Abstract
The experience of pain is characterized by tremendous inter-individual variability. Multiple biological and psychosocial variables contribute to these individual differences in pain, including demographic variables, genetic factors, and psychosocial processes. For example, sex, age and ethnic group differences in the prevalence of chronic pain conditions have been widely reported. Moreover, these demographic factors have been associated with responses to experimentally-induced pain. Similarly, both genetic and psychosocial factors contribute to clinical and experimental pain responses. Importantly, these different biopsychosocial influences interact with each other in complex ways to sculpt the experience of pain. Some genetic associations with pain have been found to vary across sex and ethnic group. Moreover, genetic factors also interact with psychosocial factors, including stress and pain catastrophizing, to influence pain. The individual and combined influences of these biological and psychosocial variables results in a unique mosaic of factors that contributes pain in each individual. Understanding these mosaics is critically important in order to provide optimal pain treatment, and future research to further elucidate the nature of these biopsychosocial interactions is needed in order to provide more informed and personalized pain care.
Introduction
It has long been appreciated that individuals differ from each other in important ways. More than 2,000 years ago Plato said: “No two persons are born exactly alike; but each differs from the other in natural endowments (360 B.C.).” Such individual differences are a hallmark of the experience of pain and have been a topic of keen interest to pain researchers for many years. Indeed, more than 70 years ago, in describing the rationale for their psychophysical study of pain sensitivity in healthy adults, Chapman and Jones [13] stated that “A striking variation in the intensity of pain, experienced in diseases with apparently similar lesions, is a common observation.” Historically, this inter-individual variability in pain response was more often viewed as a nuisance than a fruitful area of scientific inquiry; however, the genomic revolution and the ensuing promise of precision medicine have reinvigorated and legitimized scientific interest in individual differences [12; 18; 21; 52]. The purpose of this article is to provide an overview of factors contributing to individual differences in pain. Given the abundance of potential individual difference factors, I will not attempt a comprehensive review of this field, rather provide examples of individual differences from our own research as well as the work of other investigators. First, I will introduce the topic of individual differences in responses to pain and its treatment, including a biopsychosocial context for conceptualizing individual differences. Then, I will present findings regarding demographic factors that are associated with individual differences in pain. Next, I will discuss genetic and psychosocial contributions to individual differences, and I will present examples of interactions among these multiple individual difference factors. I will describe the clinical implications of individual differences in pain, followed by conclusions and recommendations for future research.
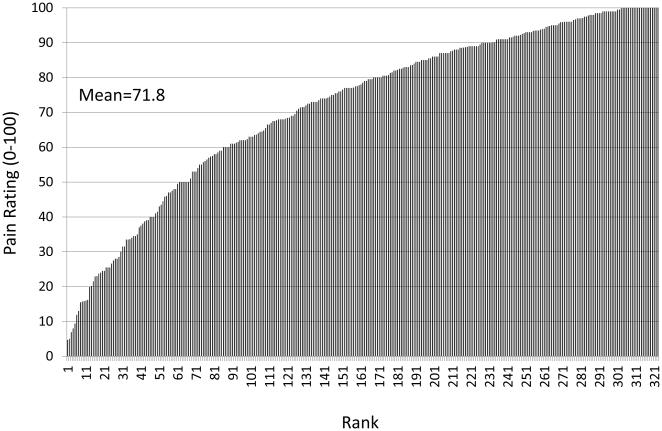
By definition pain is a subjective and highly personal experience, which presents challenges for both the researcher and clinician. A well-recognized challenge resulting from the subjective nature of pain is that direct measurement of pain is impossible, rather we must rely on individuals’ self-report, and to some extent their behavior, to provide a glimpse into their experience. However, an equally important but less often discussed challenge results from the highly personal nature of the pain experience; the experience of pain is sculpted by a mosaic of factors unique to the person, which renders the pain experience completely individualized. That is, there are pervasive and important individual differences in pain, and these individual differences produce pain experiences that are completely unique to the person experiencing them (i.e. they make the pain personal). For purposes of this paper, I will define individual differences in pain as between person differences in the pain experience that are independent of the initiating stimulus. Perhaps the simplest manifestation of individual differences is that an experimental stimulus delivered at a standardized intensity elicits subjective pain reports that vary dramatically between individuals (Figure 1), as noted decades ago by Chapman and Jones [13] and more recently by others [16; 26; 71; 86]. Interestingly, these differences in self-reported pain are corroborated by inter-individual differences in cerebral activation evoked by the same painful stimulus [14] and are in part predicted by individual differences in brain morphology [25], suggesting that these individual differences are not simply a product of idiosyncrasies in the reporting of pain. Such individual differences also emerge in the clinical environment. For example, pain reports following the same surgical procedure vary greatly across patients [7; 43; 83]. Similarly, responses to pain treatments are characterized by robust individual differences [3; 5; 11; 52]; however, a discussion of factors contributing to variability in treatment responses is beyond the scope of this article, which will focus on the individual difference factors impacting the experience of pain.
Figure 1.
Pain ratings in response to a heat stimulus (48 deg C) by 321 healthy young adults. Each line represents the pain rating (from 0 [no pain] to 100 [most intense pain imaginable]) by a single person. As can be seen, the mean pain rating was 71.8, but ratings ranged from 4 to 100. These data illustrate dramatic inter-individual differences in responses to a standardized experimental pain stimulus.
The biopsychosocial model provides an ideal framework for conceptualizing individual differences in pain. This model posits that the experience of pain is influenced by complex and dynamic interactions among multiple biological, psychological, and social factors [37]. Importantly, the ensemble of biopsychosocial factors contributing to the experience of pain and its expression varies considerably across people. Thus, pain is sculpted by a mosaic of factors that is completely unique to each individual at a given point in time, and this mosaic must be considered in order to provide optimal pain treatment.
When considering individual difference factors, it is important to distinguish characteristics of the individual that are statistically associated with pain responses (i.e. markers) from biological and psychosocial mechanisms that directly influence pain responses. Notably, some markers may reflect mechanisms underlying pain, while others do not. Examples of the former include demographic factors, such as sex, race/ethnicity and age. While each of these variables has been associated with pain responses (as discussed below), they reflect proxies for mechanisms influencing pain rather than mechanisms themselves. That is, the sex of an individual does not directly influence pain, rather sex differences in pain reflect the effects of other biological and psychosocial processes (e.g. sex hormones, inflammatory responses, gender roles, pain coping). Alternatively, a study could assess biological marker(s) related to pain, in which case the biological marker(s) represents both an individual difference factor and a potential mechanism directly influencing pain. Thus, while individual differences in pain response present challenges to the scientist and clinician, they also provide important opportunities. Indeed, investigating the factors contributing to individual differences in pain can provide important insights into pain mechanisms, which may lead to the development of novel treatments. Also, incorporating an understanding of individual differences into assessment and diagnosis of pain in the clinical setting may allow the clinician to select treatments that are tailored to the patient, thereby improving treatment outcomes.
Demographic Influences on Pain
As noted above, demographic factors do not directly influence pain, however they represent valuable individual difference factors, because they are easily measured and they provide important public health information regarding large population groups that may be at risk for increased pain. In addition, demographic associations with pain reflect the influence of underlying mechanisms, a better understanding of which can elucidate the pathophysiology of pain. That is, the prevalence of joint pain generally increases monotonically with age, and explanations for this association will enhance our mechanistic understanding of joint pain. Below, I will briefly review research examining sex differences, racial/ethnic differences, and age-related differences in pain, and the interested reader can find additional information regarding each of these topics in several recent reviews [2; 29; 32; 51; 62; 67; 78].
Sex Differences
Abundant epidemiologic evidence demonstrates that chronic pain is more prevalent among women than men [29; 67]. For example, recent findings from a large scale nationally representative study in the United States (US) found that a higher proportion of women than men reported any pain over the last 3 months [69]. Interestingly, women also were more likely to report pain that was persistent and bothersome, but only among non-Hispanic whites and non-Hispanic blacks. No such sex difference emerged for Hispanic whites. (Note: this reflects an interaction between sex and ethnic group, and such interactions among individual difference factors will be discussed further below) These findings relate to chronic pain in general, but sex differences in the prevalence of specific pain conditions have also been reported. Indeed, women are at greater risk for most common chronic pain conditions, including migraine and tension-type headaches, low back pain, fibromyalgia and widespread pain, temporomandibular disorders, irritable bowel syndrome, and osteoarthritis [29; 67]. Some studies have examined sex differences in the severity of acute and chronic pain, and in general any sex differences that have emerged have been inconsistent and small in magnitude [29; 83].
While multiple explanations for these sex differences in pain prevalence can be offered, one possibility is that fundamental differences in the functioning of female and male pain processing systems renders women at increased risk for clinical pain. This has motivated investigators to explore sex differences in responses to experimentally-induced pain. Multiple reviews of this topic are available [29; 46; 67; 78], and while some differences in interpretation of findings have emerged, the pattern of findings is indisputable. For virtually all standard measures of experimental pain sensitivity women display greater sensitivity than men, including pain threshold (the minimum stimulus intensity required to produce pain), pain tolerance (the maximum stimulus intensity an individual is willing to tolerate), and ratings of suprathreshold stimuli. Notably, the magnitude of the sex difference varies considerably across studies and across pain measures and stimulus modalities, but the direction of the difference is highly consistent. Also, women have shown greater temporal summation of pain (a measure of transient central sensitization) and less conditioned pain modulation (a measure of endogenous pain inhibition)[77], suggesting a pain modulatory balance that is tuned more strongly toward pain facilitation than pain inhibition among women. In contrast, in response to sustained and repeated thermal stimuli, females have shown greater habituation than men, suggesting a stronger pain inhibitory response to these types of stimuli [44; 45]. Multiple mechanisms have been proposed to explain these sex differences in pain, including the effects of sex hormones, differences in endogenous opioid function, cognitive/affective influences, and contributions of social factors such as stereotypic gender roles [29; 67].
Race/Ethnic Group Differences
The concepts of race and ethnicity are complex biological and social constructs that remain poorly defined. In the United States, it is typical to categorize individuals according to both ethnicity (Hispanic/Latino vs. non-Hispanic/non-Latino) and race (e.g. Asian, African-American, white), while different approaches may be taken in other parts of the world. Whether individuals from different racial and ethnic backgrounds experience pain differently has long been a topic of interest. From an epidemiologic perspective, limited evidence suggests racial or ethnic differences in pain prevalence. Nahin [69] found that pain prevalence was lowest among Asians compared to other race/ethnic groups in the US. Other studies of adults in the US have reported higher prevalence of persistent pain among whites compared to other racial/ethnic groups [53; 54]. Among older adults some studies have reported higher pain prevalence among minorities compared to whites, while others reported no differences in pain prevalence [57]. While there is conflicting information regarding pain prevalence may be lower among minority versus majority ethnic groups, studies consistently suggest that the severity and impact of pain appears to be greater among minorities who are experiencing chronic pain [2; 57; 64]. Indeed, our own studies demonstrate greater pain severity and functional limitations among African Americans compared to non-Hispanic whites with knee osteoarthritis [17]. In addition, differences in pain perception between racial/ethnic groups may contribute to differences in severity of clinical pain. A meta-analytic review of studies examining pain perception in generally healthy adults found that African Americans display greater experimental pain sensitivity compared to non-Hispanic whites [79]. Similarly, our recent findings among adults with knee osteoarthritis showed greater pain sensitivity and temporal summation of pain among African Americans [17].s These findings are largely based on work conducted in the United States, where racial and ethnic disparities in health are a substantial national concern. Similar findings have emerged in other developed countries throughout the world; however, little data related to ethnic group differences in pain have been reported from less developed countries.
The mechanisms underlying racial/ethnic group differences in the experience of pain are inevitably multifactorial, and include factors related to socioeconomic standing and access to adequate health care. For example, in most developed countries, members of minority groups on average have lower socioeconomic status, which has been associated with increased pain prevalence and more severe pain [64; 76]. In addition, considerable evidence suggests that minority patients are at greater risk for undertreatment of their pain, which could obviously contribute to the greater clinical pain severity observed among members of minority groups [2; 75]. Pain coping also differs significantly across racial/ethnic groups [48; 65], and it is possible that biological factors, such as genetic contributions, may play a role in racial/ethnic differences in pain responses [49; 79].
Age-Related Differences
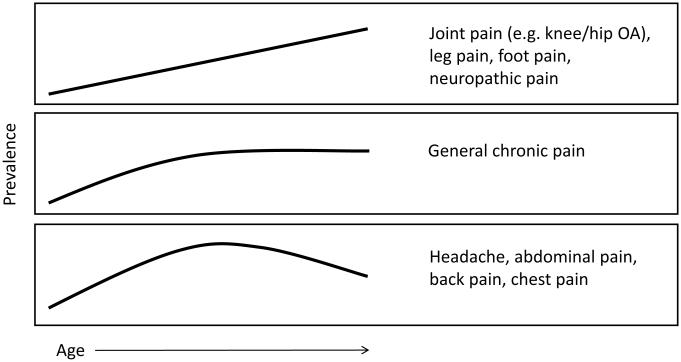
Given the aging of the world’s population, whether the experience of pain changes with age has drawn increasing attention in recent years [10; 32; 33; 62; 68; 73]. Patterns of pain prevalence across the lifespan are complex and they vary across pain conditions (see Figure 2)[32]. Briefly, the prevalence of joint pain, lower extremity pain and neuropathic pains tend to increase monotonically with age. General chronic pain increases in prevalence until middle age, at which time the prevalence plateaus. In contrast, pain conditions such as headache, abdominal pain, back pain and temporomandibular disorders show peak prevalence in the third to fifth decades of life, after which their frequency decreases. It is important to note that these epidemiologic findings are based almost exclusively on cross-sectional studies, such that cohort effects (e.g. earlier mortality among people with certain pain conditions) could influence the results. Beyond pain prevalence, multiple studies have examined age-related changes in the severity and impact of pain. Older adults have reported lower acute pain intensity in some studies [34; 83], but not others [4; 35]. Similarly, age-related differences in the intensity and impact of chronic pain have not been consistently demonstrated [32; 33].
Figure 2.
Patterns of pain prevalence across the adult lifespan. The top panel shows that prevalence increases monotonically with age for several pain conditions, including joint pain, lower extremity pain, and neuropathic pains. The middle panel shows that for general chronic pain, prevalence seems to increase until middle age, at which time it plateaus. The bottom panel shows a pattern of increasing prevalence until middle age followed by a decrease in prevalence in later life for several conditions, including headache, abdominal pain, back pain, chest pain. References supporting these patterns can be found in [32]. It is important to recognize that these prevalence patterns are based on cross-sectional rather than longitudinal data; therefore, one cannot deduce pain trajectories within people from these data.
Age-related changes in responses to experimental pain have been widely studied. Taken together these findings suggest that older adults show less sensitivity to brief, cutaneous pains (e.g. heat pain threshold); however, sensitivity to more sustained pain stimuli that impact deeper tissues increases with age [32; 55]. Moreover, several studies have demonstrated increased temporal summation of pain among older adults [23; 56; 70], while conditioned pain modulation consistently has been found to decrease with age [24; 80]. This pattern of results suggests that aging is associated with a shift in pain modulatory balance, such that older adults show enhanced pain facilitation combined with decreased pain inhibition.
A variety of biopsychosocial factors have been posited to contribute to these age-related changes in pain processing. First, many pain-related diseases increase in frequency with age (e.g. diabetes, osteoarthritis, many forms of cancer, neurological diseases), which can contribute to increased pain among older adults. Moreover, many of the biological changes that underlie aging can also contribute to increased clinical pain and altered pain modulatory balance, including systemic inflammation, oxidative stress, altered autonomic function, and changes in neuronal structure and function [32]. In addition, psychosocial changes that occur with age could also impact pain. Reductions in cognitive function, sleep quality, and social support are all common in older adults, and these factors are also associated with increased pain [78]. Notably, undertreatment of pain in older adults is common, which could further contribute to greater pain in this population [62; 74].
Interactions Among Biopsychosocial Factors
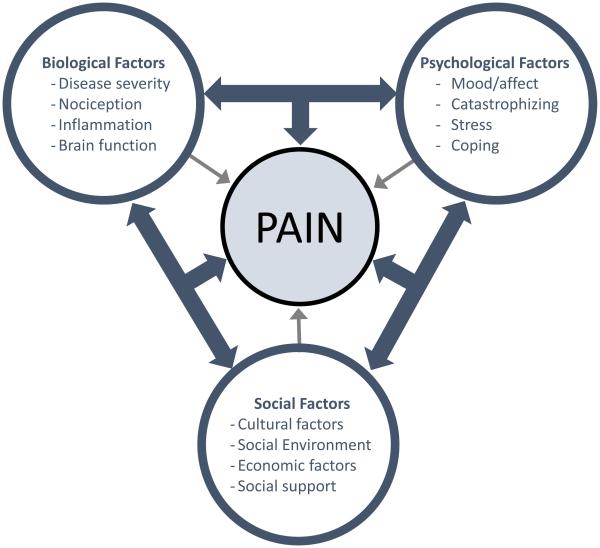
The biopsychosocial model does not simply propose that factors from biological, psychological and social domains exert important influences on pain. Perhaps the most important aspect of the model is its insistence that these different sets of factors interact to create the experience of pain. These interactions are depicted by the three-way bidirectional arrows in Figure 3. Though often neglected in pain research, identifying and ultimately understanding these interactions is critical to elucidating the mechanisms driving pain in different groups and individuals. At least three types of interactions should be appreciated: mediation, additive associations, and moderation. As a point of clarification, I use the term interaction here in a general or conceptual sense rather than a statistical one, such that some of these interactions would not necessarily emerge as statistical interactions, though some certainly do. Mediational interactions refer to the phenomenon whereby the influence of an individual difference factor from one domain on pain is mediated through a process from another domain. For example, the influence of psychological stress on pain could be mediated via specific biological processes, such as heightened sympathetic nervous system outflow or increased inflammation. Another common type of interaction is an additive association, in which combining two individual difference factors, each of which increases risk for pain, produces a stronger effect than either factor alone. For example, if both female gender and a particular genetic profile increase the risk for chronic pain, then the combination of being both female and having the genetic profile would produce greater risk than having one or the other but not both. Another type of interaction is moderation, in which the effect of one factor depends on the presence or absence of another factor. In this case, we might find that while both female sex and the genetic profile are risk factors for pain, the association between the gene and pain differs for females and males. That is, the genetic factor may increase risk for pain in females but decrease risk for pain in males. Examples of all three types of interactions will be provided below. The examples I provide are primarily limited to interactions between genetic and non-genetic factors, which is biased by my interests and the research with which I have been involved. It is important to note that many other types of factors can and do interact to shape individual differences in pain.
Figure 3.
Biopsychosocial model of pain. The figure illustrates that the experience of pain is sculpted by the influences of biological, psychological and social factors. Notably, while each of these factors can independently influence pain (as depicted by small bidirectional arrows), the more important and complex influences emerge from interactions among the factors, as depicted by the larger three-way arrows. These interactions among multiple biopsychosocial factors results in a unique mosaic of individual difference factors contributing to pain in each person.
Genetic Influences on Pain
Genetic contributions to pain have garnered considerable empirical attention in the past 20 years (see [18; 19; 31; 66] for reviews). In addition to representing identifiable individual difference variables, genetic associations with pain can reveal specific biological mechanisms that contribute to pain responses. The most commonly studied gene in pain studies has been the gene that encodes catechol-O-methyl-transferase (COMT), an enzyme that metabolizes catecholamines. COMT has been associated with pain-related mu-opioid receptor binding in the brain [87]. In addition, Diatchenko and colleagues [20] identified three COMT haplotypes that were related to global pain sensitivity and to risk of developing temporomandibular disorder. Thus, COMT has been related to clinical and experimental pain responses. The mu-opioid receptor gene (OPRM1) has also been widely studied for associations with pain phenotypes. We previously showed that the A118G single nucleotide polymorphism (SNP) of OPRM1 was associated with pressure pain sensitivity [28], and others have also demonstrated the same SNP to be related to experimental pain responses [59; 63].
Genetic associations with pain have been found to vary by sex and ethnic group, which reflects moderation as described above. Importantly, such interactions suggest that the biological pathways represented by the gene may differentially influence pain responses in different population groups. For example, COMT has been found to interact with sex in predicting pain phenotypes. Belfer and colleagues [6] reported that a haplotype coding for low COMT activity predicted increased capsaicin-induced pain among females but not males. In contrast, a COMT haplotype from a different haploblock predicted pain and pain interference following motor vehicle collision in males only [9]. Likewise, associations of OPRM1 with pain have varied across population groups. We found that the A118G SNP of OPRM1 interacted with sex to influence heat pain responses. Specifically, among males the minor allele was associated with lower rating of heat pain, while among females this allele predicted higher heat pain ratings [28]. Interestingly, a subsequent clinical study produced similar results, finding that the minor allele predicted lower pain levels one year following lumbar disc herniation among males, while females with the minor allele reported higher pain at one year [72], a finding recently replicated by others [50]. We also have reported that associations of this this SNP with experimental pain responses differ across racial/ethnic groups [49]. Among non-Hispanic whites, the minor allele conferred lower sensitivity across multiple experimental pain measures; however, among Hispanic whites the association was in the opposite direction. The study also included African Americans; however, the frequency of the minor allele was too low among African Americans to detect any particular association. Such gene X demographic interactions have profound methodological and clinical implications. Regarding the former, failure to include the interaction term in statistical analysis of the data often results in a null effect of the gene on the outcome, such that investigators fail to discover potentially important findings. Regarding the latter, a treatment targeting the biological process reflected by the gene could produce dramatically disparate outcomes in different population groups, a possibility that would only be identified had the interactions been evaluated.
Psychosocial Influences on Pain and Gene X Psychology Interactions
Abundant evidence demonstrates strong associations between psychosocial factors and the experience of pain. The examples below represent selected findings from work with which I have been involved, and there are many other important psychological processes that contribute to individual differences in pain (e.g. traumatic experiences, developmental influences, personality). On average, compared to individuals without pain, people with chronic pain conditions report increased psychological distress, greater life stress, and more non-pain somatic symptoms [22; 61; 84]. Moreover, when assessed in pain-free individuals, these psychosocial variables represent premorbid risk factors for future development of chronic pain [8; 58]. For example, in the OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) Study, we assessed multiple psychological variables in a large sample of generally healthy adults with no history of TMD pain. We found that poorer psychological functioning across two broad domains, global psychological symptoms (e.g. somatic symptoms, general psychological distress) and stress and negative affectivity (e.g. perceived stress, trait negative affect), predicted significantly increased risk for future development of TMD [30].
Importantly, psychological processes can interact with other individual difference variables, including demographic and genetic factors, to influence pain responses. George and colleagues [41] found that COMT interacted with pain catastrophizing (a maladaptive cognitive approach to pain characterized by rumination, magnification, and helplessness) to predict pain intensity in patients with chronic shoulder pain. Specifically, this additive interaction demonstrated that the subgroup of individuals who were both high in pain catastrophizing and had a high pain sensitive COMT haplotype reported greater pain than those who had only one or none of these two risk factors. These investigators subsequently replicated and extended these findings, demonstrating additional gene X psychology interactions in another clinical cohort of patients with shoulder pain as well as in healthy individuals experiencing experimentally-induced shoulder pain due to delayed onset muscle soreness [38-40; 42]. These findings suggest that the combination of genetic and psychological risk factors are associated with substantial increases in likelihood of experiencing pain of greater duration and higher intensity.
Additional findings from the OPPERA study also provide evidence of interactions between genetic and psychological factors. As noted above, perceived stress at the time of enrollment was a premorbid risk factor for development of new onset TMD. However, repeated measurements of stress also revealed that stress increased over time in those people who subsequently developed TMD but not among individuals who remained TMD-free [81]. More interestingly, increasing stress predicted TMD onset only among people who had a COMT haplotype associated with low COMT activity. Thus, increasing stress heightened risk for development of pain only in individuals with a genetic profile that rendered them more sensitive to the effects of catecholamines. While these findings emphasize risk factors, increasing research is focused on potential resilience factors that may protect against pain, which is an area of profound scientific and clinical significance [47; 82].
Clinical Implications
Individual differences in pain, and the biopsychosocial interactions that create them, have profound implications for assessment and management of pain. First, perhaps the most important implication is that awareness of individual differences in the clinical setting is critical. Those providers who approach each patient with a recognition of the importance of individual differences in pain will deliver better care and their patients will realize better outcomes. Thus, educational interventions to enhance provider understanding of individual differences in pain could enhance pain care. Second, the complexity of the biopsychosocial mosaic that influences pain demands an equally sophisticated approach to pain assessment and treatment. As recently highlighted in the ACTTION-American Pain Society Pain Taxonomy (AAPT), classification of chronic pain conditions should include not only core diagnostic features and associated symptoms, but should also incorporate information regarding biopsychosocial mechanisms and consequences [27]. This approach is also important for acute pain, which is likewise profoundly influenced by biopsychosocial factors [85], as noted in many of the examples above. Identifying the multiple biological and psychosocial processes and interactions contributing to a patient’s pain, whether acute or chronic, serves as the basis for developing an effective treatment plan. Unfortunately, the time and resource constraints that characterize most current health care environments conspire against this approach, which requires a level of time and expertise that is typically available only in the context of a multidisciplinary treatment team. Perhaps our inability to consistently achieve this standard of care partially explains why chronic pain is among the costliest health conditions in the developed world, and a leading cause of disability worldwide [1; 15; 36]. Third, pain treatment should target the multiple biopsychosocial drivers of a patient’s pain. Medical monotherapy is the norm; however, the suboptimal outcomes achieved by this approach are quite predictable given the complex and unique panoply of factors contributing to pain in each individual. The goal is to deploy personalized pain management, which is not simply pharmacotherapy based on genetic profile, rather truly personalized therapy is comprised of multiple treatment modalities designed specifically for each patient to target her or his singular mosaic. A final clinical implication is that an understanding of individual differences in pain can inform approaches to prevention of chronic pain. Indeed, identifying at risk individuals based on biopsychosocial profiles and developing prevention and early intervention programs based on those profiles has the potential to reduce the incidence of chronic pain appreciably (e.g. [60]).
Conclusions
The experience of pain is characterized by robust inter-individual differences. This article highlights multiple biopsychosocial factors that contribute to these individual differences. Demographic factors, such as sex, race/ethnicity, and age, represent easily assessed personal characteristics that are associated with pain and can have important public health implications. However, these factors themselves do not directly influence pain, rather they serve as proxies for a host of underlying processes that modulate pain. Genetic factors also represent important individual difference variables, but they have the distinct advantage of reflecting specific biological pathways that potentially directly impact pain. Psychosocial factors also contribute to individual differences in pain, and in addition to their value as risk markers, many psychological processes are modifiable and thus can be important targets for intervention. Importantly, these multiple biopsychosocial variables interact in complex ways to influence pain, and several examples of such interactions were reviewed above (e.g. sex X gene interactions, gene X psychology interactions). The many variables whose individual and combined influences drive individual differences produce a mosaic that uniquely contributes to pain in each patient. An understanding of these individual differences is critical for effective pain assessment and management, serving as the foundation for personalized pain treatment, an as yet unrealized goal. Future research is sorely needed to further illuminate the interactions among biological and psychosocial processes that importantly influence the experience of pain. In particular, identifying individual difference factors and their interactions that contribute to development and persistence of pain is a high priority. Moreover, determining individual difference factors that predict responses to pain treatments will inform future efforts toward personalized pain treatment. Such research will enhance future pain treatment efforts through identification of novel targets and better matching of therapies to patients’ needs.
Acknowledgments
Preparation of this article was supported by NIH grants R37AG033906, K07AG046371, and U01DE017018.
Dr. Fillingim holds stock in Proove Biosciences, a company that is involved in developing methods for improving the diagnosis and treatment of chronic pain conditions.
References
- [1].Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. JPain. 2009;10(12):1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [3].Angst MS, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, Lazzeroni LC, Clark JD. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153:1397–1409. doi: 10.1016/j.pain.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aubrun F, Bunge D, Langeron O, Saillant G, Coriat P, Riou B. Postoperative morphine consumption in the elderly patient. Anesthesiology. 2003;99(1):160–165. doi: 10.1097/00000542-200307000-00026. [DOI] [PubMed] [Google Scholar]
- [5].Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103(1):156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- [6].Belfer I, Segall SK, Lariviere WR, Smith SB, Dai F, Slade GD, Rashid NU, Mogil JS, Campbell CM, Edwards RR, Liu Q, Bair E, Maixner W, Diatchenko L. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain. 2013;154(8):1368–1376. doi: 10.1016/j.pain.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90(3):261–269. doi: 10.1016/S0304-3959(00)00406-1. [DOI] [PubMed] [Google Scholar]
- [8].Blyth FM, Macfarlane GJ, Nicholas MK. The contribution of psychosocial factors to the development of chronic pain: the key to better outcomes for patients? Pain. 2007;129(1-2):8–11. doi: 10.1016/j.pain.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [9].Bortsov AV, Diatchenko L, McLean SA. Complex multilocus effects of catechol-O-methyltransferase haplotypes predict pain and pain interference 6 weeks after motor vehicle collision. Neuromolecular medicine. 2014;16(1):83–93. doi: 10.1007/s12017-013-8255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Briggs AM, Cross MJ, Hoy DG, Sanchez-Riera L, Blyth FM, Woolf AD, March L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist. 2016;56(Suppl 2):S243–255. doi: 10.1093/geront/gnw002. [DOI] [PubMed] [Google Scholar]
- [11].Broderick JE, Keefe FJ, Schneider S, Junghaenel DU, Bruckenthal P, Schwartz JE, Kaell AT, Caldwell DS, McKee D, Gould E. Cognitive behavioral therapy for chronic pain is effective, but for whom? Pain. 2016;157(9):2115–2123. doi: 10.1097/j.pain.0000000000000626. [DOI] [PubMed] [Google Scholar]
- [12].Bruehl S, Apkarian AV, Ballantyne JC, Berger A, Borsook D, Chen WG, Farrar JT, Haythornthwaite JA, Horn SD, Iadarola MJ, Inturrisi CE, Lao L, Mackey S, Mao J, Sawczuk A, Uhl GR, Witter J, Woolf CJ, Zubieta JK, Lin Y. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. The journal of pain : official journal of the American Pain Society. 2013;14(2):103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chapman WP, Jones CM. Variations in cutaneous and visceral pain sensitivity in normal subjects. JClinInvest. 1944;23:81–91. doi: 10.1172/JCI101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci USA. 2003;100(14):8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collaborators USBoD The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain medicine (Malden, Mass) 2014;15(1):61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL, 3rd, King CD, Glover TL, Sotolongo A, Herbert MS, Schmidt JK, Fessler BJ, Staud R, Redden D, Bradley LA, Fillingim RB. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis & rheumatology (Hoboken, NJ) 2014;66(7):1800–1810. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. NatRevRheumatol. 2013:10. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23(12):605–613. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [20].Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. HumMolGenet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- [21].Dworkin RH, McDermott MP, Farrar JT, O'Connor AB, Senn S. Interpreting patient treatment response in analgesic clinical trials: implications for genotyping, phenotyping, and personalized pain treatment. Pain. 2014;155(3):457–460. doi: 10.1016/j.pain.2013.09.019. [DOI] [PubMed] [Google Scholar]
- [22].Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. The journal of pain : official journal of the American Pain Society. 2016;17(9 Suppl):T70–92. doi: 10.1016/j.jpain.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. The journal of pain : official journal of the American Pain Society. 2001;2(6):307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- [24].Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101(1-2):155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- [25].Emerson NM, Zeidan F, Lobanov OV, Hadsel MS, Martucci KT, Quevedo AS, Starr CJ, Nahman-Averbuch H, Weissman-Fogel I, Granovsky Y, Yarnitsky D, Coghill RC. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain. 2014;155(3):566–573. doi: 10.1016/j.pain.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fillingim RB. Individual differences in pain responses. CurrRheumatolRep. 2005;7(5):342–347. doi: 10.1007/s11926-005-0018-7. [DOI] [PubMed] [Google Scholar]
- [27].Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The ACTTION-American Pain Society Pain Taxonomy (AAPT): An Evidence-Based and Multidimensional Approach to Classifying Chronic Pain Conditions. The journal of pain : official journal of the American Pain Society. 2014;15(3):241–249. doi: 10.1016/j.jpain.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. JPain. 2005;6(3):159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [29].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. JPain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T75–90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fillingim RB, Wallace MR, Herbstman DM, Ribeiro-Dasilva M, Staud R. Genetic contributions to pain: a review of findings in humans. Oral Dis. 2008;14:673–682. doi: 10.1111/j.1601-0825.2008.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fillingim RBY, Turk RP. D.C. Pain in the elderly. In: Sierra FK, editor. Advances in Geroscience. Springer; New York: 2016. pp. 552–592. R. [Google Scholar]
- [33].Gagliese L. Pain and aging: the emergence of a new subfield of pain research. The journal of pain : official journal of the American Pain Society. 2009;10(4):343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- [34].Gagliese L, Katz J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103(1-2):11–20. doi: 10.1016/s0304-3959(02)00327-5. [DOI] [PubMed] [Google Scholar]
- [35].Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117(3):412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- [36].Gaskin DJ, Richard P. The economic costs of pain in the United States. JPain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [37].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. PsycholBull. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- [38].George SZ, Dover GC, Wallace MR, Sack BK, Herbstman DM, Aydog E, Fillingim RB. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-o-methyltransferase (COMT) diplotype predict pain ratings. ClinJPain. 2008;24(9):793–801. doi: 10.1097/AJP.0b013e31817bcb65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].George SZ, Parr J, Wallace M, Wu S, Borsa PA, Fillingim RB. Genetic and psychological risk factors are associated with pain and disability in an experimentally induced acute shoulder pain model. Journal of Pain. 2012;13(4 (Supplement 1)):s29. [Google Scholar]
- [40].George SZ, Parr JJ, Wallace MR, Wu SS, Borsa PA, Dai Y, Fillingim RB. Inflammatory Genes and Psychological Factors Predict Induced Shoulder Pain Phenotype. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, III, Sack BK, Herbstman DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136(1-2):53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].George SZ, Wallace MR, Wu SS, Moser MW, Wright TW, Farmer KW, Borsa PA, Parr JJ, Greenfield WH, 3rd, Dai Y, Li H, Fillingim RB. Biopsychosocial influence on shoulder pain: risk subgroups translated across preclinical and clinical prospective cohorts. Pain. 2015;156(1):148–156. doi: 10.1016/j.pain.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- [44].Hashmi JA, Davis KD. Women experience greater heat pain adaptation and habituation than men. Pain. 2009;145(3):350–357. doi: 10.1016/j.pain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [45].Hashmi JA, Davis KD. Effects of temperature on heat pain adaptation and habituation in men and women. Pain. 2010;151(3):737–743. doi: 10.1016/j.pain.2010.08.046. [DOI] [PubMed] [Google Scholar]
- [46].Hashmi JA, Davis KD. Deconstructing sex differences in pain sensitivity. Pain. 2014;155(1):10–13. doi: 10.1016/j.pain.2013.07.039. [DOI] [PubMed] [Google Scholar]
- [47].Hassett AL, Finan PH. The Role of Resilience in the Clinical Management of Chronic Pain. Current pain and headache reports. 2016;20(6):39. doi: 10.1007/s11916-016-0567-7. [DOI] [PubMed] [Google Scholar]
- [48].Hastie BA, Riley JL, III, Fillingim RB. Ethnic differences in pain coping: factor structure of the coping strategies questionnaire and coping strategies questionnaire-revised. JPain. 2004;5(6):304–316. doi: 10.1016/j.jpain.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [49].Hastie BA, Riley JL, III, Kaplan L, Herrera DG, Campbell CM, Virtusio K, Mogil JS, Wallace MR, Fillingim RB. Ethnicity interacts with the OPRM1 gene in experimental pain sensitivity. Pain. 2012 doi: 10.1016/j.pain.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hasvik E, Iordanova Schistad E, Grovle L, Julsrud Haugen A, Roe C, Gjerstad J. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex - OPRM1 A118G polymorphism interaction: a prospective 1-year observational study. BMC musculoskeletal disorders. 2014;15:161. doi: 10.1186/1471-2474-15-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hollingshead NA, Ashburn-Nardo L, Stewart JC, Hirsh AT. The Pain Experience of Hispanic Americans: A Critical Literature Review and Conceptual Model. The journal of pain : official journal of the American Pain Society. 2016;17(5):513–528. doi: 10.1016/j.jpain.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jimenez N, Galinkin JL. Personalizing pediatric pain medicine: using population-specific pharmacogenetics, genomics, and other -omics approaches to predict response. Anesthesia and analgesia. 2015;121(1):183–187. doi: 10.1213/ANE.0000000000000721. [DOI] [PubMed] [Google Scholar]
- [53].Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. The journal of pain : official journal of the American Pain Society. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [54].Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. The journal of pain : official journal of the American Pain Society. 2014;15(10):979–984. doi: 10.1016/j.jpain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- [55].Lautenbacher S. Experimental approaches in the study of pain in the elderly. Pain medicine (Malden, Mass) 2012;13(Suppl 2):S44–50. doi: 10.1111/j.1526-4637.2012.01326.x. [DOI] [PubMed] [Google Scholar]
- [56].Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- [57].Lavin R, Park J. A characterization of pain in racially and ethnically diverse older adults: a review of the literature. Journal of applied gerontology : the official journal of the Southern Gerontological Society. 2014;33(3):258–290. doi: 10.1177/0733464812459372. [DOI] [PubMed] [Google Scholar]
- [58].Linton SJ. A review of psychological risk factors in back and neck pain. Spine. 2000;25(9):1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- [59].Lotsch J, Stuck B, Hummel T. The human mu-opioid receptor gene polymorphism 118A > G decreases cortical activation in response to specific nociceptive stimulation. BehavNeurosci. 2006;120(6):1218–1224. doi: 10.1037/0735-7044.120.6.1218. [DOI] [PubMed] [Google Scholar]
- [60].Macfarlane GJ, Beasley M, Prescott G, McNamee P, Keeley P, Artus M, McBeth J, Hannaford P, Jones GT, Basu N, Norrie J, Lovell K. The Maintaining Musculoskeletal Health (MAmMOTH) Study: Protocol for a randomised trial of cognitive behavioural therapy versus usual care for the prevention of chronic widespread pain. BMC musculoskeletal disorders. 2016;17:179. doi: 10.1186/s12891-016-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Main CJ. The importance of psychosocial influences on chronic pain. Pain Manag. 2013;3(6):455–466. doi: 10.2217/pmt.13.49. [DOI] [PubMed] [Google Scholar]
- [62].Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825–836. doi: 10.1001/jama.2014.9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matic M, van den Bosch GE, de Wildt SN, Tibboel D, van Schaik RH. Genetic variants associated with thermal pain sensitivity in a paediatric population. Pain. 2016 doi: 10.1097/j.pain.0000000000000664. [DOI] [PubMed] [Google Scholar]
- [64].McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. BestPractResClinRheumatol. 2007;21(3):403–425. doi: 10.1016/j.berh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [65].Meints SM, Miller MM, Hirsh AT. Differences in Pain Coping Between Black and White Americans: A Meta-Analysis. The journal of pain : official journal of the American Pain Society. 2016;17(6):642–653. doi: 10.1016/j.jpain.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28(6):258–266. doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- [67].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. NatRevNeurosci. 2012;13(12):859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- [68].Molton IR, Terrill AL. Overview of persistent pain in older adults. The American psychologist. 2014;69(2):197–207. doi: 10.1037/a0035794. [DOI] [PubMed] [Google Scholar]
- [69].Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. The journal of pain : official journal of the American Pain Society. 2015;16(8):769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Naugle KM, Cruz-Almeida Y, Fillingim RB, Staud R, Riley JL., 3rd Novel method for assessing age-related differences in the temporal summation of pain. Journal of pain research. 2016;9:195–205. doi: 10.2147/JPR.S102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nielsen CS, Staud R, Price DD. Individual differences in pain sensitivity: measurement, causation, and consequences. JPain. 2009;10(3):231–237. doi: 10.1016/j.jpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- [72].Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Roe C, Gjerstad J. Pain Intensity the First Year after Lumbar Disc Herniation Is Associated with the A118G Polymorphism in the Opioid Receptor Mu 1 Gene: Evidence of a Sex and Genotype Interaction. JNeurosci. 2012;32(29):9831–9834. doi: 10.1523/JNEUROSCI.1742-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Oosterman JM, Veldhuijzen DS. On the interplay between chronic pain and age with regard to neurocognitive integrity: Two interacting conditions? Neurosci Biobehav Rev. 2016;69:174–192. doi: 10.1016/j.neubiorev.2016.07.009. [DOI] [PubMed] [Google Scholar]
- [74].Platts-Mills TF, Esserman DA, Brown DL, Bortsov AV, Sloane PD, McLean SA. Older US emergency department patients are less likely to receive pain medication than younger patients: results from a national survey. Ann Emerg Med. 2012;60(2):199–206. doi: 10.1016/j.annemergmed.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- [76].Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008 doi: 10.1016/j.pain.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain. 2010;150(2):309–318. doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- [78].Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception - part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153(3):619–635. doi: 10.1016/j.pain.2011.11.026. [DOI] [PubMed] [Google Scholar]
- [79].Rahim-Williams B, Riley JL, III, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13(4):522–540. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Riley JL, III, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150(1):153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Slade GD, Sanders AE, Ohrbach R, Bair E, Maixner W, Greenspan JD, Fillingim RB, Smith S, Diatchenko L. COMT Diplotype Amplifies Effect of Stress on Risk of Temporomandibular Pain. J Dent Res. 2015;94(9):1187–1195. doi: 10.1177/0022034515595043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sturgeon JA, Zautra AJ. Resilience: a new paradigm for adaptation to chronic pain. Current pain and headache reports. 2010;14(2):105–112. doi: 10.1007/s11916-010-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tighe PJ, Le-Wendling LT, Patel A, Zou B, Fillingim RB. Clinically derived early postoperative pain trajectories differ by age, sex, and type of surgery. Pain. 2015;156(4):609–617. doi: 10.1097/01.j.pain.0000460352.07836.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Turk DC, Fillingim RB, Ohrbach R, Patel KV. Assessment of Psychosocial and Functional Impact of Chronic Pain. The journal of pain : official journal of the American Pain Society. 2016;17(9 Suppl):T21–49. doi: 10.1016/j.jpain.2016.02.006. [DOI] [PubMed] [Google Scholar]
- [85].Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377(9784):2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- [86].Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–665. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- [87].Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]