Abstract
Objective
Impaired oxygen delivery due to reduced cerebral blood flow (CBF) is the hallmark of delayed cerebral ischemia (DCI) following subarachnoid hemorrhage (SAH). Since anemia reduces arterial oxygen content, it further threatens oxygen delivery increasing the risk of cerebral infarction. Thus, SAH may constitute an important exception to current restrictive transfusion practices, wherein raising hemoglobin could reduce the risk of ischemia in a critically hypoperfused organ. In this physiologic proof-of-principle study we determined whether transfusion could augment cerebral oxygen delivery, particularly in vulnerable brain regions, across a broad range of hemoglobin values.
Design
Prospective study measuring CBF and oxygen extraction fraction (OEF) using 15O-PET. Vulnerable brain regions were defined as those with baseline oxygen delivery < 4.5 ml/100g/min.
Setting
PET facility located within the Neurology/Neurosurgery Intensive Care Unit.
Patients
52 patients at risk for DCI after aneurysmal SAH with hemoglobin 7–13 g/dl.
Interventions
Transfusion of one unit of red blood cells (RBCs) over one hour.
Measurements and Main Results
Baseline hemoglobin was 9.7 g/dl (range 6.9–12.9) and CBF was 43±11 ml/100g/min. After transfusion, hemoglobin rose from 9.6±1.4 to 10.8±1.4 g/dl (12%, p < 0.001) and oxygen delivery from 5.0 (IQR 4.4–6.6) to 5.5 (4.8–7.0) ml/100g/min (10%, p=0.001); the response was comparable across the range of hemoglobin values. In vulnerable brain regions, transfusion resulted in a greater (16%) rise in oxygen delivery associated with reduction in OEF, independent of Hgb level (p=0.002 vs. normal regions).
Conclusions
This study demonstrates that RBC transfusion improves cerebral oxygen delivery globally and particularly to vulnerable regions in SAH patients at risk for DCI across a wide range of hemoglobin values and suggests that restrictive transfusion practices may not be appropriate in this population. Large prospective trials are necessary to determine if these physiologic benefits translate into clinical improvement and outweigh the risk of transfusion.
Keywords: RBC transfusion, anemia, subarachnoid hemorrhage, cerebral ischemia, cerebral oxygen delivery, vasospasm, delayed cerebral ischemia
INTRODUCTION
The main source of preventable disability after aneurysmal subarachnoid hemorrhage (SAH) is delayed cerebral ischemia (DCI), manifest by reductions in cerebral blood flow (CBF) and hence cerebral oxygen delivery [1]. Treatments for DCI focus on restoring oxygen delivery by increasing CBF to hypoperfused brain regions. Hemodynamic interventions are routinely employed for this purpose, including volume expansion, induced hypertension, and augmentation of cardiac output [2–4]. Still, optimal management of DCI remains largely empiric, with no therapeutic strategies being supported by clinical or even convincing physiologic proof-of-efficacy [5, 6].
An alternative but controversial approach to improving oxygen delivery in SAH is the use of red blood cell (RBC) transfusion to raise hemoglobin and thus arterial oxygen content (CaO2) [7]. p Prospective controlled trials in selected populations found no advantage to liberal compared to restrictive transfusion thresholds [8–11]. and current recommendations for use of stored blood in critically ill patients limit transfusion until hemoglobin concentration falls below 7 gm/dl [12–14]. A small prospective randomized trial of higher goal hemoglobin after SAH found higher hemoglobin targets to be safe and feasible [15]. The risks and benefits of transfusion in SAH are not well defined.
SAH, however, may represent an important exception to those general recommendations. Transfusion trials have generally excluded (or only included few) patients with acute brain injuries at risk for cerebral ischemia. As with cardiac ischemia [16, 17], the benefits of improving oxygen delivery to a critically hypoperfused organ may outweigh other risks in SAH patients. Recent guidelines have suggested higher hemoglobin targets for patients with acute coronary syndrome, severe sepsis, brain trauma and SAH [18], despite limited data to support this practice.
The question of transfusion thresholds is of particular concern in SAH patients as anemia frequently develops after SAH. Hemoglobin falls below 10 g/dl within four days of admission in half of SAH patients and almost all develop some degree of anemia during the period of highest risk for DCI [19]. The associated reduction in CaO2 additionally impairs oxygen delivery in the face of reduced CBF; studies have associated anemia after SAH with poor outcomes, including more cerebral infarction [20, 21].
However, the ability of transfusion to actually improve cerebral oxygen delivery in SAH has not been established. For decades, a policy of hemodilution was employed in an attempt to improve CBF by lowering blood viscosity; this approach has fallen out of favor with the recognition that any minimal improvement in CBF at lower hematocrit is outweighed by the fall in CaO2 [22]. We previously reported that transfusion improved oxygen delivery and reduced the number of vulnerable regions in a small group of SAH patients with hemoglobin levels of 7–10 g/dl, [23]. In this expanded cohort, we evaluated the effects of transfusion on cerebral oxygen transport in a larger number of patients across the full spectrum of anemia severity (hemoglobin levels from 7–13/dl). Our primary endpoint was the ability of transfusion to improve oxygen delivery in vulnerable brain regions. In addition, we sought to: (1) determine whether, as has been proposed [24], there is a hemoglobin threshold above which transfusion no longer improves oxygen delivery; and (2) provide physiologic evidence to guide the design and interpretation of phase III studies to determine if improving oxygen delivery with transfusion leads to less DCI and infarction and better clinical outcomes.
MATERIALS AND METHODS
Patient Selection
Patients were eligible for this study if they: (1) experienced spontaneous SAH; (2) had a ruptured cerebral aneurysm secured by endovascular or surgical means; and (3) were at risk for DCI based on clinical grade, admission CT grade [25], or angiographic vasospasm. Anemia was not an inclusion criterion for the study. Due to the concern that transfusion would increase viscosity and potentially reduce CBF and thus DO2 despite a higher hemoglobin, we first enrolled patients with hemoglobin levels <10 g/dl. After establishing that transfusion did not impair DO2 in those patients we moved on to study those with hemoglobin < 12 g/dl, and finally those >12 g/dl. Exclusion criteria included active congestive heart failure, pregnancy, or inability to obtain matched blood. Informed consent was obtained from patients or their legally authorized surrogates. The Human Research Protection Office and Radioactive Drug Research Committee of Washington University approved the study protocol. Eight of the patients included were the subject of a preliminary report [23].
Intensive Care Unit Care and Data Collection
All patients with SAH were cared for in the Neurology/Neurosurgery Intensive Care Unit (NNICU), received nimodipine and a short course of anticonvulsants. Ruptured aneurysms were repaired by surgical or endovascular means within 24 hours of admission. Patients were maintained euvolemic by adjusting intravenous fluids to preserve even fluid balance. New or worsening neurological deficits were promptly evaluated, and if no alternative cause was identified, patients underwent hemodynamic augmentation with fluids and induced hypertension, and cerebral angiography. Endovascular interventions for vasospasm, including angioplasty and/or intra-arterial vasodilators. Those remaining asymptomatic underwent screening angiography around day 7. Anemia was not routinely treated until hemoglobin fell to <7 g/dl.
Data collected included demographics, medical and social history, and neurological status at admission [26]. Admission CT was rated using the modified Fisher scale [25], and intraventricular hemorrhage was measured using the Hijdra score [27]. Cerebral angiograms were reviewed by an interventional neuroradiologist for the presence of arterial vasospasm, graded as mild, moderate, or severe in each vascular territory.
Experimental Protocol for PET Studies
All patients were studied on the Siemens/CTI ECAT EXACT HR+ PET Scanner located in the NNICU. Image acquisition to measure CBF, cerebral blood volume (CBV), oxygen extraction fraction (OEF), and cerebral metabolic rate for oxygen (CMRO2), was performed as detailed previously[28]. Scans were acquired in two-dimensional (2D) mode. Transmission scans were used for subsequent attenuation correction. All scans were calibrated for conversion of PET counts to quantitative radiotracer concentrations [29, 30]. CBF was measured by bolus injection of 15O-labeled water by using an adaptation of the Kety autoradiographic method [32]. CBV was measured by using a brief inhalation of 15O-labeled carbon monoxide [9]. CMRO2 and oxygen extraction fraction (OEF) were derived from the CBF and CBV measurements and an inhalation of 15O-labeled oxygen [31]. Arterial oxygen content (CaO2) was measured using oximetry in the hospital central laboratory. A limitation of this technique is its modest resolution which requires the assumption that OEF is uniform throughout the capillary bed, an assumption that is likely not valid.
After baseline scans, the patient remained in the scanner and a single unit of RBCs (approx. 350 ml) was transfused over 1 hour and then scans were immediately repeated. An attending neurointensivist was present throughout the study and all ongoing therapies for DCI were continued. At the time of each image acquisition, physiological data were recorded.
PET Processing
PET scans for each patient were co-registered and aligned using Automated Image Registration software (AIR, Roger Woods, University of California, Los Angeles, Calif) [32] and then co-registered to a reference brain image and resliced so data could be localized in Talairach atlas space along with the patient’s CT scan in closest temporal proximity to the PET study. Using the CT images and brain atlas coordinates, an image mask was created that included the brain below the superior sagittal sinus down to the level of the pineal gland to measure global values for each parameter. Spherical regions of 10-mm diameter were placed in 36 predetermined locations covering the major vascular territories as previously outlined [33]. Regions corresponding to hematoma, infarcted tissue, or ventricular system were excluded. Regional values were then calculated within each of the remaining spheres.
Data Analysis
Brain regions were considered hypoperfused and vulnerable if cerebral oxygen delivery (the product of CBF and CaO2) was below 4.5 ml/100 g/min (equivalent to CBF threshold of 25 ml/100 g/min with a CaO2 of 18 ml/dl). Data are presented as means ± standard deviation (if normally distributed), otherwise medians with interquartile range (IQR). Global response was assessed using paired t-tests or Wilcoxon signed rank test. The relationship between change in oxygen delivery and baseline Hgb (and covariates) was assessed using Pearson correlation coefficient.
We evaluated regional response using a linear mixed model to account for correlation among repeated measures in different brain regions from the same subject. We used low oxygen delivery as a factor and baseline hemoglobin as a covariate, evaluating the significance of the interaction of these terms with transfusion and calculating estimated marginal means for oxygen delivery and OEF pre and post-transfusion in each group. Modeling was performed using PROC MIXED and contrast and estimate statements were used. All analyses were conducted using a two-sided test at a significance level of 0.05 using SAS 9.4 (SAS Institute, Cary, NC) by an experienced biostatistician.
RESULTS
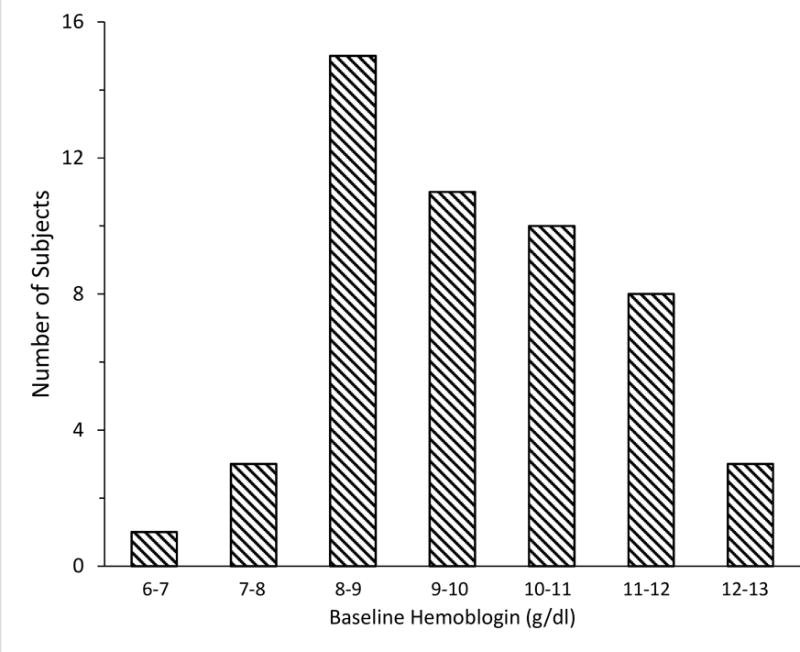
Fifty-six patients were enrolled; four were not studied due to patient instability or lack of PET scanner availability. Of 52 studies performed, 5 were excluded for technical reasons. CBF and oxygen delivery data were available in all patients. Due to logistical limitations of our PET system, measurements of OEF and CMRO2 were only able to be completed in 34 (72%). Characteristics of the study cohort are in Table 1. PET was performed a median of 9 days after SAH. Women were over-represented, both due to preponderance of women among those with SAH as well as requirement for anemia. Over half had angiographic vasospasm and over one-third were actively being treated for DCI. Baseline Hgb was median 9.7 g/dl (interquartile range 8.5–10.7) (Figure 1). Transfusion of 1 unit of RBCs over 1 hour resulted in a 10–15% rise in Hgb and CaO2, and a small increase in mean arterial pressure (MAP); other physiologic measures remained stable (Table 2).
Table 1.
Patient characteristics
| Variable | Frequency |
|---|---|
| Age (years) | 54 (48–70) |
|
| |
| Female | 42 (89%) |
|
| |
| Race, African-American | 9 (19%) |
| Caucasian | 38 (81%) |
|
| |
| WFNS Score: | |
| Grade I–III | 34 (72%) |
| Grade IV–V | 13 (28%) |
|
| |
| Modified Fisher Scale score | |
| Grade: 1–2 | 14 (33%) |
| Grade 3–4 | 33 (67%) |
|
| |
| Aneurysm location: | |
| Anterior communicating/ACA | 19 (40%) |
| Internal carotid artery branches | 11 (23%) |
| Middle cerebral artery | 9 (19%) |
| Posterior circulation | 8 (17%) |
|
| |
| Aneurysm treated: clip/coil | 24/23 |
|
| |
| Hydrocephalus (treated with EVD) | 36 (77%) |
|
| |
| Angiographic vasospasm (moderate-severe) | 25 (53%) |
|
| |
| Condition at time of PET study | |
|
| |
| Delayed ischemic deficits | 17 (36%) |
|
| |
| Mechanical ventilation | 19 (41%) |
|
| |
| Fluids and vasopressors for DCI | 15 (33%) |
|
| |
| Day of study (post SAH) | 9 (7–10) |
|
| |
| Discharge disposition | |
|
| |
| Home | 17 (36%) |
|
| |
| Rehabilitation facility | 17 (36%) |
|
| |
| Long term care/nursing home | 9 (19%) |
|
| |
| Died/Hospice care | 4 (8%) |
Continuous variables are presented as median with interquartile range; WFNS: World Federation of Neurological Surgeons; SAH: subarachnoid hemorrhage; EVD: external ventricular drainage; ACA: anterior cerebral artery
Figure 1.

Distribution of hemoglobin values prior to transfusion
Table 2.
Physiologic data during PET study
| Variable | Pre-Transfusion | Post-Transfusion | Significance† |
|---|---|---|---|
| MAP (mm Hg) | 113 ± 17 | 117 ± 16 | <0.001 |
| HR (beats per min) | 78 ± 17 | 77 ± 17 | 0.06 |
| ICP (n=32) | 10 ± 5 | 9 ± 5 | 0.29 |
| Temperature (°C) | 37.3 ± 0.7 | 37.3 ± 0.7 | 0.85 |
| PaCO2 (mm Hg) | 35.7 ± 6 | 36.2 ± 6 | 0.32 |
| Hemoglobin (g/dl) | 9.6 ± 1.4 | 10.8 ± 1.4 | <0.001 |
| Arterial Oxygen Content (g/dl) | 12.9 ± 1.8 | 14.5 ± 1.7 | <0.001 |
Data presented as mean ± SD; significance based on paired t-tests; MAP-mean arterial pressure; HR – heart rate; ICP-intracranial pressure; PaCO2-arterial partial pressure of carbon dioxide
Global response to transfusion
Mean baseline CBF was 43±11 ml/100g/min, corresponding to oxygen delivery of 5.5±1.6 ml/100g/min (median 5.0, IQR 34–47). Baseline CBF was not correlated with age, hemoglobin or GCS on admission or at time of study, but was inversely correlated with baseline OEF (r = −0.63, p<0.001). Global CBF did not differ in patients with and without DCI (41±14 vs. 44±9, p=0.4), although all but one of those with DCI were being actively treated with vasopressors during the PET study and had higher MAPs (126 vs. 105, p<0.001).
Despite a small reduction in CBF (Table 3), oxygen delivery increased 10%, from a median of 5.0 to 5.5 ml/100g/min (p=0.001). The magnitude of this response was comparable across the range of Hgb values (r=−0.17, p=0.25, Figure 2). The only variables correlated with change in oxygen delivery were baseline CBF and oxygen delivery levels (r=−0.44 and −0.48, both p=0.002), indicating that patients with lower baseline perfusion responded better to transfusion. Oxygen delivery improved after transfusion in all patients with Hgb < 8 g/dl, 84% of those with Hgb < 9 g/dl and 61% of those with Hgb ≥ 9 g/dl (p=0.08). There was no change in global OEF or CMRO2.
Table 3.
Cerebral response to transfusion
| Variable | Pre-Transfusion | Post-Transfusion | Significance† |
|---|---|---|---|
| Global | |||
| CBF | 43 ± 11 | 40 ± 9 | 0.009 |
| Oxygen delivery | 5.5 ± 1.6 | 5.9 ± 1.5 | 0.007 |
| OEF | 0.39 ± 0.11 | 0.38 ± 0.11 | 0.36 |
| CMRO2 | 2.2 ± 0.6 | 2.2 ± 0.7 | 0.98 |
| Vulnerable regions (baseline oxygen delivery < 4.5 ml/100g/min) | |||
| CBF | 31.3 ± 5.8 | 32.0 ± 7.4 | 0.014 |
| Oxygen delivery | 3.7 ± 0.5 | 4.3 ± 0.9 | < 0.001 |
| OEF | 0.49 ± .14 | 0.44 ± 0.14 | <0.001 |
| CMRO2 | 1.94 ± 0.62 | 1.89 ± 0.61 | 0.11 |
Data presented as mean ± SD; significance based on paired t-tests; CBF-cerebral blood flow; OEF-oxygen extraction fraction; CMRO2-cerebral metabolic rate for oxygen
Figure 2.
Percentage change in cerebral oxygen delivery of individual patients in relation to baseline hemoglobin levels
Responses in vulnerable brain regions
After excluding regions with infarction or hematoma, 1,580 brain regions (33.5 per subject) remained for regional analysis. 443 (28%) were classified as vulnerable based on low baseline oxygen delivery (< 4.5 ml/100g/min) and 303 of 1174 (26%) had high OEF (≥ 0.5). More vulnerable regions were seen at lower Hgb (r= −0.46, p=0.03), with a median of 13/patient (IQR 9–20) with low oxygen delivery if Hgb < 9 g/dl vs. 2 (0–11) at Hgb > 9 g/dl (p=0.014). Similarly, number of high OEF regions were greater in those with Hgb < 9 g/dl (12.5, 2–21) compared to those with higher Hgb (6, 0–11, p=0.06)
The rise in oxygen delivery was greater in vulnerable regions compared to those with normal baseline oxygen delivery (p=0.002 for interaction of transfusion and region type in mixed model). Oxygen delivery improved by 16%, from 3.7±0.5 to 4.3±0.9 ml/100g/min, in vulnerable regions compared to only a small change observed in normal regions (6.9±2.1 to 7.1±2.2, Table 3). This was independent of baseline Hgb (p=0.002 after adjusting for Hgb). There was a similar rise in regional brain oxygen delivery for those with Hgb < 10 and Hgb > 10 (estimate mean increase of 0.33–0.34 in both groups, p=0.83). OEF was also reduced significantly in hypoperfused regions (0.49±0.15 to 0.44±0.15) compared to no change in normal regions (0.39 to 0.38, p=0.0005). Again, this response was independent of baseline Hgb level. We also observed moderate inverse correlation between change in regional oxygen delivery and corresponding change in OEF (r=−0.48, p<0.001), suggesting that these changes largely occur in parallel within the same vulnerable regions. The number of vulnerable regions was reduced from a median of 9 (IQR 2–13) at baseline to 4 (1–14) after transfusion (p=0.005, Wilcoxon Signed Ranks test). Similarly, the number of regions with elevated OEF was reduced from median of 7 (IQR 1–14) to 2.5 (0–10) after transfusion (p=0.09).
DISCUSSION
This study reports the only direct multi-parametric quantitative regional measurements of cerebral oxygen delivery and extraction in a large cohort of patients with SAH at risk for DCI. Across a wide range of hemoglobin values, oxygen delivery rose globally after transfusion of one unit of RBCs, driven specifically by a selective and greater improvement in vulnerable brain regions, regardless of hemoglobin level. The results of this study establish physiologic proof-of-principle that transfusion improves cerebral oxygen delivery and challenges the paradigm that a restrictive transfusion strategy is appropriate for this subgroup of critically ill patients.
Anemia is common in SAH and has been linked to cerebral infarcts and worse outcome [20, 34–36]. Hemoglobin levels fall below 12–13 g/dl in almost all patients. Support for transfusion in SAH, however, has been modest. Studies have reported a rise in regional brain oxygen tension after transfusion [37, 38], which may or may not equate to a rise in delivery, as we have previously shown in head injury.[28] One prospective trial of 44 SAH randomized patients to a goal hemoglobin concentration of >10 or 11.5 g/dl [39]; there was a trend toward better outcome in the higher hemoglobin group.
Whether enhanced delivery translates into improved tissue outcome has yet to be determined; nonetheless, these data provide some insight. In brain regions considered vulnerable due to low baseline oxygen delivery, concomitant elevated OEF also fell, indicating better matching of delivery to metabolic demand as well as additional reserves to better tolerate any further reductions in perfusion.
In addition, our study extends our understanding of the physiology of transfusion as a means of improving cerebral tolerance to ischemia, especially in those milder degrees of anemia. We did not find, as has been proposed on theoretical grounds [24] and widely integrated into practice, a hemoglobin threshold above which the benefit of transfusion is negated by a fall in CBF due to higher viscosity. Instead we found that up to a Hgb of 13 g/dl, especially in vulnerable regions of the brain, CBF remains relatively stable, resulting in higher oxygen delivery after transfusion.
A number of factors must be considered when interpreting these data. While we have demonstrated that transfusion provides an immediate improvement in oxygen delivery, we did not determine whether this increase is sustained. This study was intended as a proof-of-concept study to test the hypothesis that transfusion would improve DO2 in order to determine if, and what further study is warranted. In order to assess the durability of the response a study where hemoglobin was maintained at particular targets over time will be required.
Without a control group we cannot state definitively say that the response was not due to a hemodynamic effect. We previously studied a cohort of SAH patients receiving a large saline bolus (15 ml/Kg) and found no effect on global CBF and a modest rise in vulnerable regions. More importantly, in the present study DO2 rose in response to the higher CaO2 and CBF did not change indicating that the response was due to the hemoglobin not the volume.
While DO2 rose and OEF fell in vulnerable regions, CMRO2 did not rise; delivery was improved but utilization did not rise as would be expected in ischemic tissue. We believe there are a number of possible explanations for this finding: 1) Our definition of vulnerable regions identifies regions with oligemia that are at risk for ischemia (reduced by sufficient DO2) which may or may not include areas with true ischemia (insufficient DO2 to meet metabolic demands). Since oligemic tissue maintains adequate DO2 at the expense of increased extraction, it has minimal reserves to be able to tolerate further insults. In that case when DO2 rises OEF will fall but CMRO2 will not change, as occurred in this study. 2) The ischemic state is relatively short lived as the tissue soon dies; the likelihood of catching that dynamic using 2 snapshot measurements of CMRO2 in each patient is quite low. 3) Due to the limited resolution of PET our regions have to be fairly large (spherical regions of 10-mm diameter) and may have blended areas of oligemia and ischemia diluting the measured impact on CMRO2.
In addition, the benefit of transfusion is relatively modest (i.e. a 10–15% rise in oxygen delivery); it remains to be determined if transfusion of more than one unit of blood would offer additional benefit. Furthermore, any physiologic benefits to oxygen delivery must be balanced against possible risks of transfusion, as suggested in observational studies of SAH patients [40, 41].
While a majority of all those studied responded with an improvement in oxygen delivery, a minority of patients did not. We explored factors that might influence the response to transfusion and found unexpectedly that this was not driven by hemoglobin level, but rather baseline perfusion was the best predictor of the magnitude of the response; the presence of hypoperfusion was associated with a more robust response. If we could predict those with persistently low CBF and regions at-risk despite routine medical therapy, perhaps transfusion could be an adjunctive intervention to improve oxygen delivery for these patients. This suggests a role for monitoring of CBF in SAH patients as an even better physiologic transfusion trigger than hemoglobin or even presence of neurological symptoms.
While this study alone does not provide adequate clinical evidence to support a change in transfusion practice, it supports and informs future clinical trials and, at least, argues against a uniformly restrictive approach to transfusion in SAH patients. Given that there remains significant uncertainty about when and to what hemoglobin to transfuse in SAH [42], our findings provide physiologic support for a less restrictive transfusion strategy if DCI is a major concern and set the stage for definitive outcome trials of transfusion in SAH patients.
Acknowledgments
The authors gratefully acknowledge the support of Doctors Michael Chicoine, Ralph Dacey, Ian Dorward, Keith Rich and Gregory Zipfel in studying their patients; as well as Michelle Allen RN, Hussain Jafri, and Ling Chen, PhD for their assistance in conducting and analyzing this study.
Source of Funding: AHA Scientist Development Grant SDG3440008 (RD), NIH/NINDS 5P01NS035966 (MND), Barnes-Jewish Hospital Foundation grant 00956-0807-01 (RD)
Footnotes
Conflicts of Interest: All authors have disclosed that they do not have any relevant conflicts of interest.
ClinicalTrials.gov registration: Effect of Red Blood Cell Transfusion on Brain Metabolism in Patients with Subarachnoid Hemorrhage; Identifier: NCT00968227
Copyright form disclosure: Dr. Dhar’s institution received funding from the Barnes-Jewish Hospital Foundation and the American Heart Association (AHA). He received support for article research from the AHA. Dr. Derdeyn received support for article research from the National Institutes of Health (NIH). He also disclosed support from Pulse Therapeutics (stock Options; acute stroke treatment device). Dr. Diringer’s institution received funding from the NIH/National Institute of Neurological Disorders and Stroke, the AHA, Barnes-Jewish Hospital Foundation, and Prolong Pharmaceuticals. He received funding from Remedy Pharmaceuticals and Pfizer. He received support for article research from the NIH. Dr. Zazulia disclosed that she does not have any potential conflicts of interest.
References
- 1.Grubb RL, Jr, Raichle MEME, Eichling JOO, et al. Effects of subarachnoid hemorrhage on cerebral blood volume, blood flow, and oxygen utilization in humans. Journal of Neurosurgery. 1977;46:446–453. doi: 10.3171/jns.1977.46.4.0446. [DOI] [PubMed] [Google Scholar]
- 2.Levy ML, Rabb CH, Zelman V, et al. Cardiac performance enhancement from dobutamine in patients refractory to hypervolemic therapy for cerebral vasospasm. Journal of Neurosurgery. 1993;79:494–499. doi: 10.3171/jns.1993.79.4.0494. [DOI] [PubMed] [Google Scholar]
- 3.Lennihan L, Mayer Sa, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage : a randomized controlled trial. Stroke. 2000;31:383–391. doi: 10.1161/01.str.31.2.383. [DOI] [PubMed] [Google Scholar]
- 4.Muench E, Horn P, Bauhuf C, et al. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Critical care medicine. 2007;35:1844–1851. doi: 10.1097/01.CCM.0000275392.08410.DD. [DOI] [PubMed] [Google Scholar]
- 5.Gathier CS, Dankbaar JW, van der Jagt M, et al. Effects of Induced Hypertension on Cerebral Perfusion in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: A Randomized Clinical Trial. Stroke. 2015;46(11):3277–3281. doi: 10.1161/STROKEAHA.115.010537. [DOI] [PubMed] [Google Scholar]
- 6.Diringer MN, Dhar R, Scalfani M, et al. Effect of High-Dose Simvastatin on Cerebral Blood Flow and Static Autoregulation in Subarachnoid Hemorrhage. Neurocrit Care. 2015 doi: 10.1007/s12028-015-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Roux PD. Anemia and transfusion after subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):342–353. doi: 10.1007/s12028-011-9582-z. [DOI] [PubMed] [Google Scholar]
- 8.Brierley RC, Pike K, Miles A, et al. A multi-centre randomised controlled trial of Transfusion Indication Threshold Reduction on transfusion rates, morbidity and healthcare resource use following cardiac surgery: study protocol. Transfusion and apheresis science: official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2014;50(3):451–461. doi: 10.1016/j.transci.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 11.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84(3):732–747. [PubMed] [Google Scholar]
- 13.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157(1):49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 14.Qaseem A, Humphrey LL, Fitterman N, et al. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(11):770–779. doi: 10.7326/0003-4819-159-11-201312030-00009. [DOI] [PubMed] [Google Scholar]
- 15.Naidech AM, Shaibani A, Garg RK, et al. Prospective, randomized trial of higher goal hemoglobin after subarachnoid hemorrhage. Neurocritical care. 2010;13:313–320. doi: 10.1007/s12028-010-9424-4. [DOI] [PubMed] [Google Scholar]
- 16.Kansagara D, Dyer E, Englander H, et al. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159(11):746–757. doi: 10.7326/0003-4819-159-11-201312030-00007. [DOI] [PubMed] [Google Scholar]
- 17.Hebert PC, Carson JL. Transfusion threshold of 7 g per deciliter–the new normal. N Engl J Med. 2014;371(15):1459–1461. doi: 10.1056/NEJMe1408976. [DOI] [PubMed] [Google Scholar]
- 18.Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160(4):445–464. doi: 10.1111/bjh.12143. [DOI] [PubMed] [Google Scholar]
- 19.Sampson TR, Dhar R, Diringer MN. Factors associated with the development of anemia after subarachnoid hemorrhage. Neurocritical care. 2010;12(1):4–9. doi: 10.1007/s12028-009-9273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidech AM, Jovanovic B, Wartenberg KE, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Critical care medicine. 2007;35:2383–2389. doi: 10.1097/01.CCM.0000284516.17580.2C. [DOI] [PubMed] [Google Scholar]
- 21.Kramer AH, Gurka MJ, Nathan B, et al. Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. Critical care medicine. 2008;36:2070–2075. doi: 10.1097/CCM.0b013e31817c1095. [DOI] [PubMed] [Google Scholar]
- 22.Ekelund A, Reinstrup P, Ryding E, et al. Effects of iso- and hypervolemic hemodilution on regional cerebral blood flow and oxygen delivery for patients with vasospasm after aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2002;144:703–712. doi: 10.1007/s00701-002-0959-9. [DOI] [PubMed] [Google Scholar]
- 23.Dhar R, Zazulia AR, Videen TO, et al. Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2009;40(9):3039–3044. doi: 10.1161/STROKEAHA.109.556159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood JH, Kee DB., Jr Hemorheology of the cerebral circulation in stroke. Stroke. 1985;16(5):765–772. doi: 10.1161/01.str.16.5.765. [DOI] [PubMed] [Google Scholar]
- 25.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–27. doi: 10.1227/01.neu.0000243277.86222.6c. [DOI] [PubMed] [Google Scholar]
- 26.Teasdale GM, Drake CG, Hunt W, et al. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. Journal of neurology, neurosurgery, and psychiatry. 1988;51:1457. doi: 10.1136/jnnp.51.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijdra A, Brouwers PJ, Vermeulen M, et al. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21(8):1156–1161. doi: 10.1161/01.str.21.8.1156. [DOI] [PubMed] [Google Scholar]
- 28.Diringer MN, Aiyagari V, Zazulia AR, et al. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- 29.Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. JNuclMed. 1983;24:782–789. [PubMed] [Google Scholar]
- 30.Raichle ME, Martin WR, Herscovitch P, et al. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. JNuclMed. 1983;24:790–798. [PubMed] [Google Scholar]
- 31.Videen TO, Perlmutter JS, Herscovitch P, et al. Brain blood volume, flow, and oxygen utilization measured with 15O radiotracers and positron emission tomography: revised metabolic computations. J Cereb Blood Flow Metab. 1987;7:513–516. doi: 10.1038/jcbfm.1987.97. [DOI] [PubMed] [Google Scholar]
- 32.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Jost SC, Diringer MN, Zazulia AR, et al. Effect of normal saline bolus on cerebral blood flow in regions with low baseline flow in patients with vasospasm following subarachnoid hemorrhage. Journal of Neurosurgery. 2005;103:25–30. doi: 10.3171/jns.2005.103.1.0025. [DOI] [PubMed] [Google Scholar]
- 34.Kramer AH, Zygun DA, Bleck TP, et al. Relationship between hemoglobin concentrations and outcomes across subgroups of patients with aneurysmal subarachnoid hemorrhage. Neurocritical care. 2009;10:157–165. doi: 10.1007/s12028-008-9171-y. [DOI] [PubMed] [Google Scholar]
- 35.Le Roux PD, Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H Anemia and transfusion after subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):342–353. doi: 10.1007/s12028-011-9582-z. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg NF, Koht A, Naidech AM. Anemia and transfusion after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2013;25(1):66–74. doi: 10.1097/ANA.0b013e31826cfc1d. [DOI] [PubMed] [Google Scholar]
- 37.Leal-Noval SR, Rincón-Ferrari MD, Marin-Niebla A, et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive care medicine. 2006;32:1733–1740. doi: 10.1007/s00134-006-0376-2. [DOI] [PubMed] [Google Scholar]
- 38.Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Critical care medicine. 2005;33:1104–1108. doi: 10.1097/01.ccm.0000162685.60609.49. [DOI] [PubMed] [Google Scholar]
- 39.Naidech AM, Shaibani A, Garg RK, et al. Prospective, randomized trial of higher goal hemoglobin after subarachnoid hemorrhage. Neurocrit Care. 2010;13(3):313–320. doi: 10.1007/s12028-010-9424-4. [DOI] [PubMed] [Google Scholar]
- 40.Smith MJ, Le Roux PD, Elliott JP, et al. Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg. 2004;101(1):1–7. doi: 10.3171/jns.2004.101.1.0001. [DOI] [PubMed] [Google Scholar]
- 41.Festic E, Rabinstein AA, Freeman WD, et al. Blood Transfusion is an Important Predictor of Hospital Mortality Among Patients with Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care. 2012 doi: 10.1007/s12028-012-9777-y. [DOI] [PubMed] [Google Scholar]
- 42.Kramer AH, Diringer MN, Suarez JI, et al. Red blood cell transfusion in patients with subarachnoid hemorrhage: a multidisciplinary North American survey. Critical care. 2011;15:R30. doi: 10.1186/cc9977. [DOI] [PMC free article] [PubMed] [Google Scholar]


