Abstract
Objective
We assessed the hypothesis that interstitial myocardial fibrosis measured by cardiac magnetic resonance imaging (CMR) predicts left ventricular (LV) beneficial remodeling in non-ischemic dilated cardiomyopathy (NIDCM) after heart failure (HF) treatment including mineralocorticoid receptor antagonists (MRA).
Background
The myocardial longitudinal relaxation time (T1) on CMR, can quantify myocardial fibrosis in the presence or absence of visually detectable late gadolinium (Gd) enhancement (LGE) MRA treatment produces beneficial remodeling in NIDCM.
Methods
Twelve patients with NIDCM, on stable beta blocker and angiotensin converting-enzyme inhibitor/angiotensin receptor blocking therapy, were studied before and after 6–29 months of MRA, with CMR assessment of LV structure, function, and T1 from standard Look-Locker sequences (T1LL).
Results
All patients had depressed cardiac function, dilated left ventricles and no visual LGE. After adding MRA to HF treatment, the LV ejection fraction increased and the LV end-systolic volume index (LVESVI (LVESV/m2)) decreased in all patients (p<0.0001). This this was inversely proportional to the baseline myocardial T1LL (r=−0.65, P=0.02).
Conclusion
Myocardial T1LL, in the absence of visually detectable LGE, was quantitatively related to the degree of beneficial LV remodeling achieved in response to adding MRA to a HF regimen.
Keywords: cardiac magnetic resonance imaging, myocardial fibrosis, heart failure
INTRODUCTION
Nonischemic dilated cardiomyopathy (NIDCM) is characterized by left ventricular (LV) dilation, systolic dysfunction, and myocardial fibrosis [1], which is associated with worse clinical status and poor prognosis[2]. Mineralocorticoid receptor antagonism (MRA) reduces morbidity and/or mortality in ischemic and nonischemic cardiomyopathies [3]. Cardiac magnetic resonance imaging (CMR), using gadolinium (Gd) contrast, is used to visually detect late Gd enhancement (LGE) and myocardial fibrosis. T1, the exponential time constant for CMR longitudinal relaxation, provides a quantitative measurement proportional to the fibrosis burden, detecting fibrosis even when the myocardium appears visually normal (LGE absent)[4]. We examined the hypothesis that myocardial T1 predicts the degree of beneficial reverse remodeling achieved by HF therapy including MRA.
METHODS
Study Design and Participants
Twelve newly diagnosed NIDCM patients underwent CMR studies before and after adding MRA to a standard HF regimen. 590 clinic visits with a diagnosis of HF were reviewed for inclusion/exclusion criteria, and 16 patients were enrolled in the study. The inclusion criteria were: age >18, NYHA Functional Class II-IV HF, echocardiographic LV ejection fraction (LVEF) of < 35%, and serum potassium below 5.0 mmol/L. Exclusion criteria were: 1) presence of or indication for cardioverter-defibrillator (ICD), 2) prior myocardial infarction on ECG, 3) stress test positive for myocardial ischemia or infarction, or 4) angiographic coronary artery disease with 50% or greater stenosis in a major epicardial artery. Further exclusion criteria included reversible causes of LV dysfunction such as myocarditis, post-partum cardiomyopathy and alcoholic cardiomyopathy, severe chronic obstructive airway disease, creatinine > 2.5 mg/dL or estimated glomerular filtration rate (eGFR) < 30 ml/min/m2 (contraindication for Gd), uncontrolled atrial fibrillation, current MRA therapy, and physician preference. Of 16 enrolled, 4 did not complete the protocol because of normal LVEF on CMR (1), bronchoconstriction with adenosine (1), patient withdrawal (1) and placement of an ICD for primary prevention (1), leaving a group of 12 subjects (8 male, 4 female). Beta-adrenergic blocking (BB) drugs and an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocking (ARB) drug were uptitrated to stable and maximally tolerated doses for at least 3 months prior to the initial CMR study. The study was approved by our IRB, and the patients gave informed consent.
Cardiac MR Imaging and T1 Analysis
CMR was performed using a 1.5-T Siemens Magnetom Avanto scanner (Erlangen, Germany), with a previously published protocol [5]. The post-contrast myocardial T1 was calculated using a TI “scout” or Look-Locker (LL) inversion recovery sequence acquired in a single mid ventricular, short axis imaging plane with progressively longer TI times. Post contrast T1 times, termed “T1 Look Locker” (T1LL), were determined using MRMAP v 1.4 software [6] based on a 3-parameter Levenberg-Marquardt curve fitting [7]. Individual pixels were manually selected in the interventricular septum, extending from the inferior to the superior right ventricular (RV) insertions, to minimize artifact secondary to cardiac motion, and to avoid the RV and LV blood pool signal (Supplementary Figure 1). Pixels were analyzed for T1LL; 12–24 measurements were made depending on wall thickness, and the results were averaged. The T1LL results were highly reproducible and are shown in the on-line supplementary material.
Statistical Analysis
Data are presented as mean values (±SD) and normalized for body surface area where applicable. LV volumes and myocardial T1LL before and after adding MRA treatment were compared using the Wilcoxon signed-rank one-sample paired test. The relationships between myocardial T1LL and LV beneficial remodeling were expressed using linear regression analysis. Statistical analyses were performed in R (R Development Core Team, Vienna, Austria). Statistical significance was judged to be two-tailed P 0.05. Graphs were produced using Graphpad Prism (GraphPad Software Inc, La Jolla California USA, www.graphpad.com).
RESULTS
Clinical data (Table 1)
Table 1.
Patient Characteristics at Baseline CMR and Medications.
| Mean (SD) | |
|---|---|
| Age yrs | 48 (11) |
| Sex (M/F) (N) | 8/4 |
| SBP mmHg | 123 (18) |
| DBP mmHg | 69 (12) |
| HR bpm | 65 (10) |
| Weeks on Medications preceding study 1: | |
| ACE-I/ARB | 22 (19) |
| BB | 22 (19) |
| MRA | |
| Spironolactone 25 mg daily (N) | 5 |
| Spironolactone 50 mg daily (N) | 6 |
| Eplerenone 50 mg daily (N) | 1 |
SBP=systolic blood pressure; DBP=diastolic blood pressure; HR=heart rate; ACE-I=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocking drug; BB=beta-adrenergic blocking drug; MRA=mineralocorticoid receptor antagonist.
The patients’ mean age was 48 years (range 27– 63 years), and the mean duration of HF was 19 (17) months, based on symptoms (range 3–57 months). The mean baseline LVEF was severely depressed, averaging 22 (7)%, with LV dilatation and reduced LV stroke volumes compared to normative data [8]. Eleven patients received spironolactone, and one received eplerenone (due to spironolactone intolerance). Repeat CMR studies were performed after 51(29) weeks of treatment (range 25–120, median 32).
Relation of baseline T1LL to LV remodeling
The mean LVEF improved significantly from 22% at baseline to 47% following the addition of MRA (P=0.007) (Table 2).
Table 2.
Patient Parameters Before and After MRA Treatment.
| N | Baseline | After MRA | Difference | P | |
|---|---|---|---|---|---|
| EF (%) | 12 | 22 (7) | 47 (7) | 25 (11) | <0.001 |
| LVEDV (mL) | 12 | 173.6 (37.5) | 153.3 (45.5) | −20.4 (13.7) | 0.001 |
| LVEDVI (mL/m2) | 12 | 83.5 (14.7) | 73.7 (19.4) | −9.8 (6.7) | 0.001 |
| LVESV (mL) | 12 | 135 (29.1) | 82 (29.1) | −53 (17.1) | <0.001 |
| LVESVI (mL/m2) | 12 | 65.3 (13.9) | 39.2 (12.6) | −26.1 (10.1) | <0.001 |
| LVSV (mL) | 12 | 38.6 (16.2) | 71.4 (20.8) | 32.8 (18.3) | <0.001 |
| LVSVI (mL/m2) | 12 | 18.1 (6.7) | 34.5 (9.5) | 16.3 (10.5) | <0.001 |
| LVM (g) | 12 | 173.2 (41.3) | 158.8 (36.7) | −14.33 (14.3) | 0.005 |
| LVMI (g/m2) | 12 | 82.1 (11.7) | 74.3 (9.7) | −7.7 (8.7) | 0.002 |
| T1 (ms) | 12 | 375 (23) | 381 (42) | 6 (37) | 0.435 |
EF = Ejection Fraction
LVEDV(I) =Left Ventricular End Diastolic Volume (Indexed for BSA)
LVESV(I) =Left Ventricular End Systolic Volume (Indexed for BSA)
LVM(I) =Left Ventricular Mass (indexed for BSA)
LVSV(I) = Left Ventricular Stroke Volume (Indexed for BSA)
MRA = mineralocorticoid antagonists
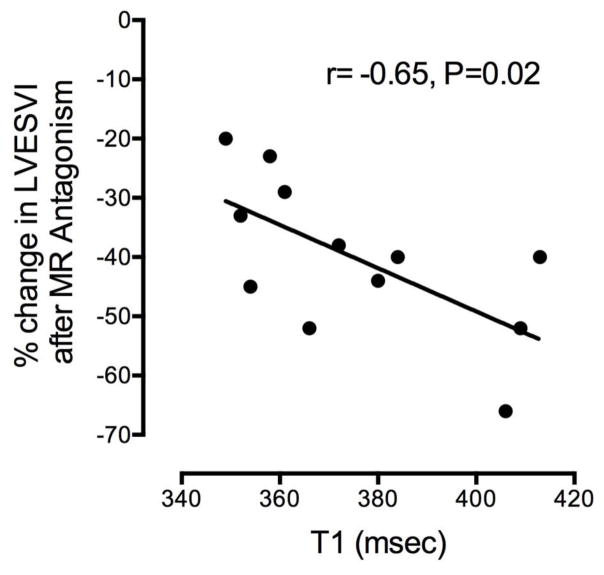
The percent decrease in LVESVI (beneficial remodeling) following MRA was related inversely to the T1LL on the baseline CMR (r=−0.65, P=0.02) (Figure).
The patients with longer baseline T1LL (consistent with less interstitial fibrosis) had greater decreases in LVESVI. Conversely, the patients with shorter baseline T1LL had lesser decreases in LVESVI. In spite of the significant beneficial remodeling, the myocardial T1LL was not modified by HF treatment, and was not significantly different from baseline (P=0.44).
DISCUSSION
This study showed that T1LL on CMR has utility for identifying dilated cardiomyopathies with the least fibrosis and the greatest potential for beneficial LV remodeling in response to anti-failure therapy that included MRA. We believe this is the first demonstration linking the therapeutic response to HF therapy with the findings of interstitial fibrosis on CMR.
The contrast agent, Gd, shortens the relaxation time of adjacent protons (and thus T1) in proportion to the tissue Gd concentration [9], is detectable as LGE on CMR [2], and is strongly associated with worse clinical status and poor outcomes [2]. In contrast, diffuse interstitial fibrosis is more difficult to detect and quantify. The T1LL technique we employed detects interstitial fibrosis even where LGE is not visually detected [4]. In patients with shorter T1LL (more fibrosis), there was less beneficial remodeling, as shown by smaller decreases in LVESVI. Conversely, patients with longer T1LL (less fibrosis), had a greater degree of beneficial remodeling, shown by greater decreases in LVESVI. This finding expands upon prior work showing the lack of myocardial LGE is an independent predictor of LV beneficial remodeling [10]. To our knowledge, the present study uniquely identifies the T1LL measurement, in the absence of visually detectable LGE, as an additional, quantitative predictor of LV remodeling by MRA. This finding may have significant prognostic value since more than half of NIDCM patients enrolled in imaging studies do not have LGE [10, 11]. We speculate that interstitial fibrosis may precede the development of overt LGE. As noted, our patients had no LGE, and the mean duration of HF symptoms at enrollment was 19 months (range 3–57), suggesting a relatively short duration of HF and greater potential for benefitting from anti-failure therapy that included MRA. Because of the relatively small patient population, this should currently be considered an hypothesis generating study.
Strengths and Limitations
The patient population was small, but the associations between T1LL and LV remodeling are based on physiologically plausible mechanisms. With the high precision of CMR, fewer patients are needed to establish a finding than with echocardiography [12]. Further, our results were aligned with previously reported systolic HF studies demonstrating beneficial remodeling in response to MRA [13].
The T1LL values were derived from conventional LL sequences available at the time of this study. Modified LL sequences (MOLLI) are now available and minimize cardiac and respiratory motion, but comparisons of LL and MOLLI demonstrate good agreement, supporting the applicability of our results [14]. We minimized cardiac and respiratory motion registration errors by measuring T1LL in the interventricular septum, with manual motion correction as needed. The Gd dose and injection-to-T1 acquisition time were tightly controlled. With this attention to detail, our results had excellent intra-observer reproducibility (see on line supplement).
The extracellular volume (ECV) index incorporates the post-contrast T1 for estimating myocardial fibrosis. We did not calculate ECV because a pre-contrast T1 time was not then in our protocol. It should be noted that significant literature supports the validity of both post-contrast T1 and ECV as quantitative measures of fibrosis. [4],[15] Thus, based on our results, we conclude that T1LL identifies patients with the greatest potential for beneficial LV remodeling, and such an evaluation may be therapeutically useful.
Supplementary Material
Supplementary Figure 1a. Method for T1 determination. Please see text for details.
Supplementary Figure 2. Reproducibility of T1 analysis. A. Intra-observer reproducibility B. Bland-Altman plot. C and D. Relation between 2 methods of T1 analysis. Please see text for details.
Figure 1.
Figure. Relation between LVESVI after adding MR antagonist and baseline T1. (Please see text for details and Table 2 for abbreviations).
Acknowledgments
Supported in part by a Discovery Grant from the Vanderbilt University Medical Center and by the Vanderbilt CTSA grant UL1RR024975 NCRR/NIH, CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences and U01HL100398. Drs. Adkisson and Bell were supported by T32 HL 07411-31. Dr. Bell is supported by K12 HD 043483-11 from NIH/NICHD, Paul B. Beeson K23AG048347 award from NIA and by the Eisenstein Women’s Heart Fund. There are no relations with industry to disclose for any authors. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
The authors thank Adam Stein, AS, RT, Francesca Sabo, BS, RT Donald CiFelli, BS, RT, Barbara Konz, RN, Amber Brock, RN, Debra Rassel, RN, Linda Howerton, RN, Brenda White, RN and Rebecca Hung MD for their contributions. The authors thank the Vanderbilt Heart Advisory Council Fund for their support. The Gd-DTPA was used off-label.
Footnotes
Clinical trials Registration Clinical Trials.Gov ID NCT00574119.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 2.Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc Imaging. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 4.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265:724–32. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell SP, Adkisson DW, Lawson MA, Wang L, Ooi H, Sawyer DB, et al. Antifailure therapy including spironolactone improves left ventricular energy supply-demand relations in nonischemic dilated cardiomyopathy. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114. pii: e000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messroghli DR, Rudolph A, Abdel-Aty H, Wassmuth R, Kuhne T, Dietz R, et al. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging. 2010;10:16. doi: 10.1186/1471-2342-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, et al. Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution--reproducibility study. Radiology. 2006;238:1004–12. doi: 10.1148/radiol.2382041903. [DOI] [PubMed] [Google Scholar]
- 8.Salton CJ, Chuang ML, O’Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–60. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 9.Nelson KL, Runge VM. Basic principles of MR contrast. Top Magn Reson Imaging. 1995;7:124–36. [PubMed] [Google Scholar]
- 10.Masci PG, Schuurman R, Andrea B, Ripoli A, Coceani M, Chiappino S, et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013;6:790–9. doi: 10.1161/CIRCIMAGING.113.000438. [DOI] [PubMed] [Google Scholar]
- 11.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 12.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 13.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1228–33. doi: 10.1016/s0735-1097(01)01116-0. [DOI] [PubMed] [Google Scholar]
- 14.Nacif MS, Turkbey EB, Gai N, Nazarian S, van der Geest RJ, Noureldin RA, et al. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34:1367–73. doi: 10.1002/jmri.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kammerlander AA, Marzluf BA, Zotter-Tufaro C, Aschauer S, Duca F, Bachmann A, et al. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc Imaging. 2016;9:14–23. doi: 10.1016/j.jcmg.2015.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1a. Method for T1 determination. Please see text for details.
Supplementary Figure 2. Reproducibility of T1 analysis. A. Intra-observer reproducibility B. Bland-Altman plot. C and D. Relation between 2 methods of T1 analysis. Please see text for details.