Abstract
Objective
To evaluate the impact of an emergency department (ED) mechanical ventilation protocol on clinical outcomes and adherence to lung-protective ventilation in patients with acute respiratory distress syndrome (ARDS).
Design
Quasi-experimental, before-after trial.
Setting
ED and intensive care units (ICU) of an academic center.
Patients
Mechanically ventilated ED patients experiencing ARDS while in the ED or after admission to the ICU.
Interventions
An ED ventilator protocol which targeted parameters in need of quality improvement, as identified by prior work: 1) lung-protective tidal volume; 2) appropriate setting of positive end-expiratory pressure (PEEP); 3) oxygen weaning; and 4) head-of-bed elevation.
Measurements and Main Results
A total of 229 patients (186 pre-intervention group, 43 intervention group) were studied. In the ED, the intervention was associated with significant changes (P < 0.01 for all) in tidal volume, PEEP, respiratory rate, oxygen administration, and head-of-bed elevation. There was a reduction in ED tidal volume from 8.1 mL/kg PBW (7.0 – 9.1) to 6.4 mL/kg PBW (6.1 – 6.7), and an increase in lung-protective ventilation from 11.1% to 61.5%, P < 0.01. The intervention was associated with a reduction in mortality from 54.8% to 39.5% (OR 0.38, 95% CI 0.17 – 0.83, P = 0.02), and a 3.9 day increase in ventilator-free days, P = 0.01.
Conclusions
This before-after study of mechanically ventilated patients with ARDS demonstrates that implementing a mechanical ventilator protocol in the ED is feasible, and associated with improved clinical outcomes.
Keywords: lung-protective ventilation, emergency department, ARDS
INTRODUCTION
Acute respiratory distress syndrome (ARDS) carries unacceptably high mortality and survivor morbidity rates.(1) Unequivocal evidence shows that lung-protective ventilation (LPV), aimed at mitigating ventilator-associated lung injury (VALI) reduces mortality in ARDS.(2) Despite this, noncompliance with LPV remains high and negatively impacts outcome.(3–9)
The emergency department (ED), where over 200,000 patients are mechanically ventilated annually in the U.S., may be important for providing LPV to improve ARDS outcome for several reasons.(10) Experimental data shows that VALI can occur shortly after initiation of mechanical ventilation; ED lengths of stay are often more than sufficient to begin the process of VALI.(11–13) Ventilator settings prior to intensive care unit (ICU) arrival influence initial ventilator settings in the ICU; this may be critically important, as delayed initiation of low tidal volume after ARDS onset appears especially influential, both for adherence to LPV and mortality.(6, 14, 15) Even if delivered for comparatively brief periods, LPV can impart significant benefit, as demonstrated by data from the operating room (OR) and in lung donation.(16, 17) Finally, cohort studies have demonstrated that adherence to LPV in the ED is poor and that ED mechanical ventilation is associated with ARDS development.(14, 18, 19) Therefore, a pre-ICU intervention aiming to improve mechanical ventilation in the ED may be an effective strategy to improve adherence to LPV and reduce ARDS mortality.
The objectives of this study were to assess the impact of an ED-based mechanical ventilator protocol on: 1) clinical outcomes in ARDS; and 2) delivery of LPV. We hypothesized that a strategy aimed at improving ED mechanical ventilation practices would reduce mortality and increase adherence to LPV in the ED and ICU.
MATERIALS AND METHODS
Study Design
This was a quasi-experimental, before-after trial performed at a tertiary, academic center. The study design included a pre-intervention period (September 2009 – January 2014), a six month run-in when LPV was implemented as the default ventilator strategy in the ED, and an intervention period (October 2014 – March 2016). During the run-in period, training consisted of a major journal club review, which introduced the scientific merit of early LPV, as well as meetings, lectures, and bedside education on how to implement the protocol. Following this implementation initiative, the intervention period commenced.
Since LPV was adopted as the standard approach in the ED in 2014, the study was approved with waiver of consent. A detailed description of the methods has been published.(20) The current report is a pre-planned sub-study on ARDS patients from the Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED) trial. The trial registration number is NCT02543554.
Participants
All mechanically ventilated patients in the ED were assessed for inclusion. To identify these patients in the pre-intervention group, a validated electronic query was used.(20) The intervention group was identified with an automated, electronic pager system, followed prospectively and enrolled consecutively, twenty-four hours per day. Inclusion criteria were: 1) aged ≥ 18 years; 2) mechanical ventilation via endotracheal tube; and 3) ARDS onset within seven days of ED presentation. Seven days was chosen because the great majority of patients that develop ARDS after admission from the ED will do so within this time period.(14, 19, 21) Exclusion criteria were: 1) extubation within 24 hours; 2) death in the ED or within 24 hours; 3) chronic mechanical ventilation; 4) presence of tracheostomy; and 5) transfer to another hospital.
Treatment Interventions
Our previous research demonstrated that mechanical ventilation in the ED was historically delivered with higher tidal volumes, low positive end-expiratory pressure (PEEP)-high fraction of inspired oxygen (FiO2) combinations, and poor adherence to head-of-bed elevation.(14, 19) To improve these practices, the intervention aimed to address: 1) low tidal volume to prevent volutrauma; 2) appropriate PEEP setting to limit atelectrauma; 3) limitation of hyperoxia with rapid oxygen weaning; and 4) head-of-bed elevation for aspiration precautions. After intubation, the ED respiratory therapist obtained patient height with a tape measure, and tidal volume was indexed to PBW. Ventilator settings were then established per protocol (Supplemental Digital Content 1, Supplemental Figure 1), and head-of-bed elevation was performed in all patients, unless contraindicated. This was a pragmatic study, designed to record data as part of usual care after intervention implementation. Therefore all interventions, including ventilator settings, were performed by ED clinical staff. The protocol specifically targeted the ED, and therefore all ICU ventilator management was at the discretion of the ICU clinical staff.
Assessments and Outcome Measures
Data on demographics, comorbid conditions, vital signs, laboratory variables, illness severity scores, ED length of stay, and indication for mechanical ventilation were collected via electronic record query for the pre-intervention group, and prospectively for the intervention group. ED treatment variables included intravenous fluids, blood products, central venous catheters, antibiotics, and vasopressors.
All ventilator settings in the ED were collected, as were airway pressures, pulmonary mechanics, and gas exchange variables. After admission, ICU ventilator settings were collected twice daily, and followed for up to two weeks (at a minimum) or for the duration of ARDS. For pressure-targeted modes of ventilation, where plateau pressure is not typically measured, peak pressure was used as a surrogate. Fluid balance was recorded daily after admission. Patients were followed until hospital discharge or death.
Definitions of comorbid conditions are in Supplemental Digital Content 2, Supplemental Text 1. Severe sepsis and septic shock were defined as described previously.(22) ARDS was defined according to the Berlin definition, and both groups’ ARDS status was adjudicated as previously described (Supplemental Digital Content 3, Supplemental Text 2).(14, 20, 23) ARDS onset was defined as the time when all ARDS inclusion criteria were met. LPV was defined as a tidal volume of ≤ 6.5mL/kg PBW (upper limit that defined adherent to lower tidal volume in ARDS Network trial). (2, 5)
The primary outcome was hospital mortality. Secondary outcomes included ventilator-, ICU-, and hospital-free days.
Statistical Analysis
Descriptive statistics, including mean (standard deviation [SD]), median (interquartile range [IQR]), and frequency distributions were used to assess patient characteristics. Spearman correlation (rs) was used to assess the relationship between ED and ICU tidal volume. To assess mortality predictors, categorical characteristics were compared using chi-square or Fisher’s exact test. Continuous characteristics were compared using independent samples t-test or Wilcoxon’s rank-sum test. A backward, stepwise, multivariable logistic regression model was used to evaluate death as a function of the intervention. Clinically relevant variables that were statistically significant in univariate analysis at P ≤ 0.10 were candidates for model inclusion. Given the prognostic significance of shock, receipt of vasopressors was also included in the model. A potential time-dependent effect of tidal volume on mortality was evaluated by including a statistical interaction of ICU tidal volume and mechanical ventilation duration. Variables for inclusion or exclusion from the model were selected in sequential fashion based on the significance level of .10 for entry and .10 for removal. Normality, statistical interactions, and collinearity were assessed, and the model used variables that were statistically independent. Model goodness of fit was assessed with the Hosmer-Lemeshow test and by examining residuals. Adjusted odds ratios (OR) and corresponding 95% confidence intervals (CI) are reported for the multivariable model, adjusted for all variables in the model.
To assess for potential secular trends (i.e. temporal changes) in outcomes, the pre-intervention cohort was divided into thirds, based on approximately equivalent periods of time (73.7 weeks), for comparison to the intervention cohort.
All tests were two-tailed, and a P value <0.05 was considered statistically significant.
RESULTS
Study population
In the pre-intervention group, a total of 2,451 patients were mechanically ventilated and assessed for eligibility, compared with 1,074 patients in the intervention group (Figure 1). A total of 229 patients experienced ARDS and were included in the final analysis. The mean (±SD) time to ARDS onset was 1.8 ± 1.7 days (Supplemental Digital Content 4, Supplemental Figure 2).
Figure 1. Flow diagram of patients in the study.
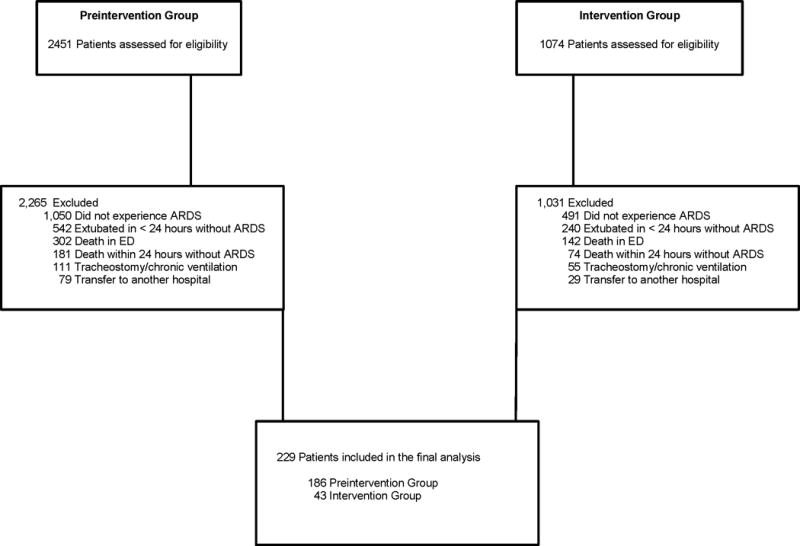
ARDS: acute respiratory distress syndrome; ED: emergency department
Table 1 presents baseline characteristics of the study population related to intervention group. ED length of stay (hours) for the pre-intervention group was 5.8 (4.0–8.2) versus 5.2 (3.6–7.5) in the intervention group, P = 0.51. Fluid balance (liters) after the first week was 5.5 (8.0) in the pre-intervention group and 5.1 (9.0) in the intervention group, P = 0.77.
Table 1.
Characteristics of mechanically ventilated emergency department patients with ARDS
| Study Group | |||
|---|---|---|---|
|
|
|||
| Baseline characteristics | Pre-intervention (n= 186) | Intervention Group (n= 43) | P value |
|
| |||
| Age (yr) | 57.7 (50.9–71.9) | 57.5 (43.5–66.3) | 0.14 |
|
| |||
| Male, n (%) | 106 (57.0) | 28 (65.1) | 0.33 |
|
| |||
| Race, n (%) | |||
| Caucasian | 97 (52.2) | 22 (51.2) | 0.91 |
| African-American | 86 (46.2) | 22 (51.2) | 0.56 |
| Other | 3 (1.6) | 0 (0.0) | 0.40 |
|
| |||
| Comorbidities, n (%) | |||
| Diabetes | 71 (38.2) | 11 (25.6) | 0.12 |
| Cirrhosis | 23 (12.4) | 7 (16.3) | 0.49 |
| CHF | 39 (21.0) | 13 (30.2) | 0.32 |
| Dialysis | 10 (5.4) | 3 (7.0) | 0.72 |
| COPD | 45 (24.1) | 9 (20.9) | 0.65 |
| Immunosuppression | 26 (14.0) | 13 (30.2) | 0.01 |
| Alcohol abuse | 25 (13.4) | 7 (16.3) | 0.63 |
| HIV/AIDS | 9 (4.8) | 2 (4.7) | 1.0 |
|
| |||
| BMI | 29.9 (11.0) | 30.5 (13.4) | 0.30 |
|
| |||
| Temperature (Celsius) | 36.8 (1.3) | 36.8 (1.5) | 0.84 |
|
| |||
| MAP | 79.3 (20.9) | 75.0 (36.0) | 0.30 |
|
| |||
| Lactate | 3.0 (1.9–6.0) | 4.7 (2.3–7.9) | 0.06 |
|
| |||
| Creatinine | 1.3 (0.9–2.4) | 1.2 (1.0–2.2) | 0.92 |
|
| |||
| Platelet | 208.3 (159.7) | 244.5 (144.6) | 0.27 |
|
| |||
| INR | 1.9 (1.7) | 1.9 (1.8) | 0.26 |
|
| |||
| Total bilirubin | 0.5 (0.3–0.9) | 0.6 (0.4–1.4) | 0.78 |
|
| |||
| Albumin | 2.9 (0.8) | 3.1 (0.8) | 0.16 |
|
| |||
| APACHE II* | 17.1 (5.5) | 23.7 (7.8) | <0.01 |
|
| |||
| SOFA* | 6.0 (4–8) | 8.0 (4–9) | 0.02 |
|
| |||
| Reason for mechanical ventilation, n (%) | |||
| Medical | 129 (69.4) | 33 (83.7) | 0.34 |
| Trauma | 20 (10.8) | 5 (11.6) | 0.87 |
| Other | 37 (19.9) | 5 (11.6) | 0.21 |
|
| |||
| Sepsis, n (%) | 101 (54.3) | 30 (69.7) | 0.17 |
|
| |||
| Process of Care Variables | |||
|
| |||
| Intravenous fluids in ED (liters) | 2.5 (2.0) | 1.7 (1.6) | 0.15 |
|
| |||
| Blood products, n (%) | 33 (17.7) | 10 (23.3) | 0.40 |
|
| |||
| Central venous catheter, n (%) | 89 (47.8) | 22 (51.2) | 0.70 |
|
| |||
| Antibiotics, n (%) | 96 (51.6) | 29 (67.4) | 0.11 |
|
| |||
| Vasopressor infusion, n (%) | 69 (37.1) | 24 (55.8) | 0.02 |
ARDS: acute respiratory distress syndrome; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; AIDS: acquired immunodeficiency syndrome; BMI: body mass index; MAP: mean arterial pressure; INR: international normalized ratio; APACHE: acute physiology and chronic health evaluation; SOFA: sequential organ failure assessment score; ED: emergency department
Continuous variables are reported as mean (standard deviation) and median (interquartile range). For measurements where more than one value was present in the ED (e.g. vital signs), the initial ED value is presented.
modified score, which excludes Glasgow Coma Scale
Ventilator characteristics
A total of 65 patients fulfilled ARDS criteria while in the ED, 164 had ARDS onset after ICU admission, and a total of 480 ED ventilator settings were analyzed. Table 2 shows the effect of the intervention on ED mechanical ventilation. There were significant changes in tidal volume, PEEP, respiratory rate, FiO2, and adherence to head-of-bed elevation. For patients with ARDS while in the ED, the intervention was associated with a reduction in tidal volume from 8.0 mL/kg PBW (7.1 – 9.1) to 6.4 mL/kg PBW (6.1 – 6.8) and an increase in LPV from 11.1% to 61.5%, P < 0.01. Supplemental Digital Content 5, Supplemental Figure 3 shows the distribution of ED tidal volume. For ventilator settings non-adherent to LPV, the intervention was associated with a reduction in tidal volume, 8.8 mL/kg PBW (8.2 – 9.6) vs. 6.9 mL/kg PBW (6.8 – 7.1), P < 0.01.
Table 2.
Emergency department ventilator variables
| Variable | Pre-intervention Group (n= 186) | Intervention Group (n= 43) | P value |
|---|---|---|---|
|
| |||
| Tidal volume, mL | 500 (500 – 550) | 434 (385 – 450) | <0.01 |
|
| |||
| Tidal volume, mL/kg PBW | 8.1 (7.0 – 9.1) | 6.4 (6.1 – 6.7) | <0.01 |
|
| |||
| PEEP | 5 (5 – 7) | 8 (5 – 10) | <0.01 |
|
| |||
| Respiratory rate | 16.5 (4.4) | 20.7 (3.8) | <0.01 |
|
| |||
| FiO2 | 100 (70 – 100) | 70 (40 – 100) | <0.01 |
|
| |||
| Head-of-bed elevation, n (%) | 151 (37.1) | 67 (91.8) | <0.01 |
|
| |||
| Lung protective ventilation, n (%)* | 12 (11.1) | 24 (61.5) | <0.01 |
|
| |||
| Ventilator Mode, n (%) | |||
| VC-AC | 347 (85.3) | 68 (93.2) | 0.07 |
| PC-AC | 39 (9.6) | 2 (2.7) | 0.05 |
| PRVC-AC | 10 (2.5) | 2 (2.7) | 0.89 |
| Other | 11 (2.7) | 1 (1.4) | 0.50 |
|
| |||
| Peak pressure, cm H2O | 33.9 (8.8) | 28.9 (7.6) | <0.01 |
|
| |||
| Plateau pressure, cmH2O | 22.3 (7.3) | 22.4 (6.4) | 0.98 |
|
| |||
| Mean airway pressure, cmH2O | 11.0 (9.0 – 14.0) | 12.0 (9.9 – 17.0) | 0.47 |
|
| |||
| Compliance respiratory system (mL/cm H2O) | 32.9 (25.0 – 42.4) | 34.5 (23.7 – 45.0) | 0.93 |
|
| |||
| Driving Pressure (cm H2O) | 14.0 (12.0 – 19.3) | 13.0 (9.0 – 17.0) | 0.04 |
|
| |||
| Oxygenation index | 7.3 (4.2 – 11.9) | 9.7 (4.8 – 16.9) | 0.04 |
|
| |||
| pH | 7.24 (0.16) | 7.21 (0.18) | 0.31 |
|
| |||
| PaO2 | 113 (78 – 198) | 85 (67 – 124) | <0.01 |
|
| |||
| PaCO2 | 46 (37 – 61) | 43 (34 – 58) | 0.64 |
|
| |||
| PaO2:FiO2 | 133 (85 – 244) | 114 (88 – 207) | 0.94 |
A total of 480 ventilator settings were analyzed (407 pre-intervention group; 73 intervention group). In the pre-intervention group, peak pressure was monitored for 322 settings (79.1%), plateau pressure for 70 settings (17.2%), and mean airway pressure for 306 settings (75.2%). In the intervention group, all pressures were monitored for each recorded ventilator setting (100%).
Sixty-five patients had ARDS while in the ED (44 pre-intervention group, 108 ventilator settings; 21 intervention group, 39 ventilator settings). Lung protective ventilation was defined as a tidal volume of ≤ 6.5mL/kg PBW.
PBW: predicted body weight; PEEP: positive end-expiratory pressure; FiO2: fraction of inspired oxygen; VC: volume control; AC: assist control; PC: pressure control; PRVC: pressure regulated volume control; PaO2: partial pressure of arterial oxygen; PaCO2: partial pressure of arterial carbon dioxide
Continuous variables are reported as mean (standard deviation) and median (interquartile range).
A total of 3,495 ICU ventilator settings were analyzed. Supplemental Digital Content 6, Supplemental Table 1 shows the comparison between ICU ventilator settings between the two groups. Following the intervention, ICU tidal volume decreased from 8.1 mL/kg PBW (7.3 – 9.1) to 7.0 (6.2 – 8.4), P < 0.01. LPV in the ICU increased from 11.4% to 35.3%, P < 0.01. In the pre-intervention group, 167 (89.8%) patients had an initial ICU tidal volume of >6.5mL/kg PBW, 37 (19.9%) patients had at least one tidal volume ≤6.5mL/kg PBW at some point, and 6 (3.2%) patients had all tidal volumes ≤6.5 mL/kg PBW. In the intervention group, 29 (67.4%) patients had an initial tidal volume of >6.5mL/kg PBW, 18 (41.9%) patients had at least one tidal volume ≤6.5mL/kg PBW, and 8 (18.6%) had all tidal volumes ≤6.5 mL/kg PBW. Additional details on changes in tidal volume and LPV over time are represented in Figure 2.
Figure 2.
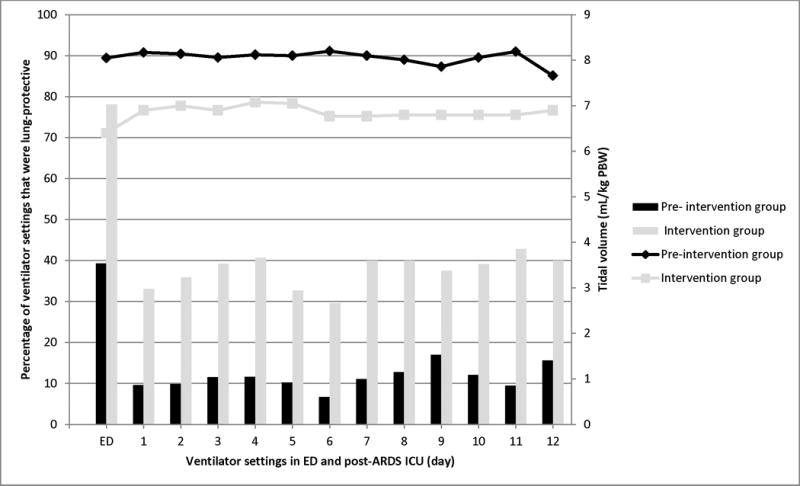
Timing of tidal volume settings and LPV in the emergency department (ED) and intensive care unit (ICU). The number of ventilator settings in the ED and ICU, respectively, were 480 and 3,495. The bars represent the percentage of ventilator settings that were lung-protective, calculated based on the number of patients alive and receiving mechanical ventilation at that point in time. The data represented by the dots and connecting lines represent the median tidal volume between the two cohorts.
After adjustment for significant covariates between the two groups (i.e. gender, age, PBW, vasopressor use), multivariable logistic regression analysis demonstrated that the ED intervention was the only significant predictor of the receipt of ICU LPV (OR 3.41, 95% CI 1.15 – 10.1, P = 0.03).
The correlation (rs) between ICU tidal volume and ED tidal volume was 0.70 in the pre-intervention group (P < 0.01) and 0.30 in the intervention group (P = 0.05).
Outcomes
The univariate comparison for the primary outcome is shown in Supplemental Digital Content 7, Supplemental Table 2. The intervention was associated with a reduction in mortality from 54.8% to 39.5%, which remained significant after multivariable logistic regression analysis (OR 0.36, 95% CI 0.16 – 0.82, P = 0.02) (Table 3). Variables included in the adjusted analysis are shown in Supplemental Digital Content 8, Supplemental Table 3.
Table 3.
Primary and secondary outcomes according to study group
| Outcome | Pre-intervention group (n= 186) | Intervention group (n= 43) | Adjusted Odds Ratio or Between-Group Difference (95% CI) | P value* |
|---|---|---|---|---|
|
| ||||
| Primary outcome, n (%) | ||||
| Mortality | 102 (54.8) | 17 (39.5) | 0.36 (0.16 – 0.82) | 0.02 |
|
| ||||
| Secondary outcomes (days) | ||||
| Ventilator-free | 7.7 (9.9) | 11.6 (10.8) | 4.0 (7.3 to 0.6) | 0.03 |
| ICU-free | 7.2 (9.4) | 9.1 (9.2) | 1.9 (5.0 to −1.2) | 0.23 |
| Hospital-free | 4.0 (6.3) | 5.7 (7.7) | 1.6 (4.2 to −0.9) | 0.20 |
P value for the primary outcome measure was a Wald test estimated using a logistic regression model accounting for age, cirrhosis, body mass index, mean arterial pressure, lactate, illness severity, sepsis, shock, oxygenation on day of ARDS onset, and intensive care unit tidal volume by time.
P values for the secondary outcomes are from the independent sample t-test
CI: confidence interval; ICU: intensive care unit
The secondary outcome analyses are shown in Table 3. Ventilator-free days were significantly higher in the intervention group than in the pre-intervention group (11.6 ± 10.8 vs. 7.7 ± 9.9 days, P = 0.03). Hospital- and ICU-free days were higher by approximately 2 days in the intervention group; this did not reach statistical significance.
Secular trends
Secular trends in tidal volume, LPV and mortality are shown in Supplemental Digital Content 9, Supplemental Table 4. The changes in tidal volume, LPV, and mortality, were a deviation from the temporal trends for the study period, and consistent with implementation of the intervention.
DISCUSSION
It has been over fifteen years since LPV showed improved survival in ARDS, yet adherence to this strategy remains poor.(2–9) Most relevant to the ED, the harm associated with delayed initiation of low tidal volume in ARDS has been established, and ventilator settings during the early course of respiratory failure are highly influential on the delivery of lung protection.(6, 14, 18) For these reasons, along with our data showing opportunity for improvement in ED mechanical ventilation practices, LPV became the default strategy in our ED in 2014. The results of the study have important additional findings.
Effective implementation of mechanical ventilation interventions in ARDS patients is feasible in the ED and associated with practice change. While endotracheal intubation has been studied extensively in the ED, mechanical ventilation has been studied little.(24, 25) Previous studies demonstrated low adherence to LPV in the ED.(14, 19) In the current study, an ED ventilator protocol was associated with a significant increase in adherence to LPV in the ED. Furthermore, amongst the ventilator settings that were by definition non-adherent to LPV, tidal volume was reduced by 1.9 mL/kg PBW. This suggests that the ED could be an important starting point for safe mechanical ventilation.
Several aspects of the ICU ventilator data deserve mention. In the pre-intervention group there was a high correlation (rs= 0.70) between ED and ICU tidal volume. In fact, comparing the ED and ICU, tidal volume (8.1 mL/kg PBW) and adherence to LPV (~11%) were virtually identical in the pre-intervention group. This is consistent with previous data showing that the initial established tidal volume after ARDS onset is highly influential in setting the course for much of the total duration of mechanical ventilation.(6, 26) However, in the intervention group, the correlation (rs= 0.30) between ED and ICU tidal volume was weaker; Figure 2 demonstrates that the first ICU tidal volume after ARDS onset was actually higher by approximately 0.6 mL/kg PBW relative to the ED. Similar to the pre-intervention group, tidal volume then remained relatively static. Between the groups, a tidal volume difference of approximately 1.2 mL/kg PBW persisted throughout the ICU stay. While seemingly small, this approximate difference in initial tidal volume has been associated with an increased incidence of ARDS in ED patients, as well as a 23% increase in mortality in ARDS.(6, 18) It is possible that the improvement in mortality associated with the intervention could have been greater had LPV been carried through from the ED to ICU at a higher rate. Furthermore, effective and consistent implementation of LPV for ARDS must emphasize: 1) early detection; 2) early implementation; and 3) short-loop feedback to revisit ventilator settings frequently. While it is encouraging that the implementation of an ED-based lung-protective protocol was associated with a decrease in ICU tidal volume and improved adherence to LPV in the ICU, significant room for improvement persists.
Finally, the intervention was associated with an improvement in mortality and resource utilization. This is consistent with previous data on tidal volume reduction in ARDS.(2, 5, 6, 27) This indicates that the use of LPV in the ED could improve clinical outcome.
Limitations
This investigation has several limitations. It was a single center study, and while implementation was effectively achieved at our center, we are unable to comment on the feasibility of implementing this protocol at other hospitals and the community as a whole. The sample size was relatively small, which can lead to an exaggeration in demonstrated benefit. However, as an exploratory study, the sample size is comparable to some randomized trials in ARDS. While causation cannot fully be established with the design, the results are consistent with randomized trials and observational studies which show that LPV improves outcome.(2, 5, 6, 28) However, unmeasured confounders could have accounted for the improved outcome in the intervention period. There was low baseline adherence to LPV and high mortality. It is possible that the intervention would have imparted less impact in the setting of greater baseline adherence to LPV or lower baseline mortality. The before-after study design can make results prone to temporal trends. Analysis of secular trends in ventilator management, as well as mortality, demonstrated that the most significant changes were isolated to the implementation of LPV in the ED. Some imbalance in baseline characteristics between the two study groups did exist. However, these imbalances should have biased our findings toward the null hypothesis (e.g. differences in immunosuppression, lactate, APACHE II, and vasopressors), and our findings remained robust after statistical adjustment. Finally, the intervention addressed several parameters; it was a bundle. Given the abundance of data regarding the importance of early LPV in ARDS, we hypothesize that a decrease in early VALI drove these findings. However, without mechanistic outcomes, we cannot elucidate where the exact benefit is coming from.
Future directions
Going forward, we must move beyond mechanical ventilation and ARDS as primarily ICU-specific entities. Timing has been established in other diseases and syndromes which span the ED-ICU interface (e.g. sepsis, trauma, stroke).(26) This has not extended to ARDS yet. Appropriate pre-ICU ventilator settings could reduce mortality by overcoming some of the existing shortfalls in the implementation of LPV.(26) In addition, as ARDS develops in a minority of mechanically ventilated patients, whether LPV is beneficial in those without ARDS remains a question to be answered by forthcoming data, including that from our at-risk cohort in the LOV-ED trial.(20)
CONCLUSIONS
This before-after study of mechanically ventilated patients with ARDS demonstrates that implementing a mechanical ventilator protocol in the ED is feasible, and is associated with a reduction in mortality.
Supplementary Material
Supplemental Digital Content 1, Supplemental Figure 1. Emergency department ventilator protocol
Supplemental Digital Content 2, Supplemental Text 1. Definitions of comorbid conditions
Supplemental Digital Content 3, Supplemental Text 2. ARDS adjudication process
Supplemental Digital Content 4, Supplemental Figure 2. ARDS Incidence by Day
Supplemental Digital Content 5, Supplemental Figure 3. Emergency department tidal volume distribution
Supplemental Digital Content 6, Supplemental Table 1. Intensive care unit ventilator variables
Supplemental Digital Content 7, Supplemental Table 2. Univariate comparison for mortality
Supplemental Digital Content 8, Supplemental Table 3. Multivariable analysis for mortality
Supplemental Digital Content 9, Supplemental Table 4. Secular trends in tidal volume, LPV, and mortality
Acknowledgments
We are indebted to the respiratory therapists and ED team at Barnes-Jewish Hospital who cared for these patients on a daily basis.
Sources of Funding: BMF and AMD were funded by the KL2 Career Development Award, and this research was supported by the Washington University Institute of Clinical and Translational Sciences (Grants UL1 TR000448 and KL2 TR000450) from the National Center for Advancing Translational Sciences (NCATS). BMF was also funded by the Foundation for Barnes-Jewish Hospital Clinical and Translational Sciences Research Program (Grant # 8041-88). AMD was also funded by the Foundation for Anesthesia Education and Research. NMM was supported by grant funds from the Emergency Medicine Foundation and the Health Resources and Services Administration. EA was supported by the Washington University School of Medicine Faculty Scholars grant and the Foundation for Barnes-Jewish Hospital. RJS was supported by the Clinical and Translational Science Award (CTSA) program of the NCATS of the National Institutes of Health (NIH) under Award Numbers UL1 TR000448 and TL1 TR000449. CCB was supported by the Short-Term Institutional Research Training Grant, NIH T35 (NHLBI). RSH was supported by NIH grants R01 GM44118-22 and R01 GM09839. MHK was supported by the Barnes-Jewish Hospital Foundation.
Copyright form disclosures: Drs. Fuller, Drewry, Stephens, Briscoe, and Hotchkiss received support for article research from the National Institutes of Health (NIH). Dr. Fuller’s institution received funding from KL2 Career Development Award KL2 TR000450 and from Foundation for Barnes-Jewish Hospital Clinical and Translational Sciences Research Program (Grant # 8041-88). Dr. Drewry’s institution received funding from Foundation for Anesthesia Education and Research and from the NIH. Dr. Mohr disclosed other support from Emergency Medicine Foundation, Iowa Health Care Collaborative, NIH, National Institute on Minority Health, and Health Disparities Children’s Miracle Network. He received funding from Illinois College of Emergency Physicians, Annals of Emergency Medicine Editorial Board, and from the University of Nebraska. Dr. Ablordeppey’s institution received funding from Washington University School of Medicine, and from Barnes Jewish Hospital Foundation. Dr. Keeperman received funding from Teleflex (provided clinical education on vascular access devices). Dr. Stephens disclosed other support from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the NIH under Award Numbers UL1 TR000448 and TL1 TR000449. He also received funding from NIH. Dr. Briscoe received funding from NIH T35 NHLBI Training Grant Grant Title: Short-Term Training in Health Professional Schools Grant Number: 5 T35 HL007815 Grant PI: Dr. Koong-Nah Chung. Dr. Hotchkiss disclosed other support: I have research grants with GSK and BMS for studies testing immunotherapeutics in mice and in patient blood.
Footnotes
This work was performed at Washington University School of Medicine in St. Louis.
Conflicts of Interest
All authors have no relevant financial disclosures or conflicts of interest.
Contributor Information
Brian M. Fuller, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
Ian T. Ferguson, School of Medicine and Medical Science, University College Dublin, Dublin 4, Ireland.
Nicholas M. Mohr, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, 200 Hawkins Drive, 1008 RCP, Iowa City, IA 52242.
Anne M. Drewry, Department of Anesthesiology, Division of Critical Care Medicine, Washington University School of Medicine in St. Louis.
Christopher Palmer, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
Brian T. Wessman, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
Enyo Ablordeppey, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
Jacob Keeperman, Departments of Emergency Medicine and Anesthesiology, Division of Critical Care, Washington University School of Medicine in St. Louis, St. Louis, MO 63110.
Robert J. Stephens, Washington University School of Medicine in St. Louis, St. Louis, MO 63110
Cristopher C. Briscoe, Washington University School of Medicine in St. Louis, St. Louis, MO 63110
Angelina A. Kolomiets, School of Public Health and Social Justice, Saint Louis University, St. Louis, MO 63104.
Richard S. Hotchkiss, Department of Anesthesiology, Division of Critical Care Medicine, Washington University School of Medicine in St. Louis.
Marin H. Kollef, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Washington University School of Medicine in St. Louis.
References
- 1.Rubenfeld G, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Oh DK, Lee MG, Choi EY, et al. Low–tidal volume mechanical ventilation in patients with acute respiratory distress syndrome caused by pandemic influenza A/H1N1 infection. J Crit Care. 2013;28(4):358–364. doi: 10.1016/j.jcrc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Lim C, Ruan S, et al. Factors associated with adherence to low-tidal volume strategy for acute lung injury and acute respiratory distress syndrome and their impacts on outcomes: an observational study and propensity analysis. Minerva Anestesiol. 2014;80(11):1158–1168. [PubMed] [Google Scholar]
- 5.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344 doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham DM, Yang T, Dinglas VD, et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med. 2003;167(10):1304–1309. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 8.Young M, Manning HL, Wilson DL, et al. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: Has new evidence changed clinical practice?*. Crit Care Med. 2004;32(6):1260–1265. doi: 10.1097/01.ccm.0000127784.54727.56. [DOI] [PubMed] [Google Scholar]
- 9.Weiss CH, Baker DW, Weiner S, et al. Low Tidal Volume Ventilation Use in Acute Respiratory Distress Syndrome. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Easter BD, Fischer C, Fisher J. The use of mechanical ventilation in the ED. Am J Emerg Med. 2012;30(7):1183–1188. doi: 10.1016/j.ajem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Webb HH, Tierney DF. Experimental Pulmonary Edema due to Intermittent Positive Pressure Ventilation with High Inflation Pressures. Protection by Positive End-Expiratory Pressure 1–4. American Review of Respiratory Disease. 1974;110(5):556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 12.Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. American Review of Respiratory Disease. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 13.Herring A, Ginde A, Fahimi J, et al. Increasing critical care admissions from US emergency departments, 2001–2009*. Crit Care Med. 2013;41(5):1197–1204. doi: 10.1097/CCM.0b013e31827c086f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller B, Mohr NM, Miller CN, et al. Mechanical ventilation and acute respiratory distress syndrome in the emergency department: a multi-center, observational, prospective, cross-sectional, study. Chest. 2015;148(2):365–374. doi: 10.1378/chest.14-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoltze AJ, Wong TS, Harland KK, et al. Prehospital tidal volume influences hospital tidal volume: A cohort study. J Crit Care. 2015;30(3):495–501. doi: 10.1016/j.jcrc.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 17.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–2627. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 18.Dettmer MR, Mohr NM, Fuller BM. Sepsis-associated pulmonary complications in emergency department patients monitored with serial lactate: An observational cohort study. J Crit Care. 2015;30(6):1163–1168. doi: 10.1016/j.jcrc.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller B, Mohr N, Dettmer M, et al. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659–669. doi: 10.1111/acem.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller BM, Ferguson I, Mohr NM, et al. Lung-protective ventilation initiated in the emergency department (LOV-ED): a study protocol for a quasi-experimental, before-after trial aimed at reducing pulmonary complications. BMJ Open. 2016;6(4):e010991. doi: 10.1136/bmjopen-2015-010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 23.Acute respiratory distress syndrome. The ARDS definition task force. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.Pallin DJ, Dwyer RC, Walls RM, et al. Techniques and trends, success rates, and adverse events in emergency department pediatric intubations: a report from the National Emergency Airway Registry. Ann Emerg Med. 2016;67(5):610–615 e611. doi: 10.1016/j.annemergmed.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Sagarin MJ, Barton ED, Chng Y-M, et al. Airway management by US and Canadian emergency medicine residents: a multicenter analysis of more than 6,000 endotracheal intubation attempts. Ann Emerg Med. 2005;46(4):328–336. doi: 10.1016/j.annemergmed.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Gong MN, Ferguson ND. Lung-protective ventilation in acute respiratory distress syndrome. How soon is now? Am J Respir Crit Care Med. 2015;191(2):125–126. doi: 10.1164/rccm.201412-2250ED. [DOI] [PubMed] [Google Scholar]
- 27.Amato M, Barbas C, Medeiros D, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 28.Villar J, Kacmarek RM, Pérez-Méndez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: A randomized, controlled trial*. Crit Care Med. 2006;34(5):1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1, Supplemental Figure 1. Emergency department ventilator protocol
Supplemental Digital Content 2, Supplemental Text 1. Definitions of comorbid conditions
Supplemental Digital Content 3, Supplemental Text 2. ARDS adjudication process
Supplemental Digital Content 4, Supplemental Figure 2. ARDS Incidence by Day
Supplemental Digital Content 5, Supplemental Figure 3. Emergency department tidal volume distribution
Supplemental Digital Content 6, Supplemental Table 1. Intensive care unit ventilator variables
Supplemental Digital Content 7, Supplemental Table 2. Univariate comparison for mortality
Supplemental Digital Content 8, Supplemental Table 3. Multivariable analysis for mortality
Supplemental Digital Content 9, Supplemental Table 4. Secular trends in tidal volume, LPV, and mortality


