 10, and 30 days after SE. MK801 blocked the SE-induced increase in [Ca2+]i. MK801 [Ca2+]i values were not statistically different from those of sham control animals at the time points tested. [Ca2+]i values for MK801-only-treated sham control animals were not different from those of sham control animals (not shown) (*, P < 0.01, compared with control). (C) The percentage of animals that developed seizures at 60 days post-SE for the control (sham control, n = 20), pilocarpine with no SE (n = 24), SE (n = 30), and SE plus MK801 (n = 15) animal-treatment groups. No seizures were detected in MK801-only-treated animals (not shown) or in the sham or pilocarpine with no SE control groups. (*, P < 0.01, compared with sham control). Pilo, pilocarpine.
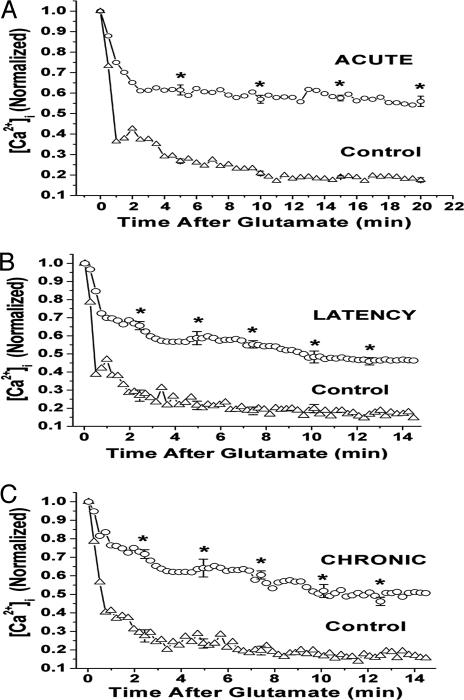
Epileptogenesis Causes Alterations in [Ca2+]i Homeostatic Mechanisms. Exposing CA1 neurons to 10 μM glutamate for 1 min produced a significant rise in [Ca2+]i to peak values that were no different in control and SE cells and averaged 1,350 nM. The quantitated values for the glutamate (10 μM) recovery curves for control and SE CA1 neurons from the injury, epileptogenesis, and chronic phases of AE are shown in Fig. 4. The decay curves were generated by calculating the mean [Ca2+]i at 15-s intervals and then normalized to a maximum value of 1.0 with sigmaplot. Control neurons returned to preglutamate baseline [Ca2+]i in <6 min, whereas the SE group in the injury, epileptogenesis, and chronic phases all exhibited a statistically significant delay in returning to baseline values. MK801 treatment during SE prevented the alterations in Ca2+ homeostatic mechanisms (data not shown). The fact that the Ca2+ homeostatic mechanisms were altered in all three phases suggests that these alterations may in part account for the elevation of [Ca2+]i observed after SE and that they may play a role in the maintenance of AE.
Fig. 4.
[Ca2+]i decay curves for hippocampal neurons from the acute (A), latency (B), and chronic (C) phases of AE. Individual decay curves were generated by calculating mean [Ca2+]i at 15-s intervals and then normalizing to a maximum value of 1.0 by using sigmaplot (14). The resultant decay curves from the highest point after the glutamate-induced [Ca2+]i load are displayed. Cells treated with glutamate recovered and did not undergo apoptosis. Standard errors and statistical significance between control (triangles) and epileptogenic (ovals) cells were determined as shown. The epileptic cells showed a statistically significant delayed decline in [Ca2+]i compared with the controls (*, P < 0.05, Student's t test).
Discussion
Ca2+ plays a pivotal role in transmitting information from the external environment to the interior of the cell by acting as a second messenger and affecting cellular function, and prolonged alterations in [Ca2+]i can produce long-term plasticity changes (26–28). The studies presented in this paper provide direct evidence that altered Ca2+ dynamics occur in the pilocarpine model of AE in the surviving CA1 neurons during the three phases in the development of AE. There was a large rise in [Ca2+]i in hippocampal neurons for several days after SE that was maintained above control indefinitely in the chronic-epileptic phase of AE. Because changes in [Ca2+]i can produce long-term plasticity changes (26, 27), the data indicate that this initial prolonged rise of [Ca2+]i in hippocampal neurons after SE may be a second-messenger signal that initiates many of the long-term plasticity changes associated with epileptogenesis (6) and may represent a major molecular signal for the induction of AE. SE caused altered Ca2+ homeostatic mechanisms, which remained significantly altered without change during epileptogenesis and through the chronic-epilepsy phase and played a role in maintaining the elevated [Ca2+]i in the chronic-epileptic phenotype. This long-term alteration in Ca2+ homeostatic mechanisms, like other plasticity changes, may be initiated by the very high and prolonged rise in [Ca2+]i immediately after SE in neurons that survive the injury of SE and contribute to the maintenance of AE. Although further experimentation is needed to understand the precise mechanisms of the effect of [Ca2+]i in the development of AE, the results indicate that Ca2+ is an ideal second-messenger system that could play a role in both the induction and maintenance of the epileptic condition.
MK801 administered during SE blocked the development of AE and prevented the increase in [Ca2+]i and altered Ca2+ homeostatic mechanisms in the acute, epileptogenesis, and chronic phases of the development of AE. These results demonstrate an association between elevated [Ca2+]i after SE and the development of epilepsy. In addition, SE without the development of AE did not result in long-term changes in [Ca2+]i, further indicating a relationship between the development of AE and the alteration in Ca2+ dynamics. These results in an intact animal model of AE support in vitro models of AE that directly demonstrated the role of elevated [Ca2+]i in producing spontaneous recurrent seizures after SE and glutamate excitotoxic injuries in hippocampal neurons (8–13). The in vitro models of AE allow for a rigorous control of environmental conditions to directly demonstrate the role of altered Ca2+ dynamics in causing AE. Although in vitro models do not truly reflect the clinical condition of AE, the combination of the results presented in this paper and the in vitro models of AE provide strong evidence that alterations in Ca2+ dynamics may play an essential role as a second messenger underlying the induction and maintenance of AE.
It is important to evaluate the possible functional consequences of altered Ca2+ dynamics in epileptogenesis. Changes in [Ca2+]i are involved in the regulation of gene expression (26–28). In the pilocarpine model of AE, the expression of the Ca2+-modulated transcription factor, serum response factor (SRF), is permanently elevated, along with its ability to bind to its DNA consensus sequence serum response element (29). Furthermore, ΔFosB expression and AP-1 (activator protein-1) complex DNA binding also were found to be permanently elevated in this model of AE (30). NMDA-receptor activation and subsequent calcium entry stimulates the expression and activity of SRF and ΔFosB (29, 30). Thus, the long-term elevation of [Ca2+]i during epileptogenesis and in the chronic-epileptic state may directly or indirectly affect the expression of these, and perhaps other, specific Ca2+-regulated transcription factors. In addition, changes in [Ca2+]i during epileptogenesis and chronic phases of AE produce changes in the second-messenger effects of [Ca2+]i that may regulate other long-term plasticity changes associated with epileptogenesis, including neurogenesis, mossy fiber sprouting, changes in the expression of proteins such as brain-derived neurotrophic factor, increased endocytosis, and many of the other changes associated with AE (15–18, 31, 32). Further studies are needed to evaluate the role of [Ca2+]i in modulating these changes in neuronal plasticity in AE.
The ability of the neuron to restore Ca2+ loads to resting [Ca2+]i is regulated by Ca2+ homeostatic mechanisms (33). Increased or prolonged entry of extracellular Ca2+ could contribute to the altered Ca2+ homeostatic mechanisms in epilepsy (33). [Ca2+]i also is regulated by calcium-induced calcium release (CICR) from intracellular stores and by uptake of Ca2+ by the sarco/endoplasmic reticulum calcium ATPase (SERCA) (33) and other systems, including calcium buffering systems such as calbindin, calretinin, and parvalbumin (34). The membrane Na+/Ca2+ exchanger (35) and mitochondrial Ca2+ uptake (36) may also play roles in altering the homeostatic mechanisms. In vitro studies in the SE-induced hippocampal neuronal culture model of AE (11) demonstrated that epileptogenesis induced inhibition of SERCA, resulting in a decreased ability to uptake [Ca2+]i into microsomes, and stimulation of the inositol 1,4,5-trisphosphate receptor stimulated CICR from microsomes (11). Both of these changes in Ca2+ dynamics induced by epileptogenesis could account in part for the altered Ca2+ dynamics observed in the epileptic phenotype. In the pilocarpine model of AE, decreased [Ca2+]i uptake and SERCA activity have been demonstrated to persist for up to 1 year in AE (37, 38). These results provide a molecular basis for the altered Ca2+ dynamics associated with epileptogenesis and AE. Although further studies are needed, it is apparent that understanding the molecular basis of altered Ca2+ regulatory mechanisms induced by epileptogenesis may provide an insight into the long-term plasticity changes associated with epilepsy and offer specific molecular targets for preventing and possibly reversing the plasticity changes associated with AE.
CA1 neurons have been shown to contribute to the development of AE: excitatory circuits develop around CA1 neurons (39), and CA1 neurons develop rhythmic synchronization (40) and manifest increased excitability (41). The observation that SE did not alter Ca2+ dynamics in frontal and occipital neurons under conditions that produced persistent alterations in hippocampal neurons indicates that this change in Ca2+ dynamics does not occur in all regions of the brain and is consistent with the central role of the hippocampus in epileptic processes. Our results also indicated that alterations in Ca2+ dynamics were occurring in other types of hippocampal neurons and interneurons. Other areas of the hippocampus and other brain regions also may be affected by the alterations in Ca2+ dynamics. Further studies on other neurons in the hippocampus and other brain regions are indicated to evaluate changes in Ca2+ dynamics during epileptogenesis. Although some of the long-term plasticity changes in AE may be permanent, the altered Ca2+ dynamics associated with AE provide a window of opportunity to intervene with novel therapeutic strategies to reverse these changes in Ca2+ dynamics and possibly prevent the development of AE and even “cure” AE by restoring normal Ca2+ dynamics.
Acknowledgments
We thank Drs. D. Sun and T. Allen Morris for their critical suggestions during the research effort. This work was supported by National Institute of Neurological Disorders and Stroke Grants RO1 NS23350 and P50 NS25630 (to R.J.D.).
Author contributions: M.R., R.E.B., S.S., D.S.C., L.S.D., and R.J.D. performed research; M.R., R.E.B., S.S., D.S.C., L.S.D., and R.J.D. analyzed data; and M.R., R.E.B., D.S.C., L.S.D., and R.J.D. wrote the paper.
Abbreviations: AE, acquired epilepsy; [Ca2+]i, intracellular calcium concentration; EEG, electroencephalogram; MK801, (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate; SE, status epilepticus.
References
- 1.Sander, J. W. & Shorvon, S. D. (1996) J. Neurol. Neurosurg. Psychiatry 61, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeGraff, A. S. (1974) Epilepsia 15, 291–299.4527786 [Google Scholar]
- 3.Hauser, W. A. & Hesdorffer, D. C. (1990) Epilepsy: Frequency, Causes and Consequences (Demos Medical Publishing, New York).
- 4.Lothman, E. W., Bertram, E. H. & Stringer, J. L. (1991) Prog. Neurobiol. 37, 1–82. [DOI] [PubMed] [Google Scholar]
- 5.McNamara, J. O. (1994) J. Neurosci. 14, 3413–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLorenzo, R. J., Sun, D. A. & Deshpande, L. S. (2004) Pharmacol. Ther., in press. [DOI] [PMC free article] [PubMed]
- 7.Lukasiuk, K., Kontula, L. & Pitkannen, A. (2003) Eur. J. Neurosci. 17, 271–279. [DOI] [PubMed] [Google Scholar]
- 8.DeLorenzo, R. J., Pal, S. & Sombati, S. (1998) Proc. Natl. Acad. Sci. USA 95, 14482–14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sombati, S. & DeLorenzo, R. J. (1995) J. Neurophysiol. 73, 1706–1711. [DOI] [PubMed] [Google Scholar]
- 10.Pal, S., Sombati, S., Limbrick, D. D. J. & DeLorenzo, R. J. (1999) Brain Res. 851, 20–31. [DOI] [PubMed] [Google Scholar]
- 11.Pal, S., Sun, D., Limbrick, D., Rafiq, A. & DeLorenzo R. J. (2001) Cell Calcium 30, 285–296. [DOI] [PubMed] [Google Scholar]
- 12.Sun, D. A., Sombati, S., Blair, R. E. & DeLorenzo, R. J. (2002) Epilepsia 43, 1296–1305. [DOI] [PubMed] [Google Scholar]
- 13.Sun, D. A., Sombati, S. & DeLorenzo, R. J. (2004) Cell Calcium 35, 155–163. [DOI] [PubMed] [Google Scholar]
- 14.Raza, M., Pal, S., Rafiq, A. & DeLorenzo, R. J. (2001) Brain Res. 903, 1–12. [DOI] [PubMed] [Google Scholar]
- 15.Mello, L. E., Cavalheiro, E. A., Tan, A. M., Kupfer, W. R., Pretorius, J. K., Babb, T. L. & Finch, D. M. (1993) Epilepsia 34, 985–995. [DOI] [PubMed] [Google Scholar]
- 16.Turski, L., Dziki, M., Parada, J., Kleinrok, Z. & Cavalheiro, E. A. (1992) Brain Res. Dev. Brain Res. 19, 137–134. [DOI] [PubMed] [Google Scholar]
- 17.Rice, A. C. & DeLorenzo, R. J. (1998) Brain Res. 782, 240–247. [DOI] [PubMed] [Google Scholar]
- 18.Rice, A., Rafiq, A., Shapiro, S. M., Jakoi, E. R., Coulter, D. A. & DeLorenzo, R. J. (1996) Proc. Natl. Acad. Sci. USA 93, 9665–9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice, A. & DeLorenzo, R. J. (1999) Neuroscience 93, 117–123. [DOI] [PubMed] [Google Scholar]
- 20.Wallace, M. J., Blair, R. E., Falenski, D. W., Martin, B. R. & DeLorenzo, R. J. (2003) J. Pharmacol. Exp. Ther. 307, 129–137. [DOI] [PubMed] [Google Scholar]
- 21.Perlin, J. B., Churn, S. B., Lothman, E. W. & DeLorenzo, R. J. (1992) Epilepsy Res. 11, 111–118. [DOI] [PubMed] [Google Scholar]
- 22.Racine, R. J. (1972) Electroencephalogr. Clin. Neurophysiol. 32, 281–294. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs, J. W., Shumate, M. D. & Coulter, D. A. (1997) J. Neurophysiol. 77, 1924–1938. [DOI] [PubMed] [Google Scholar]
- 24.Matyas, F., Freund, T. F. & Gulyas, A. I. (2004) Hippocampus 14, 460–481. [DOI] [PubMed] [Google Scholar]
- 25.Scharfman, H. E., Goodman, J. H. & Sollas, A. L. (2000) J. Neurosci. 20, 6144–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan, J. I. & Curran, T. (1989) Trends Neurosci. 12, 459–462. [DOI] [PubMed] [Google Scholar]
- 27.Bading, H., Ginty, D. D. & Greenberg, M. E. (1993) Science 260, 181–186. [DOI] [PubMed] [Google Scholar]
- 28.DeLorenzo, R. J. & Morris, T. A. (1999) Neuroscientist 5, 86–99. [Google Scholar]
- 29.Morris, T. A., Jafari, N., Rice, A. C., Vasconcelos, O. & DeLorenzo, R. J. (1999) J. Neurosci. 19, 8234–8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris, T. A., Jafari, N. & DeLorenzo, R. J. (2000) Mol. Brain Res. 79, 138–149. [DOI] [PubMed] [Google Scholar]
- 31.Scharffman, H. E. (2002) Neuroscientist 8, 154–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slovitor, R. S. (1992) Neurosci. Lett. 137, 91–96. [DOI] [PubMed] [Google Scholar]
- 33.Carafoli, E. (1987) Annu. Rev. Biochem. 56, 395–433. [DOI] [PubMed] [Google Scholar]
- 34.Nagerl, U. V., Mody, I., Jeub, M., Lie, A. A., Elger, C. E. & Beck, H. (2000) J. Neurosci. 20, 1831–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan, S. G. (1999) J. Child Neurol. 14, 58–66. [DOI] [PubMed] [Google Scholar]
- 36.Kunz, W. S., Goussakov, I. V., Beck, H. & Elger, C. E. (1999) Brain Res. 826, 236–242. [DOI] [PubMed] [Google Scholar]
- 37.Parsons, J. T., Churn, S. B., Kochan, L. D. & DeLorenzo, R. J. (2000) J. Neurochem. 75, 1209–1218. [DOI] [PubMed] [Google Scholar]
- 38.Parsons, J. T., Churn, S. B. & DeLorenzo, R. J. (2001) J. Neurochem. 79, 319–327. [DOI] [PubMed] [Google Scholar]
- 39.Shao, L. R. & Dudek, F. E. (2004) J. Neurophysiol. 92, 1366–1373. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara-Tsukamoto, Y., Isomura, Y., Nambu, A. & Takada, M. (2003) Neurosci. 119, 265–275. [DOI] [PubMed] [Google Scholar]
- 41.Telfeian, A. E., Tseng, H. C., Baybis, M., Crino, P. B. & Dichter, M. A. (2003) Epilepsia 44, 143–149. [DOI] [PubMed] [Google Scholar]