Abstract
While major advances in our understanding of the molecular underpinnings of hormone receptor-positive (HR-positive) breast cancer have led to new therapies that have substantially improved in patient outcomes, endocrine–resistant disease still remains a leading cause of breast cancer mortality. Comprehensive molecular profiling of breast cancers has highlighted tremendous tumor heterogeneity, and analysis of paired primary and metastatic tumors has shown the evolution that can occur during acquired resistance to systemic therapies. Novel techniques for monitoring tumor load under treatment pressure, including “liquid biopsy” techniques, such as circulating free tumor DNA (cfDNA) and circulating tumor cells(CTCs), have shown promise as biomarkers to direct treatment without invasive tumor biopsies. However, more research is needed to deepen our understanding of breast cancer alterations under treatment pressure in order to reveal mechanisms of drug resistance and apply precision medicine in biomarker-driven clinical trials.
Background
Despite advances in systemic therapy that have yielded higher disease free survival (DFS) for patients with early stage HR-positive breast cancer (i.e. those expressing the estrogen and/or progesterone receptor), approximately 20% of these women will still develop recurrence in 10 years (1). Breast cancer patients with distant metastases have a 5-year survival rate of approximately 26% (2), and many patients with a short disease-free interval (DFI) do poorly (3). Traditionally, patients with HR-positive/HER2-negative tumors were considered to have the best prognosis, but more recent analyses accounting for modern-day HER2-targeted therapies have reported longer survival times for patients with HR-positive/HER2-positive disease than for their counterparts with HR-positive/HER2-negative disease (4, 5). De novo and acquired resistance to endocrine therapy remains a major therapeutic problem and an active area of investigation in ongoing preclinical and clinical research.
The most recent guidelines for endocrine treatment of metastatic breast cancer (MBC) from the American Society for Clinical Oncology (ASCO) highlight new and expanded treatment options for patients with HR-positive disease (6). Briefly, in most cases, endocrine therapy is preferred as initial therapy for patients with metastatic disease without high visceral burden or HER2-positivity, and sequencing of endocrine therapies for individual patients with HR-positive MBC should reflect prior treatments and treatment response (including adjuvant therapy), DFI, disease burden, potential toxicities, and patient preference. An aromatase inhibitor (AI) should be offered as part of first-line therapy for postmenopausal women, and combination therapies can be incorporated with some added toxicity (7). Patients without prior adjuvant endocrine therapy (de novo metastatic), can be treated with a nonsteroidal AI and fulvestrant, although existing trials have shown mixed outcomes with this approach (8, 9).
Most notably, the guidelines now incorporate two targeted agents, palbociclib and everolimus, in combinations with standard agents, based on progression-free survival (PFS) benefit seen in clinical trials. Palbociclib, an oral small-molecule inhibitor of cyclin-dependent kinases (CDKs) 4 and 6, can be administered with a nonsteroidal AI as one option for first-line therapy based on the PALOMA-1 trial showing improved PFS (but not OS) of 20.2 months with the combination compared to 10.2 months with single agent letrozole (HR 0.488; p=0.0004) (10); these results were confirmed in the recently reported and definitive PALOMA-2 trial(11). Side effects of palbociclib include fatigue, leukopenia, and grade 3–4 neutropenia in over 50% of patients, necessitating monitoring of patient blood counts. These factors, along with the increased cost of combination therapy over an aromatase inhibitor alone, should be considered when considering palbociclib for individual patients. Everolimus, a sirolimus derivative that inhibits mTOR, can be given with exemestane as another option for patients who experience progression after an AI with or without one line of prior chemotherapy, based on the BOLERO 2 trial study showing improved PFS (but not overall survival) of 6.9 months with the combination compared to 2.8 months with exemestane (HR 0.43; p<0.001)(12). Central assessment of median PFS improved reported results to 10.6 months and 4.1 months with the combination vs. exemestane, respectively. The combination of fulvestrant and palbociclib is a treatment option for patients without prior exposure to CDK 4/6 inhibitors after progression on AIs with or without one line of prior chemotherapy, based on the randomized PALOMA III trial of fulvestrant with either palbociclib or placebo showing PFS improvement of 9.5 vs. 4.6 months with the combination (HR 0.46; P<0.0001) (13).
Novel state of the art biomarker monitoring strategies have emerged in parallel to this expansion in therapeutic approaches. The feasibility of “liquid biopsies” as blood-based biomarker monitors of disease status has been demonstrated, but the routine clinical applicability of these tests is not yet established. Enumeration of CTCs is prognostic for recurrence in patients with early stage breast cancer, (14, 15); and cfDNA in plasma after definitive treatment with curative intent has been shown to track with increased risk of relapse (16). However, these circulating biomarkers are not yet endorsed by ASCO, and their utility in guiding decision making for systemic therapy remains investigational (17).
There is great interest interesting in tracking CTCs and/or cfDNA as potential biomarkers for therapeutic efficacy in patients with metastatic disease as well, given their established prognostic capability in this setting(18). However, it is a sobering reality that, after many years of molecular studies of drug response and resistance, the most recent ASCO guidelines for endocrine therapy for HR-positive MBC state: “there is no routine clinical role for genomic or expression profiling in the selection of treatment for HR-positive MBC, and tumor markers or circulating tumor cells should not be used as the sole criteria for determining disease progression.” (6) Given the myriad of choices of i) endocrine therapy agents, ii) sequences for treatments, and iii) novel targeted agents now available, there is a clear emerging need for identification of biomarkers of resistance and response. This biomarker development will be necessary to accomplish truly “precision medicine,”and a discussion of progress toward this goal is the focus of this review.
On the Horizon
1. Identification of Predictive Biomarkers
In general, mechanisms of resistance can be divided into de novo resistance, such as lack or very low levels of ER, and acquired resistance which includes i) a diverse set alterations that reactivate of ER signaling, such as mutations in the ER and ii) ER-independent mechanisms, such as activation of PI3K pathways. Attempts to overcome these resistance mechanisms will be contingent on detailed understanding of mechanisms of resistance, and identification of biomarkers predictive of success in individual patients.
A common approach to the identification of biomarkers is the analysis of the primary tumor tissue, either from retrospective cohorts or from a clinical trial, using biased or unbiased genome–wide expression analyses. For example, genomic sequencing has been used to identify putative druggable targets and predictive biomarkers. A gene expression analysis of 134 primary tumor samples from the phase III CONFIRM trial comparing 250 versus 500 mg of fulvestrant has recently shown that high EGF pathway and FOXA1 transcriptional signaling were associated with decreased fulvestrant-responsiveness (19). Intriguingly, the expression of the transcription factor TFAP2C, a known regulator of ER and growth factor receptor activity, was strongly associated with response (20, 21). Such approaches to biomarker identification will continue to be expanded through the use of more sensitive methods, such as deep next generation DNA and RNA sequencing (NGS).
2. Analysis of metastatic HR-positive disease
Studies of metastatic tissues have identified mutations that are critical in endocrine resistant MBC, with mutation in ESR1 being one of the most notable to date (22–26). Mutations in the ligand binding domain of ESR1, which are associated with structural changes and ligand-independent activities, occur rarely in primary disease, but can be found in up to one-third of endocrine resistant MBC, implying evolution through selective pressure on endocrine therapy.
Studies of paired primary and metastatic HR-positive breast tumors have been limited. Shah et al reported NGS data from an estrogen receptor-positive (ER+) lobular breast tumor (27). The study identified 32 mutations; of these five were prevalent in the DNA of the primary tumor, six were present at lower frequencies, and 19 were not detected in the primary tumor, thereby providing evidence for mutational evolution during disease progression. A recent effort utilizing deep sequencing of 106 candidate genes in 11 paired ER+ primary and metastatic tumors identified several genes more frequently mutated in relapsing versus non-relapsing cases, and/or that were associated with inferior survival based upon TCGA data; two novel genes, Lysine Methyltransferase 2C (KMT2C) and EPH Receptor A7 (EPHA7), were linked to adverse outcome (28). Varešlija et al recently analyzed paired primary and local or distant metastatic tissues, and showed altered expression of ER target genes, reflecting changes in ER activity with AI treatment (29).
Another important potential mechanism of resistance is receptor switching in the metastatic tumor as compared to the primary breast tumor. A recent analysis showed discordance rates for ER, PR, and HER2 expression between primary and metastatic tumors of 18%, 26%, and 7% of patients, respectively (30). Outcome was significantly worse in patients whose HR-positive tumors became HR-negative in the recurrent sites compared to patients whose tumors did not change. Our own recent analysis of paired primary tumors and their corresponding brain metastases showed loss of ER expression in almost half of the analyzed metastatic samples (Priedigkeit, et al, under review).
Finally, testing of intrinsic subtype in HR-positive patients has been studied extensively to guide treatment of early-stage breast cancer, but is not typically incorporated into therapeutic decision making for patients with metastatic disease. A recent retrospective analysis of women with MBC showed that intrinsic subtypes were strongly correlated to prognosis, with median PFS differing across intrinsic subtypes of clinically HER2-negative patients. Importantly, clinically HER2-negative patients benefited from lapatininb if the tumor was HER2-enriched by intrinsic subtyping (31). Our own data shows increase in HER2 expression in a significant subset of brain metastases compared to their primary tumors (Priedigkeit et al, under review), and thus reinforces the notion that the re-measurement of therapeutic targets in metastatic biopsies can have critical impact on treatment decisions and ultimately outcome. Indeed the ASCO guidelines state that “the panel believes that in most settings, recurrent disease should be biopsied whenever feasible for determination of tumor ER and HER2 status” (6). It is promising to note that feasibility of HER2 FISH analysis in CTCs has been demonstrated, which could obviate the need for tumor biopsies to recheck HER2 status if validated (32). Nonetheless it is clear that increased efforts to analyze metastatic tissues are absolutely necessary to improve our understanding of the multifaceted nature of acquired hormone resistance.
3. Longitudinal measurement of biomarkers
There is increasing evidence that monitoring tumor changes to therapy via serial measurement of biomarkers during the treatment process can help determine response and resultant long term patient prognosis. Feasibility of this biomarker-driven approach has already been demonstrated in patients with early-stage disease via monitoring of dynamic changes in breast tumor indices in response to neoadjuvant therapy, as on-treatment Ki67 has been shown to be a surrogate biomarker of outcome in patients, with Ki67 suppression after short-term (2–12 weeks) therapy serving as a better predictor of outcome than baseline Ki67 in the IMPACT and P024 trials (33–35). The preoperative endocrine prognostic index (PEPI) that combines the residual Ki67 score with measures of on-treatment ER levels, pathological tumor size and nodal status, is also a valuable biomarker of outcome in response to preoperative therapy (36). It has now been incorporated into the prospective phase III ALTERNATE trial of various preoperative endocrine therapies for women with early-stage HR-positive breast cancer (NCT01953588). Results of this trial are eagerly awaited to see whether dynamic biomarker monitoring can ultimately drive therapeutic decision making in a way that improves patient outcome.
An important question for patients with disseminated disease will be which biomarker to measure, (such as cfDNA and/or CTCs), in order to best monitor and predict treatment response. Mutations of ESR1 will likely be incorporated in any future panels, given the high frequency of such mutations in endocrine resistant MBC. We (37) and others (16, 38–42), have shown that detection of monoclonal and polyclonal mutations in ESR1 is feasible in cfDNA. Longitudinal monitoring of changes in cfDNA via “liquid” biopsies has led to successful monitoring of ESR1 mutation allelic frequencies in individual patients while on treatment ((37, 40). It has yet to be demonstrated, however, whether tracking of ESR1 mutations via tumor or “liquid” biopsies can be used as a strategy to identify emergent resistant clones, or if this can be translated into directed and improved care for the individual patient. In one study patients treated with AIs in the adjuvant setting were shown to be much less likely to acquire ESR1 mutations than patients treated with AI for metastatic disease (40). The authors postulate that micrometastatic tumor “bulk” in patients on adjuvant AI therapy is simply not high enough for rare clones to be selected under pressure of therapy. Moreover, patients with ESR1 mutations had shorter PFS with subsequent AI therapy [HR, 3.1; 95% CI, 1.9 to 23.1; P = 0.0041], which may indicate potential for ESR1 mutations as a biomarker in selecting subsequent lines of therapy. In patients with HR-positive metastatic disease, a recent analysis was performed on archived baseline plasma specimens from the SoFEA and PALOMA3 trials(41). In the SoFEA trial, patients with HR-MBC with ESR1 mutations had improved PFS with fulvestrant-based therapy (as opposed to exemestane alone), while patients with wild-type ESR1 had similar PFS with either treatment. In the PALOMA3 trial, patients had improved PFS with palbociclib plus fulvestrant compared to fulvestrant alone, regardless of ESR1 mutation status, indicating that palbociclib efficacy was not affected by mutation status. Both trials illustrated a relatively high rate of ESR1 mutations, 39.1% and 25.3% in SoFEA and PALOMA-3, respectively. Moving forward, A cautionary note is that utilization of archived plasma in EDTA for ctDNA studies needs further validation, as fresh blood samples might be necessary to give the most accurate assessment of disease status. Ultimately, the therapeutic challenge will include targeting mutant ER with the right agent at the right time during the patient’s disease course, as initial increase in ligand-independent signaling can transition to complete loss of ER target gene expression and signaling over time (29).
While refining anti-estrogen approaches (both established agents and/or novel SERMs and SERDs) may help individuals with mutated ER, endocrine resistance is also conferred by altered pathway signaling, namely cross-talk between growth factor receptor pathways and ER signaling. For instance, activating mutations in PIK3CA are seen in 40% of ER-positive breast cancers and the PI3K/Akt/mTOR pathway is often upregulated in the setting of endocrine resistance. (43) Recent analyses have indicated that in patients with HR-positive tumors, PIK3CA mutations are often associated with favorable clinicopathological features such as low grade, less nodal involvement, smaller tumor size, and older age but do not add independent prognostic value beyond these features.(44). PIK3CA mutations and low PTEN was predictive of everolimus benefit in patients treated with trastuzumab and chemotherapy for advanced disease in the combined analyses of BOLERO-1 and BOLERO-3 trials(45).
Response to palbociclib was not linked to PIK3CA mutational status, amplification of cyclin D1, or p16 loss in the PALOMA trials (10, 13). More recently, an analysis of mutations in multiple individual CTCs and matched cfDNA in patients with MBC showed that different CTCs from the same patient had heterogeneous mutations (46). Multiple CTCs might therefore have to be analyzed, the authors postulated, in order to guide therapeutic decisions. The cfDNA from the blood was able to accurately detect all the mutations seen in the CTCs.
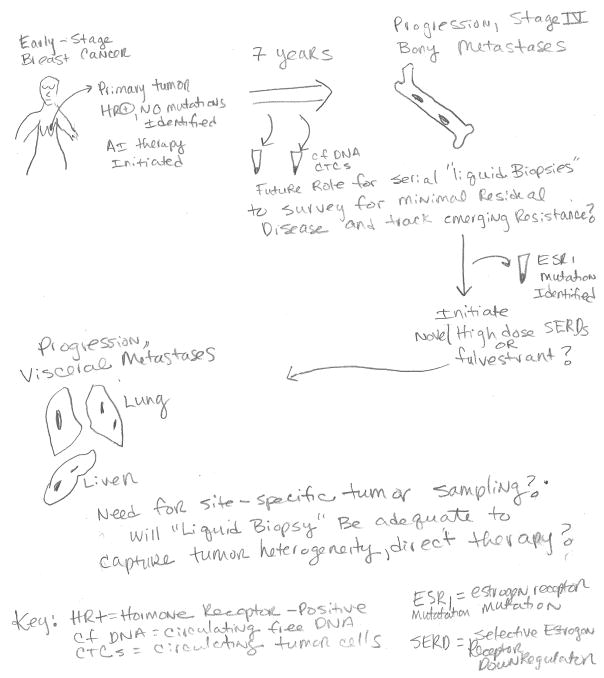
Acquired resistance underscores the need for longitudinalbiomarkers. With further investigation and validation of the various approaches, we envision a future approach that would allow for sequential assessment of biomarkers in individual patients with metastatic disease in order to guide therapeutic decision-making (Figure 1). It will be critical to determine when tissue-based biopsies are necessary, or if they will remain necessary at all in light of emerging “liquid biopsy” startegies for monitoring of disease. It is unknown to what extent “site-specific” mutations might persist/emerge on therapy in various metastatic deposits within the same patient, and whether cfDNA/CTCs will be able to capture that heterogeneity. Ongoing efforts to study these questions from patients who have donated to rapid autopsy programs could yield important answers to such questions. Although some patients are willing to undergo biopsies of metastatic sites, others are more reticent, citing fear of pain, risk of the procedure, etc(47). In addition to mutations, it is anticipated that measurement of other parameters such as DNA methylation and expression of coding or non-coding RNA is within our grasp and may add value. This would help to comprehensively follow adaptive changes of the tumor, such as changes in CDK6 expression levels, which recently were shown to have a potential association with fulvestrant resistance, as one example(48).
Figure 1.
Potential Biomarker-Driven Therapeutic Approach via Dynamic Monitoring of Tumor Burden Changes under Treatment Pressure
Summary and conclusions
The last five years have been exciting time for the study and treatment of HR-positive MBC, with emergence of several new conceptual advances and two new approved therapies (palbociclib and everolimus). Advances in technologies and sample acquisition have revealed both the complex heterogeneity of primary breast tumors and their dynamic responses to therapy as they evolve to a resistant state. Unfortunately, drug development and clinical trial investigation of novel therapeutic approaches have lagged behind our new molecular knowledge and technical abilities (49). We anticipate that comprehensive molecular profiling of primary and metastatic tumors, combined with longitudinal monitoring of tumor adaptation using cfDNA/CTCs, will reveal mechanisms to overcome de novo and acquired endocrine resistance as we move toward the promise of precision oncology medicine.
Acknowledgments
This authors are supported by grant funding from National Institutes of Health P30CA047904 (all authors), Susan G. Komen® Career Catalyst Research Award CCR14300865 (Jankowitz), Susan G. Komen® Scholar Awards (Lee and Oesterreich), Breast Cancer Research Foundation (Oesterreich, Lee, and Davidson), Fashion Footwear Association of New York (all authors). Shear Family Funds (all authors) and the Nicole Meloche Foundation (all authors).
Footnotes
Disclosures: Drs. Oesterreich, Lee, and Davidson have no disclosures. Dr. Jankowitz has participated in advisory boards for Biotheranostics and Advaxis Immunotherapies™
References
- 1.Early Breast Cancer Trialists’ Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Factsheets: Female Breast Cancer. National Cancer Institute; Bethesda MD: 2016. http://seer.cancer.gov/statfacts/html/breast.html. [Google Scholar]
- 3.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112(9):1445–51. doi: 10.1038/bjc.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–14. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 5.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–8. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 7.Ohno S. Tolerability of Therapies Recommended for the Treatment of Hormone Receptor-Positive Locally Advanced or Metastatic Breast Cancer. Clin Breast Cancer. 2016 doi: 10.1016/j.clbc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367(5):435–44. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergh J, Jonsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattstrom D, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30(16):1919–25. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 11.Finn R, Martin M, Rugo HS, Jones SE, Im S, Gelmon KA, et al. PALOMA 2: Primary Results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2- advanced breast cancer (ABC) J Clin Oncol. 2016;34(suppl) abstr 507. [Google Scholar]
- 12.Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 2014;25(12):2357–62. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 15.Janni WJ, Rack B, Terstappen LW, Pierga JY, Taran FA, Fehm T, et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res. 2016;22(10):2583–93. doi: 10.1158/1078-0432.CCR-15-1603. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302):302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 17.Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(10):1134–50. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–14. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 19.Jeselsohn R, Barry WT, Migliaccio I, Biagioni C, Zhao J, de Tribolet-Hardy J, et al. TransCONFIRM: Identification of a genetic signature of response to fulvestrant in advanced hormone receptor positive breast cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Andrade JP, Park JM, Gu VW, Woodfield GW, Kulak MV, Lorenzen AW, et al. EGFR Is Regulated by TFAP2C in Luminal Breast Cancer and Is a Target for Vandetanib. Mol Cancer Ther. 2016;15(3):503–11. doi: 10.1158/1535-7163.MCT-15-0548-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulak MV, Cyr AR, Woodfield GW, Bogachek M, Spanheimer PM, Li T, et al. Transcriptional regulation of the GPX1 gene by TFAP2C and aberrant CpG methylation in human breast cancer. Oncogene. 2013;32(34):4043–51. doi: 10.1038/onc.2012.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45(12):1446–51. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20(7):1757–67. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4(6):1116–30. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–45. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oesterreich S, Davidson NE. The search for ESR1 mutations in breast cancer. Nat Genet. 2013;45(12):1415–6. doi: 10.1038/ng.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461(7265):809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 28.Manso L, Mouron S, Tress M, Gomez-Lopez G, Morente M, Ciruelos E, et al. Analysis of Paired Primary-Metastatic Hormone-Receptor Positive Breast Tumors (HRPBC) Uncovers Potential Novel Drivers of Hormonal Resistance. PLoS One. 2016;11(5):e0155840. doi: 10.1371/journal.pone.0155840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vareslija D, McBryan J, Fagan A, Redmond AM, Hao Y, Sims AH, et al. Adaptation to AI Therapy in Breast Cancer Can Induce Dynamic Alterations in ER Activity Resulting in Estrogen-Independent Metastatic Tumors. Clin Cancer Res. 2016;22(11):2765–77. doi: 10.1158/1078-0432.CCR-15-1583. [DOI] [PubMed] [Google Scholar]
- 30.Shiino S, Kinoshita T, Yoshida M, Jimbo K, Asaga S, Takayama S, et al. Prognostic Impact of Discordance in Hormone Receptor Status Between Primary and Recurrent Sites in Patients With Recurrent Breast Cancer. Clin Breast Cancer. 2016 doi: 10.1016/j.clbc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Prat A, Cheang MC, Galvan P, Nuciforo P, Pare L, Adamo B, et al. Prognostic Value of Intrinsic Subtypes in Hormone Receptor-Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0922. [DOI] [PubMed] [Google Scholar]
- 32.Mayer JA, Pham T, Wong KL, Scoggin J, Sales EV, Clarin T, et al. FISH-based determination of HER2 status in circulating tumor cells isolated with the microfluidic CEE platform. Cancer Genet. 2011;204(11):589–95. doi: 10.1016/j.cancergen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–16. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99(2):167–70. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 35.Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat. 2007;105(Suppl 1):33–43. doi: 10.1007/s10549-007-9701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100(19):1380–8. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P, Bahreini A, Gyanchandani R, Lucas PC, Hartmaier RJ, Watters RJ, et al. Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clin Cancer Res. 2016;22(5):1130–7. doi: 10.1158/1078-0432.CCR-15-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, Inao T, Sueta A, Fujiwara S, et al. Clinical significance of monitoring ESR1 mutations in circulating cell-free DNA in estrogen receptor positive breast cancer patients. Oncotarget. 2016 doi: 10.18632/oncotarget.8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, Inao T, Sueta A, Fujiwara S, et al. Droplet digital polymerase chain reaction assay for screening of ESR1 mutations in 325 breast cancer specimens. Transl Res. 2015;166(6):540–53. e2. doi: 10.1016/j.trsl.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson A, Tarazona N, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):313ra182. doi: 10.1126/scitranslmed.aac7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fribbens C, O’Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 42.Chu D, Paoletti C, Gersch C, VanDenBerg DA, Zabransky DJ, Cochran RL, et al. ESR1 Mutations in Circulating Plasma Tumor DNA from Metastatic Breast Cancer Patients. Clin Cancer Res. 2016;22(4):993–9. doi: 10.1158/1078-0432.CCR-15-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang SX, Polley E, Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev. 2016;45:87–96. doi: 10.1016/j.ctrv.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andre F, Hurvitz S, Fasolo A, Tseng LM, Jerusalem G, Wilks S, et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J Clin Oncol. 2016;34(18):2115–24. doi: 10.1200/JCO.2015.63.9161. [DOI] [PubMed] [Google Scholar]
- 46.Shaw JA, Guttery DS, Hills A, Fernandez-Garcia D, Page K, Rosales BM, et al. Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high CTC counts. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seah DS, Scott SM, Najita J, Openshaw T, Krag K, Frank E, et al. Attitudes of patients with metastatic breast cancer toward research biopsies. Ann Oncol. 2013;24(7):1853–9. doi: 10.1093/annonc/mdt067. [DOI] [PubMed] [Google Scholar]
- 48.Alves CL, Elias D, Lyng MB, Bak M, Kirkegaard T, Lykkesfeldt AE, et al. High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1984. [DOI] [PubMed] [Google Scholar]
- 49.Andre F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER) Lancet Oncol. 2014;15(3):267–74. doi: 10.1016/S1470-2045(13)70611-9. [DOI] [PubMed] [Google Scholar]