Abstract
Background
Biased agonism of the angiotensin receptor (AT1R) is known to promote cardiac contractility. Our laboratory indicated that these effects may be due to changes at the level of the myofilaments. However, these signaling mechanisms remain unknown. As a common finding in dilated cardiomyopathy (DCM) is a reduction in the myofilament-Ca2+-response, we hypothesized that β-arrestin signaling would increase myofilament-Ca2+-responsiveness in a model of familial DCM and improve cardiac function and morphology.
Methods
We treated a DCM-linked mouse model expressing a mutant tropomyosin (Tm-E54K), for three months with either TRV120067, a β-arrestin 2 biased ligand of the AT1R, or losartan, an AT1R blocker. At the end of the treatment protocol, we assessed cardiac function using echocardiography, the myofilament-Ca2+-response of detergent-extracted fiber bundles, and used proteomic approaches to understand changes in post-translational modifications of proteins that may explain functional changes. We also assessed signaling pathways altered in vivo and using isolated myocytes.
Results
TRV120067- treated Tm-E54K mice showed improved cardiac structure and function, whereas losartan-treated mice had no improvement. Myofilaments of TRV120067-treated Tm-E54K mice had significantly improved myofilament-Ca2+-responsiveness, which was depressed in untreated Tm-E54K mice. We attributed these changes to increased MLC2v and MYPT1/2 phosphorylation seen only in TRV120067-treated mice. We found that the functional changes were due to an activation of ERK1/2-RSK3 signaling, mediated through β-arrestin, which may have a novel role in increasing MLC2v phosphorylation through a previously unrecognized interaction of β-arrestin localized to the sarcomere.
Conclusions
Long-term β-arrestin 2 biased agonism of the AT1R may be a viable approach to the treatment of DCM by not only preventing maladaptive signaling, but also improving cardiac function by altering the myofilament-Ca2+-response via β-arrestin signaling pathways.
Keywords: Biased ligand, Sarcomeres, Calcium sensitivity, TRV120067, TRV120027
Introduction
In results described here, we present novel evidence on a potential therapeutic approach in familial dilated cardiomyopathy (DCM) of ligands acting at the angiotensin II receptor (AT1R) with a bias toward promotion of β-arrestin 2 signaling. There is substantial evidence that drugs that have the net effect of reducing angiotensin II (ang II) stimulation of the AT1R, either by inhibiting the production of active octapeptide through angiotensin converting enzyme inhibitors, or antagonizing the receptor via AT1R blockers (ARBs) have therapeutic benefit in cardiovascular disease. Therefore these drugs are indicated as “first-line” in the treatment of many cardiovascular diseases.1 It is now clear that induction of maladaptive ang II signaling via the AT1R also induces adaptive signaling mediated through β-arrestin.2, 3 Our evolving understanding of the complexity of the various ligand-bound and unbound conformations of the AT1R has led to the development of biased ligands.4 β-arrestin biased ligands targeted to the AT1R act as angiotensin receptor modulators, having properties similar to ARBs in that they selectively block ang II binding to the AT1R and subsequent G-protein coupling, while simultaneously and selectively activating β-arrestin signaling pathways.5, 6
In view of evidence that a consequence of biased ligand interactions with the AT1R is a promotion of cardiac myocyte contractility with no change in the Ca2+-transient, we set out to test whether downstream β-arrestin signaling involves a modification of the myofilament-Ca2+-response.7, 8 Previously, we found that biased agonism of the AT1R in the face of chronic ang II infusion in rats was able to preserve the myofilament-Ca2+-response and prevent ang II-mediated maladaptation.9 As a decrease in myofilament-Ca2+-response is a common molecular pathology found in DCM, we hypothesized that modulating the response from the AT1R by employing biased ligands may be a viable therapeutic approach to the treatment of DCM.10, 11 In a first line of experiments we tested the effect of acute infusion of a β-arrestin biased ligand on in situ cardiac contractility in a mouse model of familial DCM. Our data revealed that unlike treatment with a conventional ARB, a 15 min infusion of a biased ligand in familial DCM model restored contractility to normal levels as determined from left ventricular pressure-volume relations.12 The mechanism of this effect requires further study. Moreover, the results of the acute study suggested that β-arrestin biased agonism of AT1R would provide benefit in chronic progression of DCM.
To test this hypothesis, we used a mouse model of DCM expressing a cardiac-directed missense mutation in the sarcomeric protein, alpha-tropomyosin (Tm), where a glutamic acid has been exchanged for a lysine at residue 54 (Tm-E54K).13 This mutation causes a disruption in the coiled-coil structure of Tm in an actin-binding region resulting in a constitutive decrease in the myofilament-Ca2+-response and a DCM-like phenotype consistent with the human presentation of the disease.14 We treated non-transgenic (NTG) littermates and Tm-E54K mice for three months beginning at one month of age, when DCM phenotypic changes were already evident, with a β-arrestin biased ligand, TRV120067 (TRV067), or an ARB, losartan.
Materials and Methods
Expanded materials and methods can be found in the Supplemental Material.
Study Objective and Design
The objective of this study was to examine the effect of chronic AT1R biased ligand treatment on the structure and function of a mouse model of familial dilated cardiomyopathy. NTG and Tm-E54K mice were randomly assigned to three experimental groups: 1) untreated, 2) TRV067-treated, or 3) losartan-treated. Echocardiography was used to assess cardiac function and morphology, in vivo, histology was used to assess cardiac structure, detergent-extracted fiber bundles were used to assess the myofilament-Ca2+-response, and Western blots were used to assess signaling pathways altered due to treatment. Neonatal rat ventricular myocytes were used in culture to confirm signaling pathways and determine protein localization using Western blotting and immunocytochemistry.
Animal Model and Institutional Approval
All experiments involving the use of animals were given prior approval by the IACUC at the University of Illinois at Chicago, AAALAC accredited. Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (National Institutes of Health, Eighth Edition, revised 2011). Male and female mice heterozygous for the Tm-E54K mutation, or NTG littermates, were treated for three months beginning at one month of age. Controls were untreated littermates. All mice were euthanized by cardiectomy under surgical anesthesia using a ketamine/xylazine (200 mg/kg, 20 mg/kg) mixture, in accordance with the American Veterinary Medical Association Panel on Euthanasia Guidelines (American Veterinary Medical Association, 2013 edition).
Statistical Analyses
Two hypotheses were tested for chronic animal studies. The first hypothesis addressed if there was a significant change due to the genotype. The second hypothesis tested was if drug treatment significantly altered a parameter within genotype. To test for a Gaussian distribution, normality was assessed using the Kolmogorov-Smirnov test with Dallal-Wilkinson-Lilliefor P-value. The Brown-Forsythe test was used to determine equal variance of the standard deviation. As indicated in figure legends, an unpaired two-tailed Student’s t-test was used for comparison of two independent variables, a one-way ANOVA was used for comparisons of one independent variable to compare three or more groups followed by Tukey’s post-hoc test for multiple comparisons, a two-way ANOVA was performed where two independent variables were considered, followed by Tukey’s post-hoc test for multiple comparisons, or a repeated measures two-way ANOVA was performed followed by Bonferroni’s post-hoc test for multiple comparisons. All data are presented as means ± standard error of the mean (SEM) and significance set at p<0.05. All statistical analysis was performed using GraphPad Prism 6.0 Software (GraphPad, Inc., La Jolla, CA).
Results
TRV067 is a β-arrestin 2 biased ligand
G-protein and β-arrestin 2 responses to activation of the AT1R were determined by measuring inositol monophosphate accumulation (IP-one) and β-arrestin 2 recruitment to the receptor respectively, in HEK-293 cells stably expressing the AT1R. Ang II stimulated maximal IP-one and β-arrestin 2 recruitment with EC50 values of 1.3 and 4.0 nM, respectively. TRV067 stimulated β-arrestin 2 recruitment to 68% of the maximum stimulated by ang II with a EC50 of 251 nM while producing no detectable stimulation of IP-one (Sup. Fig. 1A, 1B). TRV067 is a member of the family of biased ligands described earlier and exemplified by the clinical candidate TRV027.8
Cardiac structure and function is improved in Tm-E54K mice after chronic treatment with TRV067
We assessed structure and function of the hearts of NTG and Tm-E54K mice after three months treatment with TRV067 or losartan. Tm-E54K untreated mice, compared to NTG mice, had a significant decrease in systolic cardiac function as evidenced by reduced ejection fraction, fractional shortening, stroke volume, and cardiac output. Untreated Tm-E54K mice displayed a morphological phenotype that mimics human DCM, with significant left ventricular wall thinning and internal chamber dilation, as well as left atrial dilation (Sup. Table 1).14 The transgenic protein comprised approximately 23% of the Tm expressed in the Tm-E54K mice, not leading to an overall increase in Tm expression (Sup. Fig. 2A, 2B). We noted no significant sex-related functional or structural differences within genotype (Sup. Table 2). After treatment with TRV067, Tm-E54K mice had a significant improvement in systolic function compared to both Tm-E54K untreated and losartan-treated mice. Associated with TRV067 treatment in Tm-E54K mice, there were significant increases in ejection fraction, fractional shortening, velocity of circumferential fiber shortening, stroke volume and cardiac output, without an increase in heart rate. There were also increases in left ventricular anterior wall dimension at systole (LVAW;s) and increases in left ventricular posterior wall dimension at systole (LVPW;s) (Sup. Fig. 3A, 3B). Although TRV067 significantly improved cardiac performance and morphology, it was not able to reverse these measures to those of NTG animals. There were no changes in cardiac function in Tm-E54K mice treated with losartan compared with Tm-E54K untreated mice. We found no effect on cardiac function of any treatment in NTG mice.
Histological analysis revealed there was a decrease in cell cross-sectional area in hearts of Tm-E54K untreated mice compared to NTG untreated controls. TRV067 significantly increased cross-sectional cell area whereas losartan had no effect. While TRV067 had no effect in NTG mice, losartan induced a significant decrease in cross-sectional cell area (Sup. Fig. 4).
β-arrestin signaling increases cardiac myofilament-Ca2+-responsiveness
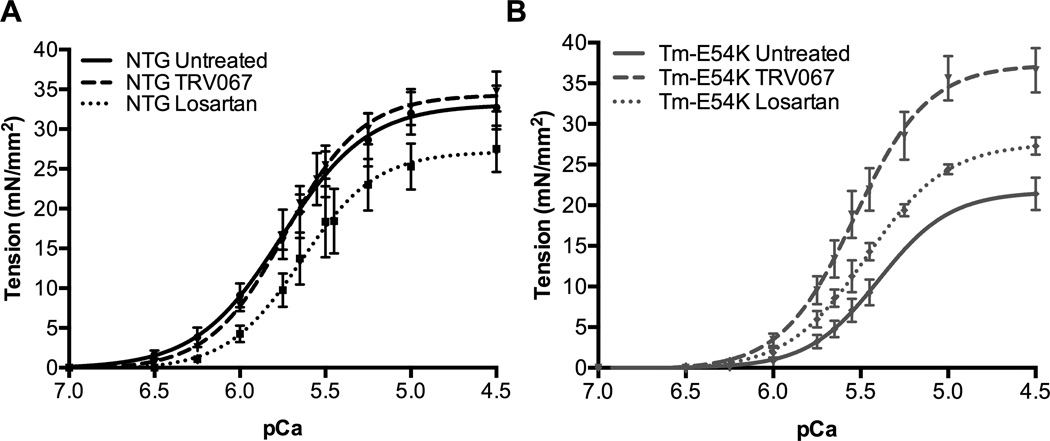
We sought to understand if biophysical changes at the level of the sarcomere could be responsible for the improvements in cardiac function seen in echocardiography. Detergent-extracted fiber bundles isolated from Tm-E54K mice had a significantly reduced myofilament-Ca2+-sensitivity, as measured by the Ca2+ concentration at half-maximal tension (pCa50), and maximum tension generation (Table 1). TRV067-treatment improved maximum tension generation to that of untreated NTG animals and significantly improved pCa50 in Tm-E54K fibers. Losartan had no significant effect on myofilament maximum tension generation or pCa50 in Tm-E54K mice (Table 1, Fig. 1A, 1B). There was no effect on pCa50, maximum tension generation, or Hill coefficient (nH) in fibers isolated from TRV067-treated NTG mice compared to untreated controls. Interestingly, fibers isolated from losartan-treated NTG mice showed a significant decrease in pCa50 compared to both TRV067 and untreated NTG groups, as well as a decrease in maximum tension generation (Table 1, Fig. 1A, 1B).
Table 1.
Assessment of the Force-Ca2+ Relationship in Detergent-Extracted Fiber Bundles.
| Parameter | NTG | NTG TRV067 |
NTG Losartan |
Tm-E54K | Tm-E54K TRV067 |
Tm-E54K Losartan |
|---|---|---|---|---|---|---|
| Sample Size (n, fibers) | 7 | 8 | 6 | 6 | 8 | 6 |
| Max. Tension (mN/mm2) | 32.72 ± 2.76 | 34.73 ± 2.51 | 25.73 ± 3.31*† | 21.49 ± 2.00* | 39.47 ± 2.77‡‡ | 27.29 ± 1.07∥ |
| pCa50 | 5.74 ± 0.04 | 5.73 ± 0.03 | 5.60 ± 0.02*† | 5.40 ± 0.03* | 5.51 ± 0.03‡ | 5.47 ± 0.04 |
| Hill Coefficient | 1.90 ± 0.14 | 1.96 ± 0.09 | 2.10 ± 0.12 | 2.47 ± 0.10* | 2.08 ± 0.06‡ | 2.00 ± 0.08‡ |
Values are Mean ± SEM.
p ≤ 0.05 vs. NTG,
p ≤ 0.05 vs. NTG TRV067
p ≤ 0.05 vs. Tm-E54K,
p ≤ 0.01 vs. Tm-E54K
p ≤ 0.05 vs. Tm-E54K TRV067
Figure 1. TRV067 improved myofilament Ca2+-responsiveness and normalized tension generation in DCM.
Force-Ca2+ relationship of fibers isolated from (A) NTG untreated, TRV067 and losartan treated animals and (B) Tm-E54K untreated, TRV067 and losartan treated animals following treatment for 3 months. Data were analyzed by two-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons and represented as means ± SEM. N = 3–4 hearts per group, 6–8 fibers per group.
β-arrestin signaling alters myofilament protein phosphorylation
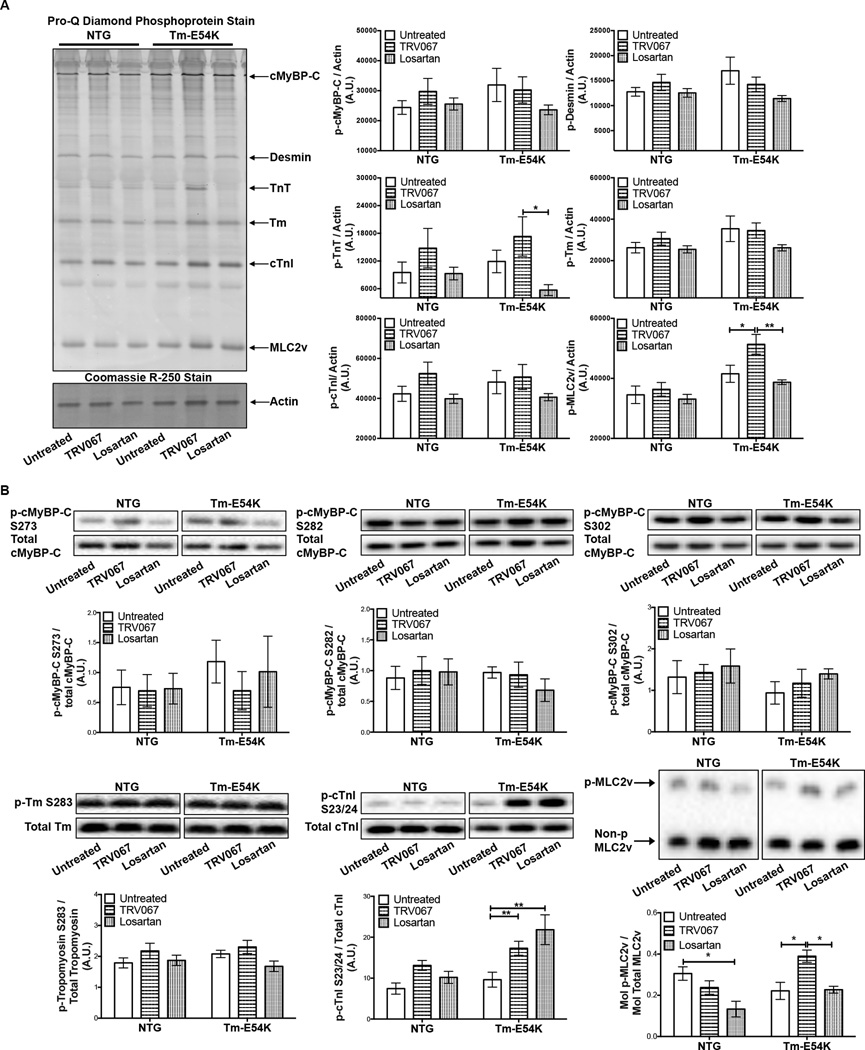
To understand the mechanism(s) for the TRV067-induced increases in the myofilament-Ca2+-response, we investigated post-translational modifications of sarcomeric proteins. We found significant alterations in phosphorylation of cardiac troponin I (cTnI), troponin T (TnT), and MLC2v.
ProQ diamond phospho-protein stain revealed no significant changes in the total phosphorylation of cardiac myosin-binding protein-C (cMyBP-C), desmin, Tm, or cTnI. Losartan-treated Tm-E54K mice displayed a significant decrease in TnT phosphorylation compared to TRV067-treated Tm-E54K mice and TRV067-treated mice displayed a significant increase in MLC2v phosphorylation (Fig. 2A).
Figure 2. TRV067 and losartan induced post-translational modification of myofilament proteins.
(A) Representative ProQ diamond and Coomassie R-250 stained gels and summarized analysis showing MLC2v phosphorylation was significantly increased in TRV067-treated Tm-E54Ks mice and decreased TnT phosphorylation in Tm-E54K losartan-treated mice. (B) Representative Western blots and summarized quantification of phospho-specific antibodies to cMyBP-C, Tm, and cTnI showing a significant increase in p-cTnI S23/24 in TRV067 and losartan-treated Tm-E54K mice and MLC2v phosphorylation significantly increased in TRV067-treated Tm-E54Ks mice and decreased in NTG losartan-treated mice. Data were analyzed by two-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons and represented as means ± SEM. N = 4–6 hearts per group. * p ≤ 0.05. ** p ≤ 0.01
We used phospho-specific antibodies, where possible, to perform a more detailed analysis of myofilament protein phosphorylation. There were no changes in site-specific phosphorylation of cMyBP-C or Tm. There was a significant increase in phosphorylation at S23/24 in both the TRV067 and losartan-treated Tm-E54K mice.
We utilized Phos-Tag gel electrophoresis to analyze phosphorylation of MLC2v. TRV067 increased MLC2v phosphorylation in Tm-E54K mouse hearts compared to myofilaments from both losartan-treated and untreated Tm-E54K mice. MLC2v phosphorylation was significantly decreased in losartan-treated NTG mice compared to NTG untreated mice (Fig. 2B).
TRV067 treatment stimulated signaling pathways associated with an improvement in cardiac function
We performed an analysis of various signaling pathways to better understand which pathways may be activated by β-arrestin and what alterations in signaling may be due solely to inhibition of Gαq coupling in our model of familial DCM.
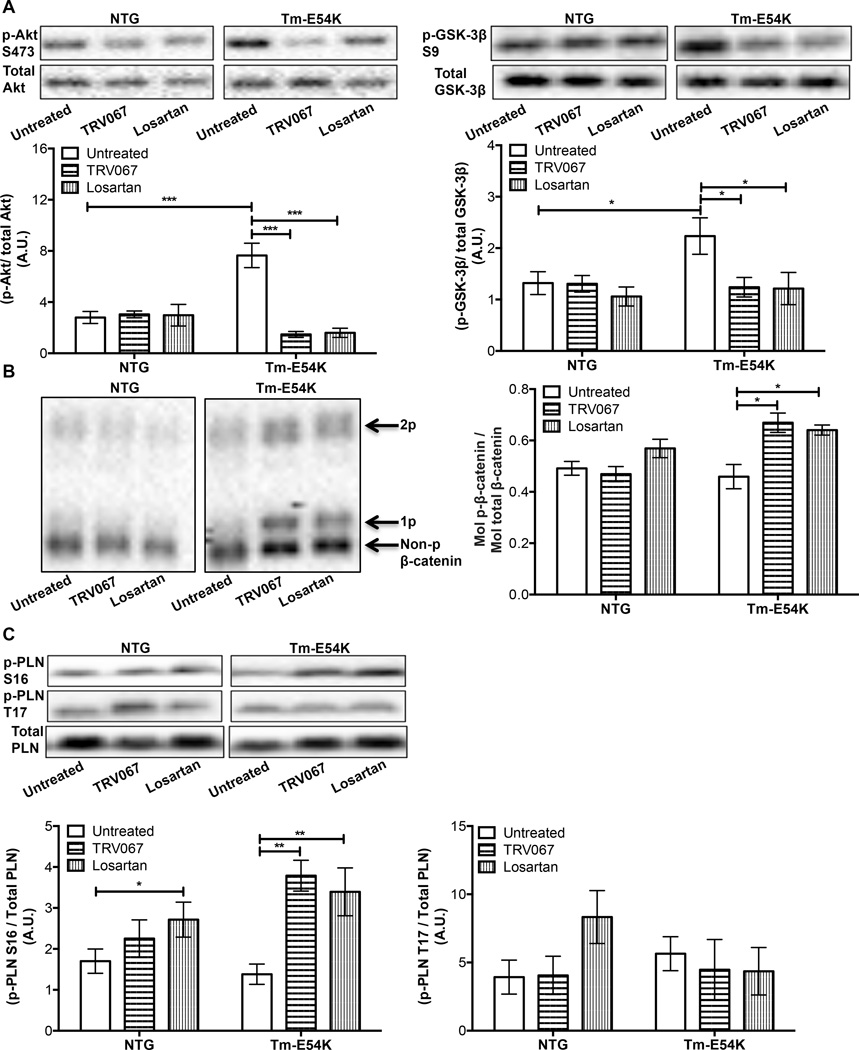
We found that both TRV067 and losartan were able to alter Akt-GSK signaling in Tm-E54K mice, indicating a common mechanism of action through the inhibition of Gαq coupling. Compared to untreated NTG hearts, Akt phosphorylation at S473 and GSK-3β phosphorylation at S9 were both increased in untreated Tm-E54K hearts (Fig. 3A). This suggests that the Tm-E54K mutation induces an increase in Akt activity and a concomitant decrease in GSK-3β activity (Fig. 3B).15 TRV067 and losartan both decreased Akt and GSK-3β phosphorylation in the Tm-E54K hearts (Fig. 3A, 3B). Evidence indicates that GSK-3β related phosphorylation of β-catenin regulates its degradation.16 While we did not observe a decrease in β-catenin phosphorylation in untreated Tm-E54K mice compared to untreated NTG mice, we did observe an increase in β-catenin phosphorylation in both TRV067 and losartan-treated Tm-E54K mice (Fig. 3C). There was no change in Akt, GSK-3β, or β-catenin phosphorylation in NTG hearts regardless of treatment (Fig. 3A, 3B, and 3C). We also found that Tm-E54K mice treated with TRV067 or losartan both showed an increase in phosphorylation of phospholamban (PLN) at the S16 site, but not the T17 site. Losartan-treated NTG mice also showed an increase in S16 phosphorylation of PLN compared to untreated controls (Fig. 3C).
Figure 3. The Akt-GSK-3β pathway is downregulated in chronic TRV067 and losartan treated Tm-E54K mice.
Representative Western blots and summarized quantification showing (A) Akt phosphorylation was decreased in TRV067 and losartan-treated Tm-E54K mice and GSK-3β phosphorylation was decreased. (B) There was a concomitant increase in β-catenin phosphorylation in these mice. (C) An increase in phosphorylation of phospholamban at S16 was also seen in both TRV067 and losartan-treated mice, but not at T17. Data were analyzed by two-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons and represented as means ± SEM. N = 4–6 hearts per group. * p ≤ 0.05 , ** p ≤ 0.01, *** p ≤ 0.001.
A common finding in mouse models of heart failure is an increase in the isoform content of β-myosin heavy chain (β-MHC), which may be due to the activation of G-proteins. Our model has been previously shown to have an increase in β-MHC expression.14 Since β-MHC expression may be regulated by downstream β-catenin target genes we compared the protein expression levels of β-MHC in treated and untreated NTG and Tm-E54K hearts.17, 18 NTG hearts demonstrated no expression of β-MHC. There was a significant re-expression of β-MHC to approximately 30% of the isoform content in the untreated Tm-E54K hearts compared to NTG control hearts. This was reduced to approximately 10% expression with both TRV067 and losartan treatment (Sup. Fig. 5).
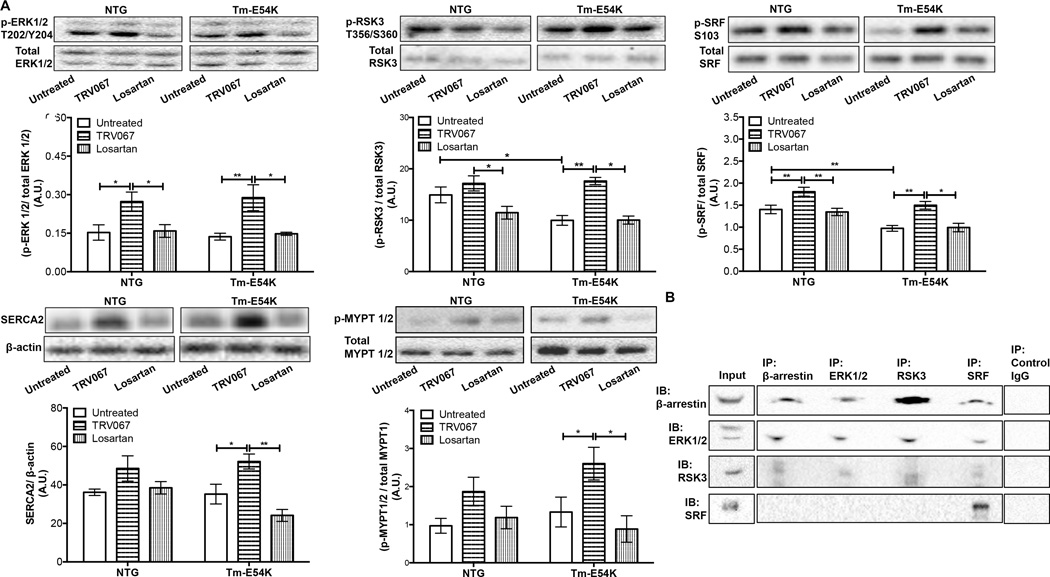
ERK1/2 phosphorylation is activated by β-arrestin 2 biased agonism of the AT1R and is used as a marker of β-arrestin activation.19 We found that ERK1/2 phosphorylation was increased in TRV067-treated Tm-E54K and NTG hearts, but not in hearts of losartan-treated mice (Fig. 4A). We hypothesized that downstream signaling from ERK1/2 may also be activated in our mice and this signaling may be an upstream signaling mediator responsible for the changes in post-translational modifications of MLC2v. We examined RSK3, a serine-threonine kinase, a known downstream target of ERK1/2, which has been implicated in the phosphorylation of MYPT1 in vascular smooth muscle cells, as well as SRF phosphorylation, which is involved in the transcription of cardiac-specific genes in the heart, such as SERCA.20–22 TRV067 increased RSK3, SRF, and MYPT1/2 phosphorylation in both NTG and Tm-E54K mice. Losartan had no effect on RSK3, SRF, or MYPT1/2 phosphorylation. This difference in response between TRV067 and losartan suggests a mechanism reliant on β-arrestin (Fig. 4A). At baseline, RSK3 phosphorylation was significantly decreased in untreated Tm-E54K mice compared to untreated NTG mice (Fig. 4A). We found a significant decrease in SRF phosphorylation in untreated Tm-E54K mice compared to untreated NTG mice (Fig. 4A). TRV067 also increased SERCA2 protein expression in Tm-E54K and NTG mice compared to untreated groups. Losartan had no effect on SERCA2 expression. (Fig. 4A).
Figure 4. TRV067, but not losartan, activated ERK1/2 and downstream signaling.
Representative Western blot images and quantification showing (A) ERK1/2, RSK3, SRF phosphorylation, SERCA2 expression, and MYPT1/2 phosphorylation were all significantly increased in TRV067-treated mice but not losartan treated mice. (B) Representative western blot images of co-immunoprecipitation experiments of β-arrestin, ERK1/2, RSK3 and SRF. Data were analyzed by two-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons and represented as means ± SEM. N = 4–6 hearts per group. * p ≤ 0.05 , ** p ≤ 0.01.
We tested for interactions between proteins altered due to TRV067-treatment using co-immunoprecipitations. Shown in Figure 4B, β-arrestin, ERK2, and RSK all co-immunoprecipitated, suggesting a signaling complex of these three proteins. β-arrestin, RSK3 and ERK2 were found in the SRF immunoprecipitation but SRF was not co-immunoprecipiated in the reverse immunoprecipitation. Perhaps suggesting a weak or localization-specific interaction with these proteins. We were unable to perform reliable immunoprecipitations of MYPT1/2, likely due to its strong interaction with insoluble proteins.23
Signaling activated by TRV067 is ERK1/2 and RSK-dependent
To confirm our hypothesized signaling pathway, we employed neonatal rat ventricular myocytes (NRVM) treated with either TRV067 or losartan in the presence of FR180204, an ERK1/2 inhibitor, or BI-D1870, a pan-RSK inhibitor. Losartan had no effect; however, NRVMs treated with TRV067 had increased RSK3, MYPT1/2, and SRF phosphorylation as seen in the chronically treated adult mice. Pre-treatment of NRVMs with either FR180204 or BI-D1870 abolished increases in the phosphorylation of these proteins with drug treatment. These data indicate that both ERK1/2 and RSK are activated downstream of β-arrestin-mediated signaling and are required for the increases in downstream phosphorylation (Sup. Fig. 6A, 6B, and 6C).
Myofilament proteins are substrates for RSK3 phosphorylation
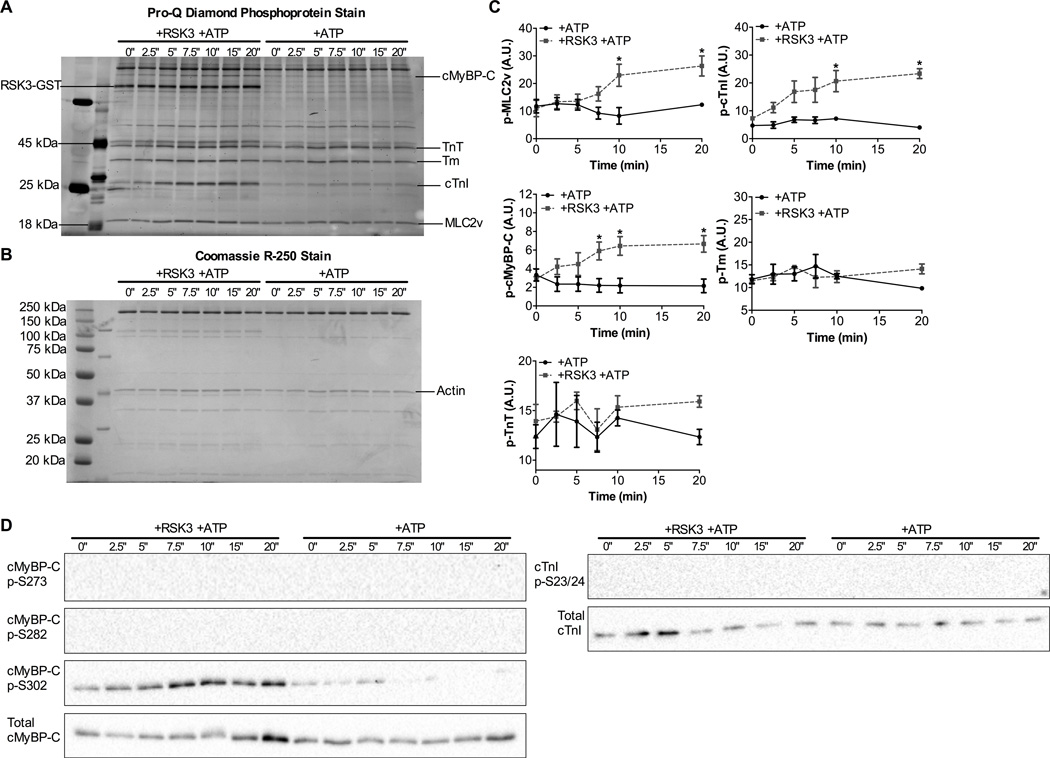
In light of results presented above, we hypothesized that RSK3 may be the most proximal kinase able to mediate the effects seen in our mice treated with TRV067. To test this hypothesis, we isolated dephosphorylated myofibrils from Langendorff-perfused mouse hearts and incubated them with recombinant, active RSK3. We found that RSK3 was able to phosphorylate not only MLC2v, but also cTnI and cMyBP-C, but not TnT or Tm (Fig. 5A, 5B, 5C). To understand what specific residues were being phosphorylated by RSK3 we used phospho-specific antibodies to cMyBP-C and cTnI. RSK3 solely phosphorylated cMyBP-C at the S302 site. We did not see an increase in cMyBP-C phosphorylation at S302 in vivo as we did in these in vitro experiments. We found that RSK3 did not phosphorylate cTnI at S23/24, consistent with our findings that this increase was not due to β-arrestin signaling (Fig. 5D). These results are consistent with our findings that MLC2v phosphorylation was increased in TRV067 treated mice.
Figure 5. MLC2v, cTnI and cMyBP-C are substrates for RSK3 in vitro.
Representative (A) Pro-Q diamond stained SDS-PAGE gel and (B) Coomassie R-250 stained SDS-PAGE gel showing an increase in MLC2v, cTnI, and cMyBPC by RSK3. (C) Summarized quantification of SDS-PAGE gels. (D) Western blot images showing phosphorylation of cMyBP-C at S302 by RSK3 but not S273 or S282 nor cTnI S23/24. Data were analyzed by repeated measures two-way ANOVA followed by Bonferroni’s post-hoc test for multiple comparisons and represented as means ± SEM. N = 4 hearts. * p ≤ 0.05.
β-arrestins localize to the M-line and A-band
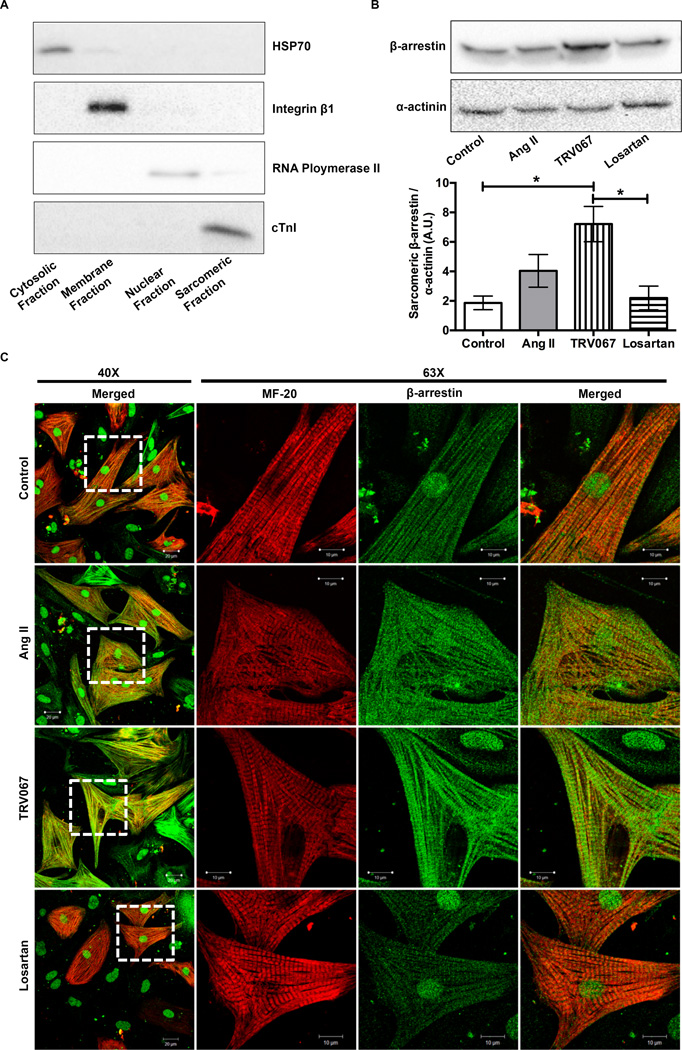
We hypothesized that β-arrestins may directly scaffold signaling proteins to the myofilaments and that this localization may be related to agonism of the AT1R. To answer this question we cultured NRVMs and, following treatment, subjected them to a subcellular fractionation protocol to remove the cytosolic, membrane, and nuclear components of the cell, leaving the sarcomeric fraction for analysis 24. Shown in Figure 6A, cytosolic, membrane, nuclear and sarcomeric markers are found only within their respective fractions. Western blot analysis of sarcomeric fractions from NRVMs treated for one hour with Ang II, TRV067 or losartan revealed a significant increase in β-arrestin localization to the sarcomere with TRV067-treatment (Fig. 6B).
Figure 6. A-band and M-line of the sarcomere show localization of β-arrestins, which was increased by TRV067.
(A) Representative Western blots showing efficiency of subcellular fractionation in neonatal rat ventricular myocytes (NRVM). (B) Representative Western blot and summarized quantification of sarcomeric fractions of NRVMs that were treated for one hour with either angiotensin II (ang II), TRV067, or losartan and probed for β-arrestin and α-actinin. There is a basal amount of β-arrestin localized to the sarcomere under basal conditions, while analysis revealed that there was a significant increase β-arrestin localized to the sarcomere with TRV067 treatment. (C) Images of NRVMs stripped of their cytosolic and membrane components were stained for MF-20, light meromyosin, (red) or β-arrestin (green). Co-localization (yellow) which was increased by ang II and TRV067 treatment was observed for β-arrestin at the M-line and A-band, No increase was seen in the losartan -treated cells. Data were analyzed by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons and represented as means ± SEM. N = 4 separate cultures. * p ≤ 0.05.
NRVMs depleted of their cytosolic and membrane compartments were stained for MF-20, an antibody that recognizes light meromyosin (red), and β-arrestin (green). Under untreated conditions there was localization of β-arrestin at the M-line and the A-band, indicated by yellow striations and diffuse staining surrounding them. When stimulated with TRV067 we found that there was an increase in this localization, which was seen to a lesser extent in ang II treated cells, and not at all in the losartan-treated cells (Fig. 6C). Taken together, these results indicate that agonism of the AT1R by biased ligands induced a rearrangement of β-arrestins to a more sarcomeric distribution at the M-line and A-band.
Discussion
A major new finding reported here is the demonstration that unlike the ARB, losartan, TRV067, a β-arrestin 2 biased ligand, is able to improve functional and morphological outcomes in a model of familial DCM. Our data indicate that increased MLC2v phosphorylation induced by TRV067 is likely to be the mechanism for the improved systolic function induced by the β-arrestin pathway. We propose that β-arrestin signaling activates a novel ERK1/2-RSK3 mediated phosphorylation of MLC2v as well as an increase in MYPT 1/2 phosphorylation. Losartan and TRV067 share the effect of inhibition of Gαq-mediated maladaptive responses in the progression of DCM. However, TRV067 not only reduced maladaptive protein expression, but also improved left ventricular structure and function. Additionally, a unique and novel finding is our demonstration that these effects may be mediated, in part, by β-arrestin localization to the sarcomere.
Our results support the hypothesis for a significant role of increased MLC2v phosphorylation in the mechanism by which a β-arrestin 2 biased ligand compensates for the depression in maximum sarcomeric tension and response to Ca2+, commonly attributed as a causative factor in the development and progression of DCM.25, 26 Previous work has indicated that increases in phosphorylation of MLC2v may result in modest increases in Ca2+-sensitivity, which may be more pronounced in various models of cardiomyopathy, as seen in the TRV067-treated Tm-E54K mice.27, 28 We also found that the increases in MLC2v phosphorylation restored maximum tension generation to that of untreated NTG fibers in TRV067-treated mice. It is expected that these changes in phosphorylation induced by TRV067, but not losartan, manifest as an improvement in systolic functional parameters. These increases in cardiac function were seen in the absence of an increase in heart rate. A decrease in afterload is known to increase cardiac output, as may be induced by vasodilating agents, such as losartan. Unloading may be necessary for the improvement in cardiac output seen in the Tm-E54K mice treated with TRV067; however, the fact that losartan-treated Tm-E54K mice did not have improved cardiac output suggests that it is not sufficient, at least in our model. This strongly suggests that modification of the myofilaments may be the source of the improved cardiac output in Tm-E54K mice. Interestingly, in losartan treated NTG mice, MLC2v phosphorylation was decreased, along with Ca2+-sensitivity and maximum tension generation. While increases in MLC2v phosphorylation can explain the increases in Ca2+-sensitivity and maximal tension found in TRV067-treated Tm-E54K mice, it is possible that MLC2v phosphorylation is not the sole mechanism for this observation. This is underscored by our finding that there was a significant increase in phosphorylation at cTnI S23/24 in both TRV067 and losartan-treated transgenic mice despite no changes in overall phosphorylation. This suggests that there is a change in the phosphorylation pattern of the phosphorylatable sites of cTnI that may affect the impact of S23/24 phosphorylation.29, 30 Despite the fact that S23/24 phosphorylation site is associated with a decrease in myofilament-Ca2+-sensitivity and no effect on maximum tension generation, no desensitization was observed and maximal tension was increased.31, 32 The missense mutation in our model has been shown to induce an uncoupling of the S23/24 phosphorylation to myofilament-Ca2+-sensitivity.33, 34 This finding, which has been demonstrated only in thin-filament mutations linked to DCM, could explain the lack of response to S23/24 phosphorylation. Studies in human tissue from heart failure and idiopathic DCM have shown that cTnI phosphorylation may be dominant over MLC2v phosphorylation.32, 35, 36 Taken together, think this requires that further studies be done to determine the effect of β-arrestin 2 biased agonism of the AT1R in other animal models and human DCM as the response may differ.
Previous work has implicated RSK family members in the phosphorylation of cTnI, as well as MYPT1 in smooth muscle, but these roles have yet to be determined in vivo.21, 37 In our current work, we report that β-arrestin signaling increases ERK1/2 phosphorylation as well as RSK3 phosphorylation, signaling to MLC2v. The role of the RSK family members in the development and maintenance of cardiac disease is not understood. Studies have shown an increase in RSK phosphorylation in many pathological states of the heart.38, 39 However, the investigation of specific RSK isoforms in many of these studies is often absent. When a RSK3 knockout mouse was subjected to trans-aortic constriction there was a blunting of the increase in cell-size induced by the pressure overload.40 While the effects of this knockout do not appear to cause cardiac insufficiency, one apparently consistent finding is a left ventricular wall thinning, and an increase in left ventricular internal dimension, as reported in the literature.40, 41 Although not investigated by the authors, the beneficial effects of RSK3 knockout in these models may be related to a decrease in myofilament-Ca2+-response, which has been implicated in the reversal of hypertrophic remodeling.41, 42 We think this indicates that a basal level of activated RSK3 may be important for normal cardiac function and morphology. Experiments carried out in a swimming model of exercise-induced hypertrophy, as well as in vitro studies where exercise was mimicked by IGF-1 stimulation, have demonstrated a potential role for an increase in RSK phosphorylation as a physiological adaptation. This up regulation of RSK phosphorylation and activity is most likely as a result of downstream activation from ERK1/2.43
Activation of RSK3 in the setting of pathological hypertrophy is most well-known for its effects at the nucleus, where it interacts with muscle A-kinase anchoring protein (mAKAP) and is believed to promote gene transcription related to hypertrophy, as well as its ability to up regulate Na+-H+ exchanger (NHE) activity in various cardiac disorders, and physiological hypertrophy.44, 45 Previous studies have suggested that nuclear-localized RSK3 is responsible for maladaptation.40 Maladaptive signaling that has previously been associated with RSK3 may be blunted in the context of β-arrestin signaling as ERK1/2 activated by biased ligands remains cytosolic, and fails to activate nuclear transcription factors.19 Since RSK3 binds to and has a greater and longer duration of activity when bound to ERK1/2, it is likely that RSK3 acts in a similar manner upon biased ligand stimulation.46 This hypothesis is supported by our novel finding of β-arrestin localization to the sarcomere, which was found under both control and treatment conditions, though to a significantly greater degree with biased ligand treatment and that a β-arrestin-ERK2-RSK3 complex exists in cardiac tissue. The role of this localization in physiological maintenance of cardiac function is unknown. The localization of these proteins to the sarcomere under control conditions suggests that they may be important in regulation of sarcomeric contraction. Though, to our knowledge, no endogenous biased ligands have been discovered, previous work has suggested that biased β-arrestin signaling may be involved in the cardiomyocyte response to stretch, suggesting a physiological role for these unique signaling pathways.47 However, β-arrestin localization, the localization of associated proteins, the subcellular interactomes in which they participate, and the functional consequences of these interactions requires extensive future study.
We show here that RSK3 is involved in the phosphorylation of SRF in the heart, which has also been described previously in other models.20 An increase in SRF phosphorylation promotes the binding to DNA and the transcription of sarcomeric and other cardiac-specific genes.22, 48, 49 We attribute our finding of an increase in SERCA2 expression in our model to this effect. Taken together with our finding that MLC2v is a RSK3 substrate, we have demonstrated a direct functional consequence of RSK3 in mediating inotropic effects via β-arrestin.
These collective pro-inotropic effects of a biased ligand add to the beneficial signaling pathways altered by losartan, where Akt phosphorylation was normalized, and β-catenin phosphorylation was increased. β-catenin has long been implicated in the development of cardiomyopathy, and long-term cardiac dysfunction after ischemia-reperfusion injury.50, 51 The effects of TRV067 treatment decreasing Akt phosphorylation differ from previous reports of membrane strain-induced β-arrestin signaling via the AT1R where Akt phosphorylation was increased.52 These reports were from acute studies using membrane strain, whereas our work is chronic using a peptide agonist. We think that this indicates the existence of discrete receptor and β-arrestin conformations induced by strain compared to ligand-bound state. This thought is supported by studies that have shown that distinct β-arrestin conformations are induced by various combinations of phosphorylated receptor “barcodes”. Recent work using nuclear magnetic resonance and X-ray crystallography has provided some structural basis for discrete signaling outcomes induced by various receptor conformations or phosphorylation states.53–55 The AT1R may not possess these phosphorylation patterns that result in discrete signaling outcomes but it is likely that cytoplasmic-facing receptor conformations may dictate discrete β-arrestin signaling pathways, though not currently understood.53
We also found that shared by TRV067 and losartan, phosphorylation of PLN at S16 and cTnI at S23/24 increased in Tm-E54K mice. The increases in PLN phosphorylation were shared in the NTG animals treated with losartan, but not TRV067. These are canonical PKA phosphorylation sites that are phosphorylated in response to b-adrenergic stimulation. Ang II is known to inhibit adenylate cyclase in a Gαi-dependent manner, and ARBs have been shown to prevent this inhibition.56–58 Perhaps TRV067 acts similarly to losartan to increase PKA activity in a β-arrestin independent manner. However, we did not observe an increase in other PKA phosphorylatable sites, such as cMyBP-C, nor an increase in heart rate in these animals treated with TRV067 or losartan treatment.
Recently, a clinical trial testing the efficacy of employing acute treatment with a beta-arrestin 2 biased AT1R modulator, TRV027, for acute decompensated heart failure (AHF) failed to meet primary and secondary endpoints. This trial entitled, Biased Ligand at the Angiotensin Receptor in Acute Heart Failure (BLAST-AHF) (NCT01966601), is unique in that it is the first to bring a cardiovascular-targeted biased ligand to any patient population.59 While AHF and DCM have a common major symptom of impaired systolic function, AHF generally results from a rapid decompensation from chronic heart failure resulting in pulmonary congestion and severely depressed left ventricular function.59 There are key differences in BLAST-AHF and our current work. We have tested the effects of TRV067 given by constant treatment over 12 weeks, whereas the effects of TRV027 were tested in the AHF population over a 48 to 96-hours via intravenous infusion, compared to placebo.59 This allowed us to investigate the impact of β-arrestin 2 biased signaling in DCM over time compared to losartan. The TRV-family AT1R biased ligands cannot be used to test the effectiveness of this treatment strategy in a chronic, clinical setting as they are peptide drugs with a very short half-life between 2–13 minutes.60 Chronic treatment will require the development of drugs with a longer half-life.
In this study we used different biased ligand compound than in BLAST-AHF. We have noted no differences in signaling indicators to suggest that the effects of TRV067 are different from its sister peptides. This does not exclude the possibility that the downstream signaling mechanisms may have nuanced differences we have yet to appreciate.
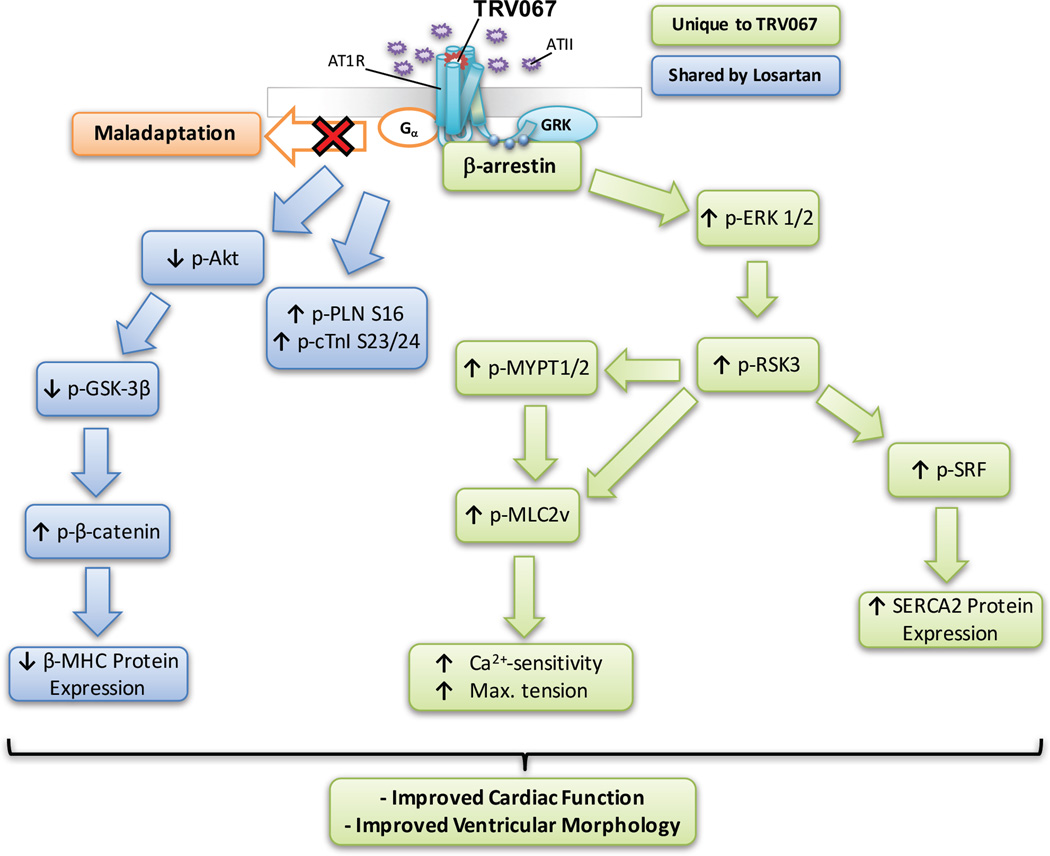
Figure 7 illustrates our hypothesis for the downstream signaling mechanism activated by biased agonism of the AT1R by TRV067. TRV067 stimulation activates ERK1/2 and subsequently RSK3. RSK3 phosphorylates MLC2v and MYPT1/2 to increase the myofilament-Ca2+-response, and improve cardiac function. RSK3 activated by β-arrestin phosphorylates SRF leading to an increase in SERCA2 expression. A pathway shared by both TRV067 and losartan results in a decrease in Akt and GSK3β phosphorylation, resulting in an increase in β-catenin phosphorylation and a decrease in β-MHC expression, and an increase in phosphorylation of cTnI and PLN. This study provides pre-clinical, mechanistic evidence that biased ligands may be a viable approach to the treatment of DCM.
Figure 7. Proposed mechanism by which TRV067 improves cardiac function and morphology in DCM.
Boxes in blue indicate pathways altered by both losartan and TRV067, whereas boxes in green indicate pathways only altered by TRV067.
Supplementary Material
Clinical Perspective.
What is new?
Chronic treatment of a DCM mouse model with a biased ligand acting at the AT1R induces activation of novel signaling pathways via β-arrestin that compensates for depressed cardiac function.
A previously unrecognized interaction of β-arrestin with the sarcomere may be involved in modulating the myofilament-Ca2+-response through alterations in MLC2v phosphorylation.
What are the clinical implications?
Modulation of the myofilament-Ca2+-response, targeted directly or by altering signaling pathways may be of therapeutic benefit in a variety of cardiomyopathies.
Novel β-arrestin signaling pathways that improve cardiac function and structure in DCM could be targeted for therapeutic intervention.
Acknowledgments
The Tm-E54K mice were generously provided by David F. Weiczorek, PhD. The authors thank Chad M. Warren, MS for his support in proteomic experiments, Sakthivel Sadayappan, PhD and Richard L. Moss, PhD for their kind gifts of the phospho-specific and total cMyBP-C antibodies, respectively, and Mark D. McCauley, MD, PhD for his helpful comments and discussion.
Sources of Funding: Our work was supported by National Institutes of Health grants P01 HL 062426 (Project 1, Project 2, Core B, and Core C) (to RJS, BR, and BMW) F31 HL 127996 (to DMR), a University of Illinois at Chicago College of Medicine Bridge Award (BMW), and American Heart Association grant 15PRE22180010 (to DMR).
Footnotes
Disclosures: TRV120067 was provided by Trevena, Inc. CLC is an employee of Trevena, Inc., a company that discovers and develops biased ligands.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Kim K-S, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303:H1001–H1010. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correll CC, McKittrick BA. Biased Ligand Modulation of Seven Transmembrane Receptors (7TMRs): Functional Implications for Drug Discovery. J Med Chem. 2014;57:6887–6896. doi: 10.1021/jm401677g. [DOI] [PubMed] [Google Scholar]
- 5.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 9.Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, Violin JD, Solaro RJ. The beta-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol. 2013;305:H856–H866. doi: 10.1152/ajpheart.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, Robinson P, Redwood C, Watkins H. Dilated Cardiomyopathy Mutations in Three Thin Filament Regulatory Proteins Result in a Common Functional Phenotype. J Biol Chem. 2005;280:28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77:659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 12.Tarigopula M, Davis RT, 3rd, Mungai PT, Ryba DM, Wieczorek DF, Cowan CL, Violin JD, Wolska BM, Solaro RJ. Cardiac myosin light chain phosphorylation and inotropic effects of a biased ligand, TRV120023, in a dilated cardiomyopathy model. Cardiovasc Res. 2015;107:226–234. doi: 10.1093/cvr/cvv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza M, Robinson P, Kremneva E, Copeland On, Nikolaeva O, Watkins H, Levitsky D, Redwood C, EL-Mezgueldi M, Marston S. The Effect of Mutations in α-Tropomyosin (E40K and E54K) That Cause Familial Dilated Cardiomyopathy on the Regulatory Mechanism of Cardiac Muscle Thin Filaments. J Biol Chem. 2007;282:13487–13497. doi: 10.1074/jbc.M701071200. [DOI] [PubMed] [Google Scholar]
- 14.Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101:205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Kuster DW, Sequeira V, Najafi A, Boontje NM, Wijnker PJ, Witjas-Paalberends ER, Marston SB, Dos Remedios CG, Carrier L, Demmers JA, Redwood C, Sadayappan S, van der Velden J. GSK3beta phosphorylates newly identified site in the proline-alanine-rich region of cardiac myosin-binding protein C and alters cross-bridge cycling kinetics in human: short communication. Circ Res. 2013;112:633–639. doi: 10.1161/CIRCRESAHA.112.275602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/β-Catenin Signaling Is Required for Cardiac Differentiation in Human Embryonic Stem Cells. PLoS ONE. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Bjørbaek C, Weremowicz S, Morton CC, Moller DE. RSK3 encodes a novel pp90rsk isoform with a unique N-terminal sequence: growth factor-stimulated kinase function and nuclear translocation. Mol Cell Biol. 1995;15:4353–4363. doi: 10.1128/mcb.15.8.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artamonov M, Momotani K, Utepbergenov D, Franke A, Khromov A, Derewenda ZS, Somlyo AV. The p90 ribosomal S6 kinase (RSK) is a mediator of smooth muscle contractility. PLoS One. 2013;8:e58703. doi: 10.1371/journal.pone.0058703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balza RO, Jr, Misra RP. Role of the serum response factor in regulating contractile apparatus gene expression and sarcomeric integrity in cardiomyocytes. J Biol Chem. 2006;281:6498–6510. doi: 10.1074/jbc.M509487200. [DOI] [PubMed] [Google Scholar]
- 23.Yin X, Cuello F, Mayr U, Hao Z, Hornshaw M, Ehler E, Avkiran M, Mayr M. Proteomics analysis of the cardiac myofilament subproteome reveals dynamic alterations in phosphatase subunit distribution. Mol Cell Proteomics. 2010;9:497–509. doi: 10.1074/mcp.M900275-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol. 2007;292:H259–H269. doi: 10.1152/ajpheart.00766.2006. [DOI] [PubMed] [Google Scholar]
- 25.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Jarcho J, Shapiro LR, Seidman CE. Mutations in Sarcomere Protein Genes as a Cause of Dilated Cardiomyopathy. N Engl J Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 26.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 27.Muthu P, Kazmierczak K, Jones M, Szczesna-Cordary D. The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts. J Cell Mol Med. 2012;16:911–919. doi: 10.1111/j.1582-4934.2011.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren SA, Briggs LE, Zeng H, Chuang J, Chang EI, Terada R, Li M, Swanson MS, Lecker SH, Willis MS, Spinale FG, Maupin-Furlowe J, McMullen JR, Moss RL, Kasahara H. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation. 2012;126:2575–2588. doi: 10.1161/CIRCULATIONAHA.112.116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nixon BR, Walton SD, Zhang B, Brundage EA, Little SC, Ziolo MT, Davis JP, Biesiadecki BJ. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol. 2014;72:177–185. doi: 10.1016/j.yjmcc.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salhi HE, Walton SD, Hassel NC, Brundage EA, de Tombe PP, Janssen PML, Davis JP, Biesiadecki BJ. Cardiac troponin I tyrosine 26 phosphorylation decreases myofilament Ca2 + sensitivity and accelerates deactivation. J Mol Cell Cardiol. 2014;76:257–264. doi: 10.1016/j.yjmcc.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Q-W, Hinken AC, Patrick SE, Solaro RJ, Kobayashi T. Phosphorylation of Cardiac Troponin I at Protein Kinase C Site Threonine 144 Depresses Cooperative Activation of Thin Filaments. J Biol Chem. 2010;285:11810–11817. doi: 10.1074/jbc.M109.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wijnker PJM, Foster DB, Tsao AL, Frazier AH, dos Remedios CG, Murphy AM, Stienen GJM, van der Velden J. Impact of site-specific phosphorylation of protein kinase A sites Ser23 and Ser24 of cardiac troponin I in human cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013;304:H260–H268. doi: 10.1152/ajpheart.00498.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Memo M, Leung MC, Ward DG, dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE. Familial dilated cardiomyopathy mutations uncouple troponin I phosphorylation from changes in myofibrillar Ca(2)(+) sensitivity. Cardiovasc Res. 2013;99:65–73. doi: 10.1093/cvr/cvt071. [DOI] [PubMed] [Google Scholar]
- 34.Messer AE, Marston SB. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca(2+)-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol. 2014;5:315. doi: 10.3389/fphys.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kooij V, Saes M, Jaquet K, Zaremba R, Foster DB, Murphy AM, Remedios Cd, van der Velden J, Stienen GJM. Effect of Troponin I Ser23/24 Phosphorylation on Ca(2+)-Sensitivity in Human Myocardium Depends on the Phosphorylation Background. J Mol Cell Cardiol. 2010;48:954–963. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, Burton PBJ, Goldmann P, Jaquet K, Stienen GJM. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 37.Itoh S, Ding B, Bains CP, Wang N, Takeishi Y, Jalili T, King GL, Walsh RA, Yan C, Abe J-i. Role of p90 Ribosomal S6 Kinase (p90RSK) in Reactive Oxygen Species and Protein Kinase C β (PKC-β)-mediated Cardiac Troponin I Phosphorylation. J Biol Chem. 2005;280:24135–24142. doi: 10.1074/jbc.M413015200. [DOI] [PubMed] [Google Scholar]
- 38.Martinez EC, Passariello CL, Li J, Matheson CJ, Dodge-Kafka K, Reigan P, Kapiloff MS. RSK3: A regulator of pathological cardiac remodeling. IUBMB Life. 2015;67:331–337. doi: 10.1002/iub.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeishi Y, Huang Q, Abe J-i, Che W, Lee J-D, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Kritzer MD, Michel JJC, Le A, Thakur H, Gayanilo M, Passariello CL, Negro A, Danial JB, Oskouei B, Sanders M, Hare JM, Hanauer A, Dodge-Kafka K, Kapiloff MS. Anchored p90 Ribosomal S6 Kinase 3 Is Required for Cardiac Myocyte Hypertrophy. Circulation Research. 2013;112:128–139. doi: 10.1161/CIRCRESAHA.112.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passariello CL, Gayanilo M, Kritzer MD, Thakur H, Cozacov Z, Rusconi F, Wieczorek D, Sanders M, Li J, Kapiloff MS. p90 ribosomal S6 kinase 3 contributes to cardiac insufficiency in α-tropomyosin Glu180Gly transgenic mice. Am J Physiol Heart Circ Physiol. 2013;305:H1010–H1019. doi: 10.1152/ajpheart.00237.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilder T, Ryba DM, Wieczorek DF, Wolska BM, Solaro RJ. N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;309:H1720–H1730. doi: 10.1152/ajpheart.00339.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeves AM, Villa-Abrille MC, Pérez NG, Medina AJ, Escudero EM, Ennis IL. Physiological cardiac hypertrophy: Critical role of AKT in the prevention of NHE-1 hyperactivity. J Mol Cell Cardiol. 2014;76:186–195. doi: 10.1016/j.yjmcc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Cuello F, Snabaitis AK, Cohen MS, Taunton J, Avkiran M. Evidence for Direct Regulation of Myocardial Na+/H+ Exchanger Isoform 1 Phosphorylation and Activity by 90-kDa Ribosomal S6 Kinase (RSK): Effects of the Novel and Specific RSK Inhibitor fmk on Responses to α1-Adrenergic Stimulation. Molecular Pharmacology. 2007;71:799–806. doi: 10.1124/mol.106.029900. [DOI] [PubMed] [Google Scholar]
- 45.Diviani D, Dodge-Kafka KL, Li J, Kapiloff MS. A-kinase anchoring proteins: scaffolding proteins in the heart. Am J Physiol Heart Circ Physiol. 2011;301:H1742–H1753. doi: 10.1152/ajpheart.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Bjørbæk C, Moller DE. Regulation and Interaction of pp90rsk Isoforms with Mitogen-activated Protein Kinases. J Biol Chem. 1996;271:29773–29779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]
- 47.Tang W, Strachan RT, Lefkowitz RJ, Rockman HA. Allosteric Modulation of beta-Arrestin-Biased AT1R Signaling by Membrane Stretch. J Biol Chem. 2014;289:28271–28283. doi: 10.1074/jbc.M114.585067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Tsui H, Ke Y, Shi Y, Li Y, Davies L, Cartwright EJ, Venetucci L, Zhang H, Terrar DA, Huang CL, Solaro RJ, Wang X, Lei M. Pak1 is required to maintain ventricular Ca(2)(+) homeostasis and electrophysiological stability through SERCA2a regulation in mice. Circ Arrhythm Electrophysiol. 2014;7:938–948. doi: 10.1161/CIRCEP.113.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern S, Haverkamp S, Sinske D, Tedeschi A, Naumann U, Di Giovanni S, Kochanek S, Nordheim A, Knoll B. The transcription factor serum response factor stimulates axon regeneration through cytoplasmic localization and cofilin interaction. J Neurosci. 2013;33:18836–18848. doi: 10.1523/JNEUROSCI.3029-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 51.Zelarayan LC, Noack C, Sekkali B, Kmecova J, Gehrke C, Renger A, Zafiriou MP, van der Nagel R, Dietz R, de Windt LJ, Balligand JL, Bergmann MW. Beta-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19762–19767. doi: 10.1073/pnas.0808393105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu JJ, Horst R, Katritch V, Stevens RC, Wüthrich K. Biased Signaling Pathways in β2-Adrenergic Receptor Characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang F, Yu X, Liu C, Qu C-X, Gong Z, Liu H-D, Li F-H, Wang H-M, He D-F, Yi F, Song C, Tian C-L, Xiao K-H, Wang J-Y, Sun J-P. Phospho-selective mechanisms of arrestin conformations and functions revealed by unnatural amino acid incorporation and 19F-NMR. Nat Commun. 2015;6:8202. doi: 10.1038/ncomms9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anand-Srivastava MB. Angiotensin II receptors negatively coupled to adenylate cyclase in rat myocardial sarcolemma. Involvement of inhibitory guanine nucleotide regulatory protein. Biochem Pharmacol. 1989;38:489–496. doi: 10.1016/0006-2952(89)90389-4. [DOI] [PubMed] [Google Scholar]
- 57.Edwards RM, Stack EJ. Angiotensin II inhibits glomerular adenylate cyclase via the angiotensin II receptor subtype 1 (AT1) J Pharmacol Exp Ther. 1993;266:506–510. [PubMed] [Google Scholar]
- 58.Hackenthal E, Aktories K, Jakobs KH. Mode of inhibition of renin release by angiotensin II. J Hypertens Suppl. 1985;3:S263–S265. [PubMed] [Google Scholar]
- 59.Felker GM, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Soergel DG, Teerlink JR, Violin JD, Voors AA, Pang PS. Heart Failure Therapeutics on the Basis of a Biased Ligand of the Angiotensin-2 Type 1 Receptor: Rationale and Design of the BLAST-AHF Study (Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure) JACC: Heart Fail. 2015;3:193–201. doi: 10.1016/j.jchf.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Soergel DG, Subach RA, Cowan CL, Violin JD, Lark MW. First Clinical Experience with TRV027: Pharmacokinetics and Pharmacodynamics in Healthy Volunteers. J Clin Pharmacol. 2013;53:892–899. doi: 10.1002/jcph.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.