Abstract
Selective estrogen receptor modulators (SERMs) are a class of compounds that interact with estrogen receptors (ERs) and exert agonist or antagonist effects on ERs in a tissue-specific manner. Tamoxifen, a first generation SERM, is used for treatment of ER positive breast cancer. Raloxifene, a second generation SERM, was used to prevent postmenopausal osteoporosis. The third-generation SERM bazedoxifene (BZA) effectively prevents osteoporosis while preventing estrogenic stimulation of breast and uterus. Notably, BZA combined with conjugated estrogens (CE) is a new menopausal treatment. The menopausal state predisposes to metabolic syndrome and type 2 diabetes, and therefore the effects of SERMs on metabolic homeostasis are gaining attention. Here, we summarize knowledge of SERMs’ impacts on metabolic, homeostasis, obesity and diabetes in rodent models and postmenopausal women.
Keywords: Selective estrogen receptor modulators, tamoxifen, raloxifene, bazedoxifene, metabolic syndrome, steatosis, energy metabolism, diabetes
1. Introduction
Increased life expectancy in developed countries indicates that most women will spend the second half of their lives in an estrogen deficient state. In addition to increasing the risks of cardiovascular diseases, estrogen deficiency also predisposes to visceral obesity, metabolic syndrome and type 2 diabetes (T2D). Therefore, the broad impact of estrogen deficiency on the pathobiology of metabolic diseases in women represents a new therapeutic challenge. From that perspective, we need to dissect and harness the beneficial effects of estrogen on metabolic homeostasis while at the same time avoiding its adverse effects.
Selective estrogen receptor modulators (SERMs) are a class of compounds that interact with estrogen receptors (ERs) and induce a unique receptor conformation that correlates with specific behaviors in estrogen-responsive tissues. SERMs exert agonist or antagonist activity on ERs in a tissue-specific manner that depends on the complexity of ER signaling, including different tissue distribution of ERs [1], ligand binding specificity [2, 3] and diverse interactions with coactivators or corepressors [4, 5].
Tamoxifen, one of the first generation of SERMs, behaves as an ER antagonist in breast tissue and is used to prevent and treat ER-positive breast cancer [6]. However, tamoxifen acts as an ER agonist in the endometrium and therefore increases endometrial carcinoma risk [7, 8]. The second-generation SERMs were developed to overcome the adverse effect of tamoxifen on endometrial proliferation. Raloxifene, for example, retains anti-estrogenic activity in breast tissue [9, 10] and also exhibits estrogenic activity in bone, thus preventing osteoporosis [11]. Bazedoxifene, a third-generation SERM, is used to prevent osteoporosis in postmenopausal women without raising the safety concerns related to endometrium and breast [12–14]. The pairing of bazedoxifene with conjugated estrogen (CE) in a tissue-selective estrogen complex is a novel menopausal therapy [15] which provides the benefits of CE treatment, and with the addition of BZA, protects breast and uterus from estrogen stimulation without using a progestin [14, 16].
In addition to the established impacts of SERMs on breast, bone and endometrium, the impact of SERMs on postmenopausal metabolic dysfunction is gaining attention. Here, we summarize the accumulated knowledge of SERMs’ impacts on diabetes, obesity and metabolic homeostasis in rodent models and postmenopausal women.
2. The effects of SERMs on glucose homeostasis and diabetes
Tamoxifen has been used for over 40 years to treat ER-positive breast cancer due to its anti-estrogenic effect on breast tissue [2]. It has been gradually recognized that tamoxifen causes metabolic side effects such as diabetes, lipid abnormities and hepatic steatosis. Independent population-based studies conducted in women with breast cancer have revealed a correlation between tamoxifen treatment and an increased incidence of diabetes [17–19] (Figure 1). For example, in a Canadian case control study of 14,360 breast cancer survivors, tamoxifen use was associated with a 24% increased risk of developing diabetes [19]. In another Asian population-based cohort study of 22,257 breast cancer patients, tamoxifen use was associated with a 31% higher diabetes risk [18]. The mechanisms of the diabetogenic effects of tamoxifen are unclear. One possibility is that tamoxifen exhibits adverse effects on pancreatic β cells. For example, in wild-type female mice, tamoxifen reversed the protective effect of estradiol on preventing insulin-deficient diabetes induced by streptozotocin (STZ), suggesting that tamoxifen acts as an ER antagonist in pancreatic β cells and impairs pancreatic islet survival [20]. In addition, tamoxifen impairs embryonic and adult mouse β-cell proliferation by antagonism of ERα [21]. Treatment with tamoxifen or genetic elimination of ERα in male mice similarly decreased the expression of the endocrine specification factor Neurogenin3 (NGN3) and β-cell proliferation in the partial duct ligation (PDL) rodent model of β-cell expansion due to pancreatic injury. Further, ERα inhibition with tamoxifen in the embryonic mouse pancreas, or its deletion as in the ERα-deficient mouse, also decreased NGN3 expression and NGN3+ progenitors at the end of gestation [21]. Thus, the generation of NGN3+ cells and the subsequent β-cell mass expansion in developing or injured mouse pancreas are both blocked by tamoxifen antagonizing ERα. Tamoxifen may also promote insulin resistance. In premenopausal women at high risk of breast cancer but with normal body weight, tamoxifen treatment did not alter insulin sensitivity (quantified with HOMA index) [22]. In contrast, in a subgroup of overweight women, tamoxifen treatment dramatically decreased insulin sensitivity [22]. The underlying mechanism by which tamoxifen increases insulin resistance remains poorly understood but could involve the development of hepatic steatosis, as we will discuss later.
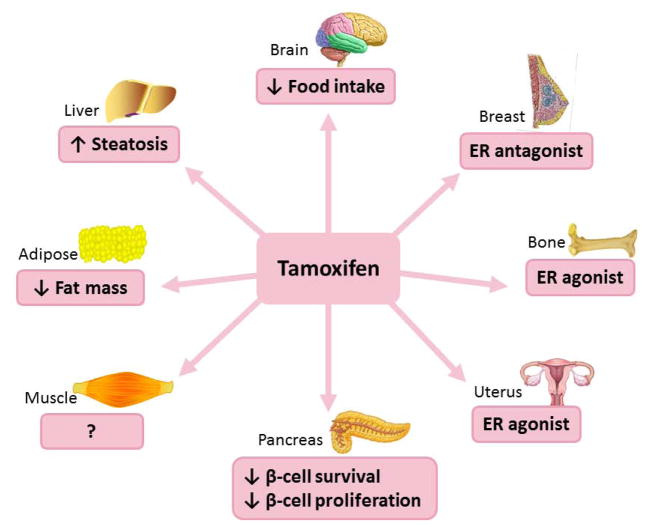
Figure 1. Summary of the effects of tamoxifen.
Tamoxifen is an ER antagonist in breast and has estrogenic effects on bone and uterus. Tamoxifen decreases food intake, body weight and fat mass in rodents. It also lowers body weight in obese women. Tamoxifen decreases β-cell survival and proliferation in rodents and increases the incidence of diabetes in patients with breast cancer. Tamoxifen also promotes hepatic steatosis in rodents and women.
Raloxifene can affect glucose homeostasis to varying degrees depending on treatment duration (Figure 2). In postmenopausal women with and without type 2 diabetes mellitus, short-term raloxifene treatment (3 or 6 months) did not alter fasting blood glucose [23–26] or insulin level [23, 24, 26]. However, in a subgroup of women with hyperinsulinemia, raloxifene reduced insulin levels by enhancing both fractional hepatic insulin extraction and peripheral insulin sensitivity [23]. Similarly to short-term treatment, long-term raloxifene treatment (12 months) did not modify fasting glucose or glucose tolerance [27]. However, in contrast to short-term treatment, long-term raloxifene treatment decreased insulin sensitivity [27]. Since the number of study subjects in this latter study was small (only 24 patients), this duration-based effect warrants further investigation. In summary, tamoxifen and raloxifene exhibit either deleterious or neutral effects on glucose homeostasis.
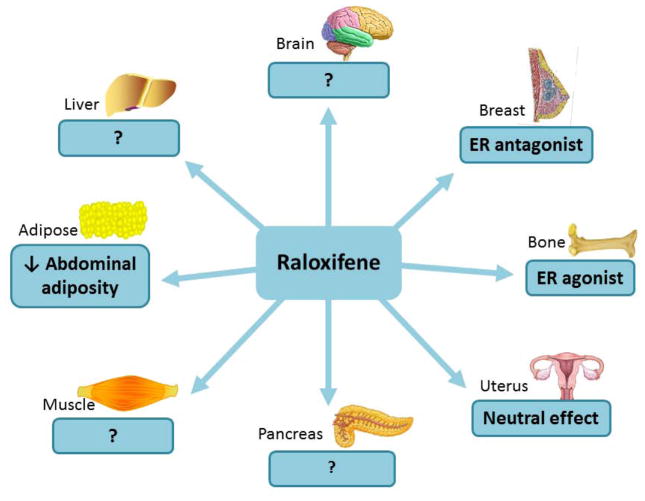
Figure 2. Summary of the effects of raloxifene.
Raloxifene is an ER agonist in bone and acts as ER antagonist in breast. It has a neutral effect in uterus. Raloxifene reduced fat mass in OVX female rodents and prevented abdominal adiposity in postmenopausal women. Raloxifene’s effects on insulin sensitivity are controversial.
The combination of bazedoxifene with CE improved glucose homeostasis in OVX mice fed a western diet (Figure 3). Blood glucose and insulin levels were significantly decreased after bazedoxifene/CE treatment under both fasting [28, 29] and fed conditions [28, 29], and mice showed improved insulin sensitivity and glucose tolerance. These effects are similar to those observed with CE alone. When systemic insulin action was studied in euglycemic, hyperinsulinemic clamp conditions, the combination bazedoxifene/CE provided the same improvement in systemic insulin action in muscle and liver than BZA alone [28]. Yet, surprisingly, fasting blood glucose and insulin levels were not significantly changed in postmenopausal women taking the combination bazedoxifene/CE [30]. The bazedoxifene/CE combination and bazedoxifene alone reduced the severity of β-cells destruction and insulin-deficient diabetes induced by STZ in OVX female mice to an extent similar to that of CE alone [31]. The prevention of STZ-induced insulin-deficient diabetes in mice is a marker of ERα agonist activity in β cells [20]. Thus, the prevention of STZ-induced diabetes by bazedoxifene suggests that in female mice, bazedoxifene acts as an ERα agonist in β-cells. In a preliminary report, the effect of bazedoxifene/CE was assessed in the Akita mouse model of β cell endoplasmic reticulum (ER) stress [32]. Bazedoxifene/CE decreased β cell destruction and helped prevent the development of diabetes in Akita mice to an extent similar to that of CE alone. In cultured islets from female mice exposed to ER-stress induced by thapsigargin, CE, BZA or bazedoxifene/CE decreased the expression of markers of ER stress [32]. Thus, the combination of bazedoxifene with CE used for menopausal hormone therapy could act as a pharmacological ER stress mitigator and protect women from estrogen deficiency-induced β-cell dysfunction and damage.
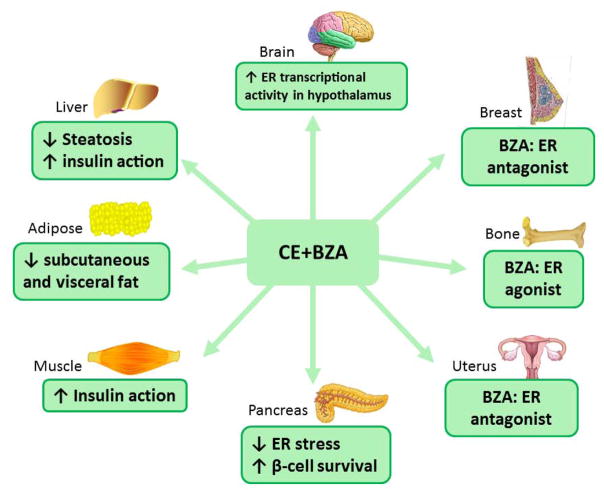
Figure 3. Summary of the effects of the combination bazedoxifene with CE.
Bazedoxifene acts as an ER antagonist in breast and uterus while it is an ER agonist in bone. In rodent models of menopause, CE/BZA prevents obesity, reduces hepatic steatosis formation, and improves liver and muscle insulin sensitivity as well as glucose homeostasis.
3. The effects of SERMs on obesity
Tamoxifen significantly reduced food intake, body weight, and fat mass in OVX rats [33] (Figure 1). In neutered female Wistar-Kyoto (WKY) rats, tamoxifen also suppressed weight gain partially by suppressing food intake [34]. This effect could reflect the activation of ERα in hypothalamic neurons which is known to suppress food intake in rodents [35].
Serum leptin positively correlates with body fat mass and plays a key role in regulating energy balance [36]. Therefore, serum leptin level serves as an indicator of obesity. Serum leptin levels were higher in breast cancer patients receiving tamoxifen than in those not taking tamoxifen [37]. Another study conducted in breast cancer patients who received short term tamoxifen treatment showed that those who developed fatty liver during the 3-month tamoxifen treatment had elevated serum leptin levels compared to those without fatty liver [38]. However, in non-cancer patients, tamoxifen decreased body weight [39]. Weight gain (expressed as BMI increase) was smaller in obese women taking tamoxifen compared to those taking placebo, indicating that tamoxifen had a predominant anorectic effect, which was also observed in rats. In these animals, tamoxifen-induced anorexia was associated with decreased FAS mRNA expression, which caused malonyl-CoA accumulation in the hypothalamic ventromedial nucleus [39]. Therefore, how tamoxifen regulates energy balance as an ER agonist in the hypothalamus deserves further investigation.
Raloxifene prevented estrogen deficiency-induced weight gain in OVX rats [40, 41] (Figure 2). In healthy postmenopausal women, a 12-month raloxifene treatment inhibited body weight gain and abdominal adiposity by changing fat distribution from an android distribution to a gynoid distribution [42]. Another study conducted in postmenopausal women reported that a 12-month raloxifene treatment failed to affect body weight, but remarkably altered body composition by increasing fat-free mass and total body water [43]. However, a 6-month raloxifene treatment was unable to increase either exercise-induced weight loss or fat-mass loss in postmenopausal women [44], which may be due to raloxifene’s effect on elevating fat-free mass and total body water. The effect of raloxifene on serum leptin concentration has been investigated in both rodents and postmenopausal women. In OVX rats, raloxifene decreased total fat mass induced by estrogen deficiency and reversed hyperleptinemia [41]. A 3- or 6-month raloxifene treatment significantly increased serum leptin levels compared to baseline in postmenopausal women [45, 46]. In contrast, another study found that serum leptin levels were not different from baseline in postmenopausal women receiving raloxifene, although serum leptin levels were significantly lower in postmenopausal women receiving raloxifene than in those without treatment [47]. Raloxifene treatment did not alter serum leptin level or body mass index (BMI) in women who underwent oophorectomy, whereas serum leptin levels significantly increased in the control group [48].
The impact of the combination bazedoxifene/CE on weight gain and fat mass has been studied in OVX mice fed a western diet compared to CE and BZA alone. Bazedoxifene/CE or even bazedoxifene alone significantly attenuated OVX-induced body weight gain [28, 29, 49] and adipose tissue accumulation (both subcutaneous and visceral) without altering lean body mass to an extend similar to that of CE alone [28, 29] (Figure 3). In a meta-analysis of five Selective Estrogens, Menopause, and Response to Therapy (SMART) randomized control trials in postmenopausal women, bazedoxifene/CE treatment for 2 years prevented the increase in body weight and BMI observed in the placebo group [50]. Studies are ongoing to address the effect of bazedoxifene/CE in preventing metabolic dysfunction in obese postmenopausal women [51, 52].
Interestingly, a novel SERM (GSK232802A) reduced body weight and adiposity in ovariectomized nonhuman primates by suppressing food intake and increasing locomotor activity, particularly in the most sedentary individuals [53]. These findings in primates suggest that SERM treatment may also suppress food intake in postmenopausal women.
4. The effects of SERMs on lipids
Estrogen can increase the concentration of high-density lipoprotein (HDL) cholesterol (“good” cholesterol) while decreasing the concentration of low-density lipoprotein cholesterol (LDL) (“bad” cholesterol) in the blood of healthy postmenopausal women [54]. On the other hand, oral estrogen can increase triglyceride levels, which may lead to hypertriglyceridemia [54]. Hypertriglyceridemia is a frequently encountered lipid abnormality in patients with uncontrolled diabetes [55]. Tamoxifen treatment produced severe hypertriglyceridemia in breast cancer patients with preexisting diabetes [56, 57] and those without known diabetes [58, 59], indicating that tamoxifen usage exacerbates lipid abnormalities.
Unlike tamoxifen, raloxifene did not increase triglyceride levels in cultured HepG2 human hepatocarcinoma cells regardless of the presence of added fatty acid [60]. In addition, raloxifene prevented triglyceride accumulation in cultured rat INS-1 insulin-producing cells under lipogenic conditions [61]. Thus, the effect of raloxifene in regulating triglyceride accumulation seems to be tissue-specific. Raloxifene significantly reduced LDL cholesterol level in postmenopausal women [24, 25, 27, 62]. Unlike estrogen, raloxifene had no impact on HDL cholesterol [24, 25] or triglyceride levels [24, 25, 63]. Furthermore, raloxifene did not modify triglyceride levels in patients with a history of oral estrogen-induced hypertriglyceridemia [64].
A 12-week bazedoxifene treatment alone significantly decreased LDL cholesterol and HDL cholesterol levels in postmenopausal women with type 2 diabetes (T2DM) but had no impact on triglyceride levels [65]. Initial studies of bazedoxifene/CE treatment showed that bazedoxifene/CE had overall favorable effects on the lipid profile with only minimal changes on coagulation [65], including reduced LDL cholesterol and total cholesterol and increased HDL cholesterol and triglyceride. A pooled analysis of the effects of bazedoxifene/CE on lipid parameters in postmenopausal women from the SMART trials (n=2796) concluded that bazedoxifene/CE reduces LDL and increases HDL, while TG levels also increased at 24 months [66]. Thus, the addition of bazedoxifene to CE does not modify the know effect CE on lipids including a reduction in LDL, an increase in HDL, and an increase in TGs [67–69].
5. The effects of SERMs on hepatic steatosis
Tamoxifen treatment promotes the development of non-alcoholic fatty liver (hepatic steatosis) in women with breast cancer [70–73] (Figure 1). The occurrence of fatty liver in these women was observed as early as 3 months following the initiation of tamoxifen treatment [70]. In healthy women who had had a hysterectomy, tamoxifen treatment was associated with the development of hepatic steatosis only in overweight and obese women with metabolic syndrome [74]. The mechanisms by which tamoxifen promotes fatty liver development were investigated in different rodent models [75–77]. Tamoxifen increased the synthesis of hepatic triglyceride without affecting fatty acid β-oxidation, thereby elevating liver triglyceride content in a rat mammary tumor model [75]. Another study found that tamoxifen increased de novo fatty acid synthesis in mouse liver at least partially via downregulating AMP-activated protein kinase (AMPK) activity, a key fatty acid oxidation activator and fatty acid synthesis suppressor, whereas fatty acid β-oxidation was not inhibited [76]. In male Wistar rats receiving tamoxifen, the formation of fatty liver was closely related to decreased expression and enzyme activity of fatty acid synthase (FAS) [77]. This resulted in the accumulation of the FAS substrate malonyl-CoA, impaired fatty acid β-oxidation and hepatic triglyceride accumulation. Consistent with these in vivo findings, tamoxifen directly upregulated intracellular triglyceride concentration in HepG2 cells cultured in medium containing oleic acid or very low density lipoprotein [60]. Several studies conducted in rodents also suggested that tamoxifen could have a neutral or even protective effect on fatty liver. For instance, tamoxifen showed a neutral effect on steatosis in the absence of high fat diet in male Sprague-Dawley (SD) rats [78]. Tamoxifen alleviated hepatic steatosis induced by high fat diet or methionine and choline deficient diet in female ICR mice by diminishing inflammatory responses [79]. Tamoxifen treatment also contributed to the prevention of fatty liver formation in female WSB/EiJ mice by upregulating key regulators of hepatic fatty acid β-oxidation, including lipocalin 13 (Lcn13) and the peroxisome proliferator-activated receptor gamma (PPARγ) [80]. In addition, tamoxifen played a hepatoprotective role against hepatotoxic compounds by increasing the hepatic protein expression of monocyte to macrophage differentiation-associated 2 (Mmd2) in an ERα dependent manner [81].
The combination bazedoxifene/CE and bazedoxifene alone had a similar favorable impact on fatty liver in OVX mice than CE alone in directly decreasing hepatic triglyceride accumulation [28, 29] (Figure 3). De novo fatty acid synthesis was suppressed following bazedoxifene/CE, CE and BZA treatments, which was secondary to reduced fatty acid synthase (FAS) expression and enzyme activity [28]. This appears to be mediated in part by inducing the expression and insulin-stimulated phosphorylation of carcinoembryonic antigen-related cell adhesion molecule (CEACAM1), which triggers CEACAM1 binding to and downregulation of FAS activity [28]. Consequently, hepatic free fatty acid accumulation was also repressed in OVX mice treated with bazedoxifene/CE as those treated with CE alone [82].
The combination bazedoxifene/CE also increased hepatic lipid oxidation through multiple critical pathways involved in lipid metabolism [28]. First, CE alone enhanced hepatic production of fibroblast growth factor-21 (FGF21) [83]. In contract, BZA did not increase hepatic production of FGF21. Instead, BZA alone increased hepatic expression and activity of metabolic activators of hepatic lipid oxidation, sirtuin 1 and its target the peroxisome proliferator-activated receptor-α, as well as the AMPK α [84–86]. Thus, in OVX mice, the combination bazedoxifene/CE promotes a state of increased FGF21 production and sensitivity without increase in FGF21 circulating concentrations [28]. Interestingly, bazedoxifene/CE also restored the physiological oscillatory activity of liver ERα that was diminished due to ovariectomy and prevented the effect of estrogen deficiency on hepatic fat disposition in OVX mice [82]. Finally, bazedoxifene/CE selectively increased ER transcriptional activity in the hypothalamic arcuate nucleus of mice, a critical area for control of energy homeostasis, suggesting bazedoxifene/CE may centrally regulate metabolism [49].
6. Conclusions
Enhancing the metabolic actions of SERMs for the prevention of metabolic dysfunction in postmenopausal women is an important avenue for clinical investigation. One solution is to target the ERs involved in energy homeostasis, using novel SERMs that will retain the beneficial metabolic effects of estrogen while antagonizing ERs in breast and uterus. An important advantage of using SERMs to prevent postmenopausal metabolic disease is the vast library of knowledge accumulated from decades of preclinical and clinical studies of estrogen and ER ligand pharmacology, efficacy and toxicity. The challenge with CE or estradiol (E2), however, is their narrow therapeutic index when administered as a chronic treatment. The inherent beauty of using bazedoxifene/CE combining estrogens with a SERM is that it retains the beneficial effects of estrogens to prevent metabolic dysfunction and other chronic degenerative diseases, while at the same time blocking ERs in breast and uterus without the use and side effects of a progestin. In fact, studies presented in this review suggest that in mice, the combination bazedoxifene with CE promote the same beneficial metabolic effects than CE alone. Further studies are needed to determine the long term beneficial effect of bazedoxifene/CE on metabolic function in postmenopausal women.
Highlights.
Tamoxifen predisposes to lipid abnormities, hepatic steatosis and diabetes in women
Tamoxifen impairs pancreatic β cell survival and proliferation in rodents.
Raloxifene has neutral effects on glucose homeostasis in women
The combination bazedoxifene with conjugated estrogens prevents obesity and improves insulin sensitivity in mice to as extend similar to conjugated estrogens
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK074970, DK107444) and the American Diabetes Association (7-13-BS-101).
Abbreviations
- SERMs
selective estrogen receptor modulators
- ER
estrogen receptor
- BZA
bazedoxifene
- CE
conjugated estrogens
- TSEC
tissue-selective estrogen complex
- T2D
Type 2 Diabetes
- STZ
streptozotocin
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- OVX
ovariectomized
- DMBA
7, 12-dimethylbenz[a]anthracene
- FAS
fatty acid synthase
- Mmd2
monocyte to macrophage differentiation-associated 2
- Lcn13
lipocalin 13
- PPARγ
peroxisome proliferator-activated receptor gamma
- FGF21
fibroblast growth factor-21
- SIRT1
sirtuin1
- PPARα
peroxisome proliferator-activated receptor α
- AMPKα
AMP-activated protein kinase α
- E2
estradiol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfaffl MW, et al. Tissue-specific expression pattern of estrogen receptors (ER): quantification of ER alpha and ER beta mRNA with real-time RT-PCR. APMIS. 2001;109(5):345–55. doi: 10.1034/j.1600-0463.2001.090503.x. [DOI] [PubMed] [Google Scholar]
- 2.Tee MK, et al. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15(3):1262–72. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 4.Bramlett KS, Burris TP. Effects of selective estrogen receptor modulators (SERMs) on coactivator nuclear receptor (NR) box binding to estrogen receptors. Mol Genet Metab. 2002;76(3):225–33. doi: 10.1016/s1096-7192(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 5.Webb P, Nguyen P, Kushner PJ. Differential SERM effects on corepressor binding dictate ERalpha activity in vivo. J Biol Chem. 2003;278(9):6912–20. doi: 10.1074/jbc.M208501200. [DOI] [PubMed] [Google Scholar]
- 6.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451–67. [PubMed] [Google Scholar]
- 7.Fornander T, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1(8630):117–20. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen FE, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343(8895):448–52. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- 9.Vogel VG. Raloxifene: a selective estrogen receptor modulator for reducing the risk of invasive breast cancer in postmenopausal women. Womens Health (Lond Engl) 2007;3(2):139–53. doi: 10.2217/17455057.3.2.139. [DOI] [PubMed] [Google Scholar]
- 10.Vogel VG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger B, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama. 1999;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 12.Komm BS, Chines AA. Bazedoxifene: the evolving role of third-generation selective estrogen-receptor modulators in the management of postmenopausal osteoporosis. Ther Adv Musculoskelet Dis. 2012;4(1):21–34. doi: 10.1177/1759720X11422602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archer DF, et al. Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause. 2009;16(6):1109–15. doi: 10.1097/gme.0b013e3181a818db. [DOI] [PubMed] [Google Scholar]
- 14.Komm BS, Mirkin S. Evolution of the tissue selective estrogen complex (TSEC) J Cell Physiol. 2013;228(7):1423–7. doi: 10.1002/jcp.24324. [DOI] [PubMed] [Google Scholar]
- 15.Pinkerton JV, et al. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16(6):1116–24. doi: 10.1097/gme.0b013e3181a7df0d. [DOI] [PubMed] [Google Scholar]
- 16.Santen RJ, et al. Current and evolving approaches to individualizing estrogen receptor-based therapy for menopausal women. J Clin Endocrinol Metab. 2014;99(3):733–47. doi: 10.1210/jc.2013-3680. [DOI] [PubMed] [Google Scholar]
- 17.Hejazi J, Rastmanesh R. Association between tamoxifen treatment and diabetes: a population-based study. Cancer. 2012;118(23):6012. doi: 10.1002/cncr.27517. author reply 6012–3. [DOI] [PubMed] [Google Scholar]
- 18.Sun LM, et al. Association of tamoxifen use and increased diabetes among Asian women diagnosed with breast cancer. Br J Cancer. 2014;111(9):1836–42. doi: 10.1038/bjc.2014.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipscombe LL, et al. Association between tamoxifen treatment and diabetes: a population-based study. Cancer. 2012;118(10):2615–22. doi: 10.1002/cncr.26559. [DOI] [PubMed] [Google Scholar]
- 20.Le May C, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103(24):9232–7. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuchi Y, et al. Estrogen Receptor alpha Regulates beta-Cell Formation During Pancreas Development and Following Injury. Diabetes. 2015;64(9):3218–28. doi: 10.2337/db14-1798. [DOI] [PubMed] [Google Scholar]
- 22.Johansson H, et al. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 2008;68(22):9512–8. doi: 10.1158/0008-5472.CAN-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cucinelli F, et al. The effect of raloxifene on glyco-insulinemic homeostasis in healthy postmenopausal women: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87(9):4186–92. doi: 10.1210/jc.2001-011302. [DOI] [PubMed] [Google Scholar]
- 24.Cagnacci A, et al. Raloxifene does not modify insulin sensitivity and glucose metabolism in postmenopausal women. J Clin Endocrinol Metab. 2002;87(9):4117–21. doi: 10.1210/jc.2002-020120. [DOI] [PubMed] [Google Scholar]
- 25.Nagamani M, et al. Effects of raloxifene on insulin sensitivity, beta-cell function, and hepatic insulin extraction in normal postmenopausal women. Fertil Steril. 2008;89(3):614–9. doi: 10.1016/j.fertnstert.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 26.Andersson B, et al. Raloxifene does not affect insulin sensitivity or glycemic control in postmenopausal women with type 2 diabetes mellitus: a randomized clinical trial. J Clin Endocrinol Metab. 2002;87(1):122–8. doi: 10.1210/jcem.87.1.8168. [DOI] [PubMed] [Google Scholar]
- 27.Lasco A, et al. Effects of a long-term treatment with raloxifene on insulin sensitivity in postmenopausal women. Diabetologia. 2004;47(3):571–4. doi: 10.1007/s00125-004-1328-4. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, et al. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Mol Metab. 2014;3(2):177–90. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrera J, et al. Bazedoxifene and conjugated estrogen prevent diet-induced obesity, hepatic steatosis, and type 2 diabetes in mice without impacting the reproductive tract. Am J Physiol Endocrinol Metab. 2014;307(3):E345–54. doi: 10.1152/ajpendo.00653.2013. [DOI] [PubMed] [Google Scholar]
- 30.Lobo RA, et al. Fertil Steril. United States: 2009. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile; pp. 1025–38. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Mauvais-Jarvis F. The combination of conjugated equine estrogens with bazedoxifene prevents streptozotocin-induced diabetes in ovariectomized female mice. Matters. 2016 [Google Scholar]
- 32.Xu B, Allard C, Mauvais-Jarvis F. Diabetes. AMER DIABETES ASSOC; 1701 N BEAUREGARD ST, ALEXANDRIA, VA 22311-1717 USA: 2015. Estrogen Complexes Improve the Unfolded Protein Response and Prevent ER Stress-induced beta-Cell Failure in Akita Mice. [Google Scholar]
- 33.Wade GN, Heller HW. Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am J Physiol. 1993;264(6 Pt 2):R1219–23. doi: 10.1152/ajpregu.1993.264.6.R1219. [DOI] [PubMed] [Google Scholar]
- 34.Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr. 2001;131(9):2351–7. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- 35.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–38. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 37.Ozet A, et al. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn J Clin Oncol. 2001;31(9):424–7. doi: 10.1093/jjco/hye097. [DOI] [PubMed] [Google Scholar]
- 38.Gunel N, et al. Serum leptin levels are associated with tamoxifen-induced hepatic steatosis. Curr Med Res Opin. 2003;19(1):47–50. doi: 10.1185/030079902125001308. [DOI] [PubMed] [Google Scholar]
- 39.Lopez M, et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55(5):1327–36. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J. 1996;10(8):905–12. doi: 10.1096/fasebj.10.8.8666168. [DOI] [PubMed] [Google Scholar]
- 41.Meli R, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145(7):3115–21. doi: 10.1210/en.2004-0129. [DOI] [PubMed] [Google Scholar]
- 42.Francucci CM, et al. Effects of raloxifene on body fat distribution and lipid profile in healthy post-menopausal women. J Endocrinol Invest. 2005;28(7):623–31. doi: 10.1007/BF03347261. [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen DE, et al. Raloxifene and body composition and muscle strength in postmenopausal women: a randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2010;162(2):371–6. doi: 10.1530/EJE-09-0619. [DOI] [PubMed] [Google Scholar]
- 44.Van Pelt RE, et al. Estrogen or raloxifene during postmenopausal weight loss: adiposity and cardiometabolic outcomes. Obesity (Silver Spring) 2014;22(4):1024–31. doi: 10.1002/oby.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cakmak A, et al. Raloxifene increases serum leptin levels in postmenopausal women: a prospective study. Am J Obstet Gynecol. 2005;193(2):347–51. doi: 10.1016/j.ajog.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Lambrinoudaki IV, et al. Circulating leptin and ghrelin are differentially influenced by estrogen/progestin therapy and raloxifene. Maturitas. 2008;59(1):62–71. doi: 10.1016/j.maturitas.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Tommaselli GA, et al. Serum leptin levels and body composition in postmenopausal women treated with tibolone and raloxifene. Menopause. 2006;13(4):660–8. doi: 10.1097/01.gme.0000227335.27996.d8. [DOI] [PubMed] [Google Scholar]
- 48.Tommaselli GA, et al. Effects of bilateral ovariectomy and postoperative hormonal replacement therapy with 17beta-estradiol or raloxifene on serum leptin levels. Menopause. 2003;10(2):160–4. doi: 10.1097/00042192-200310020-00008. [DOI] [PubMed] [Google Scholar]
- 49.Fontana R, et al. Estrogen replacement therapy regulation of energy metabolism in female mouse hypothalamus. Endocrinology. 2014;155(6):2213–21. doi: 10.1210/en.2013-1731. [DOI] [PubMed] [Google Scholar]
- 50.Black D, et al. The effect of conjugated estrogens/bazedoxifene therapy on body weight of postmenopausal women: pooled analysis of five randomized, placebo-controlled trials. Menopause. 2016;23(4):376–82. doi: 10.1097/GME.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 51.Mauvais-Jarvis F. Pilot Study of Duavee in Preventing Metabolic Dysfunction in Postmenopausal Women. 2014 Available from: https://clinicaltrials.gov/ct2/show/NCT02237079.
- 52.Ravussin E. Tissue Selective Estrogen Complex to Prevent Metabolic Dysfunction in Women (TSEC) 2014 Available from: https://clinicaltrials.gov/ct2/show/NCT02274571.
- 53.Sullivan EL, et al. Selective estrogen receptor modulator promotes weight loss in ovariectomized female rhesus monkeys (Macaca mulatta) by decreasing food intake and increasing activity. Am J Physiol Endocrinol Metab. 2012;302(7):E759–67. doi: 10.1152/ajpendo.00327.2011. [DOI] [PubMed] [Google Scholar]
- 54.Walsh BW, et al. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196–204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 55.Jialal I, Amess W, Kaur M. Management of hypertriglyceridemia in the diabetic patient. Curr Diab Rep. 2010;10(4):316–20. doi: 10.1007/s11892-010-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YA, et al. Severe acute pancreatitis due to tamoxifen-induced hypertriglyceridemia with diabetes mellitus. Chin J Cancer Res. 2014;26(3):341–4. doi: 10.3978/j.issn.1000-9604.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Artac M, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced severe hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14(3):309–11. doi: 10.1179/joc.2002.14.3.309. [DOI] [PubMed] [Google Scholar]
- 58.Hozumi Y, Kawano M, Miyata M. Severe hypertriglyceridemia caused by tamoxifen-treatment after breast cancer surgery. Endocr J. 1997;44(5):745–9. doi: 10.1507/endocrj.44.745. [DOI] [PubMed] [Google Scholar]
- 59.Elisaf MS, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11(8):1067–9. doi: 10.1023/a:1008309613082. [DOI] [PubMed] [Google Scholar]
- 60.Hozumi Y, Kawano M, Jordan VC. In vitro study of the effect of raloxifene on lipid metabolism compared with tamoxifen. Eur J Endocrinol. 2000;143(3):427–30. doi: 10.1530/eje.0.1430427. [DOI] [PubMed] [Google Scholar]
- 61.Tiano J, Mauvais-Jarvis F. Selective estrogen receptor modulation in pancreatic beta-cells and the prevention of type 2 diabetes. Islets. 2012;4(2):173–6. doi: 10.4161/isl.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrett-Connor E, et al. Post hoc analysis of data from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial on the effects of three years of raloxifene treatment on glycemic control and cardiovascular disease risk factors in women with and without type 2 diabetes. Clin Ther. 2003;25(3):919–30. doi: 10.1016/s0149-2918(03)80114-5. [DOI] [PubMed] [Google Scholar]
- 63.Mosca L, et al. Effect of raloxifene on serum triglycerides in postmenopausal women: influence of predisposing factors for hypertriglyceridemia. Clin Ther. 2001;23(9):1552–65. doi: 10.1016/s0149-2918(01)80127-2. [DOI] [PubMed] [Google Scholar]
- 64.Carr MC, et al. Effect of raloxifene on serum triglycerides in women with a history of hypertriglyceridemia while on oral estrogen therapy. Diabetes Care. 2005;28(7):1555–61. doi: 10.2337/diacare.28.7.1555. [DOI] [PubMed] [Google Scholar]
- 65.Yoshii T, et al. The Effects of Bazedoxifene on Bone, Glucose, and Lipid Metabolism in Postmenopausal Women With Type 2 Diabetes: An Exploratory Pilot Study. J Clin Med Res. 2015;7(10):762–9. doi: 10.14740/jocmr2278w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevenson JC, et al. A Pooled Analysis of the Effects of Conjugated Estrogens/Bazedoxifene on Lipid Parameters in Postmenopausal Women From the Selective Estrogens, Menopause, and Response to Therapy (SMART) Trials. J Clin Endocrinol Metab. 2015;100(6):2329–38. doi: 10.1210/jc.2014-2649. [DOI] [PubMed] [Google Scholar]
- 67.Miller V, LaRosa J, Barnabei V. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. Jama. 1995;273(3):199–208. [PubMed] [Google Scholar]
- 68.Hulley S, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 69.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 70.Liu CL, et al. Fatty liver and transaminase changes with adjuvant tamoxifen therapy. Anticancer Drugs. 2006;17(6):709–13. doi: 10.1097/01.cad.0000215056.47695.92. [DOI] [PubMed] [Google Scholar]
- 71.Nishino M, et al. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR Am J Roentgenol. 2003;180(1):129–34. doi: 10.2214/ajr.180.1.1800129. [DOI] [PubMed] [Google Scholar]
- 72.Ogawa Y, et al. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351(9104):725. doi: 10.1016/S0140-6736(05)78493-2. [DOI] [PubMed] [Google Scholar]
- 73.Coskun U, Toruner FB, Gunel N. Tamoxifen therapy and hepatic steatosis. Neoplasma. 2002;49(1):61–4. [PubMed] [Google Scholar]
- 74.Bruno S, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330(7497):932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gudbrandsen OA, Rost TH, Berge RK. Causes and prevention of tamoxifen-induced accumulation of triacylglycerol in rat liver. J Lipid Res. 2006;47(10):2223–32. doi: 10.1194/jlr.M600148-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Cole LK, Jacobs RL, Vance DE. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology. 2010;52(4):1258–65. doi: 10.1002/hep.23813. [DOI] [PubMed] [Google Scholar]
- 77.Lelliott CJ, et al. Transcript and metabolite analysis of the effects of tamoxifen in rat liver reveals inhibition of fatty acid synthesis in the presence of hepatic steatosis. FASEB J. 2005;19(9):1108–19. doi: 10.1096/fj.04-3196com. [DOI] [PubMed] [Google Scholar]
- 78.Mu Y, et al. Effects of estrogen and androgen deprivation on the progression of non-alcoholic steatohepatitis (NASH) in male Sprague-Dawley rats. Hepatol Res. 2009;39(9):910–20. doi: 10.1111/j.1872-034X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 79.Miyashita T, et al. Hepatoprotective effect of tamoxifen on steatosis and nonalcoholic steatohepatitis in mouse models. J Toxicol Sci. 2012;37(5):931–42. doi: 10.2131/jts.37.931. [DOI] [PubMed] [Google Scholar]
- 80.de Conti A, et al. Genotoxic, epigenetic, and transcriptomic effects of tamoxifen in mouse liver. Toxicology. 2014;325:12–20. doi: 10.1016/j.tox.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Yoshikawa Y, et al. Mechanisms of the hepatoprotective effects of tamoxifen against drug-induced and chemical-induced acute liver injuries. Toxicol Appl Pharmacol. 2012;264(1):42–50. doi: 10.1016/j.taap.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Villa A, et al. Tetradian oscillation of estrogen receptor alpha is necessary to prevent liver lipid deposition. Proc Natl Acad Sci U S A. 2012;109(29):11806–11. doi: 10.1073/pnas.1205797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Purushotham A, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–38. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aoyama T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273(10):5678–84. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 86.Velasco G, Geelen MJ, Guzman M. Control of hepatic fatty acid oxidation by 5′-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch Biochem Biophys. 1997;337(2):169–75. doi: 10.1006/abbi.1996.9784. [DOI] [PubMed] [Google Scholar]