Abstract
Background
Clinical heart failure (HF) occurs frequently after ST-segment elevation myocardial infarction (STEMI), and is associated with increased mortality. We assessed the impact of remote ischemic peri-conditioning (RIPC) during inter-facility air medical transport of STEMI patients on clinical HF following primary percutaneous coronary intervention (pPCI).
Methods
Data from Acute Coronary Treatment and Intervention Outcomes Network Registry®-Get With the Guidelines™ (ACTION Registry-GWTG) from two PCI-hospitals that are utilizing RIPC during inter-facility helicopter transport of STEMI patients for pPCI between March, 2013 and September, 2015 were used for this study. The analyses were limited to inter-facility STEMI patients transported by helicopter with LVEF <55% after pPCI. The outcome measures were occurrence of clinical HF and serum level of brain-type natriuretic peptide (BNP).
Results
Out of the 150 STEMI patients in this analysis, 92 patients received RIPC and 58 did not. The RIPC and non-RIPC groups were generally similar in demographic and clinical characteristics except for lower incidence of cardiac arrest in the RIPC group (3/92 [3.3%] versus 13/58 [22.4%], p=0.002). STEMI patients who received RIPC were less likely to have in-hospital clinical HF compared to patients who did not receive RIPC (3/92 [3.3%] versus 7/58 [12.1%]; adjusted OR=0.22, 95% CI 0.05–0.92, p=0.038) after adjusting for baseline differences. In subgroup analysis, RIPC was associated with lower BNP (123 [interquartile range, 17.0–310] versus 319 [interquartile range, 106–552], p= 0.029).
Conclusion
RIPC applied during inter-facility air transport of STEMI patients for pPCI is associated with reduced incidence of clinical HF and serum BNP.
Keywords: remote ischemic conditioning, acute myocardial infarction, heart failure, brain-type natriuretic peptide
INTRODUCTION
Clinical heart failure is a common complication of ST-segment elevation myocardial infarction (STEMI), leading to prolonged hospital stay, consumption of healthcare resources, and increased morbidity and mortality [1–3]. While clinical heart failure (HF) and reduced left ventricular ejection fraction (LVEF) are not synonymous, most patients that develop clinical HF after STEMI have reduced LVEF, and some patients with reduced LVEF have no clinical signs or symptoms of HF. Although improvements in STEMI treatment and prompt primary percutaneous coronary intervention (pPCI) in the last decade have reduced the incidence and/or severity of cardiac dysfunction after STEMI, some studies have indicated that a significant proportion of patients still develop clinical HF [1,2], with a reported incidence rate of about 10–40% depending on the population studied [1,2]. There is an increasing concern that the reduction in mortality of STEMI patients might lead to increased numbers of patients surviving only to suffer severe HF [4], with significant global economic burden [3]. Therefore, there is a need to develop adjunctive therapy to pPCI that can prevent or reduce clinical HF in STEMI patients.
In this regard, emerging evidence suggests that remote ischemic conditioning (RIC) elicited by non-injurious, brief episodes of ischemia and reperfusion at a distant vascular bed may protect vital organs such as the heart from subsequent injury [5,6]. This non-invasive strategy appears to confer protection when applied prior to prolonged ischemic injury (pre-conditioning), during ischemic injury (peri-conditioning) and at the end of ischemic injury or onset of reperfusion (post-conditioning). In a recent pre-clinical study in rats, RIC reduced adverse left ventricular remodeling and oxidative stress induced by myocardial infarction [7]. However, the effect of RIC on clinical HF in humans remains unknown. Accordingly, this cross-sectional study examined for the first time the impact of remote ischemic peri-conditioning (RIPC) on in-hospital clinical HF and biomarker of cardiac dysfunction, brain-type natriuretic peptide (BNP), in a contemporary population of STEMI patients with reduced LVEF after pPCI.
MATERIALS AND METHODS
Protocol Implementation and Data Sources
Data from the National Cardiovascular Data Registry® (NCDR) Acute Coronary Treatment and Intervention Outcomes Network Registry®-Get With the Guidelines™ (ACTION Registry–GWTG) from two tertiary care STEMI receiving centers (University of Pittsburgh Medical Center Presbyterian and Passavant Hospital) in the United States that are utilizing RIPC during inter-facility helicopter transport of STEMI patients for pPCI were used for this study. The ACTION Registry–GWTG is a nationwide, voluntary, quality improvement registry sponsored by the American College of Cardiology and the American Heart Association [8]. The conduct of the RIPC protocol during helicopter transport of STEMI patients transferred to our two receiving facilities has been previously published [9]. In summary, RIPC was performed during inter-facility helicopter transport by repeated 5-minute cycles of inflation to 200 mmHg and deflation of a blood pressure cuff in the upper arm. A maximum of four cycles of RIPC were performed en route, and standard medical care for these patients and timeliness of transport were prioritized over RIPC. The protocol was approved by the University of Pittsburgh Institutional Review Board, the Pennsylvania Department of Health, the state EMS advisory council, and the regional Emergency Medical Services (EMS) council.
Patient Population
Patients with STEMI (n=536) that presented to the two PCI centers for pPCI from March 2013 through September 2015 were identified. Analyses were limited to STEMI patients with inter-facility air transport within the period that the RIPC quality improvement protocol was implemented. The protocol was first implemented at two air medical base sites as a pilot and then extended to 12 additional base sites, resulting in a cohort of patients that did or did not receive RIPC. STEMI was defined as chest pain or epigastric pain for more than 30 minutes and either (a) new ST elevation at the J point in two contiguous leads with the cut-off points of ≥0.2 mV in men or ≥0.15 mV in women in leads V2-V3 or ≥0.1 mV in all other leads or (b) new or presumed new left bundle branch block on electrocardiogram. Patients were excluded if they arrived directly to the Emergency Department of the two PCI centers (n=88), were transferred from the field directly to the two PCI centers (n=177), were transported by ground ambulance (n=49), and those with persistent hypotension at the time of air transport (n=3) were excluded. These patients were excluded because during the period of this study RIPC was not routinely performed in this EMS system for those transferred by ground ambulance and those with hypotension at the time of transfer. Additionally, those transferred from the field were excluded because the current analysis is limited to inter-facility transfers where multiple cycles of RIPC can be performed during transfer of the patients without delaying pPCI at the receiving facility. Given that patients with reduced LVEF are more likely to develop clinical HF symptoms, those with LVEF ≥55% (n=69) were excluded from 219 inter-facility STEMI patients. The final population (n=150) included in this analysis were inter-facility STEMI patients that were transported by air ambulance, received coronary stenting or plain old balloon angioplasty, and with LVEF <55% after pPCI.
Study Outcomes
The main study outcome measure was the occurrence of clinical HF, defined as physician report of one or more of the following: i) unusual dyspnea on exertion or dyspnea occurring in the supine position; ii) rales or jugular venous distention on physical examination; and iii) pulmonary edema on chest x-ray presumed to be due to cardiac dysfunction. The other outcome measure was the first BNP level during hospitalization. These definitions were based on predefined criteria outlined in the ACTION Registry®–GWTG™ database. For outcomes analyses, we report i) the percentage of patients who experienced in-hospital clinical HF, and ii) the median BNP value.
Data Analysis
Descriptive characteristics of the study population are presented as n (%) for categorical variables and median (interquartile range) or mean (SD) for continuous variables. Baseline differences between patients that received RIPC and those that did not were tested using chi-squared tests for categorical variables (Fisher’s Exact test for very small cells) and Wilcoxon rank-sum tests for continuous variables.
Although this analysis was based on registry data with modest sample size, there were only trivial differences in baseline characteristics between the patients that received RIPC and those that did not receive RIPC. Therefore, the conventional approach to assessing association in non-randomized studies (performing multivariable regression to “adjust” for characteristics that differ between the treatment groups) does not require that many covariates be accounted for with multivariable modeling. Nevertheless, to assess the robustness of our results when accounting for potential confounders, we present the effect of RIPC on clinical HF with a multivariable logistic regression model adjusted for variable with significant baseline difference: cardiac arrest on first medical contact. We also performed subgroup analysis excluding patients with cardiac arrest on first medical contact.
For outcomes analyses, we report i) the percentage of patients who experienced in-hospital clinical HF, and ii) the median BNP value. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Two-sided p values <0.05 were considered as indicative of statistical significance.
RESULTS
Out of the 150 STEMI patients (median age, 62 years, interquartile range: 54–70 years; 74% male) included in this analysis, 92 patients received RIPC and 58 did not. Of the 92 patients that received RIPC, 70 patients (76.1%) received 3 or 4 cycles of RIPC while 22 patients (23.9%) received less than 3 cycles. The RIPC and non-RIPC groups were generally similar in demographic, clinical, angiographic and procedural characteristics except for significantly lower incidence of cardiac arrest in the RIPC group (3/92 [3.3%] versus 13/58 [22.4%], p=0.002) (Tables 1 and 2).
Table 1.
Baseline Clinical Characteristics and Risk Factors
| Overall | Non RIPC | RIPC | P-Value | |
|---|---|---|---|---|
| # Patients | 150 | 58 | 92 | |
| Demographics | ||||
| Age (Years) | 62.0 (54.0–70.0) | 61.5 (54.0–70.0) | 62.0 (53.5–71.0) | 0.922 |
| Gender (% Male) | 111 (74.0%) | 46 (79.3%) | 65 (70.7%) | 0.239 |
| Height (cm) | 173 (163–180) | 173 (170–178) | 173 (163–180) | 0.251 |
| Weight (kg) | 84.5 (75.0–100) | 83.5 (76.0–100) | 86.0 (73.5–100) | 0.769 |
| Body Mass Index | 29.1 (25.5–34.0) | 27.5 (24.3–34.0) | 29.5 (26.4–34.2) | 0.071 |
| Race | 0.724 | |||
| White | 145 (96.7%) | 57 (98.3%) | 88 (95.7%) | |
| Black | 3 (2.0%) | 1 (1.7%) | 2 (2.2%) | |
| Asian | 1 (0.7%) | 0 (0.0%) | 1 (1.1%) | |
| Other | 1 (0.7%) | 0 (0.0%) | 1 (1.1%) | |
| Insurance | 0.658 | |||
| Private | 77 (51.3%) | 29 (50.0%) | 48 (52.2%) | |
| Public | 59 (39.3%) | 22 (37.9%) | 37 (40.2%) | |
| None | 14 (9.3%) | 7 (12.1%) | 7 (7.6%) | |
| Medical History | ||||
| Hypertension | 85 (56.7%) | 36 (62.1%) | 49 (53.3%) | 0.289 |
| Dyslipidemia | 74 (49.3%) | 30 (51.7%) | 44 (47.8%) | 0.642 |
| Current Dialysis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Chronic Lung Disease | 7 (4.7%) | 3 (5.2%) | 4 (4.3%) | 0.845 |
| Diabetes | 30 (20.0%) | 7 (12.1%) | 23 (25.0%) | 0.054 |
| Prior Myocardial Infarction | 22 (14.7%) | 11 (19.0%) | 11 (12.0%) | 0.237 |
| Prior Heart Failure | 5 (3.3%) | 2 (3.4%) | 3 (3.3%) | 0.950 |
| Prior Percutaneous Coronary Intervention | 25 (16.7%) | 13 (22.4%) | 12 (13.0%) | 0.134 |
| Prior Coronary Artery Bypass Graft | 6 (4.0%) | 2 (3.4%) | 4 (4.3%) | 0.784 |
| Prior Atrial fibrillation | 5 (3.3%) | 1 (1.7%) | 4 (4.3%) | 0.383 |
| Prior Stroke | 5 (3.3%) | 2 (3.4%) | 3 (3.3%) | 0.206 |
| Prior Peripheral Artery Disease | 3 (2.0%) | 1 (1.7%) | 2 (2.2%) | 0.848 |
| Home Medications | ||||
| Aspirin | 45 (30.0%) | 21 (36.2%) | 24 (26.1%) | 0.188 |
| Clopidogrel | 8 (5.3%) | 3 (5.2%) | 5 (5.4%) | 0.945 |
| Prasugrel | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Ticargrelor | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Warfarin | 3 (2.0%) | 1 (1.7%) | 2 (2.2%) | 0.848 |
| Beta blocker | 31 (20.7%) | 14 (24.1%) | 17 (18.5%) | 0.405 |
| Angiotensin Converting Enzyme Inhibitor | 24 (16.0%) | 8 (13.8%) | 16 (17.4%) | 0.558 |
| Angiotensin Receptor Blocker | 9 (6.0%) | 2 (3.4%) | 7 (7.6%) | 0.289 |
| Aldosterone Blocking Agent | 3 (2.0%) | 2 (3.4%) | 1 (1.1%) | 0.314 |
| Statin | 31 (20.7%) | 13 (22.4%) | 18 (19.6%) | 0.675 |
Continuous variables presented as median (IQR) and categorical variables presented as n (%).
Table 2.
Clinical Presentation, Reperfusion Time, Biomarkers and Angiographic Characteristics
| Overall | Non RIPC | RIPC | P-Value | |
|---|---|---|---|---|
| # Patients | 150 | 58 | 92 | |
| Signs at Presentation | ||||
| Heart Rate (beats per minute) | 80.0 (69.0–94.5) | 86.5 (69.0–98.5) | 78.0 (69.5–90.5) | 0.065 |
| Systolic Blood Pressure (mmHg) | 145 (124–161) | 137 (116–168) | 146 (127–159) | 0.228 |
| Shock on First Medical Contact | 5 (3.3%) | 3 (5.2%) | 2 (2.2%) | 0.319 |
| Cardiac Arrest on First Medical Contact | 16 (10.7%) | 13 (22.4%) | 3 (3.3%) | 0.0002 |
| Diseased Vessels and Cardiac Function | ||||
| Vessels with Significant Disease | ||||
| Left Main | 4 (2.7%) | 2 (3.4%) | 2 (2.2%) | 0.637 |
| Proximal Left Anterior Descending | 34 (22.7%) | 14 (24.1%) | 20 (21.7%) | 0.733 |
| Mid/Distal Left Anterior Descending | 49 (32.7%) | 21 (36.2%) | 28 (30.4%) | 0.463 |
| Left Circumflex | 41 (27.3%) | 15 (25.9%) | 26 (28.3%) | 0.748 |
| Right coronary | 70 (46.7%) | 32 (55.2%) | 38 (41.3%) | 0.097 |
| Ramus Intermedius | 6 (4.0%) | 3 (5.2%) | 3 (3.3%) | 0.561 |
| Number of Diseased Vessels | 0.424 | |||
| 1 | 102 (68.0%) | 39 (67.2%) | 63 (68.5%) | |
| 2 | 30 (20.0%) | 14 (24.1%) | 16 (17.4%) | |
| 3 | 18 (12.0%) | 5 (8.6%) | 13 (14.1%) | |
| Left ventricular Ejection Fraction | 45.0 (38.0–53.0) | 49.0 (40.0–53.0) | 45.0 (38.0–50.0) | 0.290 |
| Culprit Vessels | 0.467 | |||
| Left Anterior Descending | 64 (42.7%) | 24 (41.4%) | 40 (43.5%) | |
| Non Left Anterior Descending | 86 (57.3%) | 34 (58.6%) | 52 (56.5) | |
| Coronary Intervention/Stent Type | 0.250 | |||
| Plain Old Balloon Angioplasty | 15 (10.0%) | 5 (8.6%) | 10 (10.9%) | |
| Bare Metal Stent | 38 (25.3%) | 19 (32.8%) | 19 (20.7%) | |
| Drug Eluting Stent | 97 (64.7%) | 34 (58.6%) | 63 (68.5%) | |
| Cardiac and Renal Biomarkers | ||||
| Initial Troponin (ng/mL) | 0.9 (0.1–8.3) | 0.7 (0.1–9.0) | 0.9 (0.1–7.9) | 0.727 |
| Peak Troponin (ng/mL) | 54.0 (18.6–112) | 46.2 (18.3–130) | 56.7 (19.8–111) | 0.749 |
| Initial Creatinine (mg/dL) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 1.0 (0.8–1.1) | 0.602 |
| Initial BUN (mg/dL) | 16.0 (13.0–20.0) | 16.0 (13.0–18.0) | 17.0 (13.0–22.0) | 0.192 |
| Initial eGFR by MDRD (ml/min/1.73m2) | 77.8 (64.4–93.9) | 76.9 (62.0–95.8) | 78.0 (65.1–93.8) | 0.649 |
| Total Contrast Used (mL) | 175 (150–200) | 175 (155–200) | 175 (138–220) | 0.879 |
| Reperfusion Time Intervals | ||||
| Door-In-Door-Out (minutes) | 54.0 (41.0–75.0) | 59.0 (43.0–97.0) | 52.0 (41.0–71.0) | 0.170 |
| Transport Time (minutes) | 29.0 (19.0–35.0) | 25.0 (16.0–33.0) | 30.0 (22.0–35.0) | 0.159 |
| Procedure Time (minutes) | 27.0 (22.6–40.0) | 26.0 (23.0–44.0) | 27.5 (22.0–37.9) | 0.543 |
| Door-To-Balloon Time (minutes) | 112 (88.0–138) | 112 (88.0–148) | 112 (88.0–130) | 0.552 |
| Door-To-Balloon Time Goal Category | 0.844 | |||
| <120 minutes | 92 (61.3%) | 35 (60.3%) | 57 (62.0%) | |
| >120 minutes | 58 (38.7%) | 23 (39.7%) | 35 (38.0%) |
Continuous variables presented as median (IQR) and categorical variables presented as n (%).
eGFR = estimated glomerular filtration rate; MDRD = modification of diet in renal disease.
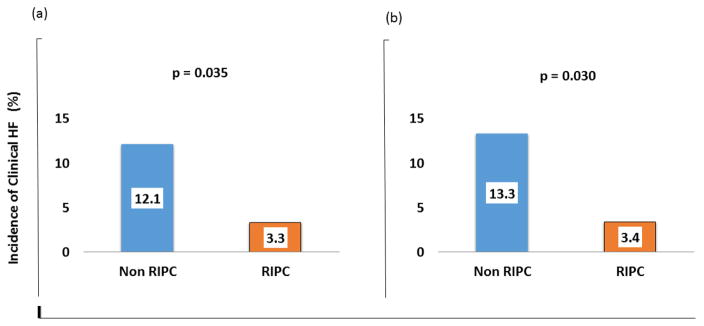
As shown in figure 1a. Despite having similar incidence of prior HF (3/92 [3.3%] versus 2/58 [3.4%], p=0.950), left main coronary artery disease (2/92 [2.2%] versus 2/58 [3.4%], p=0.637), anterior wall myocardial infarction (40/92 [43.5%] versus 24/58 [41.4%], p=0.467), left ventricular ejection fraction (45 [interquartile range, 38.0–50.0] versus 49 [interquartile range, 40–53], p=0.290), and door-to-balloon time (112 [interquartile range, 88–130] versus 112 [interquartile range, 88–130], p=0.552), STEMI patients who received RIPC were less likely to have in-hospital clinical HF compared to patients who did not receive RIPC (3/92 [3.3%] versus 7/58 [12.1%]; OR=0.25, 95% CI 0.06–0.99, p=0.035). In multivariate logistic regression analysis adjusting for baseline difference in incidence of cardiac arrest, RIPC remained significantly associated with lower incidence of clinical HF (OR=0.22, 95% CI 0.05–0.92, p=0.038).
Figure 1.
Incidence of in-hospital clinical heart failure (HF) with reduced ejection fraction after primary percutaneous coronary intervention in the overall study population (a) and in those without cardiac arrest (b) stratified by whether they received or did not receive remote ischemic peri-conditioning (RIPC).
Further subgroup analyses restricted to patients without cardiac arrest (n=134) showed similar results (3/89 [3.4%] versus 6/45 [13.3%]; OR=0.28, 95% CI 0.05–0.95, p=0.030) (Figure 1b). Similarly, in a subgroup of patients that had serum BNP measured during hospitalization (n=41), RIPC, compared to non-RIPC group, was associated with lower serum BNP level (123 [interquartile range, 17.0–310] versus 319 [interquartile range, 106–552], p= 0.029).
DISCUSSION
This cross-sectional study of contemporary STEMI patients at two PCI centers in the United States suggests an association of RIPC applied during inter-facility air transport of STEMI patients with reduced incidence of in-hospital clinical HF, and lower serum BNP among patients with reduced LVEF after PCI. To the best of our knowledge, this is the first report on the impact of RIPC applied during interfacility air transport of STEMI patients on clinical HF after pPCI.
The occurrence of clinical HF in STEMI patients results in exercise intolerance, impairs quality of life, and has been associated with high morbidity and mortality [1,2]. Although improvements in STEMI treatment by prompt revascularization have reduced the incidence and severity of clinical HF after STEMI, a significant proportion of patients still develop clinical HF [1,2]. Therefore, there is a need to develop adjunctive therapy to prevent or reduce clinical HF in STEMI patients. The present observation of reduced clinical HF and serum BNP in STEMI patients that received RIPC calls for additional study on the possible cardio-protective effects of RIPC on ischemia HF in STEMI patients.
Although, the mechanisms of these associations are not completely understood, studies have shown that that several signal transduction pathways are activated by RIPC, including generation of endogenous protective biomarkers in many organs, and specifically in the heart, nitric oxide and nitrite [10, 11]. A recent translational study in rat and humans found nitrite, the single electron oxidation product of nitric oxide, to be increased by about 50% with a single cycle of RIPC when normal reperfusion accompanied by reactive hyperemia was performed. Further experiments suggested that nitrite was both necessary and sufficient to confer cardio-protection when human plasma after RIPC was given to rat hearts in an ex vivo cardiac ischemia reperfusion model [11]. This endogenous, RIPC-induced nitrite may reduce clinical heart failure symptoms and serum BNP via vasodilatory activity in large veins and arteries resulting in reduced pre-load, after-load and ventricular wall stress. The present observation extends upon these findings and provides new insight by demonstrating that RIPC may protect against clinical HF in humans with STEMI undergoing pPCI.
The present study has some limitations. First, this is a cross-sectional analysis of a registry data and thus could not account for unmeasured cofounders including effect of medications such as ticagrelor on development of dyspnea. Second, the present study is limited to patients with reduced LVEF (<55%) after pPCI who are known to have higher incidence of clinical HF. Third, our sample size is modest and outcome events are few thus limiting adjustment for multiple covariates. However, there were only trivial differences in baseline characteristics between the patients that received RIPC and those that did not receive RIPC. Therefore, the conventional approach to assessing association in non-randomized studies (performing propensity score matching or multivariable regression to “adjust” for characteristics that differ between the treatment groups) does not require that many covariates be accounted for with multivariable modeling. Nevertheless, we observed similar findings with multivariable adjustment for cardiac arrest and subgroup analysis excluding patients with cardiac arrest. Given the significance of clinical HF and the associated morbidity and mortality in STEMI patients, the observed reduced incidence of clinical HF in the RIPC group is an important hypothesis generating finding that warrants further in-depth investigation. To the best of our knowledge, this is the first report on impact of RIPC applied during inter-facility transfer on clinical HF in STEMI patients.
Conclusions
RIPC applied during inter-facility air transport is associated with lower incidence of in-hospital clinical HF and serum BNP level in contemporary population of STEMI patients with reduced LVEF after pPCI. Large randomized clinical trials are needed to determine the effects of RIPC on clinical HF in patients with coronary artery disease, and to discern the mechanisms of such effects.
HIGHLIGHTS.
Clinical heart failure (HF) occurs frequently after ST-segment elevation myocardial infarction (STEMI), and is associated with increased mortality
This study shows that remote ischemic peri-conditioning (RIPC) applied during inter-facility helicopter transfer of STEMI patients is associated with reduced incidence of clinical HF after primary percutaneous coronary intervention (pPCI)
Large, multicenter randomized controlled studies are needed to determine the effects of RIPC on clinical HF in STEMI patients
Acknowledgments
Funding: This study was funded in part by National Institutes of Health (5K12HL109068-04 and UL1TR000005)
Footnotes
Disclosures: All authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly DJ, Gershlick T, Witzenbichler B, Guagliumi G, Fahy M, Dangas G, et al. Incidence and predictors of heart failure following percutaneous coronary intervention in ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Am Heart J. 2011;162:663–70. doi: 10.1016/j.ahj.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart. 2005;91(Suppl 2):ii7–ii13. doi: 10.1136/hrt.2005.062026. discussion ii31, ii43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–76. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Jhund PS, McMurray JJ. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118:2019–21. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 5.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 6.Lavi S, Lavi R. Conditioning of the heart: from pharmacological interventions to local and remote protection: possible implications for clinical practice. Int J Cardiol. 2011;146:311–8. doi: 10.1016/j.ijcard.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2015;178:239–46. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 8.Peterson ED, Roe MT, Rumsfeld JS, Shaw RE, Brindis RG, Fonarow GC, et al. A call to ACTION (Acute Coronary Treatment and Intervention Outcomes Network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–9. doi: 10.1161/CIRCOUTCOMES.108.847145. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Gill C, Wayne M, Guyette FX, Olafiranye O, Toma C. Feasibility of Remote Ischemic Peri-conditioning during Air Medical Transport of STEMI Patients. Prehosp Emerg Care. 2016;20:82–9. doi: 10.3109/10903127.2015.1056894. [DOI] [PubMed] [Google Scholar]
- 10.Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–95. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circulation research. 2014;114:1601–10. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]