Abstract
Objective
We tested an uncertainty self-management telephone intervention (SMI) with patients awaiting liver transplant and their caregivers.
Methods
Participants were recruited from four transplant centers and completed questionnaires at baseline, 10, and 12 weeks from baseline (generally two and four weeks after intervention delivery, respectively). Dyads were randomized to either SMI (n = 56) or liver disease education (LDE; n = 59), both of which involved six weekly telephone sessions. SMI participants were taught coping skills and uncertainty management strategies while LDE participants learned about liver function and how to stay healthy. Outcomes included illness uncertainty, uncertainty management, depression, anxiety, self-efficacy, and quality of life. General linear models were used to test for group differences.
Results
No differences were found between the SMI and LDE groups for study outcomes.
Conclusion
This trial offers insight regarding design for future interventions that may allow greater flexibility in length of delivery beyond our study’s 12-week timeframe.
Practice implications
Our study was designed for the time constraints of today’s clinical practice setting. This trial is a beginning point to address the unmet needs of these patients and their caregivers as they wait for transplants that could save their lives.
Keywords: Self-management, Intervention, Illness uncertainty, Coping skills
1. Introduction
Patients awaiting liver transplants live with significant uncertainty while suffering substantial morbidity and mortality [1–4]. Agonizing uncertainty [5,6] exacerbates chronic illness symptoms, including fatigue, muscle weakness, nausea, and weight loss, and in cases of advanced disease, encephalopathy and diminished cognitive ability [7,8]. Uncertainty includes wondering about transplant wait time, whether physical function will deteriorate before transplantation, and whether deterioration will increase dependence on caregivers. The experience of waiting may exacerbate depressive symptoms and worsen quality of life. Currently, more than 15,000 Americans await liver transplants [9] and up to 17% will die before the surgery can take place [10]. Despite this enormous impact, little attention has been paid to the experience of waiting, and few interventions address the patient’s emotional needs [11,12].
One randomized controlled trial has been conducted outside of the United States for patients awaiting liver transplants and caretakers [13]. Psycho-educational interventions were delivered to patients in three face-to-face sessions for each patient and a family member. Patients and their caregivers also participated in a 90-min group session. While results were promising, the session lengths are not realistic in the current U.S. healthcare environment.
To address patients’ needs and provide a more timely delivery of services, we designed a telephone intervention comprised of cognitive behavior-based coping skills training and symptom management strategies informed by Uncertainty in Illness Theory [14]. Mishel has proposed that over time, chronic illness causes uncertainty to spread from symptom and disease state concerns to uncertainty about broader life issues [14]. The theory has informed interventions aimed at helping patients and their family members manage uncertainty and disease symptoms associated with a variety of illnesses [15,16]. In a previous study, we evaluated the benefits of an uncertainty intervention delivered by telephone for men who chose “watchful waiting” after a prostate cancer diagnosis, and found that experimental group participants experienced less confusion and improved quality of life [14,17].
Our aim was to see if we could reproduce those positive results in a randomized controlled trial (RCT) for the efficacy of a telephone-delivered uncertainty self-management intervention designed to teach patients and their caregivers (1) cognitive-behavioral coping skills and (2) symptom management strategies. Caregivers served as coaches for their family members in between intervention sessions. This study, one of three projects in the Center for Self-Management in Life-Limiting Illness, a P01 Center funded by the National Institute of Nursing Research [18], compared this self-management intervention to an education control condition on outcomes of patient and caregiver psychological well-being (illness uncertainty, depression, anxiety), self-efficacy, symptom control, uncertainty management, and quality of life.
2. Methods
We collected data from patients awaiting liver transplant and their caregivers at baseline, 10 weeks and 12. Patients and caregivers participated in six weekly phone calls over a period of eight weeks; we allowed an additional two weeks for participants unavailable for their scheduled calls. After the intervention period, staff conducted follow-up surveys. Patients received $20 per survey; caregivers received $10 per survey.
We enrolled patients and caregivers from four liver transplant centers: Duke University Medical Center, University of North Carolina at Chapel Hill, University of Pittsburgh Medical Center, and University of Nebraska Medical Center. Eligible patients were: on a liver transplant list, 18 years or older, able to read and speak English, had not received a prior transplant (any organ), and had a caregiver willing to participate. Caregivers were: the individual who accompanied the patient to pre-transplant clinic visits, 18 years or older, and able to read and speak English. Eligible patients and caregivers had no significant cognitive impairment.
2.1. Recruitment and randomization
A letter describing the study was mailed to eligible patients with upcoming appointments. The letter was followed with a face-to-face clinic visit or telephone contact. A member of the study team obtained written consent. Dyads were randomly assigned to either the treatment or comparison condition via a computer-generated block randomization sequence stratified by Duke/non-Duke study site. The interventionist disclosed the randomization status to the dyad; study team members conducting follow-up surveys were blinded to randomization arm. Institutional Review Boards at all four sites approved the protocol.
2.2. Setting and intervention
This study builds upon our prior work by adding cognitive-behavioral coping skills training and symptom self-management based on Uncertainty in Illness Theory [14,19] for patients awaiting liver transplant. The benefits of coping skills training, used to help patients reframe how they view their symptoms, are well known [20,21]. They are beneficial in reducing pain, fatigue and psychological distress. Symptom self-management refers to the patient’s ability to manage treatment, symptoms, and psychosocial challenges [21].
2.2.1. Self-Management Intervention (SMI)
The standardized intervention was delivered to dyads by a trained interventionist, either a registered nurse or social worker, in six 30-min phone calls. Intervention components included: (a) coping skills training, based on cognitive-behavioral principles, to help patients change illness-related thoughts, emotions and behaviors; and (b) symptom management strategies, based on Uncertainty in Illness Theory, designed to provide information about symptoms and strategies to decrease their frequency and intensity.
The first session introduced the intervention and role of coping skills. Caregivers participated as coaches by learning and practicing new skills with the patients, and helping them apply the skills on a daily basis. Caregivers were also encouraged to use what they learned to manage their own stress. This portion of the intervention included progressive muscle relaxation, brief relaxation (mini-practices), pleasant imagery, activity pacing (activity-rest cycling), and cognitive restructuring. Traditionally cognitive restructuring focuses on modifying irrational thoughts, however our approach was to help the patient identify overly negative thoughts that contribute to distress (such as “I can’t do the things I used to do”) and replace them with more positive, realistic coping thoughts (e.g., “I can’t do all of the things I would like to do, but there are still things I can do”). This approach is recommended for patients with chronic illness [22] and is similar to that used in our prior study of patients with lung cancer [23]. The interventionist taught each skill by providing a description and rationale for its use, then led the patient and caregiver through practice with feedback. Subsequent sessions began with a review of the previous sessions’ content and how the new skills were being used. Sessions ended with homework assignments. In the final session, the interventionist reviewed learned skills and helped the dyad develop a sustainable plan. Participants received handouts describing the coping skills and a CD-ROM with muscle relaxation exercises.
Symptom management training included an assessment of symptoms and strategies to manage them. In the first session, the interventionist referred the patient and caregiver to the Symptom Guide for information on fatigue, nutrition, memory concerns, sleep, skin problems, and ascites, with detailed information on management. For example, discussions about fatigue focused on scheduling activities at times when the patient’s energy level would be highest. We encouraged patients to eat small meals and healthy snacks to improve nutrition and encouraged brain stimulating exercises (crosswords) or brain protecting activities (walking outside for 15-min daily).
2.2.2. Liver Disease Education (LDE) intervention
Dyads received six telephone-based education sessions about liver functions, disease etiologies, stages of liver disease, diagnosing liver disease, common treatments, transplantation, and staying healthy while waiting for a transplant. The LDE attention control intervention provided interactive sessions for the patient and caregiver, similar to the treatment group. The 30-min sessions were delivered by the same interventionists as the SMI. Participants in both groups who did not have access to a speaker phone were mailed one for the duration of the intervention.
2.2.3. Intervention fidelity
The interventions were standardized, and interventionists were trained through role play and feedback. All sessions were recorded. During the first three months of the study, two investigators (DEB and LSP) conducted fidelity checks of all calls using a 5-item rating scale with 1 (Poor) to 5 (Excellent). They continued to monitor 25% of all calls and met weekly with interventionists. Investigator ratings of fidelity ranged from 4 (Very Good) to 5 (Excellent) throughout the intervention period.
2.3. Patient measures
The Model for End-Stage Liver Disease (MELD) is a numerical scale used to rate illness severity for adult liver transplant candidates. The range is from 6 (less ill) to 40 (gravely ill). The individual score determines how urgently a patient needs a liver transplant within the next three months. The number is calculated using current laboratory tests [9,24]. MELD score was included in baseline data. Demographics collected included age, gender, ethnicity, race, marital status, employment status, financial well-being, and education.
2.3.1. Outcomes
Illness uncertainty, the primary outcome, was measured by the Mishel Uncertainty in Illness Scale (MUIS-A) [25], a 33-item scale that identifies four types of uncertainty; ambiguity, complexity, inconsistency, and unpredictability. Responses are selected on a 5-point scale ranging from strongly agree to strongly disagree. Total scale scores range from 33 to 165. Higher scores indicate greater levels of illness uncertainty. The scale has been used widely in studies involving cancer and chronic illness [26].
Depression was measured using the 10-item Center for Epidemiological Studies Depression Scale (CES-D), [27] which assesses depressive symptoms experienced in the past week [28,29]. Scores are summed across items and may range from 0 to 30, with a score of 16 or higher indicating risk for clinical depression [27]. Participants with scores of 17 or higher were further screened to determine if they were at risk for self-harm.
Anxiety was measured using the Profile of Mood States [30,31] anxiety sub-scale that consisted of five items rated on a scale from 0 to 4, based on the strength of emotion where 0 = “not at all," and 4 = “extremely." Higher scores indicate greater levels of anxiety.
Uncertainty management was measured by two subscales from the Self-Control Schedule: problem solving and cognitive reframing [32]. Problem solving was defined as the ability to identify and define concerns and generate solutions. Cognitive reframing was defined as the ability to address concerns from a positive point of view. Higher scores indicate greater levels of problem solving and cognitive reframing.
Self-efficacy for symptom management was measured by a 12-item self-efficacy scale [33]. We modified the scale to include liver disease symptoms. The scale uses ratings from 10 (very uncertain) to 100 (very certain). Items are phrased in terms of can rather than will, since can is a judgment of capability, while will is a statement of intention. Scores indicate the strength of perceived self-efficacy of patients. The self-efficacy score was the average of the 12 items, and the single item assessing stress was treated as a stand-alone item. Prior studies used this instrument to assess self-efficacy in cancer patients and their caregivers [23,34].
Patient’s Quality of Life (QoL) was measured using the Quality of Life in Chronic Illness: FACT-G. This 27-item questionnaire measures four domains of quality of life (physical, functional, social/family, and emotional well-being) [35]. A higher score indicates better quality of life.
2.4. Caregiver measures
Demographics were the same as those collected for patients with the addition of items to determine their relationship to the patient and time spent caregiving.
2.4.1. Outcomes
Caregiver uncertainty was measured with the Perception of Uncertainty Scale-Family Member (PUS-FM), an assessment of a person’s evaluation of the uncertainty experienced in another’s illness. Four datasets have been used to form the normative database for the family version of the PUS-FM [26]. Higher scores indicate greater levels of illness uncertainty.
Caregiver uncertainty management was measured by two subscales from the Self-Control Schedule: problem solving and cognitive reframing [32]. This is identical to the patient measure. Higher scores indicate greater levels of problem solving and cognitive reframing.
Caregiver self-efficacy for symptom management was measured by the 12-item self-efficacy scale modified for caregivers [33].
Caregiver reaction was assessed with a 24-item multidimensional instrument designed to evaluate reactions to caregiving for family members with a variety of chronic illnesses. Sub-scales include: caregiver esteem, family support, impact on finances, impact on schedule, and impact on health [36].
2.5. Analysis
We estimated sample size based on the primary hypothesis that patients assigned to the SMI group would have decreased illness uncertainty at follow-up compared with the LDE group [37]. The estimated baseline to follow-up correlation was 0.5 [26]. To detect a between-arm difference in the baseline to follow-up change of 7.5 with a standard deviation of 15 with 80% power, and a type I error rate of 5%, 100 patients were needed. However, to account for dropout, we enrolled and randomized 115 eligible patients.
For all outcomes, general linear models (PROC MIXED in SAS, version 9.2, SAS Institute, Inc., Cary, North Carolina) were used to test for differences in the SMI group relative to LDE group. Final models included dummy coded time (10 and 12 weeks post-baseline [baseline as referent]), intervention arm interacted with each follow-up time point, and the stratification variable Duke vs. non-Duke study site. An unstructured covariance matrix was fit to account for the correlation of patients’ repeated measures over time. Estimated mean differences between the SMI group and LDE at 10 and 12 weeks post-baseline were calculated, along with corresponding 95% confidence intervals (CIs), using SAS ESTIMATE statements. Primary and secondary outcomes were identified a priori, and no adjustments for multiple comparisons were made. A p-value less than 0.05 was considered statistically significant.
All measurements from patients, including those who discontinued the study, were used for the longitudinal analyses (n = 115 patients). Patients and caregivers who discontinued the study differed on baseline characteristics compared with those who completed the study, so a multiple imputation procedure to estimate missing values was employed [38]. Outcomes for patients who received a transplant or died were multiply imputed, yielding an unconditional mean intervention effect over time [39]. Baseline demographic characteristics differing for patient completion include state of site, employment, education, marital status, MELD score, time on transplant list, and time spent in caregiving relationship. Baseline demographic characteristics differing for caregiver completion included state of site, marital status, patient’s MELD score, patient’s time on transplant list, and time spent caregiving. Two separate imputation models (one for patients and one for caregivers) were fit; the models included baseline variables that were predictors of dropout in addition to treatment group, site, and the patient (or caregiver) outcomes at baseline, 10, and 12 weeks post-baseline. The macro IVEware (version 0.2) [40] in SAS was used to generate 10 imputed datasets via a sequential regression method. General linear models for each outcome were fit to each of these datasets, and the 10-sets of parameter estimates and standard errors were combined using the Rubin rules for multiple imputation (using PROC MIANALYZE in SAS). More information on this general analytic approach can be found elsewhere [41–43].
3. Results
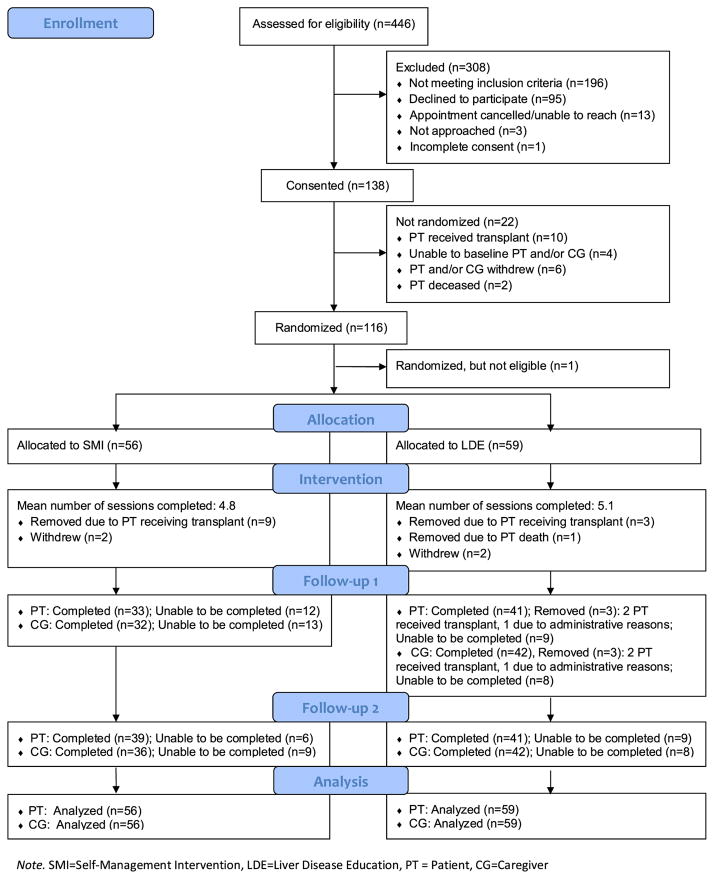
Of the 446 patient records screened for study eligibility, 250 were eligible (Fig. 1). Of these, 95 declined to participate and 17 dyads were unreachable to complete consent, resulting in a participation rate of 55% (138/250). Of the 138 consented dyads, 116 were randomized; after randomization one dyad was deemed ineligible. Of the 115 eligible dyads, 56 were assigned to the SMI group while 59 were in the LDE group. On average, dyads in the SMI group completed 4.8 intervention calls and dyads in the LDE group completed 5.1 calls. Dyads assigned to SMI completed less than the full intervention dose (six calls) as compared to LDE (29 SMI vs. 37 LDE completing 6 calls). See Table 2 for completion rates. Dyads did not complete calls because the patient had undergone a liver transplant or died; one member of the dyad had withdrawn from the study or was unable to complete follow-up due to scheduling difficulties. The first follow-up assessment was completed by 33 patients and 32 caregivers in the SMI group and 41 patients and 42 caregivers in the LDE group. The second follow-up assessment was completed by 39 patients and 36 caregivers in the SMI group and 41 patients and 42 caregivers in the LDE group.
Fig. 1.
Study enrollment.
Note. SMI=Self-Management Intervention, LDE=Liver Disease Education, PT = Patient, CG=Caregiver
Table 2.
Intervention session topic and completion rates.
| SMI | LDE | |
|---|---|---|
| N = 56 | N = 59 | |
| Session 1 | ||
| Topic | Study overview and symptom review | Study overview and liver function |
| Completion rate | 82.1% | 88.1% |
| Session 2 | ||
| Topic | Progressive muscle relaxation and symptom management | Liver disease overview |
| Completion rate | 76.8% | 88.1% |
| Session 3 | ||
| Topic | Pleasant imagery, relaxation, and symptom management | Stages of liver disease |
| Completion rate | 73.2% | 79.7% |
| Session 4 | ||
| Topic | Positive coping thoughts and symptom management | Diagnostic tests for liver disease |
| Completion rate | 67.9% | 74.6% |
| Session 5 | ||
| Topic | Pleasant activities, activity-rest cycle, and symptom management | Common treatment and liver transplantation |
| Completion rate | 62.5% | 72.9% |
| Session 6 | Review of all skills and symptom management | Staying healthy while waiting |
| Topic | ||
| Completion rate | 51.8% | 62.7% |
Note. LDE = Liver Disease Education, SMI = Self-management Intervention.
Patient characteristics are described in Table 1. On average, SMI group patients were a year older than LDE participants (56.4 vs. 55.6). The median time on the transplant wait list was 423.5 days for those in the SMI group, compared to 385.0 days for those in the LDE group.
Table 1.
Patient and caregiver characteristics at baseline.
| Patient
|
Caregiver
|
|||
|---|---|---|---|---|
| SMI | LDE | SMI | LDE | |
| N = 56 | N = 59 | N = 56 | N = 59 | |
| Demographics | ||||
| Enrollment site | ||||
| Duke University | 24 (42.9) | 28 (47.5) | 24 (42.9) | 28 (47.5) |
| UNC | 1 (1.8) | 1 (1.7) | 1 (1.8) | 1 (1.7) |
| Pittsburgh | 23 (41.1) | 21 (35.6) | 23 (41.1) | 21 (35.6) |
| Nebraska | 8 (14.3) | 9 (15.3) | 8 (14.3) | 9 (15.3) |
| Age, mean in years (SD) | 56.4 (9.9) | 55.6 (10.0) | 55.8 (11.9) | 53.8 (12.1) |
| Gender | ||||
| Male | 33 (58.9) | 37 (62.7) | 15 (26.8) | 15 (25.4) |
| Female | 23 (41.1) | 22 (37.3) | 41 (73.2) | 44 (74.6) |
| Race | ||||
| White, not of Hispanic/Latino ethnicity | 52 (92.9) | 56 (94.9) | 52 (92.9) | 57 (96.6) |
| Other | 4 (7.1) | 3 (5.1) | 4 (7.1) | 2 (3.4) |
| Marital status | ||||
| Single or never married | 5 (8.9) | 4 (6.8) | 8 (14.3) | 6 (10.2) |
| Married | 42 (75.0) | 46 (78.0) | 45 (80.4) | 49 (83.1) |
| Divorced or separated | 8 (14.3) | 5 (8.5) | 2 (3.6) | 3 (5.1) |
| Widowed | 1 (1.8) | 4 (6.8) | 1 (1.8) | 1 (1.7) |
| Highest level of educationa | ||||
| High school graduate/GED or less | 25 (44.6) | 25 (42.4) | 17 (30.9) | 27 (45.8) |
| Greater than high school | 31 (55.4) | 34 (57.6) | 38 (69.1) | 32 (54.2) |
| Relationship to patient | ||||
| Husband or wife | 38 (67.9) | 44 (74.6) | ||
| Son or daughter | 4 (7.1) | 5 (8.5) | ||
| Brother or sister | 6 (10.7) | 1 (1.7) | ||
| Father or mother | 5 (8.9) | 5 (8.5) | ||
| Other | 3 (5.4) | 4 (6.8) | ||
| Clinical Characteristics | ||||
| MELD score, mean (SD) | 15.7 (4.3) | 15.2 (4.2) | ||
| Days on transplant list, median (1st quartile, 3rd quartile) | 423.5 (132.0, 953.0) | 385.0 (108.0, 1312.0) | ||
| Uncertainty & Psychological Well Being, mean (SD) | ||||
| MUIS-A, total score | 86.1 (16.2) | 88.8 (11.5) | ||
| CES-D | 9.9 (6.1) | 9.8 (5.7) | ||
| POMS anxiety subscale, | 4.9 (4.4) | 4.7 (4.4) | ||
| SCS, cognitive reframing subscale | 76.6 (14.2) | 76.4 (16.0) | 78.8 (10.6) | 81.0 (11.0) |
| SCS, problem solving subscale, | 78.0 (15.6) | 78.5 (15.6) | 83.2 (9.7) | 83.3 (12.3) |
| Self-efficacy a | 62.5 (19.2) | 63.7 (18.3) | 63.2 (16.0) | 64.7 (19.0) |
| Fact-G, total score | 72.3 (19.1) | 72.9 (14.6) | ||
| PUS-FM, total score | 85.1 (10.1) | 81.3 (10.3) | ||
| CRA, impact on schedule subscale | 3.1 (0.9) | 3.1 (0.8) | ||
| CRA, caregiver esteem subscale | 4.2 (0.5) | 4.3 (0.5) | ||
| CRA, lack of family support subscale | 2.5 (0.9) | 2.3 (0.8) | ||
| CRA, impact on health subscale | 2.1 (0.6) | 2.2 (0.6) | ||
| CRA, impact on finances subscale | 2.7 (1.0) | 2.6 (0.9) | ||
Note. n (%) unless otherwise indicated. LDE = Liver Disease Education, SMI = Self-management Intervention, UNC = University of North Carolina at Chapel Hill, MELD = Model for End-Stage Liver Disease, CES-D = 10-item Center for Epidemiological Studies Depression Scale, MUIS-A = Mishel Uncertainty in Illness Scale, POMS = Profile of Mood States, SCS = Self-Control Schedule, Fact-G = Quality of Life in Chronic Illness, PUS-FM = Perception of Uncertainty Scale-Family Member, CRA = Caregiver Reaction Assessment.
1 caregiver has missing data for education, 1 caregiver has missing data for self-efficacy.
Caregiver characteristics are described in Table 1. The SMI caregivers were on average two years older than the LDE (55.8 vs. 53.8). The majority of caregivers in both groups were female, white, and married. Caregivers had cared for the patient for approximately 5.4 years (median number of years = 4).
3.1. Outcomes
3.1.1. Patient outcomes
There were no differences between the SMI and LDE groups in the follow-up change at 10 and 12 weeks post baseline on the primary and secondary patient outcomes of illness uncertainty, depressive symptoms, anxiety, uncertainty management, self-efficacy for symptom management, and quality of life. Mean depressive symptoms, anxiety, cognitive reframing and problem solving, aspects of uncertainty management, and QoL scores were essentially unchanged from baseline through follow up. Self-efficacy scores increased from baseline to the second follow up by 4.3 points in the SMI group as compared with a 1.2 point increase in the LDE group (Mean difference between groups = 3.1, 95% CI: −4.4, 10.7) (Table 3).
Table 3.
Estimated changes from baseline and differences between the SMI and LDE groups for patient outcomes at follow-up 1 and 2.
| Measurement and study time point | Follow-up #1 change from baseline
|
Follow-up #2 change from baseline
|
||||||
|---|---|---|---|---|---|---|---|---|
| SMI | LDE | Mean Difference Between Groups (95% CI) | P-value | SMI | LDE | Mean Difference Between Groups (95% CI) | p-value | |
| Primary outcome | ||||||||
| MUIS-A, total score | 1.4 | −0.1 | 1.5 (−2.1, 5.1) | 0.410 | −2.3 | −2.3 | 0.0 (−3.4, 3.4) | 1.000 |
| MUIS-A, ambiguity subscale | 0.5 | −0.1 | 0.6 (−2.1, 3.3) | 0.677 | −1.2 | −1.5 | 0.3 (−1.6, 2.2) | 0.739 |
| MUIS-A, complexity subscale | 0.6 | −0.003 | 0.6 (−0.5, 1.6) | 0.284 | 0.1 | 0.4 | −0.3 (−1.4, 0.8) | 0.589 |
| MUIS-A, inconsistency subscale | 0.2 | −0.1 | 0.3 (−1.1, 1.7) | 0.666 | −0.6 | −0.6 | −0.01 (−1.3, 1.3) | 0.992 |
| MUIS-A, unpredictability subscale | 0.3 | 0.02 | 0.3 (−1.2, 1.7) | 0.731 | −0.6 | −0.5 | −0.1 (−1.2, 0.9) | 0.800 |
| Secondary outcomes | ||||||||
| CES-D | −0.8 | −0.4 | −0.4 (−2.0, 1.3) | 0.669 | −0.5 | −0.1 | −0.4 (−2.2, 1.4) | 0.661 |
| POMS anxiety subscale | −0.3 | −1.1 | 0.8 (−0.7, 2.3) | 0.290 | 0.4 | 0.3 | 0.1 (−1.6, 1.8) | 0.917 |
| SCS, cognitive reframing subscale | −1.7 | −2.6 | 0.9 (−4.8, 6.5) | 0.766 | 0.4 | 0.6 | −0.2 (−5.3, 4.8) | 0.923 |
| SCS, problem solving subscale | −0.2 | −3.4 | 3.2 (−3.2, 9.5) | 0.329 | 2.7 | 1.6 | 1.1 (−4.5, 6.6) | 0.700 |
| Self-efficacy | 2.8 | 2.4 | 0.4 (−6.9, 7.7) | 0.917 | 4.3 | 1.2 | 3.1 (−4.4, 10.7) | 0.415 |
| Fact-G, total score | 0.9 | 2.0 | −1.1 (−5.0, 2.8) | 0.580 | 1.8 | 2.8 | −1.0 (−4.8, 2.9) | 0.620 |
| Fact-G, physical subscale | 0.1 | 0.03 | 0.1 (−1.9, 2.0) | 0.941 | −0.3 | 0.2 | −0.5 (−2.7, 1.6) | 0.622 |
| Fact-G, emotional subscale | 0.04 | 0.7 | −0.7 (−2.0, 0.6) | 0.286 | 1.0 | 0.9 | 0.1 (−1.2, 1.4) | 0.863 |
| Fact-G, social subscale | 0.1 | 0.3 | −0.1 (−1.8, 1.5) | 0.853 | 0.5 | 0.2 | 0.3 (−1.3, 1.9) | 0.725 |
| Fact-G, functional subscale | 0.6 | 1.1 | −0.5 (−2.1, 1.1) | 0.539 | 0.6 | 1.5 | −1.0 (−2.6, 0.7) | 0.266 |
Note. LDE = Liver Disease Education, SMI = Self-management Intervention, CI = Confidence interval, MUIS-A = Mishel Uncertainty in Illness Scale, CES-D = 10-item Center for Epidemiological Studies Depression Scale, POMS = Profile of Mood States, SCS = Self-Control Schedule, Fact-G = Quality of Life in Chronic Illness. Estimates are based on a general linear model with an unstructured covariance matrix and adjusted for study site (Duke vs. non-Duke). Results are combined across multiply imputed datasets. Improvement from baseline to respective follow-up assessments is reflected in negative values for MUIS-A (total score and subscales), CES-D, and POMS, and positive values for SCS subscales, self-efficacy, and FACT-G (total score and subscales).
3.1.2. Caregiver outcomes
There were no significant differences between caregivers in the SMI and LDE groups at 10 and 12 weeks post baseline in the caregiver outcomes of caregiver perception of illness uncertainty, uncertainty management, self-efficacy to support their loved one’s symptoms, and assessment of caregiving. Mean scores on the perception of illness uncertainty, cognitive reframing an aspect of uncertainty management, and caregiver reaction were essentially unchanged from baseline through followup. Self-efficacy scores increased nearly two points from baseline to the second followup for SMI caregivers and decreased nearly three points for the LDE group (mean difference between groups = 4.8, 95% CI: −1.4, 11.0) (Table 4).
Table 4.
Estimated changes from baseline and differences between the SMI and LDE groups for caregiver outcomes at follow-up 1 and 2.
| Measurement and study time point | Follow-up #1 change from baseline
|
Follow-up #2 change from baseline
|
||||||
|---|---|---|---|---|---|---|---|---|
| SMI | LDE | Mean Difference Between Groups (95% CI) | P-value | SMI | LDE | Mean Difference Between Groups (95% CI) | p-value | |
| PUS-FM, total score | −0.9 | −1.2 | 0.3 (−3.7, 4.3) | 0.888 | −2.0 | −0.6 | −1.5 (−4.6, 1.7) | 0.369 |
| PUS-FM, lack of clarity subscale | −0.04 | −0.7 | 0.6 (−1.3, 2.5) | 0.515 | −0.3 | 0.02 | −0.3 (−2.0, 1.4) | 0.734 |
| PUS-FM, unpredictability subscale | 0.1 | 0.2 | −0.2 (−1.3, 1.0) | 0.774 | −0.2 | −0.3 | 0.1 (−0.9, 1.1) | 0.856 |
| PUS-FM, lack of information subscale | 0.6 | 0.5 | 0.1 (−0.8, 1.0) | 0.893 | 0.2 | 0.3 | −0.1 (−0.9, 0.7) | 0.732 |
| PUS-FM, ambiguity subscale | −1.5 | −1.3 | −0.2 (−2.7, 2.3) | 0.891 | −1.9 | −0.6 | −1.3 (−3.7, 1.2) | 0.316 |
| SCS, cognitive reframing subscale | −0.2 | −1.6 | 1.4 (−3.3, 6.0) | 0.560 | −0.1 | −0.9 | 0.8 (−3.1, 4.7) | 0.694 |
| SCS, problem solving subscale | −0.4 | −2.1 | 1.8 (−2.7, 6.2) | 0.434 | −0.4 | −1.4 | 1.0 (−2.6, 4.6) | 0.578 |
| Self-efficacy | 0.1 | −2.0 | 2.2 (−4.8, 9.1) | 0.543 | 1.7 | −3.1 | 4.8 (−1.4, 11.0) | 0.125 |
| CRA, impact on schedule subscale | −0.2 | −0.2 | −0.04 (−0.4, 0.3) | 0.816 | −0.2 | −0.1 | −0.004 (−0.3, 0.3) | 0.983 |
| CRA, caregiver esteem subscale | −0.04 | −0.1 | 0.02 (−0.2, 0.2) | 0.820 | 0.03 | −0.02 | 0.05 (−0.1, 0.2) | 0.584 |
| CRA, lack of family support subscale | −0.2 | −0.2 | 0.003 (−0.3, 0.3) | 0.983 | −0.1 | −0.1 | 0.04 (−0.2, 0.3) | 0.774 |
| CRA, impact on health subscale | −0.1 | −0.04 | −0.01 (−0.2, 0.2) | 0.927 | −0.04 | 0.002 | −0.04 (−0.2, 0.2) | 0.668 |
| CRA, impact on finances subscale | −0.1 | −0.1 | −0.03 (−0.4, 0.3) | 0.878 | 0.1 | 0.05 | 0.03 (−0.3, 0.3) | 0.867 |
Note. LDE = Liver Disease Education, SMI = Self-management Intervention, CI = Confidence interval, PUS-FM = Perception of Uncertainty Scale Family Member, SCS = Self Control Schedule, CRA = Caregiver Reaction Assessment. Estimates are based on a general linear model with an unstructured covariance matrix and adjusted for study site (Duke vs. non-Duke). Results are combined across multiply imputed datasets.
Improvement from baseline to respective follow-up assessments is reflected in negative values for PUS-FM (total score and subscales), and positive values for SCS subscales, self-efficacy, and CRA subscales.
4. Discussion and conclusion
4.1. Discussion
A self-management intervention (SMI) delivered by telephone to patients waiting for liver transplant and their caregivers did not result in significant differences when compared to dyads receiving a liver disease education intervention (LDE). These results differ from a previous psychoeducational trial for patients with end-stage liver disease and their caregivers which reported an improvement in QoL including symptom management [13].
Several factors likely contributed to the difference in our results. In our study the intervention dose was six sessions for both SMI and LDE participants; the sessions lasted approximately 30-min. In contrast, the other study offered fewer sessions (four) of longer duration (90-min) and focused on teaching coping skills and educational content [13]. Participants and their family members received three individual sessions and then participated in a final group session. Also, our SMI intervention dose was considerably less than previous programs, which have utilized 12–14 sessions and positive effects among patients with lung cancer and their caregivers [34] and patients awaiting lung transplant and their caregivers [44]. Thus, it is possible that we diluted the effects of the SMI intervention. Another possibility is that these patients and their caregivers could benefit from a combined SMI/LDE intervention similar to prior work [13].
Another factor contributing to our null findings may have been the mode of intervention delivery. We called patients via telephone to deliver our intervention. Sharif et al. (2005) used face-to-face educational sessions and included information on liver disease, coping strategies, relaxation, exercise and diet [13]. The intensity and efficacy of in-person encounters for patients and caregivers with end-stage liver disease and in need of transplant might be superior to telephone-delivered interventions in this patient population. While telephone-delivered interventions have been successful in other patient populations [15], it is important to determine what content is appropriate for teaching remotely.
Lastly, we recognize that patients awaiting liver transplants deteriorate, and that our interventions might have prevented their decline resulting in null findings. However it is possible that identifying their uncertainty heightened feelings of ambiguity and the unpredictability of their situation at least in the short term thereby diluting benefit.
4.2. Conclusion
A strength of our study was the use of an active control group that controlled for interventionist time and attention. In Sharif’s study, participants in the control group only completed questionnaires and did not receive interventions [13]. In our study, we provided LDE as a comparison condition. Even so, participants might have benefited from the supportive interventionist and sought out additional information that could have influenced study outcomes.
Both LDE and SMI groups improved in patient self-efficacy. This suggests that SMI, when compared to a non-active control condition, might have been effective. More participants in the LDE group completed all six sessions than in the SMI group. This might imply that participants found the LDE helpful and that it diminished the effect of the SMI. Overall, based on their total scores on the MUIS-A, CES-D, and POMS Anxiety subscale, participants regardless of group assignment had moderate to low levels of illness uncertainty, depressive symptoms, and anxiety. This might explain why we did not find significant differences and improvement after intervention delivery.
4.3. Practice implications
Our study was designed for the time constraints of today’s practice setting. We limited intervention calls to 30-min or less, selected relevant coping skills and symptom management strategies, and enrolled patients recently listed for transplant and those who had been waiting more than a year. Prior coping skills training studies involved a larger dose of intervention [45], and there is evidence that some patients benefitted more than others (i.e., participants with later-stage lung cancer benefited more from coping skills training and those with earlier disease benefited more from education) [34].
The experience of waiting exacerbates uncertainty, depression, and poor QoL. Future studies could identify patients with high uncertainty as those in most need of intervention. Understanding how educational interventions might neutralize the effect of coping skills and self-management interventions will be important in designing future trials. This trial begins to address the unmet needs of these patients and their caregivers.
Acknowledgments
The authors thank Iris Pounds, Margaret Falkovic, Melanie Paige, Sarah Garrigues, and Sophia Duong for their contributions to this study. We also extend our gratitude to the clinic staff and all study participants for their time and effort.
The study was supported by the National Institute of Nursing Research (NIH/NINR: P01 NR010948). The study is registered with ClinicalTrials.gov, identifier is NCT02006823. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Conflicts of interest
None.
References
- 1.Brown J, Sorrell JH, McClaren J, Creswell JW. Waiting for a liver transplant. Qual Health Res. 2006;16:119–1136. doi: 10.1177/1049732305284011. http://dx.doi.org/10.1177/1049732305284011. [DOI] [PubMed] [Google Scholar]
- 2.Goetzmann L, Wagner-Huber R, Klaghofer R, Muellhaupt B, Clavien PA, Buddeberg C, Scheuer E. Waiting for a liver transplant: psychosocial well-being, spirituality, and need for counselling. Transplant Proc. 2006;38:2931–2936. doi: 10.1016/j.transproceed.2006.08.171. doi: http://dx.doi.org/10.1016/j.transproceed.2006.08.171. [DOI] [PubMed] [Google Scholar]
- 3.Streisand RM, Rodrigue JR, Perri SF, Jr, Sears MG, Davis GL, Banko CG. A psychometric normative database for pre-liver transplantation evaluations. The Florida cohort 1991–1996. Psychosomatics. 1999;40:479–485. doi: 10.1016/S0033-3182(99)71185-0. [DOI] [PubMed] [Google Scholar]
- 4.Surman OS. Psychiatric aspects of organ transplantation. Am J Psychiatry. 1989;146:972–982. doi: 10.1176/ajp.146.8.972. [DOI] [PubMed] [Google Scholar]
- 5.Mishel MH. Living with Chronic Illness: Living with Uncertainty. Springer; New York: 1993. [Google Scholar]
- 6.Mishel MH. Uncertainty in chronic illness. Annu Rev Nurs Res. 1999;17:269–294. [PubMed] [Google Scholar]
- 7.Sargent S. Pathophysiology and management of hepatic encephalopathy. Br J Nurs. 2007;16:335–339. doi: 10.12968/bjon.2007.16.6.23003. [DOI] [PubMed] [Google Scholar]
- 8.Wright G, Jalan R. Management of hepatic encephalopathy in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:95–110. doi: 10.1016/j.bpg.2006.07.009. doi: http://dx.doi.org/10.1016/j.bpg.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 9. [accessed 2016. April 22];United Network for Organ Sharing, Organ Procurement and Transplantation Network, Current U. S. Waiting list – Liver. 2016 < https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#>.
- 10.Cox-North P, Doorenbos A, Shannon SE, Scott J, Curtis JR. The transition to end-of-life care in end-stage liver disease. J Hosp Palliat Nurs. 2013;15:209–215. doi: http://dx.doi.org/10.1097/NJH.0b013e318289f4b0. [Google Scholar]
- 11.Stilley CS, Miller DJ, Gayowski T, Marino IR. Psychological characteristics of candidates for liver transplantation. Clin Transplant. 1998;12:416–424. [PubMed] [Google Scholar]
- 12.Miyazaki ET, Dos Santos R, Jr, Miyazaki MC, Domingos NM, Felicio HC, Rocha MF, Arroyo PC, Jr, Duca WJ, Silva RF, Silva RC. Patients on the waiting list for liver transplantation: caregiver burden and stress. Liver Transplant. 2010;16:1164–1168. doi: 10.1002/lt.22130. [DOI] [PubMed] [Google Scholar]
- 13.Sharif F, Mohebbi S, Tabatabaee HR, Saberi-Firoozi M, Gholamzadeh S. Effects of psycho-educational intervention on health-related quality of life (QOL) of patients with chronic liver disease referring to Shiraz University of Medical Sciences. Health Qual Life Outcomes. 2005;3:81. doi: 10.1186/1477-7525-3-81. doi: http://dx.doi.org/10.1186/1477-7525-3-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishel MH. Uncertainty in illness. Image: J Nurs Scholarsh. 1988;20:225–232. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Germino BB, Mishel MH, Crandell J, Porter L, Blyler D, Jenerette C, Gil KM. Outcomes of an uncertainty management intervention in younger African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2013;40:82–92. doi: 10.1188/13.ONF.82-92. doi: http://dx.doi.org/10.1188/13.onf.82-92. [DOI] [PubMed] [Google Scholar]
- 16.Mishel MH, Belyea M, Germino BB, Stewart JL, Bailey DE, Jr, Robertson C, Mohler J. Helping patients with localized prostate carcinoma manage uncertainty and treatment side effects: nurse-delivered psychoeducational intervention over the telephone. Cancer. 2002;94:1854–1866. doi: 10.1002/cncr.10390. [DOI] [PubMed] [Google Scholar]
- 17.Bailey DE, Mishel MH, Belyea M, Stewart JL, Mohler J. Uncertainty intervention for watchful waiting in prostate cancer. Cancer Nurs. 2004;27:339–346. doi: 10.1097/00002820-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Bailey DE, Jr, Steinhauser K, Hendrix C, Tulsky JA. Pairing self-management with palliative care: intervening in life-limiting illness. J Nurs Healthc Chronic Illn. 2011;3:1–3. doi: 10.1111/j.1752-9824.2011.01083.x. doi: http://dx.doi.org/10.1111/j.1752-9824.2011.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishel MH. Reconceptualization of the uncertainty in illness theory. Image: J Nurs Scholarsh. 1990;22:256–262. doi: 10.1111/j.1547-5069.1990.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage. 2010;39:126–138. doi: 10.1016/j.jpainsymman.2009.05.022. doi: http://dx.doi.org/10.1016/j.jpainsymman.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 22.White CA. Cognitive behavioral principles in managing chronic disease. West J Med. 2001;175:338–342. doi: 10.1136/ewjm.175.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter LS, Keefe FJ, Garst J, Baucom DH, McBride CM, McKee DC, Sutton L, Carson K, Knowles V, Rumble M, Scipio C. Caregiver-assisted coping skills training for lung cancer: results of a randomized clinical trial. J Pain Symptom Manage. 2011;41:1–13. doi: 10.1016/j.jpainsymman.2010.04.014. doi: http://dx.doi.org/10.1016/j.jpainsymman.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates: too well for transplant, too sick for life. J Am Med Assoc. 2006;295:2168–2176. doi: 10.1001/jama.295.18.2168. doi: http://dx.doi.org/10.1001/jama.295.18.2168. [DOI] [PubMed] [Google Scholar]
- 25.Mishel MH. The measurement of uncertainty in illness. Nurs Res. 1981;30:258–263. [PubMed] [Google Scholar]
- 26.Mishel MH. Uncertainty in Illness Scales manual. 1997. Available from M. Mishel at the University of North Carolina-Chapel Hill, School of Nursing. [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.Given CW, Stommel M, Given B, Osuch J, Kurtz ME, Kurtz JC. The influence of cancer patients' symptoms and functional states on patients' depression and family caregivers' reaction and depression. Health Psychol. 1993;12:277–285. doi: 10.1037//0278-6133.12.4.277. [DOI] [PubMed] [Google Scholar]
- 29.Hall LA, Gurley DN, Sachs B, Kryscio RJ. Psychosocial predictors of maternal depressive symptoms parenting attitudes, and child behavior in single-parent families. Nurs Res. 1991;40:214–220. [PubMed] [Google Scholar]
- 30.Shacham S. A shortened version of the profile of mood states. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 31.Curran SL, Andrykowski MA, Studts JL. Short-form of the profile of mood states (Poms-Sf) – psychometric information. Psychol Assess. 1995;7:80–83. doi: http://dx.doi.org/10.1037/1040-3590.7.1.80. [Google Scholar]
- 32.Rosenbaum M. Learned resourcefulness as a behavioral repertoire for the self-regulation of internal events: issues and speculations. In: Rosenbaum M, Franks CM, Jaffe Y, editors. Perspectives on Behavior Therapy in the Eighties. Springer Pub. Co; New York: 1983. pp. 54–73. [Google Scholar]
- 33.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 34.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137:306–315. doi: 10.1016/j.pain.2007.09.010. doi: http://dx.doi.org/10.1016/j.pain.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 36.Given CW, Given B, Stommel M, Collins C, King S, Franklin S. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 37.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. doi: http://dx.doi.org/10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–96. [Google Scholar]
- 39.Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal data with follow-up truncated by death: match the analysis method to research aims. Stat Sci: Rev J Inst Math Stat. 2009;24:211. doi: 10.1214/09-STS293. doi: http://dx.doi.org/10.1214/09-sts293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.University of Michigan, Institute for Social Research, Survey Research Center. [accessed 2014. November 25];Survey Methodology Program, IVEware Version 0.2 User Guide and Installation Instructions. 2011 < ftp://ftp.isr.umich.edu/pub/src/smp/ive/ive21_user.pdf>.
- 41.Olsen MK, Stechuchak KM, Edinger JD, Ulmer CS, Woolson RF. Move over LOCF: principled methods for handling missing data in sleep disorder trials. Sleep Med. 2012;13:123–132. doi: 10.1016/j.sleep.2011.09.007. doi: http://dx.doi.org/10.1016/j.sleep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 42.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. doi: http://dx.doi.org/10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 43.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6:330–351. [PubMed] [Google Scholar]
- 44.Blumenthal JA, Babyak MA, Keefe FJ, Davis RD, Lacaille RA, Carney RM, Freedland KE, Trulock E, Palmer SM. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74:535–544. doi: 10.1037/0022-006X.74.3.535. doi: http://dx.doi.org/10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigue JR, Mandelbrot DA, Pavlakis M. A psychological intervention to improve quality of life and reduce psychological distress in adults awaiting kidney transplantation. Nephrol Dialysis Transplant. 2010;26:709–715. doi: 10.1093/ndt/gfq382. [DOI] [PMC free article] [PubMed] [Google Scholar]